Published online Dec 20, 2025. doi: 10.5493/wjem.v15.i4.110917
Revised: July 25, 2025
Accepted: November 17, 2025
Published online: December 20, 2025
Processing time: 184 Days and 2.9 Hours
Oral squamous cell carcinoma (OSCC) poses a major health burden, with frequent late-stage diagnoses and limited prognostic tools. Palmitoyl-protein thioesterase (PPT)1, a lysosomal enzyme, has been implicated in tumor biology. This study investigates PPT1 expression in OSCC and its association with clinicopathological features.
To evaluate the immunohistochemical expression of PPT1 in OSCC and assess its correlation with patient age, gender, tumor grade, and TNM staging using H-score quantification.
Immunohistochemistry for PPT1 was performed on 43 histopathologically con
PPT1 expression showed a significant positive correlation with tumor grade (ρ = 0.48, P = 0.0015), while a weak, nonsignificant negative correlation was noted with patient age (ρ = -0.27, P = 0.083). No significant differences were found by gender or tumor stage. Given the small sample size, these findings should be interpreted as preliminary.
PPT1 expression is associated with histological tumor grade in OSCC and may reflect tumor aggressiveness. These initial results highlight the potential of PPT1 as a prognostic biomarker, warranting further validation in larger, multicenter studies.
Core Tip: This study explored the expression of palmitoyl-protein thioesterase (PPT)1 in oral squamous cell carcinoma (OSCC) using immunohistochemistry and H-score quantification. A strong positive correlation was observed between PPT1 expression and tumor grade, while a negative correlation was seen with age. These findings suggest that PPT1 serves as a novel prognostic and therapeutic biomarker, especially in younger patients and advanced OSCC cases. The study highlights the potential of integrating molecular biomarkers like PPT1 with conventional histopathological evaluation to guide personalized treatment strategies in OSCC.
- Citation: Kumar KCP, Sahoo S, Adhya AK, Gaikwad MR, Ganapathy A, Ravi PK. Evaluating palmitoyl-protein thioesterase 1 in oral squamous cell carcinoma: A novel indicator of tumor behavior and therapeutic response. World J Exp Med 2025; 15(4): 110917
- URL: https://www.wjgnet.com/2220-315x/full/v15/i4/110917.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i4.110917
Oral squamous cell carcinoma (OSCC) is a significant public health concern, accounting for > 90% of oral cancers globally. According to recent epidemiological data, OSCC ranks among the top 10 most common cancers worldwide, with particularly high incidence rates in South Asia due to prevalent risk factors such as tobacco chewing, smoking, and alcohol use[1]. In India, OSCC contributes significantly to the overall cancer burden, with the Indian Council of Medical Research (ICMR) reporting a steadily rising trend in incidence and mortality.
Despite advances in surgical techniques, chemoradiotherapy, and adjuvant therapies, the 5-year survival rate for OSCC remains low, primarily due to late-stage presentation and tumor recurrence[2]. While surgical resection remains the cornerstone of treatment and enables accurate pathological staging, predicting patient outcomes and tailoring treatment remain challenging. Traditional staging methods like the TNM classification lack integration of tumor biology, which limits their prognostic accuracy.
