Published online Jun 20, 2025. doi: 10.5493/wjem.v15.i2.100443
Revised: November 19, 2024
Accepted: January 7, 2025
Published online: June 20, 2025
Processing time: 242 Days and 18.9 Hours
Gallbladder cancer (GBC) is a highly aggressive malignant tumor originating from the biliary tract. As one of the most common malignancies in the biliary system, GBC is particularly challenging due to its tendency to remain asymp
To establish a novel GBC cell line to investigate the molecular mechanisms underlying GBC progression and evaluate potential therapeutic targets.
We developed a unique GBC cell line, named OCUG-2, derived from a metastatic peritoneal implant, and verified its authenticity using short tandem repeat (STR) profiling. RT-PCR and RNA sequencing (RNA-seq) were performed to assess gene expression profiles, with functional enrichment analyzed through Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses. The MTT cell proliferation assay and an invasion assay were performed to evaluate response to nine inhibitors. Immunohistochemistry (IHC) was conducted on 34 GBC samples to analyze insulin-like growth factor 1 receptor (IGF1R) expression.
OCUG-2 cells displayed adhesive growth with dendritic morphology and a 30-hour doubling time. Subcutaneous inoculation of OCUG-2 cells into mice confirmed their tumorigenic potential. STR analysis authenticated the cell line, and there was high mRNA and protein expression of IGF1R in OCUG-2 cells. The IGF1R inhibitor picropodophyllotoxin significantly inhibited OCUG-2 cell proliferation, yielding an IC50 of 0.49 μM. RNA-seq analysis identified gene fusions, and GO/KEGG functional enrichment analyses revealed pathways implicated in cancer progression. IHC analysis showed IGF1R positivity in 18 of 34 GBC cases, with significant association between IGF1R expression and poor prognosis. In invasion assays, an IGF1R inhibitor effectively reduced OCUG-2 cell invasiveness.
IGF1R might be a promising target for GBC. The newly established OCUG-2 cell line serves as a valuable model for investigating the molecular mechanisms of GBC and evaluating therapeutic strategies.
Core Tip: We established a new gallbladder cancer (GBC) cell line derived from a metastatic peritoneal implant of GBC. These cells, named OCUG-2, showed adhesive growth with a dendritic morphology. Doubling time of OCUG-2 cells was 30 hours. OCUG-2 cells were positive for insulin-like growth factor 1 receptor (IGF1R), and an IGF1R inhibitor reduced the proliferation of OCUG-2 cells. Immunohistochemistry found IGF1R positivity in 18 of 34 cases of GBC, and IGF1R expression was associated with poor prognosis. These findings suggest that IGF1R may be a promising target for GBC and OCUG-2 might be useful for analysis of GBC.
- Citation: Wang Q, Fan C, Tsujio G, Sakuma T, Maruo K, Yamamoto Y, Imanishi D, Kawabata K, Nishikubo H, Kanei S, Aoyama R, Kushiyama S, Ohira M, Yashiro M. Establishment and characterization of a new human gallbladder cancer cell line, OCUG-2. World J Exp Med 2025; 15(2): 100443
- URL: https://www.wjgnet.com/2220-315x/full/v15/i2/100443.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i2.100443
Gallbladder cancer (GBC) accounts for approximately two-thirds of all biliary tract cancers[1]. Most early-stage GBC cases are identified incidentally by cholecystectomy performed for gallbladder stones, and they have relatively good prognosis. By contrast, patients with GBC diagnosed at a late stage show poor survival[1-3], possibly because these tumors present limited sensitivity to chemotherapy. The development of a novel therapy based on the biological behaviors of GBC is urgently being sought to improve the prognosis of late-stage GBC; however, the mechanisms responsible for the characteristic progression of GBC have not been clearly understood. While GBC cell lines would be useful in studying the biological behavior of GBC[4,5], the number of GBC cell lines remains small. To the best of our knowledge, only nine GBC cell lines, have been developed to date, namely OCUG-1, NOZ, TYGBK-1, TYGBK-8, G-415, EH-GB2, ZJU-0430, and TJGBC2[6-9].
Insulin-like growth factor 1 receptor (IGF1R), a tyrosine kinase receptor, is crucial for various biological functions such as cell proliferation, differentiation, and metabolism. Dysregulated expression of molecules in the IGF1R pathways has been detected across a spectrum of cancers, including brain tumors, head and neck cancer, breast cancer, and gastrointestinal cancer[10]. Despite promising preclinical evidence supporting the potential of IGF1R-targeted therapies as anticancer treatments, clinical trials have yet to yield definitive results[11]. Research on the expression of IGF1R in GBC is scarce, and the role of IGF1R-targeted therapies in GBC remains uncertain.
