Published online Jun 20, 2025. doi: 10.5493/wjem.v15.i2.99065
Revised: November 17, 2024
Accepted: December 12, 2024
Published online: June 20, 2025
Processing time: 277 Days and 20.4 Hours
Incisional hernias are a common complication of previous surgeries and remain a persistent issue in clinical practice, posing a significant burden on healthcare systems despite advances in education and technology. Surgical techniques, primarily involving the use of mesh to cover the abdominal wall gap, are widely used as a standard intervention strategy.
To examine the regeneration of the aponeurosis defect in the anterior abdominal wall in rats using regenerative mimetic factors of the extracellular matrix [ReGeneraTing Agent (RGTA)], adipose tissue micrografts (ATM), and platelet rich plasma (PRP) as regenerative agents.
Regenerative agents such as RGTA, ATM, and PRP are gaining popularity. ATM involves autologous adipose tissue cells with mesenchymal stem cell markers and a high percentage of stromal vascular fraction cells. RGTAs are heparan sulfate (HS) mimetics that replace degraded HSs in damaged tissue, enhancing the quality and speed of repair. PRP is a concentrated plasma preparation containing seven fundamental proteins responsible for tissue production. An acellular dermal matrix is a biological implant free of cellular or antigenic components, making it an excellent material for reconstructive surgery. Polyglactin is a synthetic, absorbable mesh that loses 50% of its strength after fourteen days, providing initial support for new tissue regeneration before being completely absorbed.
Rats will undergo a laparotomy with a precise 2 cm by 2 cm excision of the anterior abdominal wall fascia below the umbilicus. They will be divided into sixteen groups, each receiving different combinations of regenerative factor injections into the denervated area in both non-contaminated and contaminated environments. A collagen-elastin matrix will be used to join the aponeurosis edges, with an absorbable polyglactin mesh anchored over it. Samples will be taken for macroscopic, histological, and immunohistochemical evaluation of tissue regeneration.
Our study aims to demonstrate how these factors promote cell proliferation and healing of the denervated anterior abdominal wall, potentially reducing the frequency and complications of incisional hernias. This approach could offer a more economical and efficient treatment option compared to current costly methods.
Core Tip: This study protocol explores innovative regenerative strategies for abdominal wall defect repair using a combination of platelet rich plasma, adipose tissue micrografts, and regenerative glycosaminoglycan mimetics (ReGe
- Citation: Zapsalis K, Ioannidis O, Xylas C, Siozos K, Gemousakakis G, Anestiadou E, Symeonidis S, Bitsianis S, Kotidis E, Cheva A, Bekiari C, Loukousia A, Angelopoulos K, Pramateftakis MG, Mantzoros I, Tserkezidis F, Driagka B, Angelopoulos S. Platelet rich plasma, adipose tissue micrografts, and regenerative mimetic factors for abdominal wall defect reconstruction: Experimental study protocol. World J Exp Med 2025; 15(2): 99065
- URL: https://www.wjgnet.com/2220-315x/full/v15/i2/99065.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i2.99065
Acquired abdominal wall defects can arise due to surgical interventions, injuries, or infections that compromise the integrity of the abdominal wall. These defects can include hernias, in which a portion of the intestine protrudes through a weak spot in the abdominal muscles. The prevalence of incisional hernias, particularly following midline abdominal incisions, is notably high at 9.9% after laparotomy, and these defects increase morbidity rates to a range of 2% to 20%[1]. Each year, over 20 million hernia repair surgeries are conducted worldwide, with costs projected to reach 6.3 billion United States dollars by 2027[2]. Incisional hernias can severely impact a patient's quality of life and may lead to serious complications such as strangulation, obstruction, or gangrene[3]. Treating incisional hernias typically involves surgically repairing the abdominal wall defects, a procedure that has increasingly utilized mesh placement in recent years. Unlike temporary suturing, using meshes is advantageous because it reduces tension on the abdominal walls[4].
