Published online Jun 20, 2025. doi: 10.5493/wjem.v15.i2.105798
Revised: March 12, 2025
Accepted: March 24, 2025
Published online: June 20, 2025
Processing time: 68 Days and 14.7 Hours
Sodium fluoride (NaF) is a daily necessity consumed as the major ingredient of fluorinated drinking water, milk, salts, mouthwashes, toothpaste, and dentistry medications. However, the use of NaF products has also been associated with increased fluoride anion distribution in the body, leading to hypertension.
This study evaluated the antihypertensive effect of sweet orange peels-enriched white melon seed protein concentrate (WSP) biscuit meal in eight-week-old albino rats exposed to NaF for 14 days.
Forty-two (42) male Wistar albino rats were assigned at random into 7 groups of 6 rats per group (control group and six experimental groups). The experimental groups received various treatments that lasted for two weeks. Twenty-four hours after the last administration, hemodynamic parameters were evaluated, rats were sacrificed, blood samples were collected, and the heart was harvested. Blood serum was assessed for cardiac troponin I (cTnI), creatine kinase-MB (CK-MB), and lactate dehydrogenase (LDH). At the same time, the heart homogenate was assayed for angiotensin-1 converting enzyme (ACE) activity, proinflammatory cytokines, nitric oxide concentrations, and antioxidant status. Cardiac tissues were stained with Hematoxylin and Eosin, Masson’s Trichrome, and cTnI. Also, the safety of the WSP biscuit diet was evaluated.
Results obtained showed that NaF administration elevated the collagen content of cardiac tissues, activities of ACE, and concentrations of cTnI, CK-MB, LDH, tumor necrosis factor-alpha, and interleukin 1 beta, while there was a reduction in the concentration of nitric oxide and antioxidants; however, their alterations were significantly prevented in WSP-biscuit-fed rats. The WSP biscuit meal is safe for consumption and possesses dose-dependent antihypertensive ability at 10% and 20% inclusion.
The WSP biscuit diet may be recommended in diet formulation for the management of individuals or communities that are predisposed to NaF contaminations.
Core Tip: The present study thus evaluates the antihypertensive effect of sweet orange peels-enriched white melon seed protein concentrate (WSP) biscuit meal in sodium fluoride (NaF)-exposed rats. Hypertension is mainly dependent on the biological mechanisms of the heart’s renin-angiotensin-aldosterone system which have also been implicated to be significantly modulated in NaF toxicity. Exposure to NaF also results in hypertension via excessive production of reactive oxygen species, damage of DNA, and depletion of antioxidant defense systems in cardiac tissues. Results obtained showed that NaF administration elevated the collagen content of cardiac tissues, activities of angiotensin-1 converting enzyme and concentrations of cardiac troponin I, creatine kinase-MB, and lactate dehydrogenase, tumor necrosis factor-alpha, and interleukin 1 beta, while there was a reduction in the concentration of nitric oxide and antioxidant; however, their alterations were significantly prevented in WSP biscuits-fed rats.
- Citation: Fasakin OW, Awosika A, Ogunsanya ST, Benson IO, Olopoda AI. Anti-hypertensive effect of enriched white melon seed protein concentrate biscuit on sodium fluoride exposed rats. World J Exp Med 2025; 15(2): 105798
- URL: https://www.wjgnet.com/2220-315x/full/v15/i2/105798.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i2.105798
The use of sodium fluoride (NaF), the major ingredient of daily inevitable products such as fluorinated drinking water, milk or salts, mouthwashes, toothpaste, and dentistry medications, has resulted in its ever-increasing presence globally[1]. Increased NaF products are associated with elevated fluoride (F) anion’ distribution in the environment due to occupational exposure and leaching into drinking and household waters[2]. As a result, over 200 million individuals are estimated to yearly consume water with fluoride contents above the 1.5 mg/L recommended by the World Health Organization (WHO) standard guidelines with a resultant 65% of global endemic fluorosis prevalence attributed to fluoride-contaminated water intake[3-6]. This elevated level of fluoride content in drinking water is a national burden in most developing countries that depend on underground water for drinking due to the menace of potable water scarcity. Several studies have established a strong link between concentrations of NaF in drinking water and the prevalence of hypertension in the same population[5-8].
Hypertension is mainly dependent on the biological mechanisms of the heart’s renin-angiotensin-aldosterone system (RAAS), which have also been implicated to be significantly modulated in NaF toxicity[9]. Inhibitors of nitric oxide synthase such as Nw-nitro-L-arginine-methyl-ester hydrochloride have been implicated in inducing hypertension by suppressing nitric oxide levels in the heart, a condition that has been established in prolonged NaF-exposed individuals[10,11]. NaF affects the RAAS through complex mechanisms involving direct renal toxicity, oxidative stress, and electrolyte imbalance. Fluoride directly affects the renal tissue, resulting in nephrotoxicity or impairment of the kidney’s ability to regulate renin release effectively. Exposure to fluoride causes electrolyte imbalance, such as reduced magnesium or increased calcium levels, which can influence renin secretion and overall RAAS activity. The possibility of NaF altering the secretion of RAAS components has also been established[1,11].
Exposure to NaF also results in hypertension via excessive production of reactive oxygen species (ROS), damage of DNA, and depletion of antioxidant defense systems in cardiac tissues[12]. This process also results in nuclear factor-kappa B (NF-κB) pathway activation. The NF-κB translocates into the nucleus to enhance the expression of genes encoding for proinflammatory cytokines [Tumor necrosis factor-alpha (TNF-α), interleukin (IL) 1 beta (IL-1β), IL-6], adhesion molecules (Vascular cell adhesion molecule, endothelial-leukocyte adhesion molecule 1, intercellular adhesion molecule), chemokines [Monocyte chemoattractant protein-1, IL-8, Chemokine (C-C motif) ligand 5], growth factors (epidermal growth factor and fibroblast growth factor), inducible enzymes (inducible nitric oxide synthase and cyclooxygenase-2), immune receptors (toll-like receptors and high-affinity IgE receptor), and acute phase proteins (C-reactive protein and serum amyloid A); which play major roles in mediating critical cardiac inflammatory processes[13,14].
Diverse pharmacological agents have been developed to manage the resultant hypertensive conditions; however, they come with adverse side effects, high prices, and suboptimal controls, thereby deterring individuals from employing them in managing the condition[11,15]. Most developing countries are blessed with plants that serve as medicine and food. Some of these plants are underutilized, e.g., Cucumeropsis mannii (C. mannii) (white melon) seeds, while others are considered waste products, e.g., peels, that are of medicinal value[16,17]. Protein concentrate from C. mannii seed has been established to prevent atherosclerosis formation in blood arteries via enhancement of high-density lipoprotein (HDL) cholesterol concentrations or depletion of low-density lipoprotein cholesterol concentrations in the blood[16]. The study also evaluated the modulatory effects of the seed protein concentrate on the RAAS, the key mediator of normal blood pressure and fluid balance, and reported its inhibitory activity on the angiotensin-1 converting enzyme (ACE). White melon seed protein concentrate (WSP) inhibition on RAAS was implicated to primarily occur through the suppression of ACE activity, resulting in reduced angiotensin II production and lowered vasoconstriction. The protein’s antioxidant ability to mitigate oxidative stress and nitric oxide systems further explains its inhibitory effects on RAAS. The hydro
Furthermore, despite sweet orange peel being a waste product, it is a substantial natural source of phytochemicals and antioxidants, which have been implicated in managing cardiovascular diseases and related complications[17]. However, there is still a dearth of research on the in-vivo evaluation of commonly consumed food products (biscuit meal) made of safe-to-consume local seeds (white melon seed) and food waste products (sweet orange peel) in Africa as an antihypertensive agent. Given this, the present study seeks to evaluate the antihypertensive effect of sweet orange peels-enriched WSP biscuit meal in NaF-exposed rats for 14 days.