In this study, we established a new GBC cell line, OCUG-2, and introduce further evidence suggesting that IGF1R-targeted therapies hold promise for patients with GBC.
The cell line was isolated from metastatic peritoneal implants of GBC in a 78-year-old male. This tumor was cultured in Dulbecco's Modified Eagle Medium (DMEM; Fujifilm Wako Pure Chemical Co., Osaka, Japan) supplemented with 10% fetal bovine serum (FBS; Nichirei Biosciences, Inc., Tokyo, Japan), 0.5 mmol/L sodium pyruvate (Sigma-Aldrich, St. Louis, MO, United States), and a penicillin-streptomycin solution (Fujifilm Wako Pure Chemical Corporation). Adherent cell lines, identified within the tumor tissue culture, were termed OCUG-2. OCUG-2 cells were harvested using trypsin (Fujifilm Wako Pure Chemical Co.). The cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2. The OCUG-2 cells had been maintained for over 24 months and had undergone more than 100 passages. All experiments utilized cells confirmed to be free from mycoplasma contamination. This study was approved by the Osaka Metropolitan University Ethics Committee (Reference No. 0924, 2022-110). Informed consent was obtained from the patient. This study was conducted according to the principles of the Declaration of Helsinki.
A suspension of approximately 1 × 107 OCUG-2 cells was subcutaneously inoculated into BALB/c nude mice (provided by Oriental Kobo, Osaka, Japan). After 6 weeks, tumor incidence was assessed. The subcutaneous tumors were fixed in 10% formalin for paraffin embedding and sectioning. All animal experiments were conducted in accordance with the guidelines of the Ethics Committee.
Fifty thousand OCUG-2 cells were seeded in 24-well plates with 1 mL DMEM containing 10% FBS, and cell numbers were counted every 24 hours for 11 days with the Coulter Counter Z2 (Beckman Coulter, Inc., Sykesville, MD, United States). The doubling time was determined from the growth curve.
To authenticate the cell line, short tandem repeat (STR) profiling was conducted by the Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan).
Total cellular RNA was extracted from OCUM-2 cells using TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, United States). Subsequently, 0.4 μg RNA was reverse transcribed into cDNA using the ReverTra Ace qPCR RT Master Mix (TOYOBO, Osaka, Japan). Then, the synthesized cDNA was amplified by PCR in 40 cycles, each cycle consisting of an annealing step at 60 °C for 30 seconds, an extension step at 72 °C for 60 seconds, and a final extension step at 72 °C for 7 minutes. PCR amplification was performed in a thermal cycler using AmpliTaq Gold 360 Master Mix (Thermo Fisher Scientific).
Twelve molecular target drugs were employed: Picropodophyllotoxin (IGF1R inhibitor; Sigma), CP-673451 (PDGFR inhibitor; MedChemExpress, Monmouth Junction, NJ, United States), afatinib (EGFR inhibitor; MedChemExpress), SU11274 (c-Met inhibitor; Selleckchem, Houston, TX, United States), infigratinib (FGFR1/2/3 inhibitor; Med
Prior to initiating the Transwell assay, the membrane was pre-coated with Matrigel to simulate the extracellular matrix. Cells were subsequently suspended in serum-free DMEM and seeded into the upper compartment, which contained a membrane with 12-μm pores (Corning Inc., Corning, NY, United States). The lower compartment contained DMEM supplemented with 10% FBS, serving as a chemoattractant, along with nine molecular target drugs: Panitumumab, cetuximab, bevacizumab, ramucirumab, SU11274, infigratinib, FGF401, sapitinib, and trastuzumab. After incubation for 72 hours, cells that migrated to the lower compartment were stained with Diff-Quick (Sysmex Co., Kobe, Japan) and counted.
A total of 34 GBC tissue samples were obtained from Osaka Metropolitan University Hospital (Osaka, Japan), with all patients providing written informed consent. The tissue slides were subjected to heating for deparaffinization. After blocking the endogenous peroxidase activity, samples underwent overnight incubation at 4 °C with anti-IGF1R antibody (1:200; Santa Cruz Biotechnology, Dallas, TX, United States). This step was followed by incubation with a biotinylated secondary antibody and application of streptavidin-peroxidase complex. Counterstaining was performed using Mayer's hematoxylin. Immunohistochemistry (IHC) staining results were evaluated based on both the intensity and area of positive staining, with two independent pathologists conducting reviews independently. Positive cell percentages were scored as: < 5%, 0; 5%-25%, 1; 26%-50%, 2; 51%-75%, 3; and 76%-100%, 4. Staining intensity was graded as follows: 0 for no visible staining, 1 for pale yellow, 2 for tan, and 3 for brown. The final IHC score for each sample was calculated by multiplying the scores of positive cell percentage and staining intensity. A score of ≥ 6 was deemed indicative of positive expression.
