Published online Jun 20, 2025. doi: 10.5493/wjem.v15.i2.102969
Revised: March 3, 2025
Accepted: April 1, 2025
Published online: June 20, 2025
Processing time: 163 Days and 21.9 Hours
The gut microbiome, a complex ecosystem of microorganisms, has a significant role in modulating pain, particularly within orthopaedic conditions. Its impact on immune and neurological functions is underscored by the gut-brain axis, which influences inflammation, pain perception, and systemic immune responses. This integrative review examines current research on how gut dysbiosis is associated with various pain pathways, notably nociceptive and neuroinflammatory mecha
Core Tip: Modulating the gut microbiome may offer novel pain management strategies for orthopaedic conditions by reducing inflammation and influencing pain pathways through the gut-brain axis. Probiotics, prebiotics, dietary adjustments, and faecal microbiome transplantation show potential in controlling pain and limiting opioid dependence, though personalized approaches are crucial due to microbiome variability and the need for clinical validation.
- Citation: Jeyaraman N, Jeyaraman M, Dhanpal P, Ramasubramanian S, Nallakumarasamy A, Muthu S, Santos GS, da Fonseca LF, Lana JF. Integrative review of the gut microbiome’s role in pain management for orthopaedic conditions. World J Exp Med 2025; 15(2): 102969
- URL: https://www.wjgnet.com/2220-315x/full/v15/i2/102969.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i2.102969
The gut microbiota, co-evolving with humans, is vital from birth through death, influencing immune, metabolic, and neurological functions. It aids in developing the gut's immune system and establishing immune tolerance to prevent overreactions to harmless antigens[1]. Top of Form This microbiota helps shape our immune system and fosters tolerance to avoid undue reactions to safe antigens[2]. Moreover, the gut-brain axis, connecting our digestive and nervous systems, regulates critical brain activities such as stress management and memory[3]. The production of short-chain fatty acids (SCFAs) by gut bacteria influences learning, memory, serotonin levels, and thus, our mood, well-being, and how we manage pain[4].
On the other hand, the management of pain in various spectrum of orthopaedics faces hurdles due to its persistent nature, associated health issues, immune response and the growing limitations of usage of opioids. Recognizing sex-based differences is key for effective therapies. Diagnoses are complex due to subjective pain and lack of specific markers. Thus, creating personalized treatments and better translating lab findings to clinical practice for pain management is essential[5].
The connection between gut microbiota and the modulation of pain has increasingly captured the interest of medical professionals as the field of medical science progresses. An expanding range of studies has demonstrated that bacteria can directly stimulate nociceptors through their products and intrinsic component. Signalling molecules from the gut microbiota, like metabolites, neurotransmitters, and neuromodulators, influence the activity of primary nociceptive neurons, contributing to chronic pain development[6]. Furthermore, these gut-derived mediators regulate neuroinflammation within the central nervous system. They affect the operation of the blood-brain barrier, microglia, and immune cells, thereby playing a crucial role in both initiating and sustaining central sensitization, a heightened pain response mechanism[7-9].
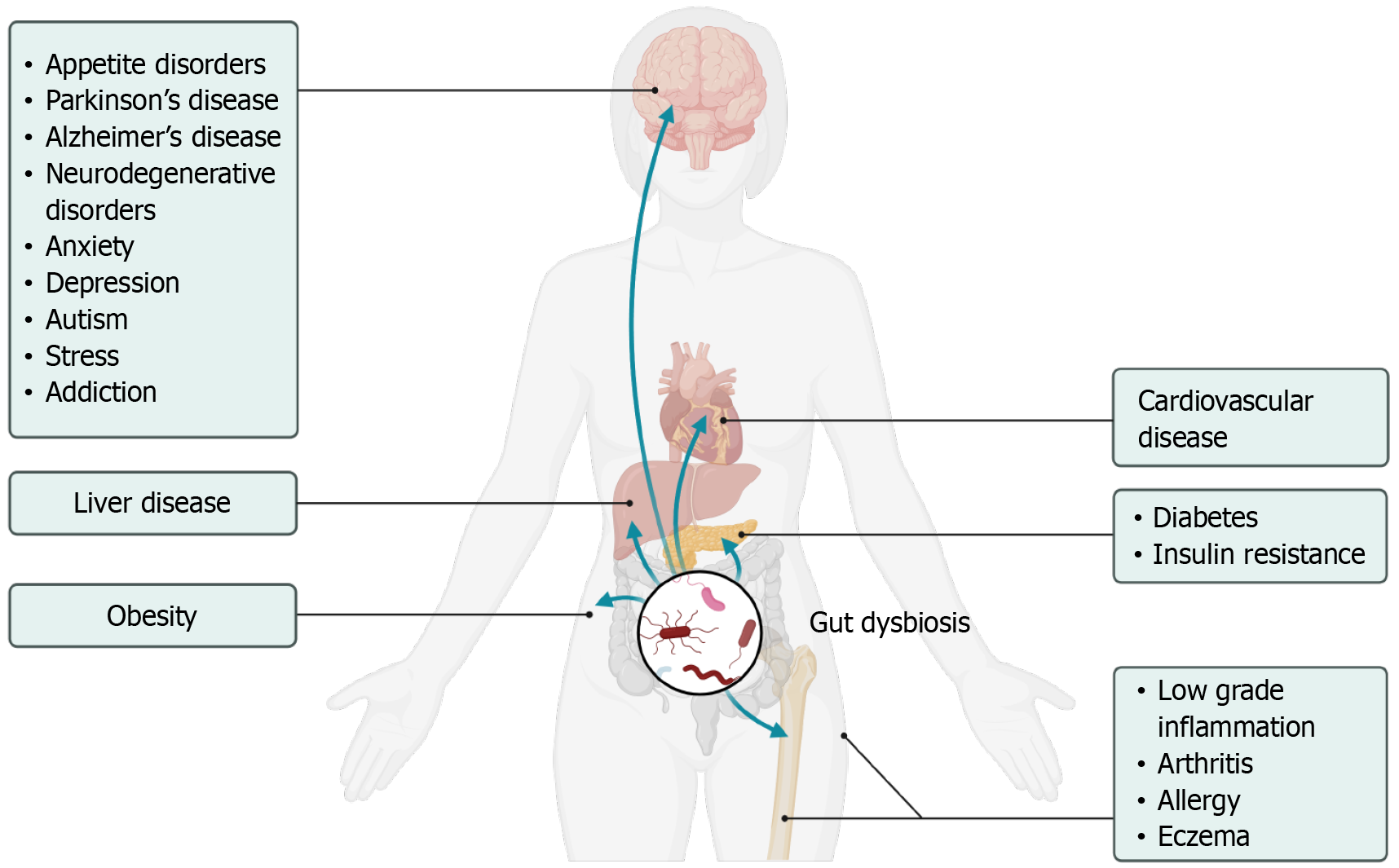
The composition of the gut microbiome is a complex and dynamic ecosystem, significantly influencing human health. Research has established that the gut microbiome is predominantly composed of bacteria, along with archaea, viruses, and fungi, with Firmicutes and Bacteroidetes being the most prevalent bacterial phyla in healthy adults[10]. Research into the gut microbiome underscores its crucial role in health, particularly in digesting dietary carbohydrates and producing beneficial SCFAs like butyrate, propionate, and acetate[10,11]. These SCFAs are known for their positive effects, including anti-inflammatory properties and immune system modulation[12]. Additionally, the diversity of gut microbial communities is linked to health and longevity, indicating the potential benefits of dietary adjustments, prebiotics, and probiotics for extending life span and their dysbiosis is linked to various diseases as shown in Figure 1[1].