ACE, butanol, hydrogen peroxide, thiobarbituric acids (TBA), L-arginine, Coomassie blue G, nitrate, Ellman’s reagent, and NaF were obtained from Sigma Chemical Co, St. Louis, MO, United States, Organon Limited, Kolkata, India, and Cadilla Healthcare Limited, Daman, India. All reagents used were of analytical grade.
C. mannii (white melon) seeds and Citrus sinensis (sweet orange) peels were obtained from local farmers in Akure South Local Government of Ondo State, Nigeria. The seeds and fruits were authenticated at the Department of Crop, Soil and Pest Management, Federal University of Technology, Akure, Nigeria. White melon seeds were dehulled manually, blended with a Warring Commercial Heavy-Duty Blender (Model 37BL18; 24ØCB6), and defatted according to the procedure reported by Ijarotimi et al[18]. The defatted seed flour was dissolved in distilled water (1:20 w/v). Then, the resulting mixture was adjusted to pH 10 with sodium hydroxide and stirred continuously for 2 hours at a maintained temperature of 37 °C. The mixture was centrifuged at 3500 rpm and 4 °C for 30 min after the alkaline solubilization. The obtained supernatant was adjusted with 2M HCl to the isoelectric point at pH 4 to precipitate proteins. Precipitated protein was then centrifuged (3500 rpm at 4 °C for 30 minutes), washed severally with distilled water, adjusted with 0.1M NaOH to pH 7, and then freeze-dried to obtain WSP using Labconco Freeze Dryer–Stoppering tray, United States[19].
WSP was mixed with wheat flour (WWF) and sweet orange as a flavour at different percentage ratios to obtain composite flour for biscuit production. The composite flour was in the ratio WSP: WWF (%) as follows: 0:100 without sweet orange peels (100% WWF flour), 10:90 with sweet orange peels (10% WSP flour), and 20:80 with sweet orange peels (20% WSP flour). The WSP-WWF composite flours were then mixed with other ingredients (skimmed milk, egg albumin, and cocoa butter) to augment the nutritional content of the flour to meet up for a composite meal nutritional requirement based on the previous reports of Ijarotimi et al[16]. The WSP-WWF blends were then mixed with water, aspartame, baking powder, salt, and baking fat until the desired texture of the dough was attained and then kneaded on a rolling table to obtain the desired thickness. The obtained dough was cut into round shapes with a biscuit cutter and baked in a mechanized oven for 10 minutes at 200 °C. Afterward, the baked biscuits were cooled and packed for subsequent use[20].
Forty-two (42) male Wistar albino rats weighing an average of 201 ± 13 g were obtained from the animal house, Biochemistry department, Federal University of Technology, Akure, Nigeria. The handling and use of the experimental animals were strictly according to the National and Institutional Guidelines for Animal Protection and Welfare with approval from the Animal Ethical Committee, Centre for Research and Development of the Federal University of Technology, Akure, with the ethical number FUTA/ETH/2020/016. The hypertensive condition was induced in experimental animals by orally administering 300 mg/L NaF for two weeks[21]. Experimental animals were grouped at random into 7 groups of 6 rats each[10]. Group A was fed with normal grains and received normal saline; Group B was fed with 10% WSP biscuit meal and received normal saline; Group C was fed with 20% WSP biscuit meal and received normal saline; Group D received 300 mg/L NaF in drinking water orally and was fed with normal grains[21]; Group E received 300 mg/L NaF + Lisinopril (10 mg/kg bwt) and was fed with normal grains; Group F received 300 mg/L NaF + 10% WSP biscuit meal; Group G received 300 mg/L NaF and 20% WSP biscuit meal. The treatment lasted for two weeks.
The body weights of experimental animals were taken using a top loader weighing balance (OHAUS MODEL Cs 5000, Capacity 500 × 2 g).
On the 15th day of the experiment, hemodynamic [systolic blood pressure (SBP) and diastolic blood pressure (DBP)] were measured noninvasively in conscious experimental rats using tail-cuff plethysmography under stable signal while maintaining a consistent 30-minute stabilization interval before each recording via Kent Scientific: RTBP1001 Rat Tail Blood Pressure System for rats, Litchfield, United States[17]. Mean arterial blood pressure (MABP) was evaluated using the following formula[22]: MABP = DBP + 0.412 (SBP-DBP).
After measuring hemodynamic parameters, experimental rats were sacrificed and dissected, and blood samples were obtained via cardiac puncture and centrifuged for 10 min at 3500 rpm in microtubes to obtain blood serum. About 1 mL of serum was obtained from each group blood sample and evaluated for cardiac troponin I (cTnI), creatine kinase-MB (CK-MB), and lactate dehydrogenase (LDH) concentrations using highly specific enzyme-linked immunosorbent (ELISA) kits, as well as lipid profiles using kits obtained from Randox Laboratories Ltd, United Kingdom according to manufacturer manual protocols. The heart tissues were harvested, washed in normal saline, homogenized in 0.1 M physiological buffer (pH 7.4), and centrifuged in a 10000-rpm refrigerated centrifuge for 10 min. Supernatants were obtained and evaluated as follow: ACE according to the methods of Cushman and Cheung[23]; Griess reagent [0.1% N-(1-naphthyl) ethylenediamine dihydrochloride, 1% sulphanilamide, and 2.5% phosphoric acid] was used in determining the nitrite level by spectrophotometry method[24]; ROS concentration was assessed using N-N-diethyl-para-phenylenediamine (6 mg/mL) and ferrous sulphate (4.37 μmol/L) prepared in 0.1 M sodium acetate (pH 4.8), with H2O2 production been employed in determining ROS concentrations at 505 nm[25]; Lipid peroxidation was determined as the formation of TBA-reactive substances[26]; superoxide dismutase (SOD) activity was determined by measuring the inhibition of autoxidation of epinephrine by the method of Misra and Fridovich[27]; catalase (CAT) activity was determined as reported by Ademosun et al[28]; Myeloperoxidase activity was measured as the quantity of the enzyme degrading 1 μmol peroxide/min using 1.2 mmol/L tetramethylbenzidine and 100 mmol/L H2O2 prepared in NaH2PO4 (43 mmol/L, pH 5.4) and assessed at 450 nm[29]; NF-κB, TNF-α and IL-1β were determined using commercially available ELISA kits; Protein content was evaluated using Coomassie blue with bovine serum albumin according to the method of Bradford[30].
Low-density lipoprotein (LDL) content was evaluated according to the method of Friedewald et al[31], while very LDL was evaluated according to the method reported by Oguntuase et al[17].
LDL = Total cholesterol–(Triglycerides/5)–HDL.
The atherogenic index was evaluated according to the method of Yokozawa et al[32].