Total RNA was extracted from OCUG-2 cells. Then, cDNA libraries were generated and sequenced on the Illumina NovaSeq 6000 platform (Illumina, Inc., San Diego, CA, United States), producing 150 bp paired-end reads. Clean RNA sequencing (RNA-seq) reads were mapped to the human reference genome (GRCh38) using the STAR aligner (version 2.7.10b), incorporating gene annotation data from the National Center for Biotechnology Information (NCBI, Bethesda, MD, United States) RefSeq database obtained from NCBI. Gene fusions were detected using STAR-Fusion (version 1.10.0) and Arriba (version 2.4.0). The results were obtained by taking the intersection of the findings from both tools.
Biological functional enrichment was analyzed using Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis with the R package "ClusterProfiler", utilizing the “org.Hs.eg.db” package for human gene annotation. The cutoff criterion for significance was defined as P < 0.05.
Each experiment was carried out independently and in triplicate, except when noted otherwise. Data are presented as the mean ± SD. The graphs and statistical analyses were conducted using RStudio version 2023.06.0+421 and Python 2.7.3. Standard two-tailed unpaired student’s t-tests or Fisher's exact test were employed for statistical evaluations. P < 0.05 was considered statistically significant. The significance levels are denoted as follows: Not significant (NS; P > 0.05, aP < 0.05, bP < 0.01, and cP < 0.001). The statistical methods used in this study were reviewed by Tomoya Sano, a biomedical statistician from Osaka Metropolitan University Graduate School of Medicine Department of Gastroenterological Surgery, and by Qiang Wang from Osaka Metropolitan University Graduate School of Medicine.
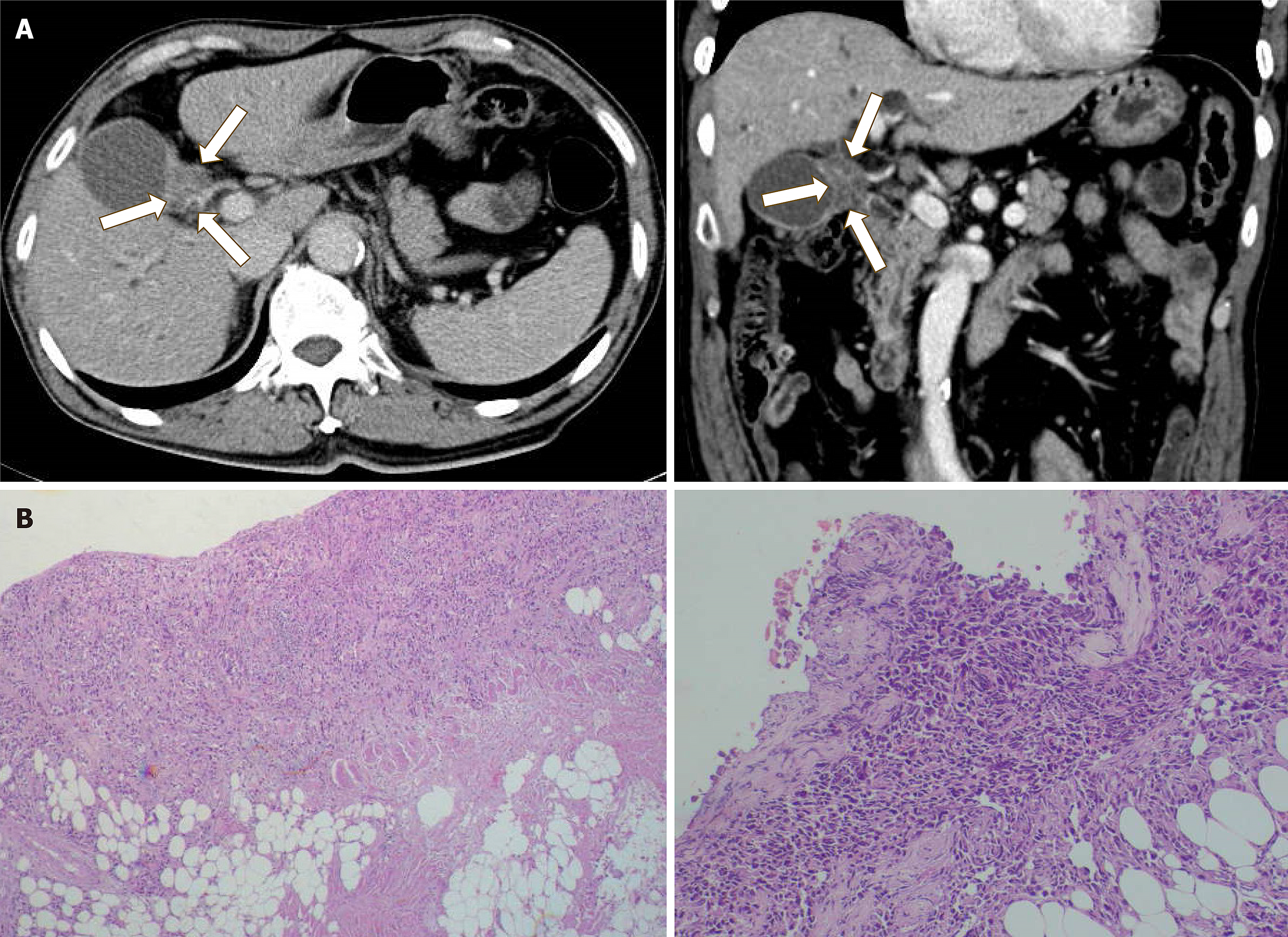