Advancements in meta-omics technologies have revolutionized the analysis of host-microbiome interactions. By combining insights from metagenomics, metatranscriptomics, and metaproteomics, researchers can better understand microbial functions and their interplay with the host, shedding light on their roles in health and disease[1].
Shotgun metagenomics and 16S rRNA sequencing represent two distinct approaches for microbiome analysis. Although shotgun metagenomics captures a wider array of species, it generally provides fewer taxonomic details at the family and genus levels when compared to 16S rRNA sequencing. On the other hand, 16S rRNA sequencing provides greater within-sample diversity, particularly at the genus level. Both methods have distinct advantages and limitations, with the choice between them depending on the research question and the available resources for the study[13]. Recent research has examined the relationship between gut bacteria and brain activities, investigating how the gut's microbiome might affect mental processes and contribute to neurological diseases[14]. The production of SCFAs by these microbes, for instance, is thought to potentially influence brain activities through altering brain inflammation signaling pathways[15].
Ongoing research into the gut microbiome has investigated the role of genetic factors, uncovering the ways in which variations in human DNA influence the microbiome's activities. This research has pinpointed specific genetic variations, known as single nucleotide polymorphisms, that are associated with certain characteristics, health issues, and diseases of the microbiome, highlighting the significant influence of human genetics on gut microbiome functions[16,17]. The application of artificial intelligence and bioinformatics in analyzing large datasets has also emerged as a crucial tool, enabling the identification of patterns and associations between the microbiome composition and various health conditions[18].
Microbiota plays a crucial role in maintaining host health and regulating immune function. Dysbiosis of microbiota can lead to various diseases, including cardiovascular diseases, cancers, and respiratory diseases[19]. Probiotics have shown potential as adjuvant therapeutic agents in the treatment of colorectal cancer, intestinal inflammatory disorders, and heart diseases[20,21]. However, their efficacy in other conditions such as inflammatory bowel disease (IBD), rotavirus diarrhoea, non-steroidal anti-inflammatory drugs enteropathy, and irritable bowel syndrome (IBS) is still controversial[22]. Modulation of the gut microbiota through probiotics may have potential therapeutic strategies for lung diseases and could potentially reduce hyperinflammation in coronavirus disease 2019[23]. However, the safety of these interventions needs further investigation. Lifestyles, especially diet, have a significant impact on the gut-brain axis and the composition of the microbiota. The Mediterranean diet has shown beneficial effects on neurovegetative disorders, psychiatric conditions, cancer, and cardiovascular diseases[24-27]. The ketogenic diet has been associated with changes in microbiota abundance and protection against acute epileptogenic seizures[28]. Medications, including antibiotics and nonantibiotic drugs, can also affect the gut microbiota.
The evolution of a mechanism-based classification of pain identifies five primary mechanisms: Central sensitization, which involves increased sensitivity in the central nervous system; peripheral neuropathic, nociceptive, pain from physical damage or potential damage to the body; sympathetically maintained pain, which is pain influenced by the sympathetic nervous system; and cognitive-affective, highlighting the role of cognitive and emotional factors in the perception of pain. This classification aids in understanding and targeting treatment for different pain types.
Osteoclasts, compared to osteoblasts and osteocytes, have gained more focus due to their significant impact on skeletal pain and nerve sensitivity[29-31]. Conditions that increase osteoclastic activity, leading to bone resorption, are frequently associated with pain experiences[32]. Pathologies in bones or joints cause the release of substances that sensitize and activate sensory nerves, enhancing pain signals. Nociceptors discharge glutamate and peptidergic neurotransmitters like calcitonin gene related peptide, substance P, into the spinal cord, activating certain neurons and amplifying pain. Moreover, peripheral sensitization is driven by local tissue factors, such as ATP, ADP, endothelin, growth factors and bradykinin, which reduce the activation threshold for neurons, leading to increased depolarization and stronger pain signals[33].
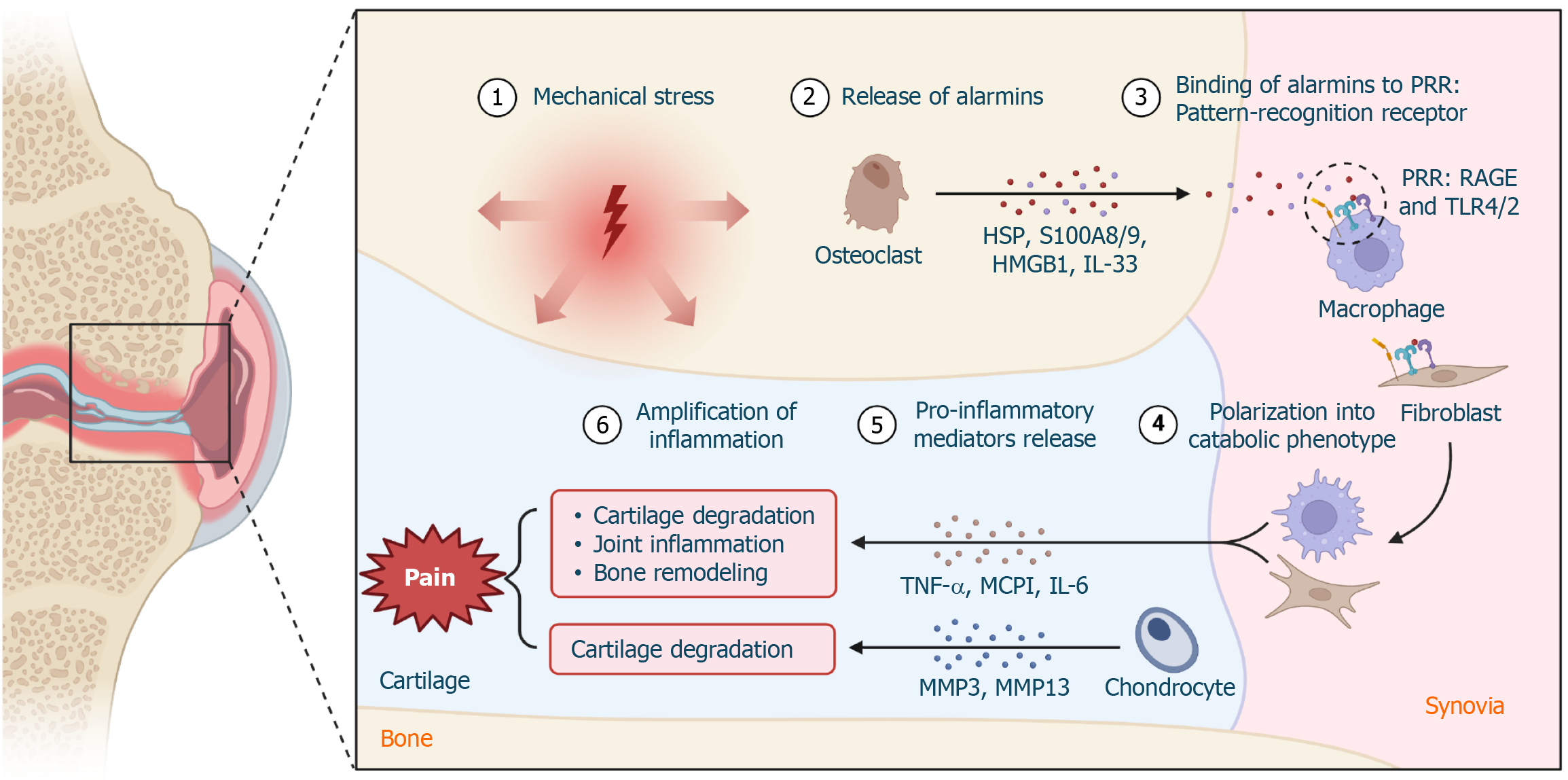
The concept of "osteoimmunology" underscores the significant interaction between the skeletal and immune systems, revealing how immune processes substantially impact bone health and the development of diseases[34,35]. This intricate relationship is prominently observed in several conditions, including bone fractures, rheumatoid arthritis, and osteoporosis. In these situations, immune cells, particularly macrophages, play a vital role throughout the healing phases. These cells are not only essential for defending against pathogens but also for secreting diverse effectors that influence bone modelling. This action demonstrates the immune system's direct involvement in managing and sometimes contributing to the development of pathological and chronic bone conditions as shown in Figure 2[36,37].