Despite the fact that meshes yield better results compared to primary suturing, they still present certain issues. The use of synthetic mesh materials has been shown to increase the risk of surgical infections and dense adhesions[5]. In surgical practice, managing and treating infections is crucial. When foreign materials like mesh become contaminated, they typically need to be removed, resulting in a significantly larger gap than the pre-existing hernia. This often necessitates further interventions, increasing the morbidity of the initial repair. Synthetic meshes are particularly associated with these complications. Moreover, heavy-weight synthetic mesh can cause chronic pain due to intense foreign body responses and fibrosis[6]. The disadvantages of intraperitoneal meshes include enterocutaneous fistulas, mesh erosion, and mesh infection[5].
Biological prostheses, such as biological meshes or bioprosthetic materials, can be used in hernia repairs, particularly for complex or contaminated abdominal hernia repairs. They may cause less inflammation and fibrosis compared to synthetic meshes, making them suitable for infected or potentially infected fields[7]. These biological prostheses are typically derived from human (allogenic) or animal tissues (xenogenic), such as porcine or bovine, and are processed to remove cellular components, leaving behind a collagen scaffold[8]. However, their main disadvantage is their high cost and a reduced inflammatory response, which leads to decreased collagen production and, consequently, reduced tissue regeneration.
Major complications associated with any mesh placement include hernia recurrence, complications of subsequent surgery, adhesive bowel obstruction, mesh contraction, mesh infection, and catastrophic enterocutaneous fistula, as well as protracted medicolegal proceedings[3]. The challenge of managing fascia deficits remains, as a universally accepted method has not yet been found. Despite the development of multiple techniques for postoperative hernia repair and various types of meshes, none have achieved the desired results, with recurrence rates over a decade reaching 32%[9].
Adipose tissue micrografts (ATM), platelet rich plasma (PRP), and Matrix ReGeneraTing Agent (RGTA) are factors known to promote tissue proliferation and repair. In preventing incisional hernias, these factors represent a novel strategy focused on the regenerative process of the abdominal wall, rather than merely covering the gap with a mesh, which is the current conservative approach.
A common goal is to reduce the incidence of postoperative hernias and their associated complications by combining an absorbable matrix as a scaffold and an absorbable mesh with a regenerating factor. These materials were selected because two of them can be isolated from the subject immediately during the surgical intervention, while the third is a commercially available product that is readily accessible. By using an absorbable matrix and mesh, no foreign body remains in the patient.
To our knowledge, current literature does not include a study that investigates the role of PRP, RGTA, and ATM in addressing abdominal wall deficits. The aim of this study is to assess whether the combination of PRP, ATM, and RGTA with an acellular collagen-elastin matrix and absorbable polyglactin mesh can aid in the repair of abdominal wall deficits.
The animals will be housed in groups under fully controlled conditions in individually ventilated cages at the Accredited Animal Facility of the Laboratory of Anatomy, Histology and Embryology of AUTH's Veterinary School (No. EL54BIO23). All procedures will comply with the revised European and National Regulations (European Community Council directive 86/609/EEC; Presidential Degree 56/2013). The experiment will receive approval from the Federal Authorities (Veterinary Directorate of Thessaloniki; AUTH's Ethics Committee). The study design will adhere to the ARRIVE guidelines[10], including all recommended steps.
For the purposes of this research, an experimental control trial will be conducted using Wistar rats aged 4-6 months and weighing 250-400 g. The rats will be randomly divided into 16 experimental groups to ensure that each rat has an equal chance of being placed in any group. Randomization will be conducted using pre-generated random number sequences. Concealment of allocation will be achieved using sealed envelopes or central allocation procedures, ensuring blindness during the treatment assignment phase.
Prior to intervention, researchers will compare key baseline characteristics between groups (age, weight, and health status). If there are any significant differences, these will be reported and considered in data analysis, as they could impact the results.