A computed tomography scan delineated the site of the primary gall bladder tumor (Figure 1A) from the patient whose metastatic peritoneal implants we harvested to establish the novel GBC cell line, OCUG-2. Histopathological analysis of the peritoneal tumor revealed it to be poorly differentiated (Figure 1B). The OCUG-2 cells did not require immortalization procedures. STR analysis demonstrated that approximately 70% of the loci matched those in STR profile databases, identifying OCUG-2 as a unique cell line originating from GBC (Supplemental Figure 1; Table 1). Under microscopic examination, OCUG-2 cells displayed a dendritic morphology (Figure 2A) and were found to proliferate with a doubling time of 30 hours (Figure 2B). Histological examination of the xenografted tumors following the subcutaneous injection of 1.0 × 107 OCUG-2 cells into mice revealed a poorly differentiated tumor with moderate stromal cells (Figure 2C).
| Microsatellite (chromosome number) | OCUG-2 cells |
| D3S1358 (3) | 16 |
| TH01 (11) | 7, 9 |
| D21S11 (21) | 30 |
| D18S51 (18) | 13 |
| Penta E (15) | 12 |
| D5S818 (5) | 12 |
| D13S317 (13) | 12 |
| D7S820 (7) | 9, 12 |
| D16S539 (16) | 9, 10 |
| CSF1PO (5) | 12 |
| Penta D (21) | 11 |
| Amelogenin (X Y) | X |
| vWA (12) | 16 |
| D8S1179 (8) | 13, 15 |
| TPOX (2) | 8, 12 |
| FGA (4) | 20, 21 |
In OCUG-2 cells, the mRNA expression of FGFR1, FGFR3, FGFR4, VEGFR2, PDGF-Rβ, EGFR, ERBB2, ERBB3, IGF1R, FMS-like tyrosine kinase 3, and c-Met was detected, whereas the expression of FGFR2, VEGFR1, VEGFR3, PDGFRα, ERBB4, c-Kit, and EGFR was not observed (Figure 3A).
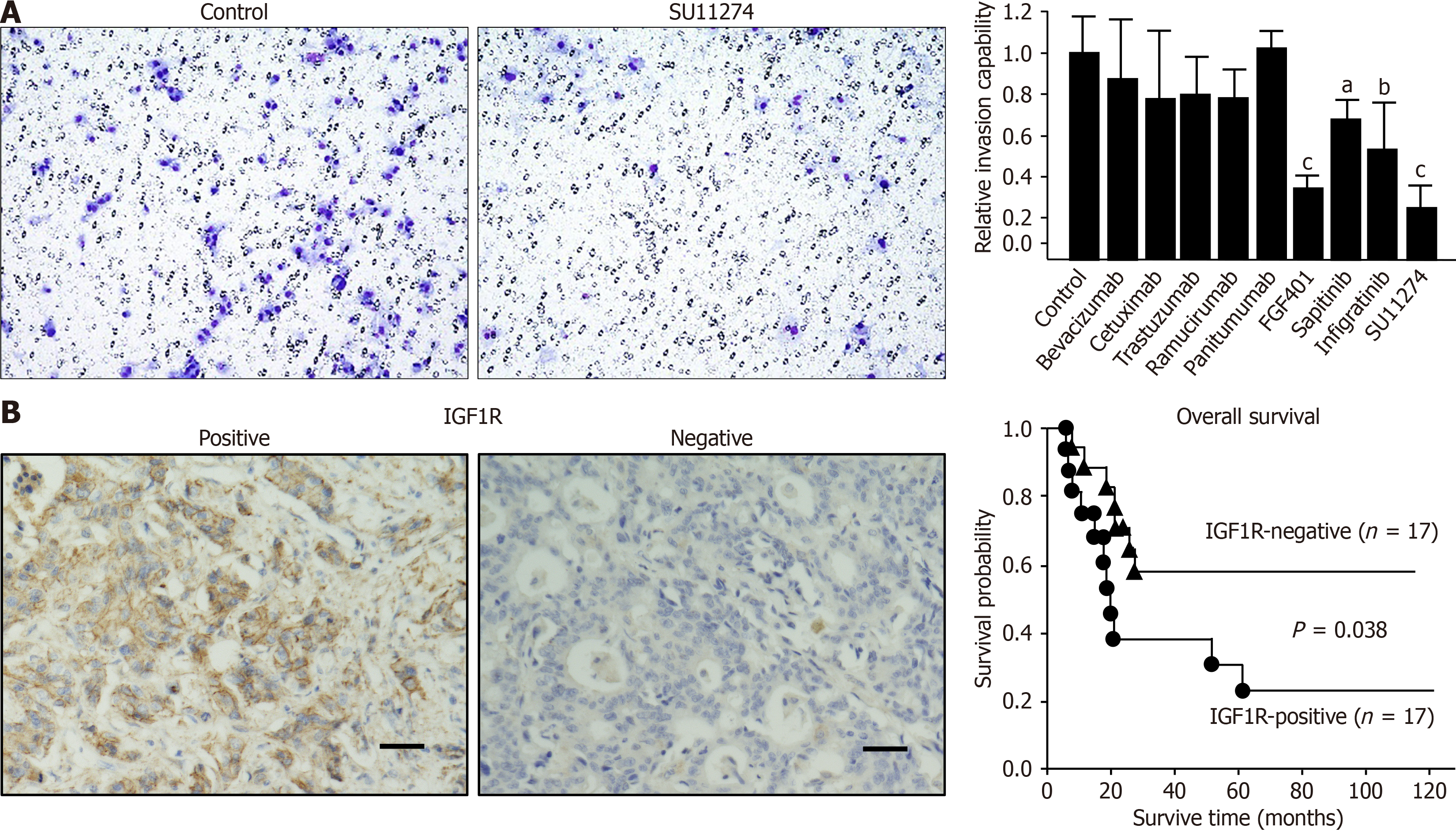