The current management of pain in various orthopaedic procedures is Multimodal analgesia, a modality that combines various medications and delivery methods[38]. It aims to enhance pain relief, support recovery, and minimize opioid use and its side effects. Techniques include preemptive analgesia, neuraxial anaesthesia, peripheral nerve blockade, patient-controlled analgesia (PCA), local infiltration, and oral medications. PCA allows patients to self-dose analgesics, utilizing opioids like oxycodone and morphine, to manage pain effectively[39,40].
Opioids, a key component of multimodal analgesia, come with inherent limitations due to adverse effects like nausea, respiratory depression, and urinary retention, restricting their routine use in clinical practice[41]. Additionally, there is no established consensus on the ideal composition or infiltration technique for local infiltration analgesia (LIA), an integral part of multimodal analgesia. The role of liposomal bupivacaine in extending the duration of LIA remains contentious[42]. The choice of anaesthesia, whether general or spinal, can affect pain management. While spinal anaesthesia is associated with lower rates of complications and shorter hospital stays compared to general anaesthesia, there are still differences in outcomes between the two approaches. The use of COX-2 inhibitors in multimodal analgesia may reduce pain and opioid consumption, but their long-term effects and potential complications need further investigation[43].
The role of the gut microbiome in modulating pain, particularly through central and peripheral mechanisms, is a burgeoning area of research that underscores the complex interplay between our gut and overall health.
The interaction between microbiota-derived mediators like LPS and flagellin with toll-like receptors triggers pro-inflammatory mediators, influencing neuropathic pain development[44,45]. The gut-brain axis further highlights the microbiome's impact on pain perception, with dysregulation linked to conditions like visceral hypersensitivity and inflammatory pain[46-49]. Probiotics show promise in pain management, suggesting the gut microbiome's modulation could offer new therapeutic strategies[44]. The link between the gut microbiome and its role in modulating orthopaedic pain is gaining traction in scientific research. Studies have demonstrated that the gut microbiome can influence inflammation and pain in common orthopaedic conditions as cited in the Table 1 below. Table 1 summarizes the existing studies investigating the gut microbiome composition in patients with different orthopaedic conditions[50-54].
| Ref. | Orthopedic condition | Key findings on microbiome composition | Main conclusion |
| Liu et al[50], 2021 | Sarcopenia | Decreased lactobacillus and bifidobacterium | Correlation between microbiome and muscle mass |
| Chakraborty et al[51], 2022 | Coronavirus disease | Elevated gut microbes such as Rothia mucilaginosa, Granulicatella spp, Collinsella tanakaei, Collinsella aerofaciens, Morganella morganii, and Streptococcus infantis | Correlation between microbiome and inflammation |
| Collins et al[52], 2017 | Post menopausal osteoporosis | Lactobacillus, Enterococcus, Bacillus, Escherichia, and Bifidobacterium | Improvement in condition by supplementation |
| Ramires et al[53], 2022 | Osteoarthritis | Lower proportion of Bacteroidetes and higher Firmicutes bacterial populations | Correlation between microbiome and bone mass |
Strategies such as the use of probiotics, prebiotics, synbiotics, and anti-inflammatory foods and spices have been explored as evidence-based approaches to target inflammation and pain, potentially minimizing the need for non
The gut microbiome's influence on neuropathic pain caused by chronic constriction injury has been established, with evidence showing that antibiotic pretreatment can mimic the protective effects of gut microbiome depletion[63]. This effect is associated with alterations in intestinal SCFAs levels, microglial activation, and the concentrations of inflammatory cytokines in both the spinal cord and hippocampus[64]. Dietary interventions focusing on anti-inflammatory foods and spices offer a fascinating research domain due to their potential to modulate the gut-brain axis, thereby affecting the gut microbiome's makeup and functioning. This approach proposes a natural, side-effect-free avenue to address orthopaedic pain and inflammation, emphasizing the critical link between diet, microbial health, and overall well-being[63].
As research continues to unravel the intricate relationships between the gut microbiome and orthopaedic pain, it becomes increasingly clear that the gut microbiome holds significant therapeutic potential. The implications for patient care are profound, offering hope for more effective pain management strategies that harness the natural mechanisms of the body's microbiome. This research trajectory not only deepens our understanding of the body's interconnected systems but also opens new possibilities for treating pain, one of the most challenging aspects of orthopaedic medicine.
Diet plays a crucial role in modulating the gut microbiome, influencing both its composition and function. Research has demonstrated that dietary patterns rich in fibre, such as fruits, vegetables, and whole grains, can promote the growth of beneficial bacterial species within the gut[65]. These beneficial bacteria are key players in the production of SCFAs, which have been shown to exert anti-inflammatory effects and strengthen the intestinal barrier[66]. Conversely, diets high in processed foods and sugars can lead to dysbiosis, a harmful imbalance of gut microbiota associated with various diseases[67]. The Mediterranean diet has been specifically recognized for enhancing gut microbiota diversity, which may provide protective benefits against inflammatory diseases while supporting overall health[68-71].