The excised left ventricles were fixed in neutral buffered formalin for 24 hours, and processed using paraffin wax embedding method. Sections of 5 μm and 7 μm thicknesses were produced from the paraffin-embedded tissues. Haematoxylin and Eosin method was used to demonstrate the general histoarchitecture; Masson’s Trichrome was used to demonstrate collagen fiber, and cTnI was used to quantify Troponin. The immunohistochemical studies were conducted using myocardium tissue samples obtained from transverse slices. These tissue samples were fixed in a 10% neutral-buffered formalin solution for 24 to 36 hours. Subsequently, the samples were processed and embedded in paraffin using standard procedures. Tissue sections with a thickness of four microns were treated with xylene for five minutes to remove the paraffin. The slides were soaked in a series of alcohols with increasing concentration. The endogenous peroxidase activity was suppressed by immersing it in a solution of 3% hydrogen peroxide in methanol for 15 minutes, and then rinsing it twice for 5 minutes each in phosphate-buffered saline (PBS). The sections were subsequently treated with 3% normal horse serum (NHS), diluted with PBS, for 30 minutes. This was followed by an incubation period of 1.5-2 hours with the primary antibody, cTnI 3302, at a dilution of 1: 200. Following the elimination of the primary antibody through three 5-minute rinses in PBS, the sections were then exposed to a 40-minute incubation period with biotinylated horse anti-mouse IgG (Vector) diluted to a ratio of 1:200 in a solution containing 1% NHS. Following three 5-minute washes in PBS, the sections were then incubated for 30 minutes with horseradish peroxidase avidin D (HRP, Vector) diluted 1:1000 with PBS. Following three 5-minute washes with PBS, the sections were treated with a DAB kit (Vector) for development and then stopped by rinsing with double-distilled water. The slides were stained with a weak solution of haematoxylin, washed with ammonia, and then rinsed with tap water. The sections were desiccated using a series of ethanol solutions of increasing concentration, then treated with xylene to remove any remaining moisture, and finally covered with a coverslip. Two observers reviewed each immunostained slide. The areas of ischemic damage were classified into peripheral and central regions. The peripheral region comprised multiple layers of myocytes located directly adjacent to the viable myocardium. The central region comprised the myocardium that remained within the ischemic zone. Cardiac troponin staining loss was evaluated for each region, and immunoquantification of the stained slides was carried out using Image J software.
At sacrifice, the heart weight was determined using a top loader-sensitive balance (Mettler-Toledo Garvens GmbH, Giesen, Germany). The relative weight of the heart (%) to the body weight at sacrifice was evaluated.
Stained sections were imported into the digital slide scanner (Leica Aperio CS2_Digital Slide Scanner), and digital photomicrographs were taken at various magnifications using the digital slide assistant (Motic Images Plus, Version 2.0 software; Motic China Group Co. Ltd, Shenzhen, China).
Photomicrographs of hematoxylin and eosin-stained sections were imported onto the Motic Images Plus, Version 2.0 software (Motic China Group Co. Ltd, Shenzhen, China) for histomorphometric analysis to measure the ventricular thickness.
Photomicrographs of stained sections were analyzed and processed using image analysis and processing for JAVA (Image J), the public domain software. Photomicrographs were loaded into Image J software, macros were created. The images were calibrated, and segmented (using threshold).
Obtained data were checked for normality patterns using the Kolmogorov–Smirnov test before statistical analysis. The data were then analyzed using GraphPad Prism, version 8.0.2. for Windows using a one-way analysis of variance followed by Duncan multiple tests. Data were expressed as mean ± SEM of six (6) rats per group. The statistically significant difference was set at P < 0.05.
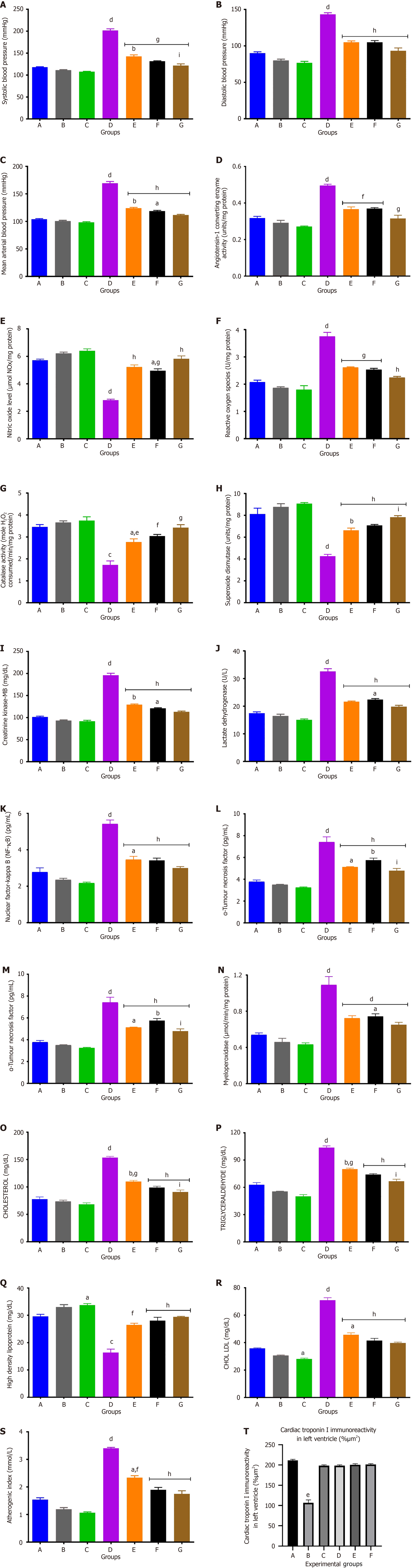
Results of the present study showed a significant elevation in hemodynamic parameters after oral exposure to NaF for 14 days (Figure 1A–C). In the NaF group, there was a significant elevation of the systolic (P < 0.0001; F(6, 35) = 162.9), diastolic (P < 0.0001; F(6, 35) = 86.06) and mean arterial (P < 0.0001; F(6, 35) = 213.9) blood pressure which was significantly reduced in 10% and 20% WSP biscuit diet-administered groups. The activity of ACE and level of nitric oxide was observed to be significantly elevated and depleted, respectively in NaF-exposed rats (Figure 1D and E). However, the dose-dependent-protective abilities of WSP biscuit diets were significantly displayed in the inhibition of the activity of ACE (P < 0.0001; F(6, 35) = 42.89) and reduction of nitric oxide levels (P < 0.0001; F(6, 35) = 79.99) in the WSP biscuit diet groups after NaF exposure.
It was also observed that, NaF oral administration significantly elevated ROS (P < 0.0001; F(6, 35) = 56.67) production while significantly depleting the CAT (P < 0.0001; F(6, 35) = 28.57) and SOD (P < 0.0001; F(6, 35) = 39.89) activities in experimental animals (Figure 1F–H). Interestingly, there was protection against oxidative stress in WSP biscuit diet-treated groups with exposure to NaF. The effects of 10% and 20% WSP biscuit diets were evaluated in the serum of experimental animals exposed to oral NaF for cardiotoxicity (Figure 1I and J). Significant elevation was observed in CK-MB (P < 0.0001; F(6, 35) = 241.3) and LDH (P < 0.0001; F(6, 35) = 114.7) activities of experimental animals exposed to oral NaF only. However, at both doses, WSP biscuit diets were able to protect against the elevation of the serum markers of cardiotoxicity.