Among the tested compounds, only the IGF1R inhibitor picropodophyllotoxin exhibited potent inhibitory effects on tumor growth, with an IC50 of 0.49 μM in OCUG-2 cells. CP673451 and afatinib demonstrated moderate inhibition of OCUG-2 cell proliferation, with IC50 values of 2.6 μM and 3.1 μM, respectively. The inhibitory effects of SU11274 (IC50 = 10.5 μM), infigratinib (IC50 = 10.6 μM), FGF401 (IC50 = 32.7 μM), and sapitinib (IC50 = 21.1 μM) on OCUG-2 cell growth were notably weak, while the remaining drugs exhibited no significant inhibitory effect at all (Figure 3B).
In the invasion assays, nine molecularly targeted drugs were evaluated following their performance in growth inhibition assessments: Panitumumab, cetuximab, bevacizumab, ramucirumab, SU11274, infigratinib, FGF401, sapitinib, and trastuzumab. Of these, SU11274 and FGF401 demonstrated notable inhibitory effects on tumor invasion (Figure 4A). The inhibitory effects of infigratinib and sapitinib were comparably modest, while the other compounds appeared to exert no significant inhibitory effect on the invasion capabilities of OCUG-2 cells.
Analysis of GBC tissues from the 34 patients revealed that 17 cases had positive IGF1R expression, while the other 17 were negative for IGF1R expression. Results of IHC facilitated the division of these patients into two distinct groups. Analysis of clinical data revealed no significant distinction between the groups with IGF1R-positive and IGF1R-negative expression (Table 2); however, Kaplan-Meier survival analysis demonstrated a significant difference (P = 0.038) in overall survival between patients with positive and negative IGF1R expression (Figure 4B). Univariate regression analysis for overall survival indicated that factors such as chemotherapy status and tumor-node-metastasis staging, together with IGF1R expression, were associated with the overall survival of patients with GBC, while multivariate regression analysis identified IGF1R expression as the sole factor significantly associated with overall survival (Table 3).