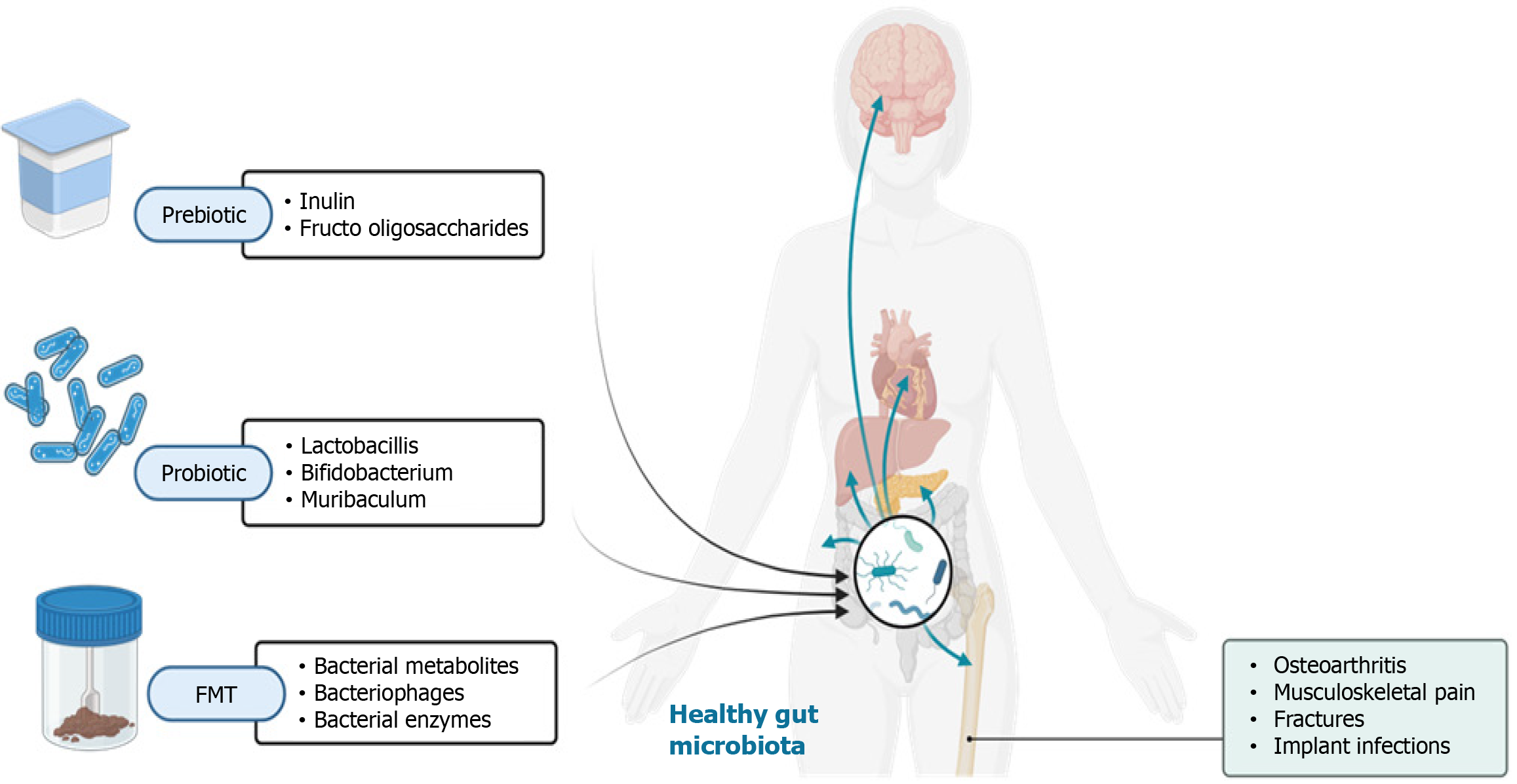
Prebiotics and probiotics are targeted strategies to enhance gut health by modulating the gut microbiome, as illustrated in Figure 3. Prebiotics, such as inulin and fructo-oligosaccharides, are non-digestible food components that act as nourishment for beneficial gut bacteria, fostering their growth and activity[72-76]. Probiotics, in contrast, are live microorganisms that, when consumed in sufficient quantities, provide health benefits to the host[77-81]. Probiotic consumption has been linked to numerous health advantages, including better digestive health, stronger immune function, and a lower risk of certain infections. Moreover, clinical trials and meta-analyses have highlighted the efficacy of specific probiotic strains in treating and influencing various orthopedic conditions, as referenced below[82,83].
Table 2 lists various probiotic strains that have been studied for their potential in managing various orthopaedic conditions. For each probiotic strain, the table outlines the proposed mechanism of action, the orthopaedic condition investigated, reference to the study[84-87].
| Probiotic strain | Mechanism of action | Orthopedic condition | Outcome | Ref. |
| Lactobacillus | Autophagy and control of inflammatory cell death of chondrocytes | Osteoarthritis | The daily supply of butyrate showed a tendency to decrease necroptosis by inducing autophagy and reversing impaired autophagy by the inflammatory environment | Cho et al[84], 2022 |
| Lactobacillus | Prevention of growth of Pseudomonas aeruginosa | Orthopedic implant infections | Supplementation with cell-free supernatant demonstrated antiadhesive, antibiofilm, and toxic properties to Pseudomonas aeruginosa | Jeyanathan et al[85], 2021 |
| Bifidobacterium and muribaculum | Reduction in pro-inflammatory cytokines | Fractures | Aging exacerbates the inflammatory response to fracture leading to high levels of pro-inflammatory cytokines and disruption of the intestinal microbiota | Roberts et al[86], 2023 |
| Cumulative | Nociceptive stimulus, neurotransmitters and hormones | Musculoskeletal pain | Modifiable and non-modifiable factors that are known to contribute to changes to the gut microbiome affects musculoskeletal pain | Tonelli Enrico et al[87], 2022 |
Faecal microbiota transplantation (FMT) is a direct approach to reshaping the gut microbiome, involving the transfer of stool from a healthy donor to a recipient with a dysbiotic microbiome[88]. It has shown significant success in treating recurrent Clostridium difficile infections[89]. Beyond this, FMT has been explored as a potential treatment for conditions associated with gut microbiome imbalances, such as IBD, IBS, and metabolic syndrome. Although the precise mechanisms underlying FMT's benefits are not fully understood, it is believed to restore microbial diversity and functionality, thereby reestablishing a healthy gut ecosystem. Current research and clinical trials continue to assess the safety, effectiveness, and long-term outcomes of FMT for these and other applications[68,90].
Dietary modifications, prebiotics and probiotics supplementation, and FMT represent innovative strategies for gut microbiome modulation. These approaches utilize the intricate relationship between diet, gut microbes, and overall health to potentially prevent and treat various diseases, emphasizing the critical role of the gut microbiome in maintaining human health and addressing disease[90].
Considering the growing interest in the gut microbiome and its role in modulating pain, there are several potential research directions that could further elucidate this relationship and explore novel therapeutic strategies:
Efficacy of probiotic strains in chronic pain management: Investigating specific probiotic strains known to influence the gut-brain axis and their effectiveness in reducing chronic pain conditions. This could involve randomized controlled trials comparing the pain relief provided by these probiotics against placebo treatments in individuals with conditions like fibromyalgia, osteoarthritis, or neuropathic pain. Prebiotics and pain perception: Conducting studies on the impact of prebiotic supplementation on pain perception and quality of life in patients with chronic pain. Prebiotics could modulate the gut microbiota in a way that affects systemic inflammation and pain signalling pathways.