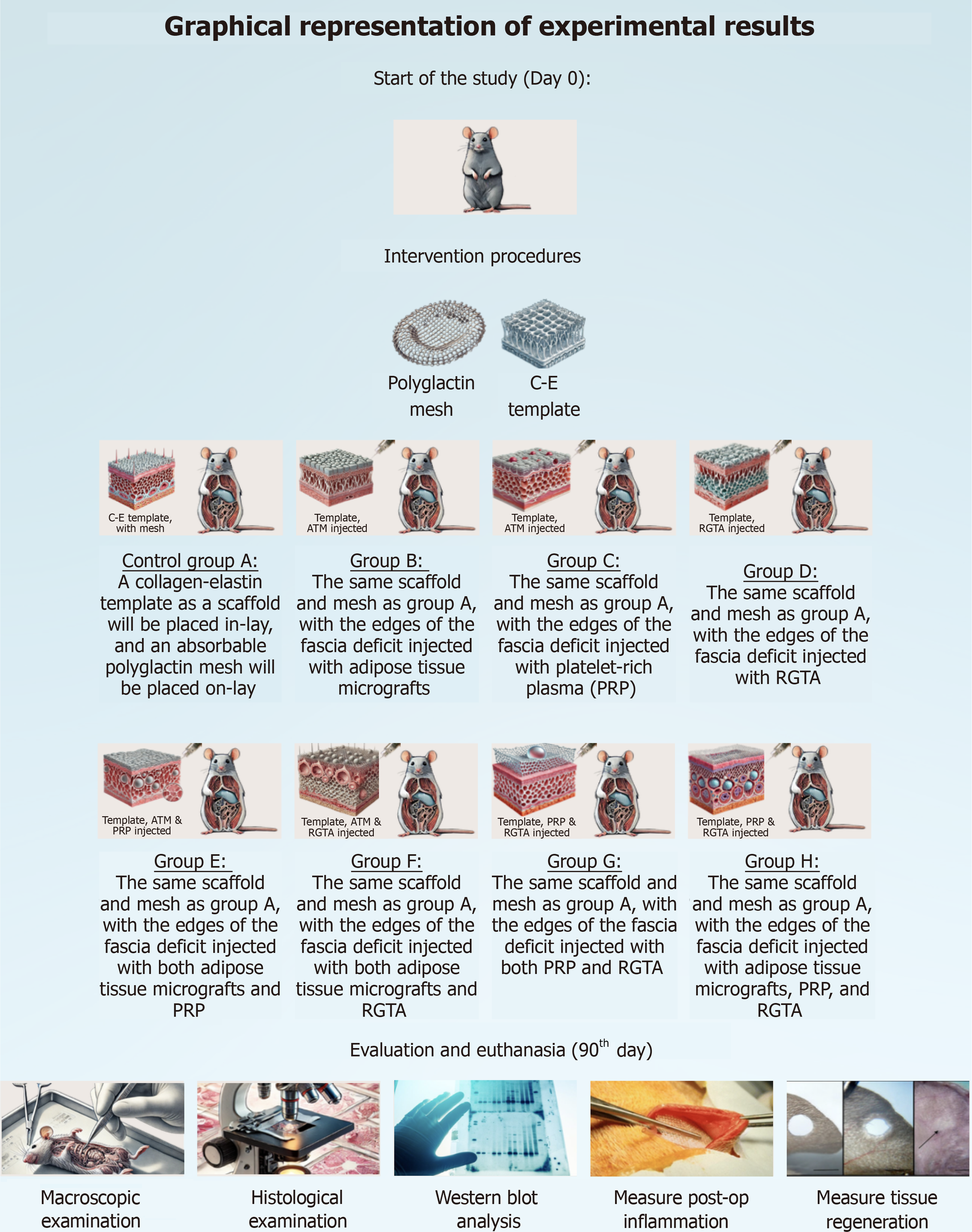
After randomization, the following groups of mice will consist of: (1) Control Group A: A collagen-elastin template as a scaffold will be placed inlay, and an absorbable polyglactin mesh will be placed onlay; (2) Group B: The same scaffold and mesh as in Group A, with the edges of the fascia deficit injected with ATM; (3) Group C: The same scaffold and mesh as in Group A, with the edges of the fascia deficit injected with PRP; (4) Group D: The same scaffold and mesh as in Group A, with the edges of the fascia deficit injected with RGTA; (5) Group E: The same scaffold and mesh as in Group A, with the edges of the fascia deficit injected with both ATM and PRP; (6) Group F: The same scaffold and mesh as in Group A, with the edges of the fascia deficit injected with both ATM and RGTA; (7) Group G: The same scaffold and mesh as in Group A, with the edges of the fascia deficit injected with both PRP and RGTA; (8) Group H: The same scaffold and mesh as in Group A, with the edges of the fascia deficit injected with ATM, PRP, and RGTA; (9) Control Group A2: A collagen-elastin template as a scaffold will be placed inlay, and an absorbable polyglactin mesh will be placed onlay, in a contaminated environment; (10) Group B2: The same scaffold and mesh as in Group A, with the edges of the fascia deficit injected with ATM, in a contaminated environment; (11) Group C2: The same scaffold and mesh as in Group A, with the edges of the fascia deficit injected with PRP, in a contaminated environment; (12) Group D2: The same scaffold and mesh as in Group A, with the edges of the fascia deficit injected with RGTA, in a contaminated environment; (13) Group E2: The same scaffold and mesh as in Group A, with the edges of the fascia deficit injected with both ATM and PRP, in a contaminated environment; (14) Group F2: The same scaffold and mesh as in Group A, with the edges of the fascia deficit injected with both ATM and RGTA, in a contaminated environment; (15) Group G2: The same scaffold and mesh as in Group A, with the edges of the fascia deficit injected with both PRP and RGTA, in a contaminated environment; and (16) Group H2: The same scaffold and mesh as in Group A, with the edges of the fascia deficit injected with ATM, PRP, and RGTA, in a contaminated environment.