This limitation has led to a growing emphasis on the role of molecular and immunohistochemical biomarkers in OSCC prognosis and therapy. Markers such as p16, programmed death protein ligand 1, epidermal growth factor receptor, Ki-67, and cyclin D1, have shown potential in stratifying risk and guiding treatment[3]. However, their clinical adoption remains limited due to variability in expression patterns and technical standardization challenges.
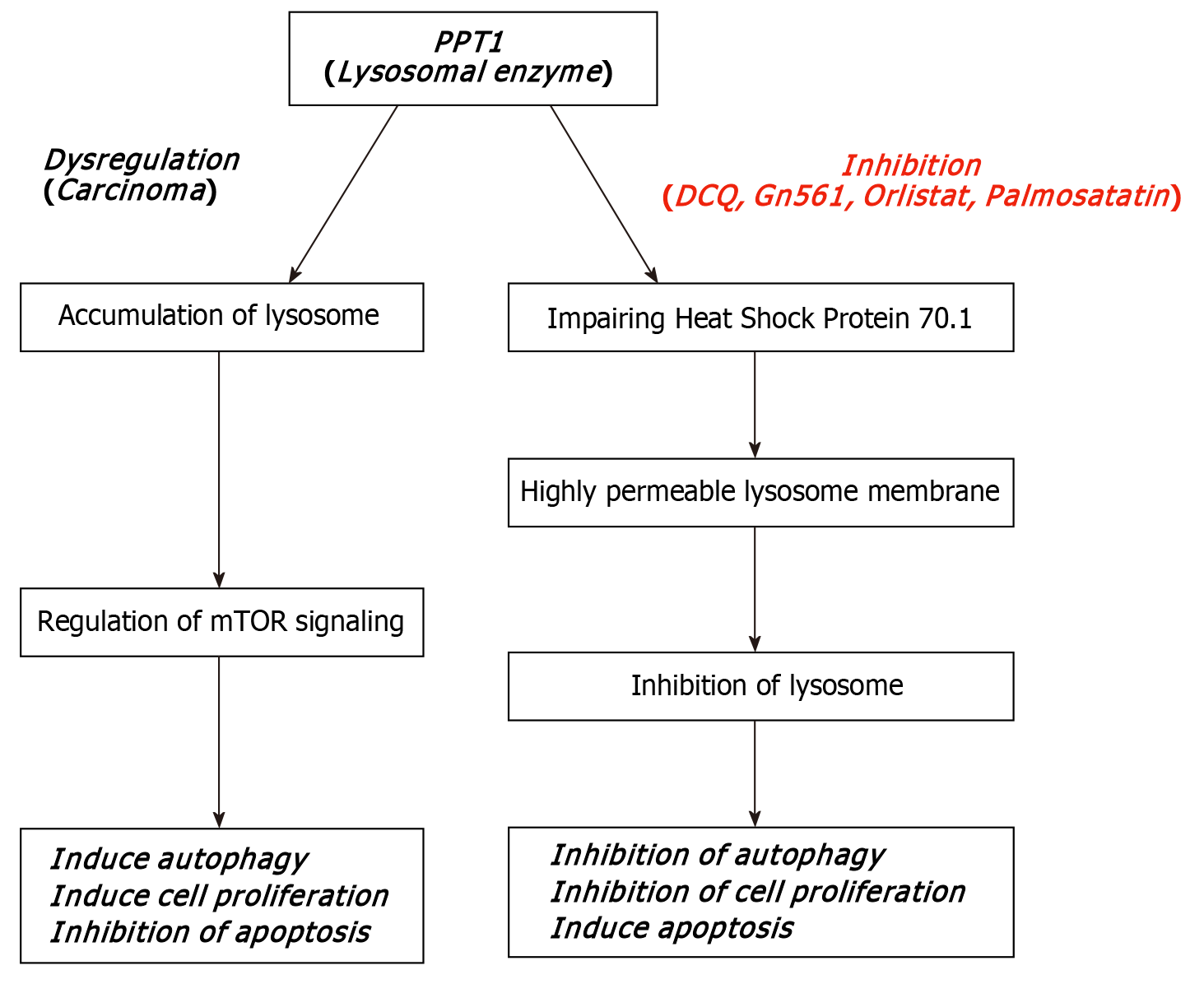
Recent studies have drawn attention to palmitoyl-protein thioesterase (PPT)1, a lysosomal depalmitoylating enzyme implicated in mTOR signaling, autophagy regulation, and cancer progression (Figure 1)[4]. Overexpression of PPT1 has been associated with poor prognosis in multiple malignancies. In vitro studies using OSCC cell lines have suggested that PPT1 inhibition can reduce cell viability and induce apoptosis, making it a promising therapeutic target[5]. However, the prognostic role of PPT1 in tissue samples of OSCC patients has not yet been fully explored.
PPT1 was selected due to its dual role in lysosomal regulation and autophagy; both of which are central to tumor sur
This study aimed to bridge this translational gap by evaluating PPT1 expression in histologically confirmed OSCC tissue samples and examining its correlation with key clinicopathological parameters such as tumor grade, stage, age, and gender. By quantifying PPT1 expression using the H-score method, this research seeks to determine whether PPT1 could serve as a reliable prognostic biomarker in OSCC.
This observational cross-sectional study was conducted at a tertiary care teaching hospital in eastern India. Institutional ethical clearance was obtained from the Institute Ethics Committee of All India Institute of Medical Sciences Bhuba
The study included patients > 18 years of age who underwent surgical resection for histopathologically confirmed OSCC. Patients with a history of neoadjuvant chemotherapy or radiotherapy were excluded. A total of 43 patients were enrolled, based on a sample size calculation using the estimated incidence of OSCC (21%) in India from ICMR 2021 data, assuming a 95% confidence interval and 15% precision.
Surgically excised specimens were immersion-fixed in 10% neutral-buffered formalin and processed using an automated tissue processor. Paraffin-embedded blocks were sectioned at 4 μm. One section from each block was stained with hematoxylin and eosin for histological evaluation, and another was subjected to immunohistochemistry for PPT1.
Antigen retrieval was performed using Tris–EDTA buffer at pH 9.0 in a microwave oven at 100 °C for 10 min. Sections were incubated with primary anti-PPT1 antibody (1:100 dilution; Biotech Desk Pvt. Ltd., Cat No: Bs-6619R). Detection was done using the PolyExcel HRP/DAB detection system (PathnSitu Biotechnologies). Normal human lung tissue was used as a positive control.
PPT1 expression was evaluated semi-quantitatively using the H-score method, defined as:
H-score = ∑ (Pi × i) Where:
i = intensity score (0 = no staining, 1 = mild, 2 = moderate, 3 = strong).
Pi = percentage of tumor cells showing that intensity.
This yields a total score from 0 to 300.
To minimize observer bias, all slides were scored independently by two blinded observers, unaware of each other's assessments and clinical data. Interobserver reliability was calculated using the intraclass correlation coefficient (ICC), which showed excellent agreement (ICC = 0.89).
To facilitate categorical analysis, H-scores were grouped into the following categories: Category 0 (no expression): H-score = 0; Category 1 (low expression): H-score 1-100; Category 2 (moderate expression): H-score 101-200; Category 3 (high expression): H-score > 200.