FMT for neuropathic pain: Exploring the use of FMT from healthy donors to patients suffering from neuropathic pain to assess changes in pain intensity, gut microbiota composition, and inflammatory markers. Such studies could help determine altering the gut microbiome through FMT can offer a viable treatment for neuropathic pain. Dietary intervention and their impact on orthopaedic pain: Investigating how various dietary patterns (e.g., Mediterranean diet, high-fibre diet) that are known to influence the gut microbiome composition can affect the development and progression of orthopaedic pain, such as osteoarthritis pain. This could involve longitudinal studies tracking diet, gut microbiome changes, and pain outcomes.
Gut microbiome’s role in mediating the effects of physical therapy: Researching how physical therapy interventions for pain management might interact with the gut microbiome to produce beneficial effects. This could include studies on how exercise-induced changes in the gut microbiome contribute to reductions in chronic pain.
Mechanistic studies in gut microbiota derived metabolites and pain management: Delving into the mechanistic pathways through which metabolites produced by the gut microbiota, such as SCFAs, influence pain pathways. This could involve both in vitro and in vivo studies to map out the biochemical and signalling pathways involved.
Exploring these research avenues holds promise for revealing further complexities in how the gut microbiome interacts with pain mechanisms, paving the way for more advanced and minimally invasive approaches to pain relief. Such investigations aim not just to enrich our grasp of the connections between the gut and the brain but also to uncover potential markers and microbial targets that could revolutionize pain treatment strategies.
Translating preclinical findings related to the gut microbiome and pain management into clinical practice presents several challenges. One major hurdle is the variability in microbiome composition across individuals, which complicates the development of universal therapeutic strategies[91-93]. This variability can be influenced by numerous factors, including diet, genetics, and lifestyle, making it difficult to predict therapeutic outcomes based on animal models alone. Additionally, while animal studies have provided valuable insights into the gut-brain axis and its potential for pain modulation, these models do not fully capture the complexity of the human microbiome or the multifactorial nature of pain[44].
Ensuring the safety and effectiveness of interventions such as FMT and probiotic supplementation presents a significant challenge[94-98]. Although these approaches show promise, comprehensive clinical trials are essential to evaluate their therapeutic potential and identify any potential side effects in humans. Furthermore, the regulatory framework for these treatments remains undefined, adding complexity to their integration into clinical practice[99]. Furthermore, the mechanisms through which the gut microbiome influences pain are still not fully understood. More research is needed to delineate these pathways and how they can be targeted therapeutically. This includes identifying specific microbial strains or metabolites that play key roles in pain modulation and understanding how changes in the gut microbiome can lead to lasting impacts on pain perception[7,100-102].
The gut microbiome influences bone, joint, tendon, nerve, and muscle health through mechanisms involving intestinal barrier permeability and systemic inflammation. However, the findings are not consistent across individuals and population[103].
Modulating the composition of gut microbiome offers an innovative approach to orthopaedic pain management, leveraging the link between gut bacteria, inflammation, and pain. Studies suggest changes in gut microbiota may affect conditions like osteoarthritis through immune modulation and the gut-brain axis. Approaches such as probiotics, prebiotics, dietary changes, FMT show promise in altering the gut to reduce inflammation and pain. Despite promising findings, translating these insights into clinical practice faces hurdles like individual microbiome variability and the necessity for rigorous clinical trials to confirm safety and effectiveness. Nonetheless, gut microbiome modulation holds exciting potential for advancing orthopaedic pain treatment, meriting further research. A multidisciplinary approach is key to fully understanding and leveraging the gut microbiota for orthopaedic pain management. Combining knowledge from microbiology, immunology, neurology, and orthopaedics can unveil how the gut influences pain through inflammation and the gut-brain axis. Such collaboration is vital for creating innovative, personalized pain management strategies, potentially including diet, probiotics, and FMT. This comprehensive research effort promises to enhance patient care by offering more effective and targeted pain solutions.
| 1. | Kho ZY, Lal SK. The Human Gut Microbiome - A Potential Controller of Wellness and Disease. Front Microbiol. 2018;9:1835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 451] [Cited by in RCA: 694] [Article Influence: 86.8] [Reference Citation Analysis (20)] |
| 2. | Takiishi T, Fenero CIM, Câmara NOS. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers. 2017;5:e1373208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 708] [Article Influence: 78.7] [Reference Citation Analysis (2)] |
| 3. | Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203-209. [PubMed] |
| 4. | Dicks LMT. Gut Bacteria and Neurotransmitters. Microorganisms. 2022;10:1838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 275] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 5. | Schoenfeld AJ. Special Considerations in Pain Management in Orthopaedic Subspecialties. J Bone Joint Surg Am. 2020;102 Suppl 1:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Defaye M, Gervason S, Altier C, Berthon JY, Ardid D, Filaire E, Carvalho FA. Microbiota: a novel regulator of pain. J Neural Transm (Vienna). 2020;127:445-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Guo R, Chen LH, Xing C, Liu T. Pain regulation by gut microbiota: molecular mechanisms and therapeutic potential. Br J Anaesth. 2019;123:637-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 276] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 8. | Bostick JW, Schonhoff AM, Mazmanian SK. Gut microbiome-mediated regulation of neuroinflammation. Curr Opin Immunol. 2022;76:102177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 9. | Gan Y, Chen Y, Zhong H, Liu Z, Geng J, Wang H, Wang W. Gut microbes in central nervous system development and related disorders. Front Immunol. 2023;14:1288256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 10. | Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787-8803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1421] [Cited by in RCA: 1970] [Article Influence: 179.1] [Reference Citation Analysis (79)] |
| 11. | Facchin S, Bertin L, Bonazzi E, Lorenzon G, De Barba C, Barberio B, Zingone F, Maniero D, Scarpa M, Ruffolo C, Angriman I, Savarino EV. Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications. Life (Basel). 2024;14:559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 98] [Reference Citation Analysis (0)] |
| 12. | Liu XF, Shao JH, Liao YT, Wang LN, Jia Y, Dong PJ, Liu ZZ, He DD, Li C, Zhang X. Regulation of short-chain fatty acids in the immune system. Front Immunol. 2023;14:1186892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 184] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 13. | Wensel CR, Pluznick JL, Salzberg SL, Sears CL. Next-generation sequencing: insights to advance clinical investigations of the microbiome. J Clin Invest. 2022;132:e154944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 262] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 14. | Tiwari P, Dwivedi R, Bansal M, Tripathi M, Dada R. Role of Gut Microbiota in Neurological Disorders and Its Therapeutic Significance. J Clin Med. 2023;12:1650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (1)] |