Additionally, exposure to oral NaF resulted in significant elevations of cardiac NF-κB (P < 0.0001; F(6, 35) = 51.01), TNF-α (P < 0.0001; F(6, 35) = 47.01), and IL-1β (P < 0.0001; F(6, 35) = 62.30) concentrations, as well as myeloperoxidase (P < 0.0001; F(6, 35) = 26.95) activities, which were interestingly all protected from significant alteration in 10% and 20% WSP biscuit diet-fed groups (Figure 1K–N). Furthermore, the concentrations of cholesterol, triglycerides, HDL and LDL-cholesterol, and atherogenic index in experimental rats are shown in Figure 1O–T. There was an increase in the concentrations of cholesterol (P < 0.0001; F(6, 35) = 103.3), triglycerides (P < 0.0001; F(6, 35) = 110.8), and LDL (P < 0.0001; F(6, 35) = 162), and atherogenic index (P < 0.0001; F(6, 35) = 127.3) alongside a concomitant decrease in HDL (P < 0.0001; F(6, 35) = 44.65) in NaF orally administered rats at the end of the 14 days experiment in comparison to control group. However, the lipid metabolism system was protected from alteration in 10% and 20% WSP biscuit diet-fed groups.
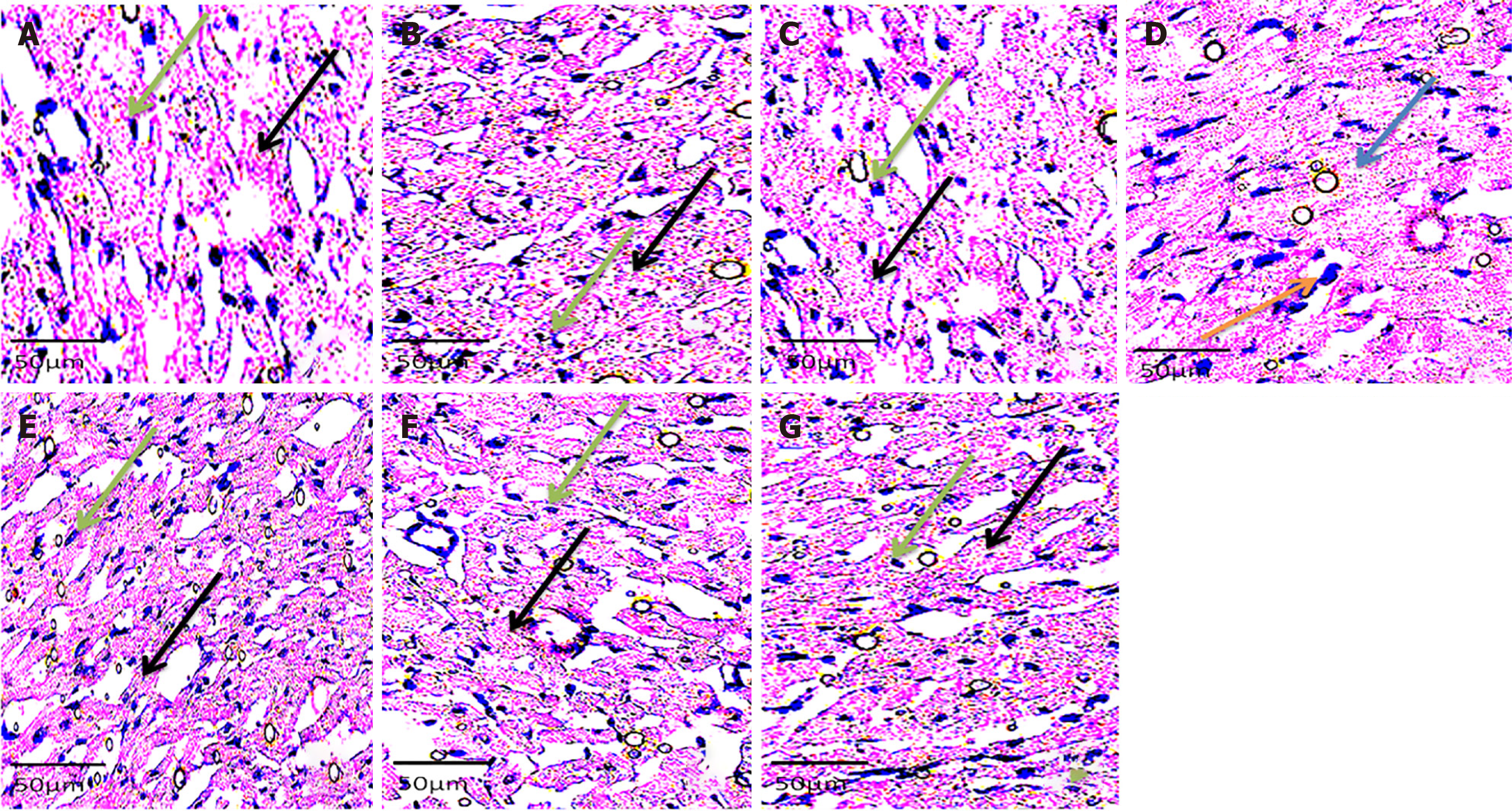
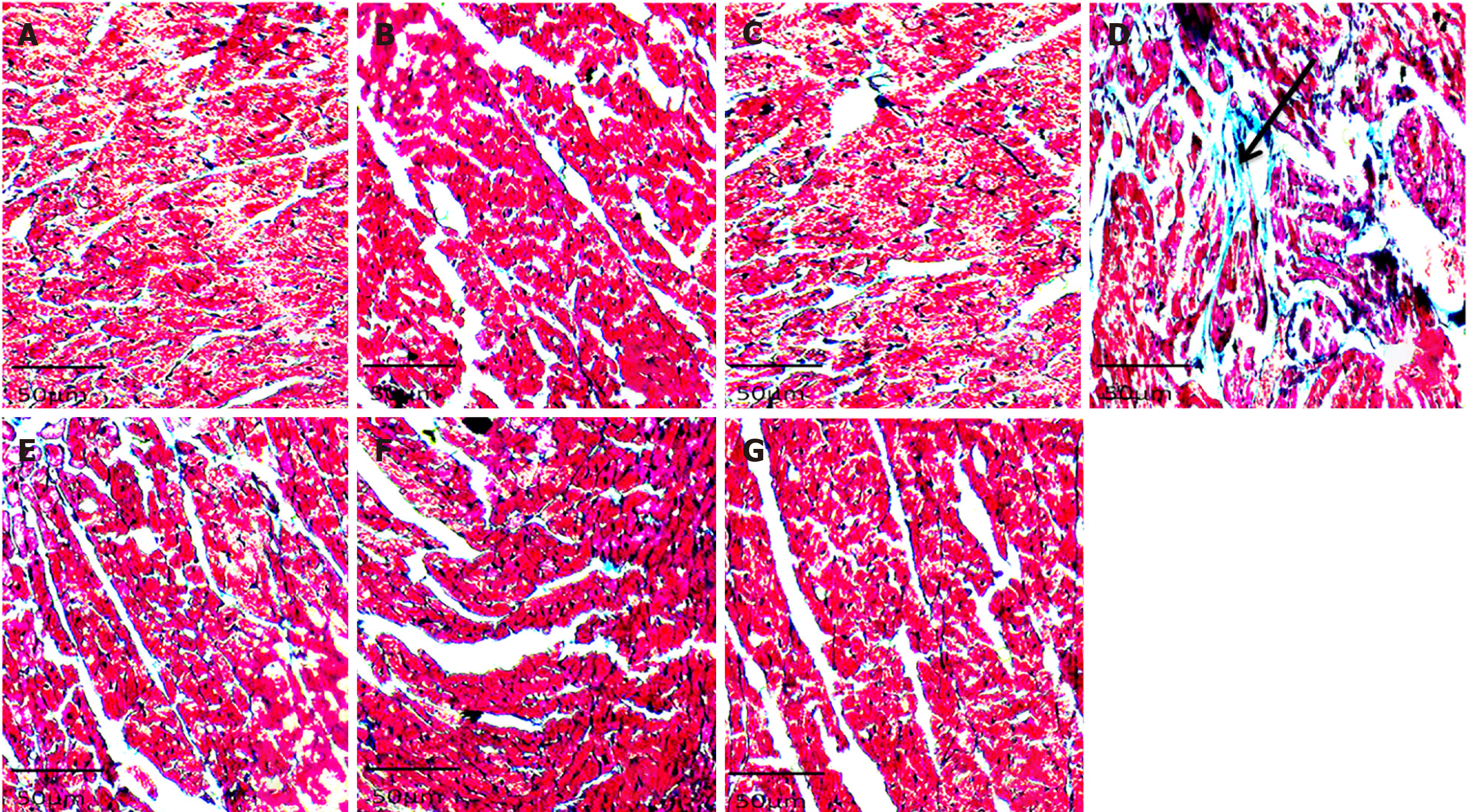
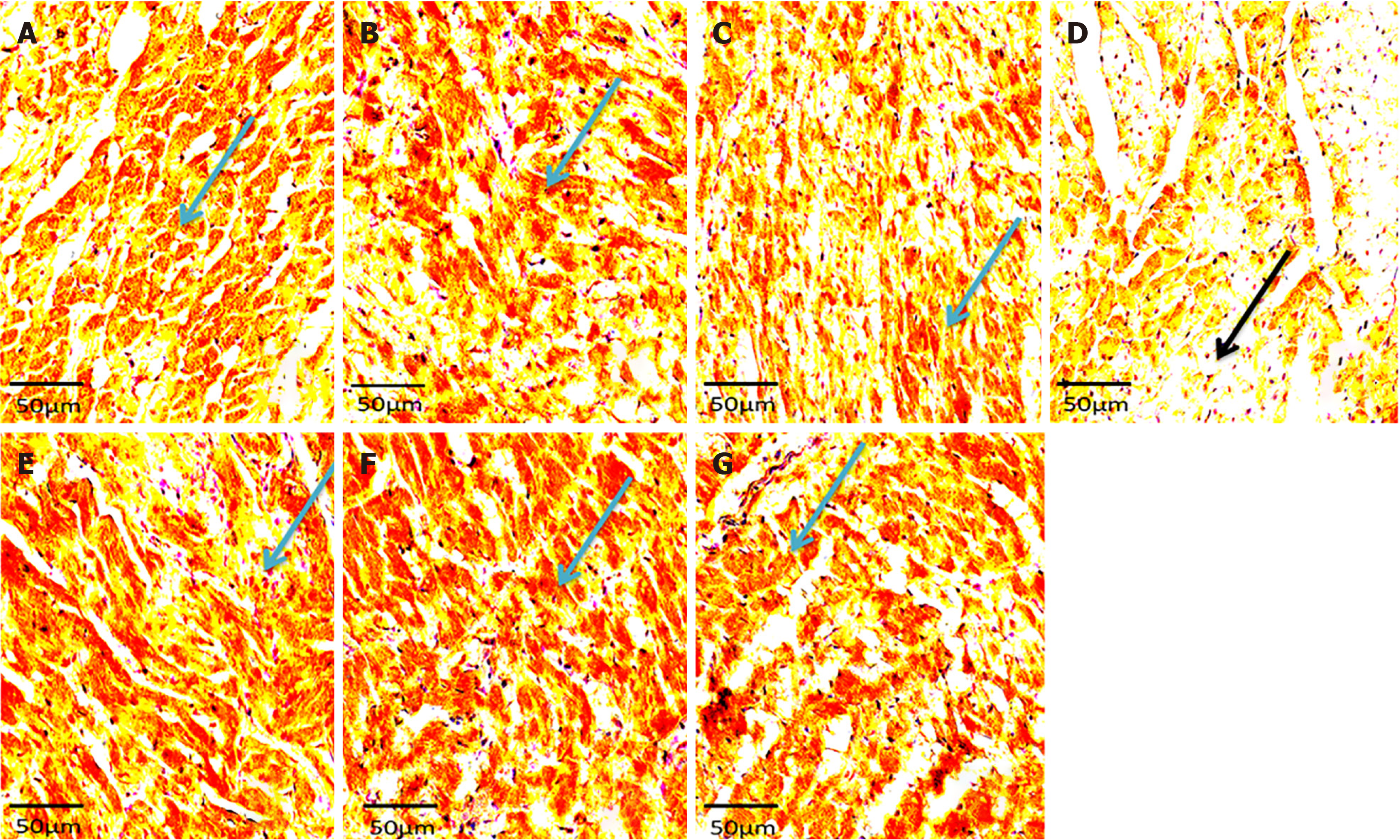
Histology showed well-arranged branched myocardial fiber with a well-placed nucleus and no alterations in groups A, B, C, E, F, and G (Figure 2). The cross-banding pattern and branching of cardiac fiber were distorted. There was a degeneration of myocardial fiber and nuclear displacement in the induced without treatment group (group D). Increased collagen fiber deposit was evident on histological sections of the left ventricle of the rats in group D (NaF only group) (Figure 3). There were no increments in collagen deposit in the WSP biscuit-treated groups. Immunohistochemical studies revealed loss of Troponin in the induced without treatment group while Troponin losses were protected in WSP biscuit diet-treated groups (Figure 4). Results of the present study also revealed that exposure to NaF significantly (P < 0.05) increased left ventricular thickness, which was significantly (P < 0.05) protected in WSP biscuit diet-treated groups (Table 1). Finally, the results of the present study revealed that exposure to NaF significantly (P < 0.0001) elevated collagen deposits, which were significantly (P < 0.0001) protected in WSP biscuit diet-treated groups (Table 2).