The contaminated environment will be created using suspensions derived from rat feces. Under sterile conditions, the meshes will be immersed in these suspensions for the eight groups of rats in which the experiments will be conducted in a contaminated environment (Control Group A2, B2, C2, D2, E2, F2, G2, H2).
For the next 90 days, the healing parameters of the abdominal wall defect will be monitored. Throughout the study period, the cause of death will be noted. If death occurs due to reasons other than the planned sacrifice, the rat will be removed from the study. The rats will be euthanized on a scheduled basis over the 90-day period, following all bioethical guidelines concerning analgesia and proper living conditions until their euthanasia (Figure 1).
An a priori statistical power analysis will be conducted using GPower 3.1 software to estimate the number of animals required for the experiments, ensuring a high level of statistical power for the hypothesis tests. Based on the power analysis performed, each group should include 7 rats (sample size n = 112).
The measured variables will be checked for the normality of their distribution using the Shapiro–Wilk test. Normally distributed, continuous variables will be expressed by the arithmetic mean ± SD, while continuous variables with non-parametric distribution will be expressed by the median and intra-quadratic range. Qualitative variables, categorical or ordinal, will be presented as numbers and percentages per 100. The confidence interval is set at 95% which means that the differences between the groups will be considered statistically significant when P < 0.05. To compare the variables in two independent study groups, a t-test will be used for parametric distributable data, whereas the Mann–Whitney U test will be used for non-parametric distributable data. If normality and variance assumptions are met, an analysis of variance test will be performed to evaluate parametric differences between multiple groups. If these assumptions about variability or normality are violated, a Kruskal-Wallis test will be performed. Statistical analysis of the results will be performed using the statistical program Jamovi version 1.2.27.0 (Figure 2).
A macroscopic examination of the area where the synthetic mesh was applied will be conducted, noting the presence of contamination, hernia, edema, granulation tissue, and the characteristics of any observed exudate (serous, bloody, or purulent).