| 15. | Zhang X, Li L, Butcher J, Stintzi A, Figeys D. Advancing functional and translational microbiome research using meta-omics approaches. Microbiome. 2019;7:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 207] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 16. | Kumar S, Mahajan S, Kale D, Chourasia N, Khan A, Asati D, Kotnis A, Sharma VK. Insights into the gut microbiome of vitiligo patients from India. BMC Microbiol. 2024;24:440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | Peddinti V, Avaghade MM, Suthar SU, Rout B, Gomte SS, Agnihotri TG, Jain A. Gut instincts: Unveiling the connection between gut microbiota and Alzheimer's disease. Clin Nutr ESPEN. 2024;60:266-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 18. | Bergamaschi M, Tiezzi F, Howard J, Huang YJ, Gray KA, Schillebeeckx C, McNulty NP, Maltecca C. Gut microbiome composition differences among breeds impact feed efficiency in swine. Microbiome. 2020;8:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 148] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 19. | Hou K, Wu ZX, Chen XY, Wang JQ, Zhang D, Xiao C, Zhu D, Koya JB, Wei L, Li J, Chen ZS. Microbiota in health and diseases. Signal Transduct Target Ther. 2022;7:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 1833] [Article Influence: 458.3] [Reference Citation Analysis (9)] |
| 20. | Petrariu OA, Barbu IC, Niculescu AG, Constantin M, Grigore GA, Cristian RE, Mihaescu G, Vrancianu CO. Role of probiotics in managing various human diseases, from oral pathology to cancer and gastrointestinal diseases. Front Microbiol. 2023;14:1296447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 70] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 21. | Zhao J, Liao Y, Wei C, Ma Y, Wang F, Chen Y, Zhao B, Ji H, Wang D, Tang D. Potential Ability of Probiotics in the Prevention and Treatment of Colorectal Cancer. Clin Med Insights Oncol. 2023;17:11795549231188225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Gallo A, Passaro G, Gasbarrini A, Landolfi R, Montalto M. Modulation of microbiota as treatment for intestinal inflammatory disorders: An uptodate. World J Gastroenterol. 2016;22:7186-7202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 84] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | de Oliveira GLV, Oliveira CNS, Pinzan CF, de Salis LVV, Cardoso CRB. Microbiota Modulation of the Gut-Lung Axis in COVID-19. Front Immunol. 2021;12:635471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 162] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 24. | AlAufi NS, Chan YM, Waly MI, Chin YS, Mohd Yusof BN, Ahmad N. Application of Mediterranean Diet in Cardiovascular Diseases and Type 2 Diabetes Mellitus: Motivations and Challenges. Nutrients. 2022;14:2777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 25. | Armeli F, Bonucci A, Maggi E, Pinto A, Businaro R. Mediterranean Diet and Neurodegenerative Diseases: The Neglected Role of Nutrition in the Modulation of the Endocannabinoid System. Biomolecules. 2021;11:790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Ventriglio A, Sancassiani F, Contu MP, Latorre M, Di Salvatore M, Fornaro M, Bhugra D. Mediterranean Diet and its Benefits on Health and Mental Health: A Literature Review. Clin Pract Epidemiol Ment Health. 2020;16:156-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 128] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 27. | Randeni N, Xu B. Critical Review of the Cross-Links Between Dietary Components, the Gut Microbiome, and Depression. Int J Mol Sci. 2025;26:614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 28. | Fan Y, Wang H, Liu X, Zhang J, Liu G. Crosstalk between the Ketogenic Diet and Epilepsy: From the Perspective of Gut Microbiota. Mediators Inflamm. 2019;2019:8373060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 29. | Zhen G, Fu Y, Zhang C, Ford NC, Wu X, Wu Q, Yan D, Chen X, Cao X, Guan Y. Mechanisms of bone pain: Progress in research from bench to bedside. Bone Res. 2022;10:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 30. | Marahleh A, Kitaura H, Ohori F, Noguchi T, Mizoguchi I. The osteocyte and its osteoclastogenic potential. Front Endocrinol (Lausanne). 2023;14:1121727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 31. | Xu H, Wang W, Liu X, Huang W, Zhu C, Xu Y, Yang H, Bai J, Geng D. Targeting strategies for bone diseases: signaling pathways and clinical studies. Signal Transduct Target Ther. 2023;8:202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 95] [Reference Citation Analysis (0)] |
| 32. | Mantyh PW. Mechanisms that drive bone pain across the lifespan. Br J Clin Pharmacol. 2019;85:1103-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 33. | Havelin J, King T. Mechanisms Underlying Bone and Joint Pain. Curr Osteoporos Rep. 2018;16:763-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Jones D, Glimcher LH, Aliprantis AO. Osteoimmunology at the nexus of arthritis, osteoporosis, cancer, and infection. J Clin Invest. 2011;121:2534-2542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 35. | Mi B, Xiong Y, Knoedler S, Alfertshofer M, Panayi AC, Wang H, Lin S, Li G, Liu G. Ageing-related bone and immunity changes: insights into the complex interplay between the skeleton and the immune system. Bone Res. 2024;12:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 48] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 36. | Xu M, Bennett DLH, Querol LA, Wu LJ, Irani SR, Watson JC, Pittock SJ, Klein CJ. Pain and the immune system: emerging concepts of IgG-mediated autoimmune pain and immunotherapies. J Neurol Neurosurg Psychiatry. 2020;91:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 37. | Guder C, Gravius S, Burger C, Wirtz DC, Schildberg FA. Osteoimmunology: A Current Update of the Interplay Between Bone and the Immune System. Front Immunol. 2020;11:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 128] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 38. | Chunduri A, Aggarwal AK. Multimodal Pain Management in Orthopedic Surgery. J Clin Med. 2022;11:6386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 39. | Sampognaro G, Harrell R. Multimodal Postoperative Pain Control After Orthopaedic Surgery. 2023 Jan 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2025. [PubMed] |
| 40. | Bhatia A, Buvanendran A. Anesthesia and postoperative pain control-multimodal anesthesia protocol. J Spine Surg. 2019;5:S160-S165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 41. | O'Neill A, Lirk P. Multimodal Analgesia. Anesthesiol Clin. 2022;40:455-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 42. | Lavand'homme PM, Kehlet H, Rawal N, Joshi GP; PROSPECT Working Group of the European Society of Regional Anaesthesia and Pain Therapy (ESRA). Pain management after total knee arthroplasty: PROcedure SPEcific Postoperative Pain ManagemenT recommendations. Eur J Anaesthesiol. 2022;39:743-757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 163] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 43. | Li JW, Ma YS, Xiao LK. Postoperative Pain Management in Total Knee Arthroplasty. Orthop Surg. 2019;11:755-761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 243] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 44. | Lin B, Wang Y, Zhang P, Yuan Y, Zhang Y, Chen G. Gut microbiota regulates neuropathic pain: potential mechanisms and therapeutic strategy. J Headache Pain. 2020;21:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 45. | Shatunova S, Aktar R, Peiris M, Lee JYP, Vetter I, Starobova H. The role of the gut microbiome in neuroinflammation and chemotherapy-induced peripheral neuropathy. Eur J Pharmacol. 2024;979:176818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 46. | Morreale C, Bresesti I, Bosi A, Baj A, Giaroni C, Agosti M, Salvatore S. Microbiota and Pain: Save Your Gut Feeling. Cells. 2022;11:971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 47. | Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol Stress. 2017;7:124-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 494] [Cited by in RCA: 788] [Article Influence: 87.6] [Reference Citation Analysis (0)] |