With over 200 million individuals being estimated to yearly consume water with fluoride contents above the 1.5 mg/L recommended by the WHO standard guidelines, as well as the resultant 65% of global endemic fluorosis prevalence attributed to fluoride-contaminated water intake, the effect of fluoride on individuals and/or communities predisposed to fluoride toxicity remains a public health burden[5,6]. Some studies have investigated the relationship between fluoride intake and hypertension (a silent global killer) with related complications[21]; these complications have been suggested to be caused by the generation of free radicals and pro-inflammatory cytokines, as well as the depletion of nitric oxide and endogenous antioxidant defense systems[21,33,34]. Treating long-term illnesses like hypertension is expensive, thus putting a strain on healthcare finances and resources, especially when combined with the possible need to treat other health problems linked to fluoride[35].
Typically, the present study showed a significant elevation of blood pressure, ACE activities, pro-inflammatory cytokines concentrations, ROS and TBA productions, nitric oxide concentrations as well as depleted organo-somatic index were noticed in rats after exposure to NaF; these features are suggestive of hypertensive condition and cardiac tissue damage. The latter was further confirmed in plates A, B, and C, where hematoxylin and eosin staining showed degeneration of myocardial tissue, displaced nucleus, and disruption of the normal branching pattern of cardiac muscle. The result of the present study corroborates with the findings of Davoudi et al[7], which established the relationship between the prevalence of high blood pressure in a population and a concomitant fluoride concentration in the groundwater of the same sample size. However, co-administration of sweet orange peel-enriched WSP of biscuit meal normalized the hypertensive potentials of NaF exposure to control levels as evident with obtained hemodynamic parameters (Figures 1A–C). These histological findings in the aforementioned plates were in tandem with the works of Alharbi et al[36], who found similar results. The possible pointer to such an occurrence could be linked to the rupture of the cardiac membrane owing to an increase in ROS production as a result of cardiomyopathy, with the WSP dietary intake possibly managing the menace.