| Feature | IGF1R-positive (n = 17) | IGF1R-negative (n = 17) | P value |
| Sex | |||
| Male | 6 | 10 | |
| Female | 11 | 7 | 0.17 |
| Chemotherapy | |||
| Yes | 11 | 10 | |
| No | 6 | 7 | 0.72 |
| T stage | |||
| T1/T2 | 8 | 10 | |
| T3/T4 | 9 | 7 | 0.49 |
| N stage | |||
| Negative | 8 | 12 | |
| Positive | 9 | 5 | 0.16 |
| M stage | |||
| Negative | 14 | 16 | |
| Positive | 3 | 1 | 0.28 |
| Clinical stage | |||
| I/II | 6 | 10 | |
| III/IV | 11 | 7 | 0.16 |
| Feature | Univariate analysis | Multivariate analysis | ||
| Hazard ratio (95%CI) | P value | Hazard ratio (95%CI) | P value | |
| Sex | 0.658 (0.259-1.672) | NS | ||
| Chemotherapy | 3.039 (1.077-8.578) | 0.03 | 1.969 (0.594-6.517) | NS |
| T | 1.771 (1.165-2.692) | 0.005 | ||
| N | 2.833 (1.2-6.687) | 0.01 | ||
| M | 4.905 (1.516-15.88) | 0.003 | ||
| Stage | 1.767 (1.146-2.723) | 0.006 | 1.595 (0.950-2.679) | 0.077 |
| IGF1R expression | 2.654 (1.102-6.913) | 0.04 | 3.302 (1.208-9.023) | 0.02 |
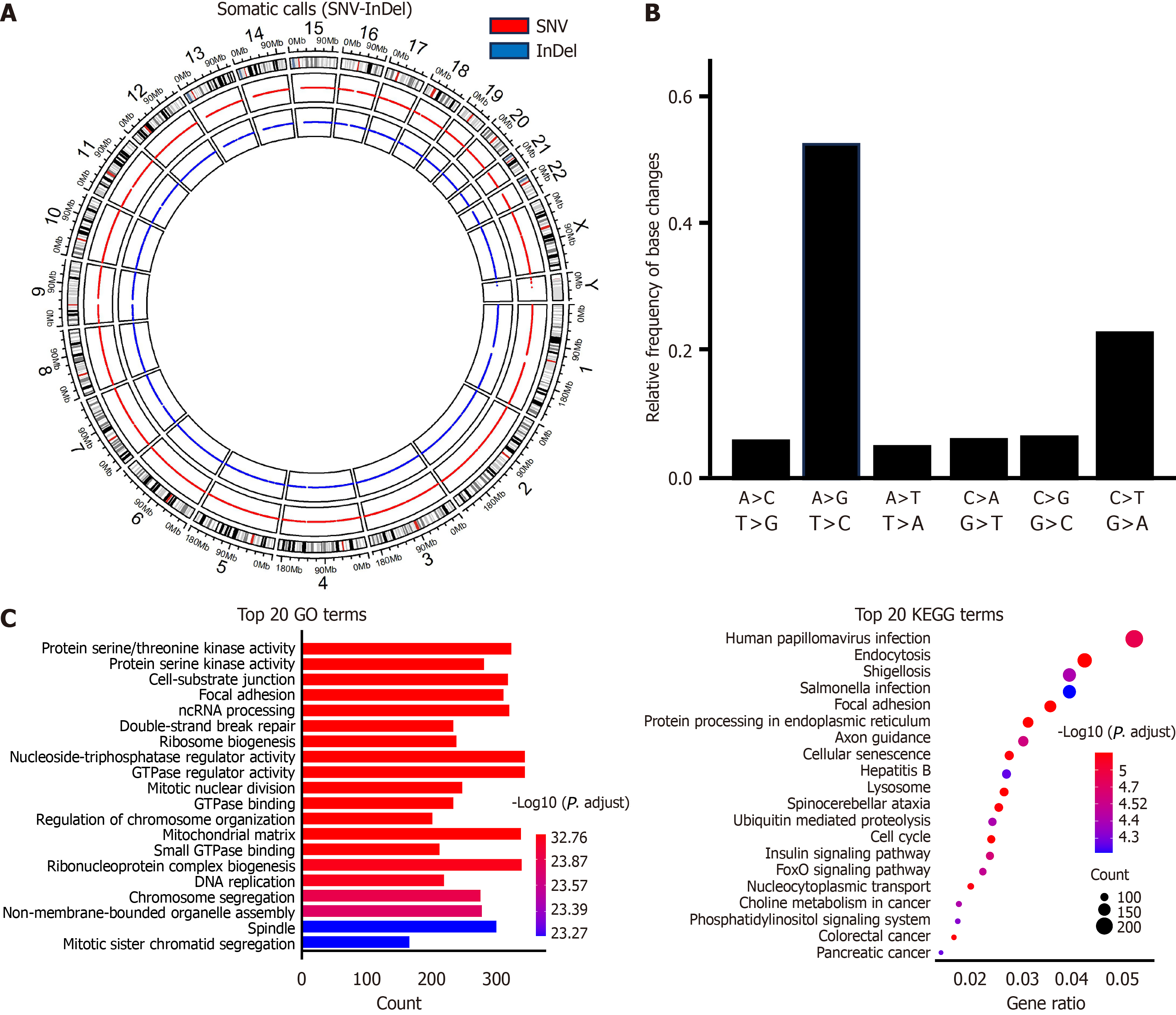
RNA-seq of OCUG-2 cells yielded 133346877 paired sequences. SnpEff initially identified 509702 variants, and after stringent filtering to remove instances where the reference matched the alternative, indicating non-variants, a total of 507657 variants were retained for in-depth analysis. Among these, 311622 variants, accounting for 61.4%, were classified as known variants. The analysis further revealed 5186 multi-allelic Variant Call Format entries, underscoring a variant rate of one variant every 6083 bases and emphasizing the rich genomic variation within the OCUG-2 cells. The variant breakdown included 377093 single nucleotide polymorphisms, 69993 insertions, and 60571 deletions (Figure 5A). Among the characterized variant effects, 221030 (2.2%) were identified as having high impact, while 208325 (2.0%) exhibited low impact. Most of the effects, 95%, were categorized as modifiers, with a smaller fraction, 0.48%, being moderate. Missense mutations accounted for 47.1% of these effects. Additionally, nonsense mutations made up 1.1%, and silent mutations represented 51.9 of the total variant effects. Notably, the most prevalent nucleotide substitutions in OCUG-2 cells were A↔G and C↔T transitions (Figure 5B).