The histological examination will include a section of the abdominal wall area where the mesh was placed and will assess the inflammatory response (number of white blood cells), fibroblasts, collagen, and neovascularization. These elements will be recorded based on the Ehrich/Hunt scale (modified by Philips) quantitatively on a scale from 0 to 4 as follows: (1) 0 = no elements; (2) 1 = occasional elements; (3) 2 = slightly increased; (4) 3 = frequent; and (5) 4 = confluent cells or fibers. In this way, a thorough investigation of neovascularization, inflammation, fibroblasts and neo collagen formation will be conducted after staining with hematoxylin and eosin and Masson's trichrome. Morphometrical analysis and stereological counting of certain cell populations will be comparatively performed and quantitative data will be comparatively examined for all experimental groups. Finally, all specimens will be sent for Western Blot analysis.
The degree of postoperative inflammation will be assessed using markers such as interleukin (IL)-6, IL-8, nuclear factor-kappa B, CD86, and CD163. The degree of tissue regeneration will be evaluated using CD31, CD146, fibroblast growth factor, vascular endothelial growth factor, vascular endothelial cadherin, and platelet-derived growth factors (PDGF) staining. Finally, the extracellular matrix will be estimated by measuring collagen, laminin, fibronectin, pro
From the combination of these regenerative factors, we expect positive outcomes, including increased tissue regeneration, reduced inflammation, and reconstruction of the extracellular matrix.
In all groups, the rats will undergo laparotomy under appropriate anesthesia. A segment of the fascia of the anterior abdominal wall, measuring 2 cm in length and 2 cm in width, will be excised below the navel. The defect will be repaired using a collagen-elastin template as a scaffold, placed inlay, and an absorbable polyglactin mesh, placed onlay. The collagen-elastin template will be secured with three sutures on each side, and the absorbable polyglactin mesh will also be secured with three sutures on each side. The mesh will be impregnated with the regeneration factors as follows: (1) Group A (Control Group): The fascia will not be injected with any regenerating factor; (2) Group B: The edges of the fascia deficit will be injected with PRP; (3) Group C: The edges of the fascia deficit will be injected with RGTA; (4) Group D: The edges of the fascia deficit will be injected with ATM; (5) Group E: The edges of the fascia deficit will be injected with both PRP and RGTA; (6) Group F: The edges of the fascia deficit will be injected with both PRP and ATM; (7) Group G: The edges of the fascia deficit will be injected with both RGTA and ATM; and (8) Group H: The edges of the fascia deficit will be injected with PRP, RGTA, and ATM.
The exact same procedure is going to be followed in the groups of rats exposed to a contaminated environment (Control Group A2, B2, C2, D2, E2, F2, G2, H2).
Adipose tissue samples will be collected for the preparation of ATM, and blood samples will be taken for PRP preparation. Adipose tissue sampling will be performed using a punch biopsy tool with a diameter of 3 mm or 4 mm from the inguinofemoral region. The adipose tissue samples will be placed inside the Adipecons device, which will then be connected to the Sicurdrill device. After five operational cycles (each cycle lasts one minute), the micrograft solution will be collected through a specific hole at the end of the Adipecons using a syringe without a needle.
Half of the obtained micrograft solution will be injected into the wound and its edges (0.1 mL injection per cm²), while the remaining half will be injected into the collagen-elastin template. The injections will be performed at a depth of 4 mm using a 30G needle.
Regarding PRP, the entire process of collection, processing, and injection takes about 20 minutes. The initial goal is to prepare autologous fibrin glue by cryoprecipitating fibrinogen with ethanol, using autologous PRP lysate as a thrombin source, and activating the coagulation mechanism with calcium chloride.