Hypertension etiology involves aggravated angiotensin-II production in the presence of ACE, an active vasoconstrictor[9,17]. Elevated ACE activities stimulate excessive angiotensin-II production, which results in RAAS alteration, a cascade that has been established in NaF exposures[21,37]. Elevated angiotensin-II concentrations coupled with oxidative stress can stimulate the expressions of NF-κB, a ubiquitous transcriptional factor, which is actively involved in the progression of hypertension via cardio-renal dysfunction, pulmonary vasoconstriction, and structural remodeling as evident in the NaF-treated group which showed nuclear displacement, disruption in normal branching pattern and degeneration of myocardial fibers as well as increased collagen deposit in myocardial tissue (Plate A, B, and C). Interestingly, WSP diets prevented the stimulation of the RAAS and NF-κB, a key therapeutic tactic for the management of glomerular hypertension[17].
Additionally, the cascade is characterized by the production of TNF-α and IL-1β, the two major cytokines implicated in cardiac inflammation and damage, which may further compound hypertensive conditions[14,21]. However, the inhibition of the RAAS by an ACE activity-inhibitor or angiotensin-II receptor antagonist lowers blood pressure and prevents the stimulation of the NF-κB cascade, thereby inhibiting the progression of NaF-induced hypertension. Interestingly, the WSP biscuit meal comprises antioxidant properties and amino residues such as hydrophobic, acidic, and branched-chain amino acids that possess strong inhibitory potentials against the RAAS and ACE cascades[16].
Also, at the inflammatory sites, myeloperoxidase has been reported to be released into cellular fluids that consume endothelial-derived NO (an endogenous vasodilator), thereby depleting NO bioavailability and resultantly impairing the anti-inflammatory and vasodilatory activities of the NO[12]. Interestingly, myeloperoxidase was elevated after NaF exposure, which is indicative of myocardial damage mediated by oxidative stress, endothelial dysfunction, modulation of protease cascade, lipid peroxidation, and elevated thrombogenicity[38]. Concomitantly, the reduction in NO level after the NaF exposure is indicative of the progression of hypertension-as NO is tasked with the maintenance of continuous vasodilation toning required for the regulation of blood flow and pressure, and platelet vasodilation and aggregation[39]. Therefore, the protective effects of white melon seed protein biscuit diets in preventing the aggravation of myeloperoxidase and depletion of NO may indicate antihypertensive abilities.
The protective effects of WSP biscuits on ACE activity, NO levels, ROS production, and oxidative stress markers suggest a multifaceted mechanism of action. One potential mechanism is the inhibition of ACE by bioactive peptides derived from white melon seed protein. These peptides may act as competitive inhibitors, binding to the active site of ACE and preventing the conversion of angiotensin I to angiotensin II, thereby reducing vasoconstriction and lowering blood pressure. Studies have shown that plant-derived peptides, particularly those containing hydrophobic and branched-chain amino acids, can effectively inhibit ACE activity and regulate blood pressure[16,18]. Additionally, WSP biscuits may enhance NO bioavailability by mitigating oxidative stress. The antioxidant properties of white melon seed protein and sweet orange peels help to scavenge free radicals, thereby preventing NO degradation by ROS, such as superoxide anions. This preservation of NO supports endothelial function and vasodilation[17]. Furthermore, polyphenolic compounds present in sweet orange peels contribute to the suppression of NF-κB activation, thereby reducing the production of pro-inflammatory cytokines like TNF-α and IL-1β[14,17].
A significant increase in ROS and TBA production was observed in the present study after exposure to NaF. The increased ROS and TBA production correlate with the studies of Ahmed et al[40] and Adetunji et al[41] that established elevated reactive species production and membrane lipid peroxidation as one of the principal consequences of oxidative damage during NaF exposure. Inferentially, the elevated oxidative damage may have been mediated by enhanced accumulation of O2- and H2O2, coupled with depleted CAT and SOD activities usually associated with NaF exposures[34,42]. However, WSP biscuit meals were able to significantly protect against elevated oxidative damage, which may be attributed to the potent antioxidant and free radical scavenging ability of the WSP, which are very active in their free states[16]. Additionally, the white melon seed protein and sweet orange peels may likely exert their antioxidant effects through other pathways. A possible mechanism involves the activation of the Nrf2-ARE (nuclear factor erythroid 2-related factor 2-antioxidant response element) pathway, a critical regulator of cellular antioxidant defenses[14]. Bioactive compounds in WSP biscuits may upregulate Nrf2 expression, leading to increased transcription of antioxidant enzymes. This upregulation enhances the ability of cells to neutralize ROS, reducing oxidative stress and preventing lipid peroxidation[27]. The peroxisome proliferator-activated receptor (PPAR) pathway may have also been employed. PPAR-α activation has been linked to improved lipid profiles by promoting the β-oxidation of fatty acids and reducing LDL cholesterol levels[43]. The protein and polyphenol components of WSP biscuits could have activated PPAR-α, thereby reducing hyperlipidemia, a major risk factor for hypertension. Furthermore, inhibition of NF-κB signaling by WSP antioxidants would prevent chronic inflammation, maintaining endothelial integrity and NO bioavailability. These molecular pathways may have collectively contributed to the cardioprotective effects of WSP biscuits, making them a promising dietary intervention for hypertension management.
The immunohistochemical findings showed loss of Troponin in NaF exposed only group (group D) (Plate C and Figure 1P) could be explained by the rupturing of the cardiac membrane characterized by elevated concentrations of cytosolic enzymes (CK-MB and LDH) and cardiac regulatory proteins (e.g., troponin I) in the serum and its loss in the tissues of experimental rats in group D (Plates A – C) has been used to established cardiac tissue damage following NaF exposure[44]. Reduced Troponin I expression in the cardiac tissue suggests ongoing cardiomyopathy within the myocardium due to increased ROS production that is attributed to the cardiac assault of NaF exposure. Also, excessive ROS production during NaF exposure results in elevated conversion of LDL-cholesterol to oxidized LDL cholesterol, a key player in the pathogenesis of plaque formation, and the enhancement of vascular smooth muscle cell migration, adhesion, and proliferation[34]. Additionally, total cholesterol and triglyceride concentrations were observed to be significantly elevated in the present study, a system termed hyperlipidemia, another key player in the development of cardiovascular diseases and atherosclerosis[45]. However, the observed effect of the WSP diet may be attributed to the potential of the diet to regulate the specific receptors related to lipid profile synthesis[17].
Furthermore, the atherogenic index, another predictor of cardiovascular diseases and atherosclerosis, was observed to be high, an indication of impaired cholesterol metabolism and aggravated oxidized lipoprotein levels, which correlate with the high ratios of LDL-c/HDL-c and TC/HDL-c[17,46]. However, WSP biscuit diets were effective in their preventive and protective roles against the loss of cardiac tissue integrity, hyperlipidemia, and atherosclerosis. Inferentially, their ability to protect cardiac tissue integrity and deplete triglycerides and cholesterol concentrations could be linked to their ability to improve HDL-c concentrations and bile flows and inhibit hepatic apolipoprotein (apo) B secretion and HMG-CoA reductase activities[44]. Therefore, the WSP diet may be an essential therapeutic approach to managing lipid profile disorders associated with hypertensive conditions.