| 48. | Loh JS, Mak WQ, Tan LKS, Ng CX, Chan HH, Yeow SH, Foo JB, Ong YS, How CW, Khaw KY. Microbiota-gut-brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct Target Ther. 2024;9:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 530] [Article Influence: 265.0] [Reference Citation Analysis (0)] |
| 49. | Mukhtar K, Nawaz H, Abid S. Functional gastrointestinal disorders and gut-brain axis: What does the future hold? World J Gastroenterol. 2019;25:552-566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 105] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (3)] |
| 50. | Liu C, Cheung WH, Li J, Chow SK, Yu J, Wong SH, Ip M, Sung JJY, Wong RMY. Understanding the gut microbiota and sarcopenia: a systematic review. J Cachexia Sarcopenia Muscle. 2021;12:1393-1407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 250] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 51. | Chakraborty C, Sharma AR, Bhattacharya M, Dhama K, Lee SS. Altered gut microbiota patterns in COVID-19: Markers for inflammation and disease severity. World J Gastroenterol. 2022;28:2802-2822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (5)] |
| 52. | Collins FL, Rios-Arce ND, Schepper JD, Parameswaran N, McCabe LR. The Potential of Probiotics as a Therapy for Osteoporosis. Microbiol Spectr. 2017;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 53. | Ramires LC, Santos GS, Ramires RP, da Fonseca LF, Jeyaraman M, Muthu S, Lana AV, Azzini G, Smith CS, Lana JF. The Association between Gut Microbiota and Osteoarthritis: Does the Disease Begin in the Gut? Int J Mol Sci. 2022;23:1494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 54. | Simon E, Călinoiu LF, Mitrea L, Vodnar DC. Probiotics, Prebiotics, and Synbiotics: Implications and Beneficial Effects against Irritable Bowel Syndrome. Nutrients. 2021;13:2112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 55. | Iriah SC, Rodriguez N, Febo M, Morrissette M, Strandwitz P, Kulkarni P, Ferris CF. The microbiome's influence on the neurobiology of opioid addiction and brain connectivity. Brain Res Bull. 2025;220:111159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Ren M, Lotfipour S. The role of the gut microbiome in opioid use. Behav Pharmacol. 2020;31:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 57. | García-Cabrerizo R, Cryan JF. A gut (microbiome) feeling about addiction: Interactions with stress and social systems. Neurobiol Stress. 2024;30:100629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 58. | Corriero A, Giglio M, Inchingolo F, Moschetta A, Varrassi G, Puntillo F. Gut Microbiota Modulation and Its Implications on Neuropathic Pain: A Comprehensive Literature Review. Pain Ther. 2024;13:33-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 59. | Ramakrishna C, Corleto J, Ruegger PM, Logan GD, Peacock BB, Mendonca S, Yamaki S, Adamson T, Ermel R, McKemy D, Borneman J, Cantin EM. Dominant Role of the Gut Microbiota in Chemotherapy Induced Neuropathic Pain. Sci Rep. 2019;9:20324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 60. | Pane K, Boccella S, Guida F, Franzese M, Maione S, Salvatore M. Role of gut microbiota in neuropathy and neuropathic pain states: A systematic preclinical review. Neurobiol Dis. 2022;170:105773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 61. | Li S, Zhu S, Yu J. The role of gut microbiota and metabolites in cancer chemotherapy. J Adv Res. 2024;64:223-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 50] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 62. | Biver E, Berenbaum F, Valdes AM, Araujo de Carvalho I, Bindels LB, Brandi ML, Calder PC, Castronovo V, Cavalier E, Cherubini A, Cooper C, Dennison E, Franceschi C, Fuggle N, Laslop A, Miossec P, Thomas T, Tuzun S, Veronese N, Vlaskovska M, Reginster JY, Rizzoli R. Gut microbiota and osteoarthritis management: An expert consensus of the European society for clinical and economic aspects of osteoporosis, osteoarthritis and musculoskeletal diseases (ESCEO). Ageing Res Rev. 2019;55:100946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 63. | Minerbi A, Shen S. Gut Microbiome in Anesthesiology and Pain Medicine. Anesthesiology. 2022;137:93-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 64. | Dahshan D, Gallagher N, Workman A, Perdue J, Aikens J, Schmicker T, Shuler FD. Targeting the Gut Microbiome for Inflammation and Pain Management in Orthopedic Conditions. Orthopedics. 2022;45:e226-e234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 65. | Aziz T, Hussain N, Hameed Z, Lin L. Elucidating the role of diet in maintaining gut health to reduce the risk of obesity, cardiovascular and other age-related inflammatory diseases: recent challenges and future recommendations. Gut Microbes. 2024;16:2297864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 110] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 66. | Markowiak-Kopeć P, Śliżewska K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients. 2020;12:1107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 754] [Article Influence: 125.7] [Reference Citation Analysis (0)] |
| 67. | Brown K, DeCoffe D, Molcan E, Gibson DL. Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients. 2012;4:1095-1119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 486] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 68. | Chen P, Wang C, Ren YN, Ye ZJ, Jiang C, Wu ZB. Alterations in the gut microbiota and metabolite profiles in the context of neuropathic pain. Mol Brain. 2021;14:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 69. | Nagpal R, Shively CA, Register TC, Craft S, Yadav H. Gut microbiome-Mediterranean diet interactions in improving host health. F1000Res. 2019;8:699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 70. | Barber TM, Kabisch S, Pfeiffer AFH, Weickert MO. The Effects of the Mediterranean Diet on Health and Gut Microbiota. Nutrients. 2023;15:2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 103] [Reference Citation Analysis (0)] |
| 71. | Tsigalou C, Paraschaki A, Karvelas A, Kantartzi K, Gagali K, Tsairidis D, Bezirtzoglou E. Gut microbiome and Mediterranean diet in the context of obesity. Current knowledge, perspectives and potential therapeutic targets. Metabol Open. 2021;9:100081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 72. | Kaur AP, Bhardwaj S, Dhanjal DS, Nepovimova E, Cruz-Martins N, Kuča K, Chopra C, Singh R, Kumar H, Șen F, Kumar V, Verma R, Kumar D. Plant Prebiotics and Their Role in the Amelioration of Diseases. Biomolecules. 2021;11:440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 73. | Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ, Berenjian A, Ghasemi Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods. 2019;8:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 604] [Cited by in RCA: 878] [Article Influence: 125.4] [Reference Citation Analysis (1)] |
| 74. | Ballini A, Charitos IA, Cantore S, Topi S, Bottalico L, Santacroce L. About Functional Foods: The Probiotics and Prebiotics State of Art. Antibiotics (Basel). 2023;12:635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 52] [Reference Citation Analysis (0)] |