To prepare the fibrin glue, 2 mL of the experimental animal's blood is centrifuged in a tube containing citrate-phosphate-dextrose (CPD). The supernatant, which contains fibrinogen, is collected, and PRP lysate and calcium chloride are added. Initially, 2.8 mL of CPD is transferred into a sterile 50 mL Falcon tube, and the identification of the experimental animal is recorded. Blood is drawn from the tail vein of the experimental animal and immediately transferred to the Falcon tube containing the anticoagulant. A small sample is taken to examine the platelet count, with an expected concentration of over 130000 cells/µL. The sample undergoes centrifugation for 15 minutes, and the plasma is collected in a new Falcon tube. The plasma is centrifuged two more times until the platelet count is measured, and then the remaining plasma [platelet poor plasma (PPP)] is passed through a 0.2-micron sterilization filter for the isolation of fibrin by precipitation with ethanol. Absolute ethanol (10% of the collected PPP) is added to the sterilized PPP, and the Falcon tube is placed at -80°C for 30 minutes. The supernatant is collected in a new Falcon tube after passing through a 0.2-micron sterilization filter, which constitutes the PRP lysate. The PRP lysate is then mixed with the fibrin sediment and homogenized to form the fibrin glue. Finally, the fibrin glue is injected into the edges of the abdominal fascia to promote regeneration (Figure 3).
Incisional hernias are a persistent issue in clinical practice, and despite advancements in surgical techniques and technological materials, their prevalence has not significantly decreased in the Western world. The incidence of postoperative hernias increases even further after emergency and prolonged surgeries. Although numerous conventional techniques have been developed, none have yielded the desired results.
The treatment of incisional hernias involves the surgical repair of abdominal wall defects, a procedure that has increasingly utilized mesh placement in recent years. Unlike temporary suturing, the use of meshes is advantageous because it reduces tension on the abdominal walls[4].
ATM are often associated with methods that use a patient's own cells to promote healing and tissue repair. This technology involves single-use devices known as Adipecons, which work alongside various activators like the Sicurdrill device[11]. Adipecons mechanically disrupt biological tissues, specifically skin and fat, to collect micrografts in a minimally invasive, autologous, and homologous manner. Autologous cell treatments are increasingly popular in various medical fields because they reduce the risk of immune rejection and other complications associated with donor tissues or cells. The entire process, including disassembly and grafting, is performed during a surgical operation that lasts about 30 minutes, with the patient serving as both donor and recipient. Studies have shown that the cells obtained after mechanical disassembly are positive for mesenchymal stem cell markers, indicating that these cells are progenitor cells with a viability range of 70%-90%[12]. Further analysis reveals that the micrografts contain a high percentage of stromal vascular fraction cells and progenitor cells, which are crucial for tissue regeneration, promoting revascularization and new growth[13]. However, the impact of ATM on the abdominal wall has not yet been studied.
PRP is a concentrated form of plasma that is particularly rich in platelets, which are essential for the body's natural healing process. PRP contains seven key proteins: (1) PDGF; (2) Transforming growth factor-β; (3) Vascular endothelial growth factor; (4) Epidermal growth factor; and (5) Adhesive proteins—fibrin, fibronectin, and vitronectin[11]. These proteins are angiogenic factors responsible for tissue proliferation. PRP is primarily used in orthopedic trauma, hair regeneration, cosmetic procedures, and dermatology and has been shown to significantly aid in wound healing and the regeneration of injured tissues. Early results suggest that PRP has promising potential for tissue regeneration. However, its effects on the fascia defects of the abdominal wall have not been sufficiently studied.
Recently, Matrix Therapy with a RGTA has gained significant attention. RGTA is a bioengineered structural analog of heparan sulfate (HS) glycosaminoglycans, offering a minimally invasive approach in regenerative medicine[14]. Its primary aim is to reconstruct the cellular microenvironment after tissue damage to promote tissue regeneration. At the site of a lesion, HSs are degraded, leading to a disorganized extracellular matrix and tissue destruction. RGTAs replace the damaged HS, restoring the extracellular matrix architecture, which is crucial for cell communication and for protecting heparin-binding growth factors, cytokines, and chemokines from proteolysis, thus facilitating tissue repair and regeneration[14]. Despite its popularity, no studies have yet explored RGTA's role in regenerating the abdominal wall.