Fourteen potential fusions were detected in OCUG-2 cells. Notably, protocadherin alpha 3 (PCDHA3)-PCDHAC2, ERC1-phosphodiesterase 3A, and PCDHGB2-PCDHGA8 fusions were identified as in-frame (Table 4). Specifically, the tetraspanin 14-AL392185.1 fusion spanned two chromosomes (chromosome 10 and chromosome 9) but was characterized as out-of-frame. The analyses of functional biological processes and KEGG pathway enrichments for mutated genes in OCUG-2 cells are presented in Figure 5C.
| Gene fusion | Breakpoint1 | Breakpoint2 | Confidence | Reading frame |
| LEPROT: LEPR | chr1:65425378 | chr1:65565546 | High | Out-of-frame |
| LEPROT: LEPR | chr1:65430048 | chr1:65565546 | Medium | Out-of-frame |
| TSPAN14: AL392185.1 | chr10:80504778 | chr9:95602159 | High | Out-of-frame |
| TSPAN14: AL392185.1 | chr10:80489314 | chr9:95602159 | High | Out-of-frame |
| TSPAN14: AL392185.1 | chr10:80506862 | chr9:95602874 | Medium | |
| TSPAN14: AL392185.1 | chr10:80504778 | chr9:95600165 | Medium | Out-of-frame |
| RNF115: ITGA10 | chr1:145823772 | chr1:145907465 | High | Out-of-frame |
| PCDHA3: PCDHAC2 | chr5:140803591 | chr5:140978949 | High | In-frame |
| AC092807.3: DDAH1 | chr1:85496166 | chr1:85358847 | High | |
| AC092807.3: DDAH1 | chr1:85494729 | chr1:85358847 | Medium | |
| AC092807.3: DDAH1 | chr1:85577984 | chr1:85358847 | Medium | |
| ERC1: PDE3A | chr12:1141787 | chr12:20556660 | High | In-frame |
| TVP23C: CDRT4 | chr17:15540433 | chr17:15440285 | High | Out-of-frame |
| PRR16: AC114284.1 | chr5:120464645 | chr5:120789954 | High | Out-of-frame |
| PCDHGB2: PCDHGA8 | chr5:141362556 | chr5:141494807 | Medium | In-frame |
| MYO9A: PKM | chr15:72117680 | chr15:72219110 | Medium | |
| RASSF8: SSPN | chr12:26055446 | chr12:26224293 | Medium | Out-of-frame |
| RASSF8: SSPN | chr12:26055446 | chr12:26179996 | Medium | Out-of-frame |
| AC104123.1: CAST | chr5:96379652 | chr5:96675539 | Medium | |
| MGST1: AC007529.2 | chr12:16383604 | chr12:16661766 | Medium | |
| LDLRAD3: PRR5L | chr11:36098461 | chr11:36296290 | Medium | Out-of-frame |
We previously reported the establishment a GBC cell line named OCUG-1. In this study, we successfully established a novel GBC cell line named OCUG-2, derived from the metastatic peritoneal implants of a patient with GBC. OCUG-2 cells exhibit an adhesive growth pattern with a dendritic morphology. Subcutaneous tumors in mice inoculated with OCUG-2 cells displayed poor differentiation, which resembled the histopathological features of the original primary GBC, suggesting the validity of the OCUG-2 cell line as a representation of primary gallbladder tumors. STR analysis demonstrated that OCUG-2 did not match any STR profile databases of reported cell lines, indicating that it is an original cell line. These findings might suggest that OCUG-2 is a valuable and unique cell line for the characterization of GBC.