The present study established the safety of a WSP biscuit diet to normotensive rats and the potential of NaF exposure to induce hypertension using a rat model, which therefore calls for caution in the daily use of NaF. It further showed the dose-dependent protective ability of WSP biscuit diet to protect against hypertensive conditions via reduction of high blood pressure and oxidative stress, improvement of antioxidant defense and nitric oxide systems, and the inhibition of renin-angiotensin-aldosterone and inflammatory systems. Protection against hypertensive conditions and cardiac complications is of paramount priority as hypertension is one of the silent killers globally. Considering WSP’s reported nutritional values, the WSP biscuit diet can be recommended to manage individuals or communities predisposed to NaF contamination from mouthwash, mouth rinse, and toothpaste usage. However, the effect of temperature on the polyphenol structures and contents of WSP and sweet orange peels should be further investigated to provide reasonable and scientific explanations behind this perception. Furthermore, molecular studies and clinical trials to evaluate the effect of the WSP biscuit in individuals predisposed to NaF contaminations or hypertensive individuals are also recommended to establish the results of the present study.
All the authors wish to show their profound gratitude to the Federal University of Technology, Akure, Nigeria.
| 1. | Zhang J, Mylonas P, Banerjee A. Mineralizing agents to manage early carious lesions. Part II: clinical application. Dent Update. 2023;50:572-582. [DOI] [Full Text] |
| 2. | Kabir H, Gupta AK, Tripathy S. Fluoride and human health: Systematic appraisal of sources, exposures, metabolism, and toxicity. Crit Rev Environ Sci Technol. 2020;50:1116-1193. [DOI] [Full Text] |
| 3. | Shaji E, Sarath K, Santosh M, Krishnaprasad P, Arya B, Babu MS. Fluoride contamination in groundwater: A global review of the status, processes, challenges, and remedial measures. Geosci Front. 2024;15:101734. [DOI] [Full Text] |
| 4. | Lacson CFZ, Lu M, Huang Y. Fluoride-containing water: A global perspective and a pursuit to sustainable water defluoridation management -An overview. J Cleaner Prod. 2021;280:124236. [DOI] [Full Text] |
| 5. | Yadav A, Kumari N, Kumar R, Kumar M, Yadav S. Fluoride distribution, contamination, toxicological effects and remedial measures: a review. Sustain Water Resour Manag. 2023;9:150. [DOI] [Full Text] |
| 6. | Al Sabti B, Samayamanthula DR, Dashti FM, Sabarathinam C. Fluoride in Groundwater. Hydrogeochemistry Aquat Ecosyst. 2023;. [DOI] [Full Text] |
| 7. | Davoudi M, Barjasteh-Askari F, Sarmadi M, Ghorbani M, Yaseri M, Bazrafshan E, Mahvi AH, Moohebati M. Relationship of fluoride in drinking water with blood pressure and essential hypertension prevalence: a systematic review and meta-analysis. Int Arch Occup Environ Health. 2021;94:1137-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Taher MK, Momoli F, Go J, Hagiwara S, Ramoju S, Hu X, Jensen N, Terrell R, Hemmerich A, Krewski D. Systematic review of epidemiological and toxicological evidence on health effects of fluoride in drinking water. Crit Rev Toxicol. 2024;54:2-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 9. | Oyagbemi AA, Adejumobi OA, Jarikre TA, Ajani OS, Asenuga ER, Gbadamosi IT, Adedapo ADA, Aro AO, Ogunpolu BS, Hassan FO, Falayi OO, Ogunmiluyi IO, Omobowale TO, Arojojoye OA, Ola-Davies OE, Saba AB, Adedapo AA, Emikpe BO, Oyeyemi MO, Nkadimeng SM, McGaw LJ, Kayoka-Kabongo PN, Oguntibeju OO, Yakubu MA. Clofibrate, a Peroxisome Proliferator-Activated Receptor-Alpha (PPARα) Agonist, and Its Molecular Mechanisms of Action against Sodium Fluoride-Induced Toxicity. Biol Trace Elem Res. 2022;200:1220-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Ajeigbe OF, Oboh G, Ademosun AO, Umar HI. Fig (Ficus exasperata and Ficus asperifolia)-Supplemented diet improves sexual function, endothelial nitric oxide synthase and suppresses tumour necrosis factor-alpha genes in hypertensive rats. Andrologia. 2022;54:e14289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 11. | Gbadamosi I, Okolo N, Oyagbemi A, Ajibade T, Omobowale T. Antihypertensive effect of Harungana madagascariensis LAM. ex poir. On sodium fluoride-induced hypertension and Associated cardiorenal dysfunctions. Ife J Sci. 2020;22:237-249. [DOI] [Full Text] |
| 12. | Muderrisoglu S, Cenesiz S, Yarim M. Determination of the effect of Quercetinon oxidant- antioxidant parameters in the blood and liver tissues of rats given sodium fluoride experimentally. J Indian Chem Soc. 2022;99:100486. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Ribeiro D, Freitas M, Lima JL, Fernandes E. Proinflammatory Pathways: The Modulation by Flavonoids. Med Res Rev. 2015;35:877-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 14. | Zhu C, Gu W, Sun D, Wei W. The mechanism underlying fluoride-induced low-renin hypertension is related to an imbalance in the circulatory and local renin-angiotensin systems. Toxicol Lett. 2023;381:36-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 15. | Ng FL, Lobo MD. Investigation and management of adult hypertension. Heart. 2018;104:1543-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Ijarotimi OS, Wumi-adefaye OA, Oluwajuyitan TD, Oloniyo OR. Processed white melon seed flour: Chemical composition, antioxidant, angiotensin-1-converting and carbohydrate-hydrolyzing enzymes inhibitory properties. Appl Food Res. 2022;2:100074. [DOI] [Full Text] |
| 17. | Oguntuase SO, Fasakin OW, Oyeleye SI, Oboh G. Effects of dietary inclusion of Bambara groundnut and sweet orange peels on streptozotocin/HFD type-2 induced diabetes mellitus complications and related biochemical parameters. J Food Biochem. 2022;46:e14373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 18. | Ijarotimi OS, Oyinloye AC, Adenugba MO, Ikhazabor SO, Oluwajuyitan TD. Comparative study on amino acids, fatty acids, functional properties and blood cholesterol status of rats fed on raw, germinated and fermented white melon seed (Cucumeropsis mannii naudin) flour. Ann Food Sci Technol. 2019;20: 402-414. |
| 19. | Girgih AT, Nwachukwu ID, Hasan F, Fagbemi TN, Gill T, Aluko RE. Kinetics of the inhibition of renin and angiotensin I-converting enzyme by cod (Gadus morhua) protein hydrolysates and their antihypertensive effects in spontaneously hypertensive rats. Food Nutr Res. 2015;59:29788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Aderinola TA, Lawal OE, Oluwajuyitan TD. Assessment of Nutritional and Microbiological Properties of Biscuit Supplemented With Moringa Oleifera Seed Protein Concentrate. J Food Eng Tech. 2020;9:22-29. [DOI] [Full Text] |