Acellular dermal matrix (ADM) has significantly advanced modern tissue engineering. ADM is a biological implant devoid of cellular or antigenic components, reducing immunogenicity and making it an excellent alternative for reconstructive surgery[15]. Derived from human, bovine, and porcine tissue, ADM consists of collagen fibers, fibronectin, elastin, laminin, glycosaminoglycans, and hyaluronic acid[16]. This material serves as a scaffold that is gradually vascularized and cellularized by the host[17]. ADM is primarily used in cosmetic and reconstructive surgery of the nasal cavity, oral cavity, burns, and diabetic wounds. International studies have shown promising results following the use of this technology in the abdominal wall, demonstrating reduced mesh infections and cost-effectiveness[18].
Polyglactin is a synthetic absorbable mesh that is inert, porous, and flexible. It loses 50% of its original strength after 14 days, serving as an initial scaffold for new tissue regeneration before being completely absorbed[19]. Made of synthetic polyfilament suture, polyglactin meshes have lower mesh infection rates and reduced postoperative adhesion formation compared to non-absorbable meshes[17]. Additionally, these meshes blend high tensile strength with high flexibility, providing excellent tissue support and allowing easy movement through tissue with minimal resistance, tissue fragility, and trauma. They are well-received and easily tolerated[18].
With regards to the pathophysiological background, incisional hernias are characterized by weakened or disrupted abdominal wall integrity, often resulting from impaired collagen metabolism, poor tissue healing, or inadequate vascularization following surgery. The proposed regenerative techniques each have specific properties that align with the pathophysiology of such defects. By targeting cellular signaling pathways, these factors may optimize integration of the reconstructed tissue, enhance tensile strength, and reduce the risk of recurrence.
Together, these techniques align with the pathophysiological mechanisms underlying hernia formation by promoting a favorable microenvironment for healing, modulating inflammation, and strengthening tissue integrity. This synergistic approach may address critical aspects of abdominal wall physiology that contribute to hernia recurrence, thereby supporting the long-term efficacy of defect reconstruction. In this manner, restoration of the continuity of the linea alba can be more easily achieved through abdominal wall regeneration, thereby avoiding techniques such as bridging, which are associated with poor outcomes[20].
Therefore, these factors employ an innovative strategy that focuses on regenerating the abdominal wall to prevent incisional hernias, setting them apart from the conventional approach that involves merely covering the gap with a mesh. This is why we consider our project part of regenerative medicine.
Our study will investigate the effect of PRP, ATM, and RGTA on wound remodeling and tissue integration in abdominal wall deficits. These regenerative factors are expected to act as anti-inflammatory agents and accelerate wound healing. The results of our study will provide a better understanding of the underlying regulatory role of these regenerative factors in wound healing.
| 1. | Sekhar S, Ekka NM, Nair R, Pratap V, Mundu M, Kumar A. Effect of Suture Length on the Incidence of Incisional Hernia and Surgical Site Infection in Patients Undergoing Midline Laparotomy: A Systematic Review and Meta-Analysis. Cureus. 2023;15:e34840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Serrano-Aroca Á, Pous-Serrano S. Prosthetic meshes for hernia repair: State of art, classification, biomaterials, antimicrobial approaches, and fabrication methods. J Biomed Mater Res A. 2021;109:2695-2719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Jensen KK, Emmertsen KJ, Laurberg S, Krarup PM. Long-term impact of incisional hernia on quality of life after colonic cancer resection. Hernia. 