Data were compiled in Microsoft Excel and analyzed using SPSS version 29. Continuous variables were expressed as mean ± SD. Spearman’s rank correlation was used to assess associations between H-score and clinicopathological variables such as age, grade, and stage. The Kruskal–Wallis test was used for between-group comparisons of tumor grade. P < 0.05 was considered statistically significant.
All cases with incomplete demographic or pathological data were excluded prior to analysis. No imputation was performed.
A total of 43 OSCC specimens were included in the study, comprising 38 males and five females. The demographic details are presented in Table 1.
| Clinicopathological features | Number of patients |
| Age | |
| < 40 yr | 11 |
| 40–60 yr | 23 |
| > 60 yr | 9 |
| Gender | |
| Male | 5 |
| Female | 38 |
| Staging | |
| 1 | 7 |
| 2 | 3 |
| 3 | 7 |
| 4 | 13 |
| Grading | |
| 1 | 20 |
| 2 | 9 |
| 3 | 14 |
| Intensity | |
| 0 | 12 |
| 1 | 17 |
| 2 | 10 |
| 3 | 4 |
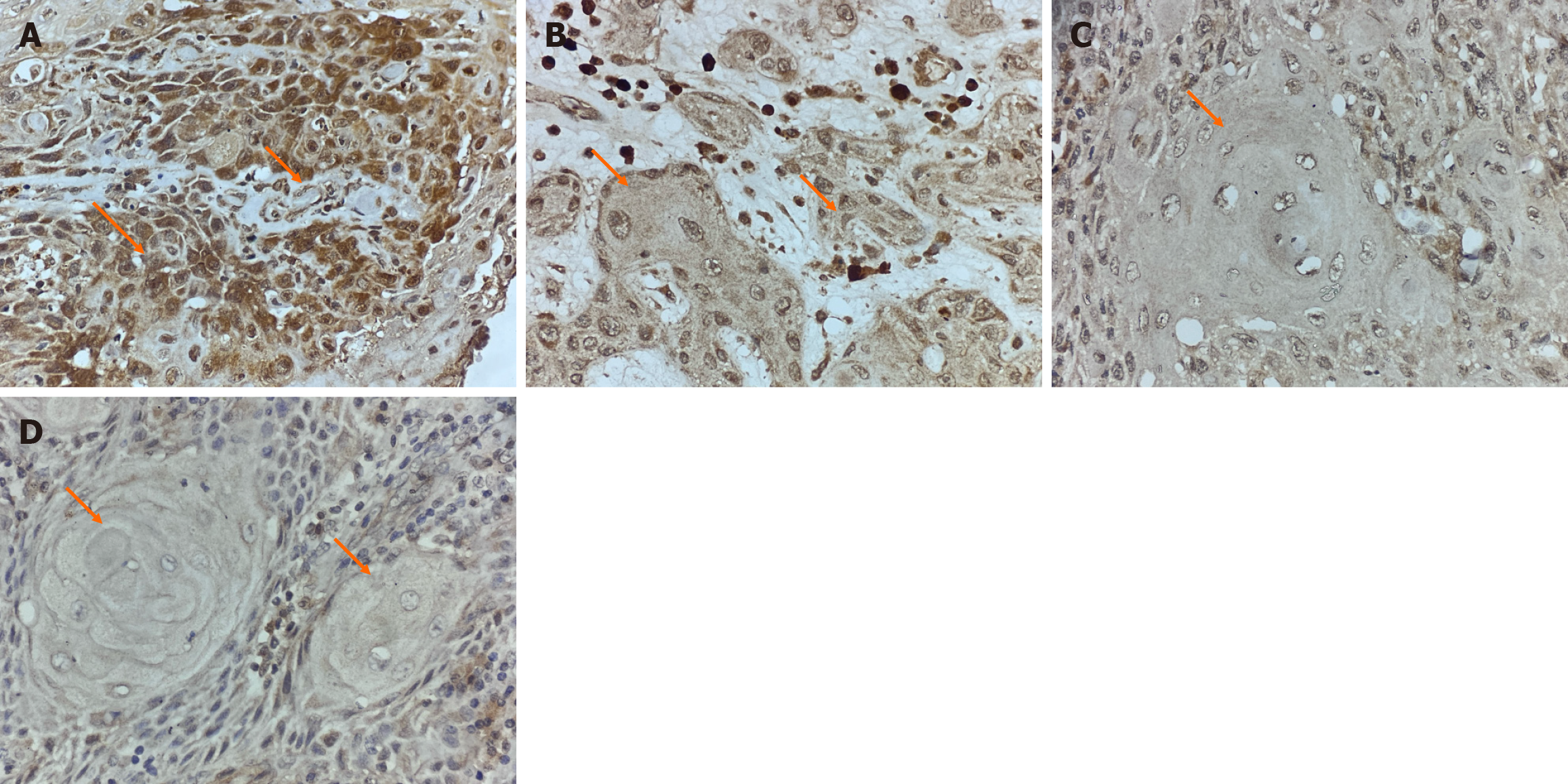
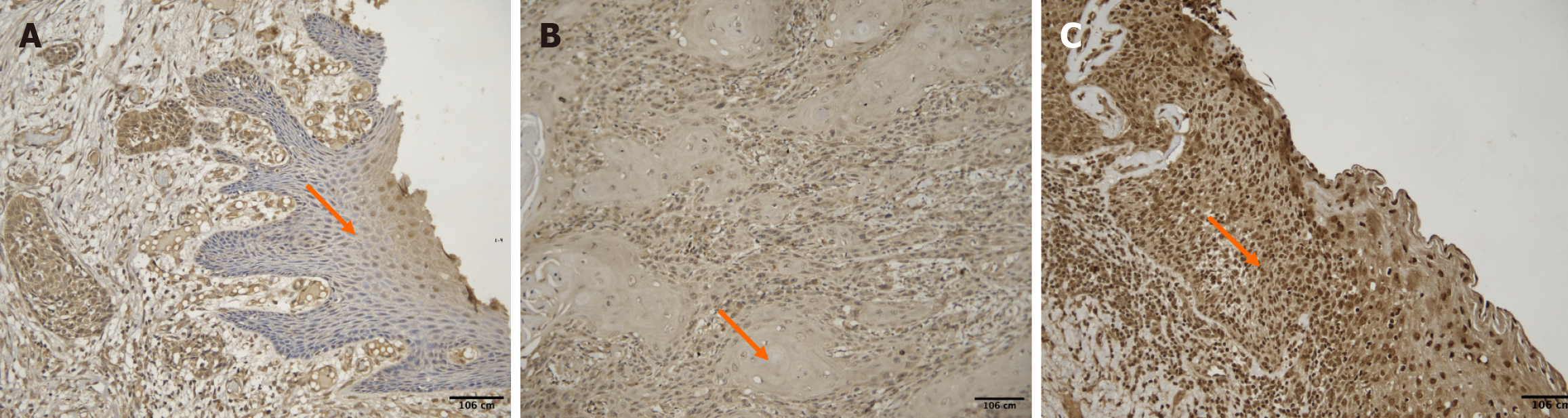
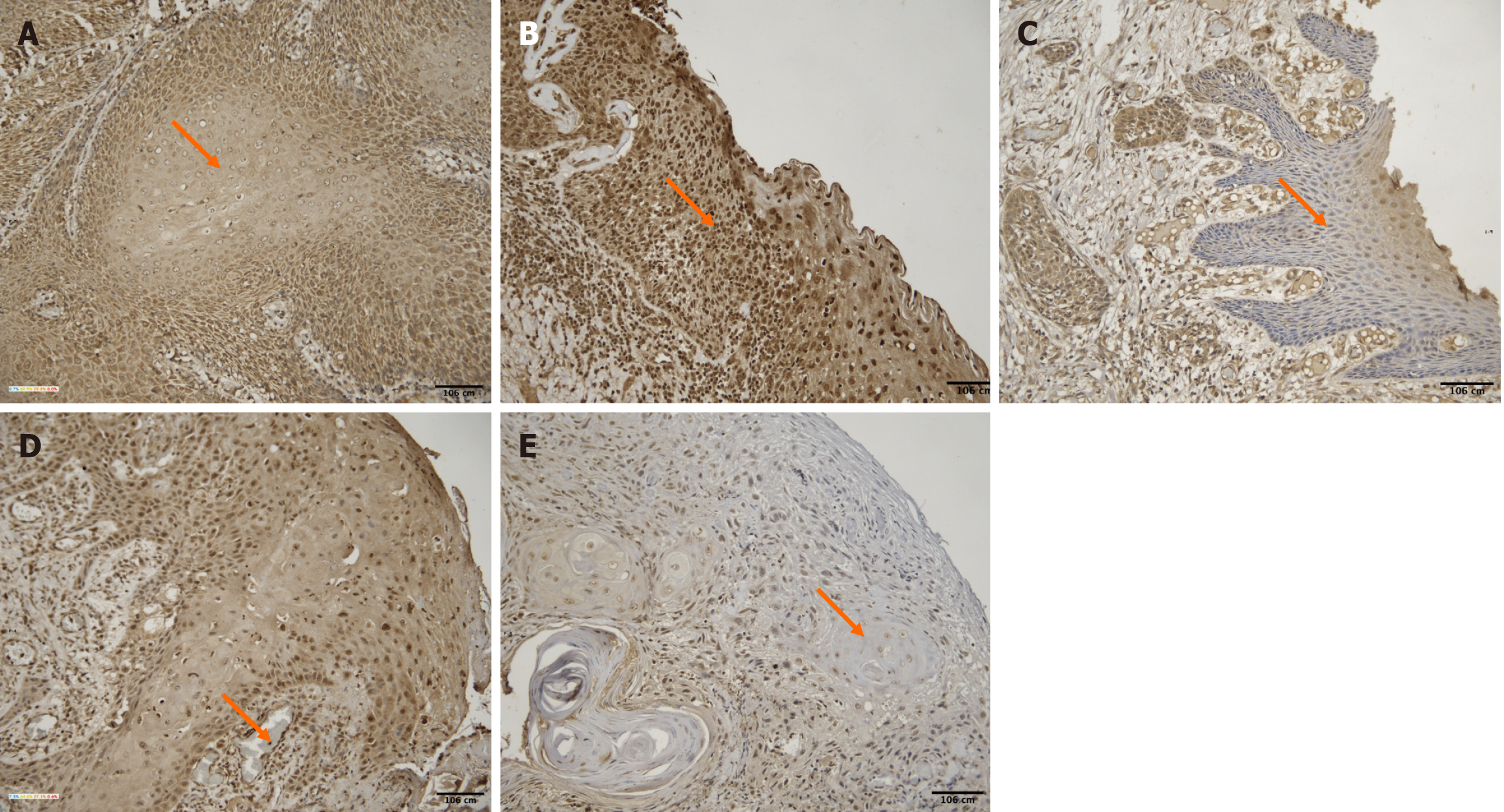
Based on the H-score method, PPT1 expression was classified as: High expression (H-score > 200): 4 cases; moderate expression (H-score 101-200): 10 cases; low expression (H-score 1-100): 17 cases; no expression (H-score = 0): 12 cases. As shown in Figure 2, representative images of all expression categories are presented.