| 21. | Oyagbemi AA, Omobowale TO, Asenuga ER, Adejumobi AO, Ajibade TO, Ige TM, Ogunpolu BS, Adedapo AA, Yakubu MA. Sodium fluoride induces hypertension and cardiac complications through generation of reactive oxygen species and activation of nuclear factor kappa beta. Environ Toxicol. 2017;32:1089-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Meaney E, Alva F, Moguel R, Meaney A, Alva J, Webel R. Formula and nomogram for the sphygmomanometric calculation of the mean arterial pressure. Heart. 2000;84:64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 138] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Fasakin OW, Oboh G, Ademosun AO. Alkaloids-rich extracts from Cannabis sativa, Datura stramonium, and Nicotiana tabacum modulate sexual behavior and key enzymes relevant to sexual function in rats. Comp Clin Pathol. 2022;31:397-407. [DOI] [Full Text] |
| 24. | Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8653] [Cited by in RCA: 9152] [Article Influence: 208.0] [Reference Citation Analysis (0)] |
| 25. | Hayashi I, Morishita Y, Imai K, Nakamura M, Nakachi K, Hayashi T. High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat Res. 2007;631:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 305] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 26. | Karaoz E, Oncu M, Gulle K, Kanter M, Gultekin F, Karaoz S, Mumcu E. Effect of chronic fluorosis on lipid peroxidation and histology of kidney tissues in first- and second-generation rats. Biol Trace Elem Res. 2004;102:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170-3175. [PubMed] |
| 28. | Ademosun AO, Popoola TV, Oboh G, Fasakin OW. Parquetina nigrescens and Spondias mombin protects against neurochemical alterations in the scopolamine model of cognitive dysfunction. J Food Biochem. 2022;46:e14213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Eiserich JP, Hristova M, Cross CE, Jones AD, Freeman BA, Halliwell B, van der Vliet A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1198] [Cited by in RCA: 1163] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 30. | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189576] [Cited by in RCA: 159162] [Article Influence: 3183.2] [Reference Citation Analysis (1)] |
| 31. | Friedewald WT, Levy RI, Fredrickson DS. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin Chem. 1972;18:499-502. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20005] [Cited by in RCA: 21073] [Article Influence: 390.2] [Reference Citation Analysis (0)] |
| 32. | Yokozawa T, Cho EJ, Sasaki S, Satoh A, Okamoto T, Sei Y. The protective role of Chinese prescription Kangen-karyu extract on diet-induced hypercholesterolemia in rats. Biol Pharm Bull. 2006;29:760-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Khan MU, Basist P, Gaurav, Zahiruddin S, Penumallu NR, Ahmad S. Ameliorative effect of traditional polyherbal formulation on TNF-α, IL-1β and Caspase-3 expression in kidneys of wistar rats against sodium fluoride induced oxidative stress. J Ethnopharmacol. 2024;318:116900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 34. | Oyagbemi AA, Ajibade TO, Esan OO, Adetona MO, Awoyomi OV, Omobowale TO, Ola-davies OE, Saba AB, Adedapo AA, Nkadimeng SM, Mcgaw LJ, Kayoka-kabongo PN, Yakubu MA, Nwulia E, Oguntibeju OO. Cardioprotective and renoprotective effects of melatonin and vitamin E on fluoride-induced hypertension and renal dysfunction in rats. Comp Clin Pathol. 2023;33:33-45. [DOI] [Full Text] |
| 35. | Ahmad S, Singh R, Arfin T, Neeti K. Fluoride contamination, consequences and removal techniques in water: a review. Environ Sci: Adv. 2022;1:620-661. [DOI] [Full Text] |
| 36. | Alharbi KS, Afzal M, Alzarea SI, Khan SA, Alomar FA, Kazmi I. Rosinidin Protects Streptozotocin-Induced Memory Impairment-Activated Neurotoxicity by Suppressing Oxidative Stress and Inflammatory Mediators in Rats. Medicina (Kaunas). 2022;58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Awosika A, Cho Y, Bose U, Omole AE, Adabanya U. Evaluating phase II results of Baxdrostat, an aldosterone synthase inhibitor for hypertension. Expert Opin Investig Drugs. 2023;32:985-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 38. | Omóbòwálé TO, Oyagbemi AA, Alaba BA, Ola-Davies OE, Adejumobi OA, Asenuga ER, Ajibade TO, Adedapo AA, Yakubu MA. Ameliorative effect of Azadirachta indica on sodium fluoride-induced hypertension through improvement of antioxidant defence system and upregulation of extracellular signal regulated kinase 1/2 signaling. J Basic Clin Physiol Pharmacol. 2018;29:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Ajuwon OR, Adeleke TA, Ajiboye BO, Lawal AO, Folorunso I, Brai B, Bamisaye FA, Falode JA, Odoh IM, Adegbite KI, Adegoke OB. Fermented Rooibos tea (Aspalathus linearis) Ameliorates Sodium Fluoride-Induced Cardiorenal Toxicity, Oxidative Stress, and Inflammation via Modulation of NF-κB/IκB/IκKB Signaling Pathway in Wistar Rats. Cardiovasc Toxicol. 2024;24:240-257. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 40. | Ahmed NF, Sadek KM, Soliman MK, Khalil RH, Khafaga AF, Ajarem JS, Maodaa SN, Allam AA. Moringa Oleifera Leaf Extract Repairs the Oxidative Misbalance following Sub-Chronic Exposure to Sodium Fluoride in Nile Tilapia Oreochromis niloticus. Animals (Basel). 2020;10:626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 41. | Adetunji JB, Adebisi OA, Adeyomoye IO, Oyeleye SI, Adebayo DO, Ejidike IP. Hibiscus sabdariffa fractions attenuate oxidative stress and some cardiac biomarkers in sodium fluoride(NaF)-induced cardiotoxicity rat. J Taibah Univ Sci. 2023;17. [DOI] [Full Text] |
| 42. | Zhang C, Wang Y, Huang F, Zhang Y, Huang M, Liu H, Liu Y, Wang Q, Liu C, Angwa L, Gao Y, Sun D, Jiang Y. Novel mechanism of fluoride induced cardiovascular system injury by regulating p53/miR200c-3p during endothelial dysfunction. Environ Res. 2025;271:121102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 43. | Bougarne N, Weyers B, Desmet SJ, Deckers J, Ray DW, Staels B, De Bosscher K. Molecular Actions of PPARα in Lipid Metabolism and Inflammation. Endocr Rev. 2018;39:760-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 656] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 44. | Abdel-baky ES, Abdel-rahman ON. Cardioprotective effects of the garlic (Allium sativum) in sodium fluoride-treated rats. JoBAZ. 2020;81:7. [DOI] [Full Text] |
| 45. | Azab AE, Ammar Algridi M. Antidyslipidemic Effect of Fenugreek Seeds Powder against Sodium Fluoride-Induced Dyslipidemia in Male Rabbits. BTBP. 2021;2:01-08. [DOI] [Full Text] |
| 46. | Fawwad A, Mahmood Y, Askari S, Butt A, Basit A, Rehman Abro MU, Ahmed KI, Ahmed K, Ali SS, Bilal A, Butt A, Devrajani BR, Hayder I, Humayun Y, Irshad R, Khan RA, Khan A, Khowaja AA, Khowaja R, Masroor Q, Mehmood M, Moin H, Mustafa N, Noor W, Qureshi H, Rafique I, Rasool T, Sabir R, Saqib MAN, Said PA, Shaikh A, Younus BB, Tahir B, Tanveer S, Zafar J. NDSP 12: Atherogenic index of plasma as a useful marker of cardiovascular disease risk among Pakistani individuals; a study from the second National Diabetes Survey of Pakistan (NDSP) 2016–2017. Clin Epidemiol Glob Health. 2023;19:101202. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/