2020;24:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Liang MK, Holihan JL, Itani K, Alawadi ZM, Gonzalez JR, Askenasy EP, Ballecer C, Chong HS, Goldblatt MI, Greenberg JA, Harvin JA, Keith JN, Martindale RG, Orenstein S, Richmond B, Roth JS, Szotek P, Towfigh S, Tsuda S, Vaziri K, Berger DH. Ventral Hernia Management: Expert Consensus Guided by Systematic Review. Ann Surg. 2017;265:80-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 283] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 5. | Rastegarpour A, Cheung M, Vardhan M, Ibrahim MM, Butler CE, Levinson H. Surgical mesh for ventral incisional hernia repairs: Understanding mesh design. Plast Surg (Oakv). 2016;24:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Deerenberg EB, Henriksen NA, Antoniou GA, Antoniou SA, Bramer WM, Fischer JP, Fortelny RH, Gök H, Harris HW, Hope W, Horne CM, Jensen TK, Köckerling F, Kretschmer A, López-Cano M, Malcher F, Shao JM, Slieker JC, de Smet GHJ, Stabilini C, Torkington J, Muysoms FE. Updated guideline for closure of abdominal wall incisions from the European and American Hernia Societies. Br J Surg. 2022;109:1239-1250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 153] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 7. | Wang See C, Kim T, Zhu D. [Hernia Mesh and Hernia Repair: A Review]. Zaisheng Gongcheng. 2020;1:19-33. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Bhanot P, King KS, Albino FP. Biologic mesh for abdominal wall reconstruction. Chronic Wound Care M. 2014;2014:57-65. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Köckerling F. Recurrent Incisional Hernia Repair-An Overview. Front Surg. 2019;6:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG; NC3Rs Reporting Guidelines Working Group. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3050] [Cited by in RCA: 3243] [Article Influence: 202.7] [Reference Citation Analysis (0)] |
| 11. | Jain NK, Gulati M. Platelet-rich plasma: a healing virtuoso. Blood Res. 2016;51:3-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Trovato L, Monti M, Del Fante C, Cervio M, Lampinen M, Ambrosio L, Redi CA, Perotti C, Kankuri E, Ambrosio G, Rodriguez Y Baena R, Pirozzi G, Graziano A. A New Medical Device Rigeneracons Allows to Obtain Viable Micro-Grafts From Mechanical Disaggregation of Human Tissues. J Cell Physiol. 2015;230:2299-2303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Purpura V, Bondioli E, Graziano A, Trovato L, Melandri D, Ghetti M, Marchesini A, Cusella De Angelis MG, Benedetti L, Ceccarelli G, Riccio M. Tissue Characterization after a New Disaggregation Method for Skin Micro-Grafts Generation. J Vis Exp. 2016;e53579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Barritault D, Gilbert-Sirieix M, Rice KL, Siñeriz F, Papy-Garcia D, Baudouin C, Desgranges P, Zakine G, Saffar JL, van Neck J. RGTA(®) or ReGeneraTing Agents mimic heparan sulfate in regenerative medicine: from concept to curing patients. Glycoconj J. 2017;34:325-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Haney NM, Huang MM, Liu JL, Hawksworth DJ, Burnett AL. Acellular Dermal Matrix Tissues in Genitourinary Reconstructive Surgery: A Review of the Literature and Case Discussions. Sex Med Rev. 2021;9:488-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Quilty BJ, Diamond C, Liu Y, Gibbs H, Russell TW, Jarvis CI, Prem K, Pearson CAB, Clifford S, Flasche S; CMMID COVID-19 working group, Klepac P, Eggo RM, Jit M. The effect of travel restrictions on the geographical spread of COVID-19 between large cities in China: a modelling study. BMC Med. 2020;18:259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Deeken CR, Matthews BD. Characterization of the Mechanical Strength, Resorption Properties, and Histologic Characteristics of a Fully Absorbable Material (Poly-4-hydroxybutyrate-PHASIX Mesh) in a Porcine Model of Hernia Repair. ISRN Surg. 2013;2013:238067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 18. | Belianskiĭ LS, Todurov IM. [Interpretation of recommendations of European Association of Herniologists for the problems of inguinal hernia treatment]. Klin Khir. 2010;5-9. [PubMed] |
| 19. | Allahdin S, Glazener C, Bain C. A randomised controlled trial evaluating the use of polyglactin mesh, polydioxanone and polyglactin sutures for pelvic organ prolapse surgery. J Obstet Gynaecol. 2008;28:427-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Huang Y, Wang Z, Liu Y, Xiong H, Zhao Y, Wu L, Yuan C, Wang L, Hou Y, Yu G, Huang Z, Xu C, Chen Q, Wang QK. αB-Crystallin Interacts with Nav1.5 and Regulates Ubiquitination and Internalization of Cell Surface Nav1.5. J Biol Chem. 2016;291:11030-11041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/