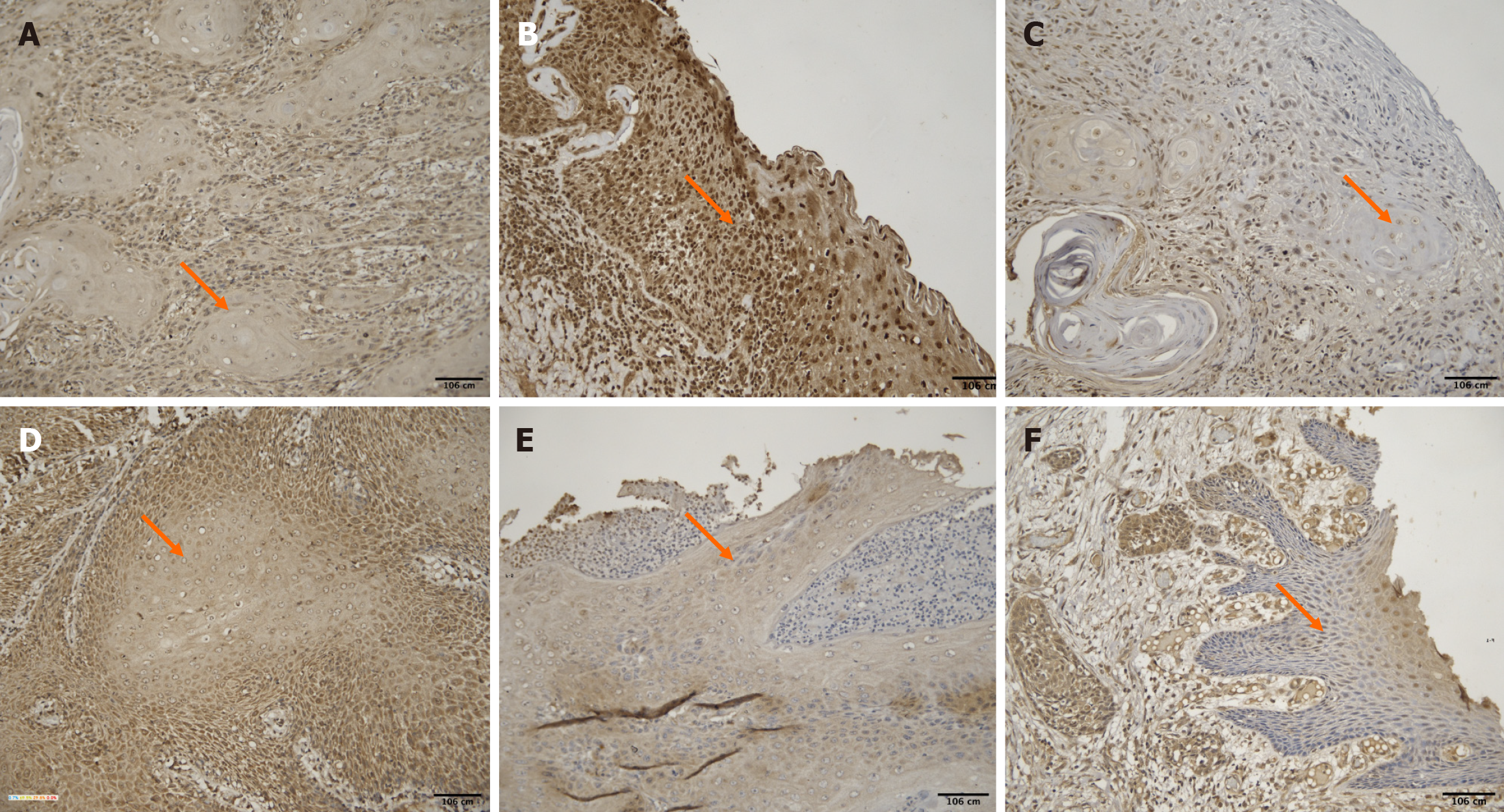
The age of the patients ranged from 28 to 78 years (mean: 48.3 ± 12.8 years). A weak negative correlation was observed between age and H-score [ρ = -0.27, 95%CI (-0.52 to 0.03), P = 0.083]. Younger patients exhibited higher PPT1 staining intensity (Figure 3).