| 75. | Bamigbade GB, Subhash AJ, Kamal-Eldin A, Nyström L, Ayyash M. An Updated Review on Prebiotics: Insights on Potentials of Food Seeds Waste as Source of Potential Prebiotics. Molecules. 2022;27:5947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 76. | Yoo S, Jung SC, Kwak K, Kim JS. The Role of Prebiotics in Modulating Gut Microbiota: Implications for Human Health. Int J Mol Sci. 2024;25:4834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 95] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 77. | Martín R, Langella P. Emerging Health Concepts in the Probiotics Field: Streamlining the Definitions. Front Microbiol. 2019;10:1047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 208] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 78. | Rau S, Gregg A, Yaceczko S, Limketkai B. Prebiotics and Probiotics for Gastrointestinal Disorders. Nutrients. 2024;16:778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 55] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 79. | Binda S, Hill C, Johansen E, Obis D, Pot B, Sanders ME, Tremblay A, Ouwehand AC. Criteria to Qualify Microorganisms as "Probiotic" in Foods and Dietary Supplements. Front Microbiol. 2020;11:1662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 259] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 80. | Maftei NM, Raileanu CR, Balta AA, Ambrose L, Boev M, Marin DB, Lisa EL. The Potential Impact of Probiotics on Human Health: An Update on Their Health-Promoting Properties. Microorganisms. 2024;12:234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 149] [Article Influence: 74.5] [Reference Citation Analysis (0)] |
| 81. | Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4055] [Cited by in RCA: 5999] [Article Influence: 499.9] [Reference Citation Analysis (4)] |
| 82. | Zheng Y, Bonfili L, Wei T, Eleuteri AM. Understanding the Gut-Brain Axis and Its Therapeutic Implications for Neurodegenerative Disorders. Nutrients. 2023;15:4631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 85] [Reference Citation Analysis (0)] |
| 83. | Zhu S, Jiang Y, Xu K, Cui M, Ye W, Zhao G, Jin L, Chen X. The progress of gut microbiome research related to brain disorders. J Neuroinflammation. 2020;17:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 297] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 84. | Cho KH, Na HS, Jhun J, Woo JS, Lee AR, Lee SY, Lee JS, Um IG, Kim SJ, Park SH, Cho ML. Lactobacillus (LA-1) and butyrate inhibit osteoarthritis by controlling autophagy and inflammatory cell death of chondrocytes. Front Immunol. 2022;13:930511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 50] [Reference Citation Analysis (0)] |
| 85. | Jeyanathan A, Ramalhete R, Blunn G, Gibbs H, Pumilia CA, Meckmongkol T, Lovejoy J, Coathup MJ. Lactobacillus cell-free supernatant as a novel bioagent and biosurfactant against Pseudomonas aeruginosa in the prevention and treatment of orthopedic implant infection. J Biomed Mater Res B Appl Biomater. 2021;109:1634-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 86. | Roberts JL, Chiedo B, Drissi H. Systemic inflammatory and gut microbiota responses to fracture in young and middle-aged mice. Geroscience. 2023;45:3115-3129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 87. | Tonelli Enrico V, Vo N, Methe B, Morris A, Sowa G. An unexpected connection: A narrative review of the associations between Gut Microbiome and Musculoskeletal Pain. Eur Spine J. 2022;31:3603-3615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 88. | Sahle Z, Engidaye G, Shenkute Gebreyes D, Adenew B, Abebe TA. Fecal microbiota transplantation and next-generation therapies: A review on targeting dysbiosis in metabolic disorders and beyond. SAGE Open Med. 2024;12:20503121241257486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 89. | Porcari S, Severino A, Rondinella D, Bibbò S, Quaranta G, Masucci L, Maida M, Scaldaferri F, Sanguinetti M, Gasbarrini A, Cammarota G, Ianiro G. Fecal microbiota transplantation for recurrent Clostridioides difficile infection in patients with concurrent ulcerative colitis. J Autoimmun. 2023;141:103033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 90. | Fortuna R, Wang W, Mayengbam S, Tuplin EWN, Sampsell K, Sharkey KA, Hart DA, Reimer RA. Effect of prebiotic fiber on physical function and gut microbiota in adults, mostly women, with knee osteoarthritis and obesity: a randomized controlled trial. Eur J Nutr. 2024;63:2149-2161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 91. | Schupack DA, Mars RAT, Voelker DH, Abeykoon JP, Kashyap PC. The promise of the gut microbiome as part of individualized treatment strategies. Nat Rev Gastroenterol Hepatol. 2022;19:7-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 125] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 92. | Aggarwal N, Kitano S, Puah GRY, Kittelmann S, Hwang IY, Chang MW. Microbiome and Human Health: Current Understanding, Engineering, and Enabling Technologies. Chem Rev. 2023;123:31-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 238] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 93. | Kumar P, Sinha R, Shukla P. Artificial intelligence and synthetic biology approaches for human gut microbiome. Crit Rev Food Sci Nutr. 2022;62:2103-2121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 94. | Merenstein D, Pot B, Leyer G, Ouwehand AC, Preidis GA, Elkins CA, Hill C, Lewis ZT, Shane AL, Zmora N, Petrova MI, Collado MC, Morelli L, Montoya GA, Szajewska H, Tancredi DJ, Sanders ME. Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes. 2023;15:2185034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 233] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 95. | Merrick B, Allen L, Masirah M Zain N, Forbes B, Shawcross DL, Goldenberg SD. Regulation, risk and safety of Faecal Microbiota Transplant. Infect Prev Pract. 2020;2:100069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 96. | Liwinski T, Elinav E. Harnessing the microbiota for therapeutic purposes. Am J Transplant. 2020;20:1482-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 97. | Ruxton CHS, Kajita C, Rocca P, Pot B. Microbiota and probiotics: chances and challenges - a symposium report. Gut Microbiome (Camb). 2023;4:e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 98. | Karimi M, Shirsalimi N, Hashempour Z, Salehi Omran H, Sedighi E, Beigi F, Mortezazadeh M. Safety and efficacy of fecal microbiota transplantation (FMT) as a modern adjuvant therapy in various diseases and disorders: a comprehensive literature review. Front Immunol. 2024;15:1439176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 58] [Reference Citation Analysis (0)] |
| 99. | Chen J, Wang A, Wang Q. Dysbiosis of the gut microbiome is a risk factor for osteoarthritis in older female adults: a case control study. BMC Bioinformatics. 2021;22:299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 100. | Brenner D, Shorten GD, O'Mahony SM. Postoperative pain and the gut microbiome. Neurobiol Pain. 2021;10:100070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 101. | Ustianowska K, Ustianowski Ł, Machaj F, Gorący A, Rosik J, Szostak B, Szostak J, Pawlik A. The Role of the Human Microbiome in the Pathogenesis of Pain. Int J Mol Sci. 2022;23:13267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 102. | Ding W, You Z, Chen Q, Yang L, Doheny J, Zhou X, Li N, Wang S, Hu K, Chen L, Xia S, Wu X, Wang C, Zhang C, Chen L, Ritchie C, Huang P, Mao J, Shen S. Gut Microbiota Influences Neuropathic Pain Through Modulating Proinflammatory and Anti-inflammatory T Cells. Anesth Analg. 2021;132:1146-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 103. | Hiltzik DM, Goodwin AM, Kurapaty SS, Inglis JE, Pagadala MS, Edelstein AI, Hsu WK. The Role of the Gut Microbiome in Orthopedic Surgery-a Narrative Review. Curr Rev Musculoskelet Med. 2024;17:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/