No significant difference was found in H-scores between males (mean ± SD: 1.13 ± 0.96) and females (1.20 ± 0.83) with a P value of 0.872 (unpaired t test). PPT1 expression did not vary notably between sexes (Figure 4). However, the interpretation of gender-based differences is limited due to the small number of female participants (n = 5), and findings should be considered preliminary.
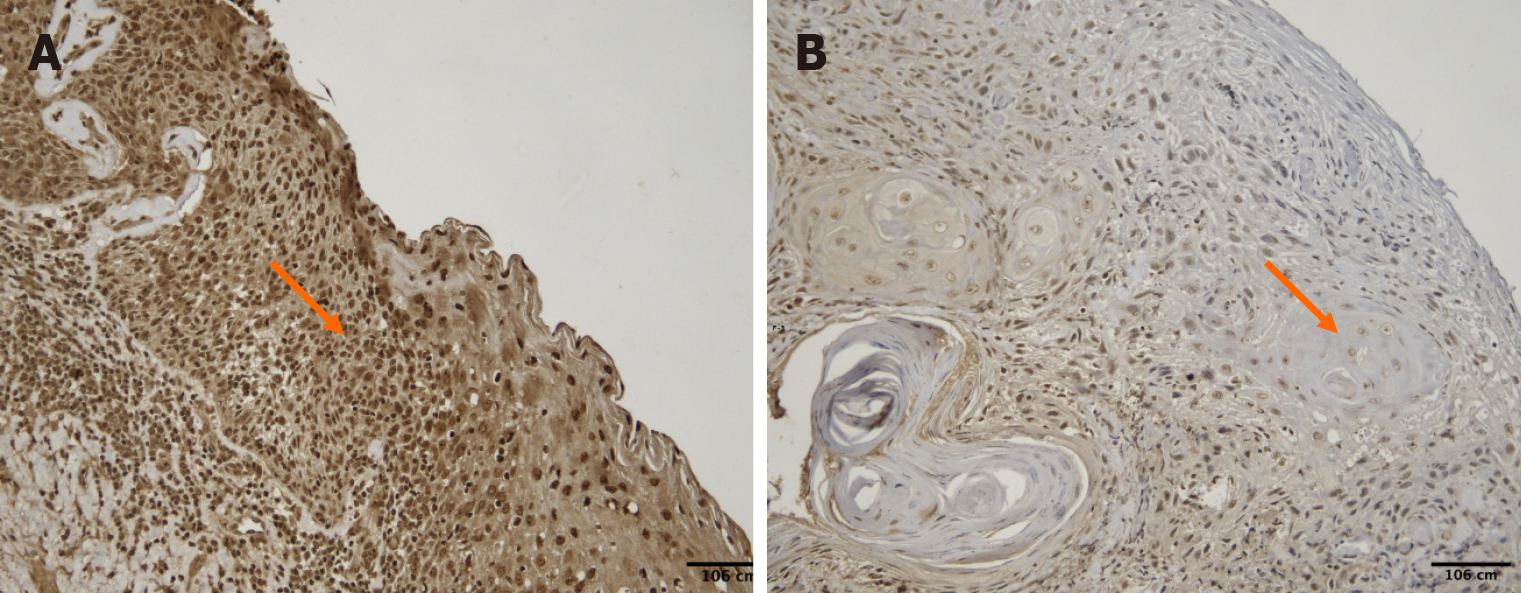
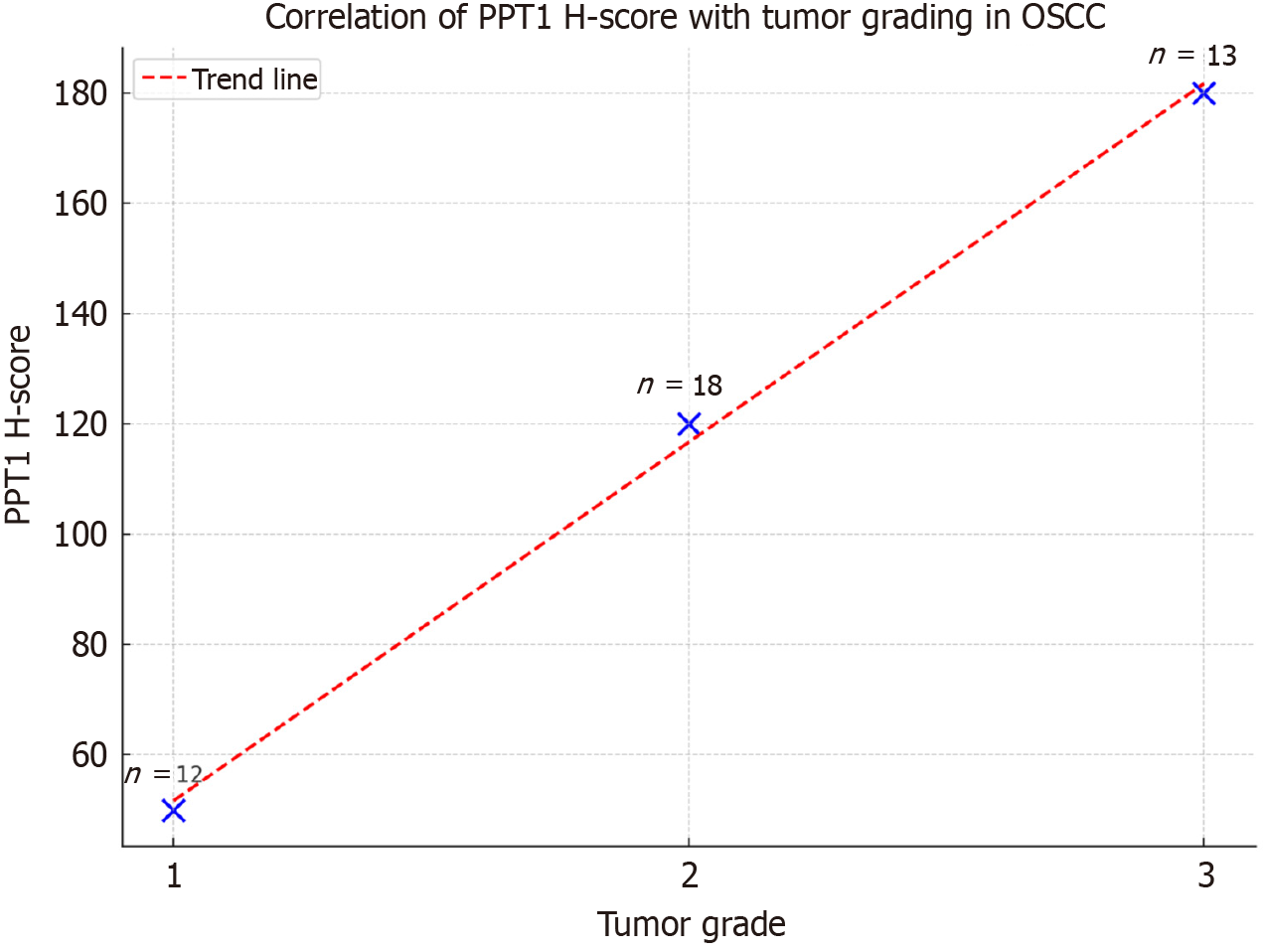
A significant positive correlation was identified between tumor grade and PPT1 expression [ρ = 0.48, 95%CI (0.21-0.68), P = 0.0015]. The Kruskal–Wallis test also revealed significant variation in H-score across tumor grades (P = 0.012). Higher tumor grades exhibited greater PPT1 expression (Figures 5 and 6).
Tumor stages ranged from I to IV (mean stage = 2.09 ± 1.6). A weak and nonsignificant positive correlation was found between stage and H-score [ρ = +0.124, 95%CI (-0.18 to 0.40), P = 0.506]. Staining intensity did not consistently correlate with stage progression (Figure 7).
This study evaluated the immunohistochemical expression of PPT1 in 43 OSCC tissue samples using H-score quantification. A significant positive correlation was found between PPT1 expression and tumor grade, while a weak negative correlation with age was observed. No significant associations were noted with gender or tumor stage.
Age-related findings in this study showed a trend toward higher PPT1 expression in younger patients. Although not statistically significant, this is consistent with earlier studies on p53 and TP53 mutations in young OSCC patients[6-8]. These studies demonstrated greater biomarker activity in younger cohorts and associated this with more aggressive tumor phenotypes[9,10].
The significant correlation between PPT1 expression and tumor grade aligns with its known role in lysosomal regulation and tumor survival mechanisms[4,5]. Poorly differentiated tumors tend to rely more heavily on autophagy and mammalian target of rapamycin (mTOR) signaling, both of which are regulated by lysosomal enzymes like PPT1[11,12]. The influence of PPT1 on protein depalmitoylation may alter signaling and metabolic flexibility, supporting proliferation in hostile tumor microenvironments.
PPT1 contributes to cellular homeostasis through the depalmitoylation of lipid-modified proteins, facilitating their degradation in lysosomes. Overexpression of PPT1 has been shown to promote cancer cell survival by modulating autophagy, lysosomal activity, and mTOR signaling[12,13]. In OSCC, these pathways are often upregulated in high-grade tumors, suggesting that PPT1 functions as a survival enhancer rather than a mere bystander.
However, whether PPT1 acts as a driver of tumor progression or reflects downstream metabolic adaptation remains unclear. Functional studies in OSCC models are required to elucidate its precise biological role. Recent research suggests PPT1 promotes cancer cell survival not only via autophagy regulation but also through lipid raft trafficking and modulation of intracellular redox balance. These functions collectively support aggressive tumor behavior, particularly in metabolically demanding microenvironments such as those found in poorly differentiated OSCC.
Given the correlation between PPT1 and tumor grade, PPT1 could serve as a potential prognostic biomarker for OSCC. Its detection through immunohistochemistry is feasible in most pathology labs, supporting its potential for clinical use. Small-molecule PPT1 inhibitors like DC661 and GNS561 have shown promising antitumor effects in preclinical settings[13]. If validated, such inhibitors might augment treatment for high-grade or therapy-resistant OSCCs.
That said, caution is warranted. Translational claims must be grounded in robust preclinical and clinical evidence. At present, PPT1 should be considered a candidate biomarker with prognostic value, pending further validation.
Several limitations should be acknowledged. First, the sample size was small (n = 43), which may have reduced the statistical power and generalizability of findings. Second, survival or outcome data were not included, preventing correlation between PPT1 expression and patient prognosis. Additionally, the gender imbalance in the sample (88.4% male) may have obscured potential sex-based expression differences. Future studies with larger, more diverse cohorts and longitudinal follow-up are necessary to validate these findings. Future research should consider multicenter recruitment to enhance generalizability and incorporate longitudinal survival and recurrence data to assess the true prognostic value of PPT1.
This study demonstrates that increased PPT1 expression, quantified by H-score, is significantly associated with higher tumor grade and shows a trend toward increased expression in younger OSCC patients. These findings suggest that PPT1 serves as a potential prognostic biomarker, particularly in aggressive tumor phenotypes. Given its role in lysosomal regulation and emerging therapeutic implications, PPT1 may contribute to risk stratification and targeted treatment approaches in OSCC. However, these results are preliminary, and larger, multicentric studies with prospective validation and survival data are essential to establish its clinical utility and translational relevance.
| 1. | Pekarek L, Garrido-Gil MJ, Sánchez-Cendra A, Cassinello J, Pekarek T, Fraile-Martinez O, García-Montero C, Lopez-Gonzalez L, Rios-Parra A, Álvarez-Mon M, Acero J, Diaz-Pedrero R, Ortega MA. Emerging histological and serological biomarkers in oral squamous cell carcinoma: Applications in diagnosis, prognosis evaluation and personalized therapeutics (Review). Oncol Rep. 2023;50:213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 54] [Reference Citation Analysis (0)] |
| 2. | Montero PH, Patel SG. Cancer of the oral cavity. Surg Oncol Clin N Am. 2015;24:491-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 418] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 3. | Pillai J, Chincholkar T, Dixit R, Pandey M. A systematic review of proteomic biomarkers in oral squamous cell cancer. World J Surg Oncol. 2021;19:315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 4. | Luo Q, Li X, Gan G, Yang M, Chen X, Chen F. PPT1 Reduction Contributes to Erianin-Induced Growth Inhibition in Oral Squamous Carcinoma Cells. Front Cell Dev Biol. 2021;9:764263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 5. | Puhl AC, Raman R, Havener TM, Minerali E, Hickey AJ, Ekins S. Identification of New Modulators and Inhibitors of Palmitoyl-Protein Thioesterase 1 for CLN1 Batten Disease and Cancer. ACS Omega. 2024;9:11870-11882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Adduri R Sr, Kotapalli V, Gupta NA, Gowrishankar S, Srinivasulu M, Ali MM, Rao S, Uppin SG, Nayak UK, Dhagam S, Chigurupati MV, Bashyam MD. P53 nuclear stabilization is associated with FHIT loss and younger age of onset in squamous cell carcinoma of oral tongue. BMC Clin Pathol. 2014;14:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Atula S, Grénman R, Laippala P, Syrjänen S. Cancer of the tongue in patients younger than 40 years. A distinct entity? Arch Otolaryngol Head Neck Surg. 1996;122:1313-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Siegelmann-Danieli N, Ben-Izhack O, Hanlon A, Ridge JA, Stein ME, Khandelwal V, Langer CJ. P53 alteration in oral tongue cancer is not significantly associated with age at diagnosis or tobacco exposure. Tumori. 2005;91:346-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Garavello W, Spreafico R, Gaini RM. Oral tongue cancer in young patients: a matched analysis. Oral Oncol. 2007;43:894-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Szewczyk M, Pazdrowski J, Golusiński P, Więckowska B, Golusiński W. Oral cancer in young adults: should we approach these patients differently? Front Oncol. 2024;14:1297752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 11. | Yuan C, Xiong Z, Shi J, Peng J, Meng X, Wang C, Hu W, Ru Z, Xie K, Yang H, Chen K, Zhang X. Overexpression of PPT2 Represses the Clear Cell Renal Cell Carcinoma Progression by Reducing Epithelial-to-mesenchymal Transition. J Cancer. 2020;11:1151-1161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Gahlot P, Kravic B, Rota G, van den Boom J, Levantovsky S, Schulze N, Maspero E, Polo S, Behrends C, Meyer H. Lysosomal damage sensing and lysophagy initiation by SPG20-ITCH. Mol Cell. 2024;84:1556-1569.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 13. | Brun S, Bestion E, Raymond E, Bassissi F, Jilkova ZM, Mezouar S, Rachid M, Novello M, Tracz J, Hamaï A, Lalmanach G, Vanderlynden L, Legouffe R, Stauber J, Schubert T, Plach MG, Courcambeck J, Drouot C, Jacquemot G, Serdjebi C, Roth G, Baudoin JP, Ansaldi C, Decaens T, Halfon P. GNS561, a clinical-stage PPT1 inhibitor, is efficient against hepatocellular carcinoma via modulation of lysosomal functions. Autophagy. 2022;18:678-694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/