OCUG-2 cells showed high mRNA expression of IGF1R and positive expression of IGF1R. The IGF1R inhibitor picropodophyllotoxin significantly reduced the proliferation of OCUG-2 cells, with an IC50 of 0.49 μM, suggesting that IGF1R is a key oncogene in OCUG-2 cells. IHC found IGF1R positivity in 18 of 34 GBC cases, and IGF1R expression was significantly associated with poor prognosis of patients with GBC. Multivariate regression analysis identified IGF1R expression as an independent prognostic factor. Kornprat et al[12] previously reported that IGF1R expression in 57 cases of GBC was correlated with lymph node metastases and hepatic metastases. These findings suggest that IGF1R signaling may be a promising target for GBC.
The amelogenin gene, located in the Xp22.1-Xp22.3 region of the X chromosome and the Yp11.2 region of the Y chromosome, is utilized in sex-typing tests. Observations across global populations have revealed variable rates of amelogenin on the Y chromosome (AMELY) dropout: 8.3% in Sri Lankans[13], 3.6% in Indian-Malays, 0.88% in Malays[14], and 0.02% in Australians[15]. Although the exact frequency of AMELY deletion in the Japanese population remains undetermined, occurrences have been documented[16]. Despite the common observation of Y nullisomy in various human malignancies[17], RNA-seq analysis in this study detected the expression of genes located on the Y chromosome in OCUG-2 cells, confirming the chromosome's presence. These findings suggest that the AMELY dropout observed in this study may be inherent to the patient from whom the OCUG-2 cell line was derived.
KEGG pathway analysis of mutated genes in OCUG-2 cells indicated human papillomavirus (HPV), Shigellosis, and Salmonella infections as three of the top four enriched terms. While HPV infection is reportedly linked with anogenital and oropharyngeal cancer[18], the association between HPV infection and GBC has not been documented. Kirkegård et al[19] conducted a cohort study involving 83008 women who underwent cervical conization, which served as an indicator of chronic HPV infection. The authors observed increased risks of certain types of cancer, including those of the gallbladder and biliary tract, with standardized incidence ratios of 1.3. Unlike the case with esophageal tumors[20,21], however, direct evidence connecting the presence of HPV DNA to GBC is still lacking. By contrast, an association of Salmonella infection with GBC has been confirmed[22,23]. Pathways such as MAPK/extracellular signal-regulated kinase, PI3K/Akt/mTOR, CREB/SP-1, and BSG (CD147) have been proposed to mediate Salmonella-induced gallbladder carcinogenesis[24]. In our study, the detection of protein serine/threonine kinase activity from GO analysis indicated that the PI3K/Akt, Ras/MAPK/mTOR, and protein kinase C signaling pathways may play pivotal roles in OCUG-2 cells.
In summary, we successfully established OCUG-2, a novel cell line derived from the peritoneal metastases of a patient with GBC. IGF1R signaling is presumed to be one of promising targets for OCUG-2 cells. Thus, OCUG-2 cells might be useful for the analysis of characteristic features of GBC.
We would like to acknowledge the Research Support Platform at Osaka Metropolitan University Graduate School of Medicine for providing the necessary infrastructure for our study.
| 1. | Roa JC, García P, Kapoor VK, Maithel SK, Javle M, Koshiol J. Gallbladder cancer. Nat Rev Dis Primers. 2022;8:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 247] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 2. | Juengpanich S, Li S, Yang T, Xie T, Chen J, Shan Y, Lee J, Lu Z, Chen T, Zhang B, Cao J, Hu J, Yu J, Wang Y, Topatana W, Gu Z, Cai X, Chen M. Pre-activated nanoparticles with persistent luminescence for deep tumor photodynamic therapy in gallbladder cancer. Nat Commun. 2023;14:5699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 3. | Zhou Y, Yuan K, Yang Y, Ji Z, Zhou D, Ouyang J, Wang Z, Wang F, Liu C, Li Q, Zhang Q, Li Q, Shan X, Zhou J. Gallbladder cancer: current and future treatment options. Front Pharmacol. 2023;14:1183619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 4. | Lai J, Yang S, Lin Z, Huang W, Li X, Li R, Tan J, Wang W. Update on Chemoresistance Mechanisms to First-Line Chemotherapy for Gallbladder Cancer and Potential Reversal Strategies. Am J Clin Oncol. 2023;46:131-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Pavlidis ET, Galanis IN, Pavlidis TE. New trends in diagnosis and management of gallbladder carcinoma. World J Gastrointest Oncol. 2024;16:13-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (6)] |
| 6. | Yamada N, Chung Y, Ohtani H, Ikeda T, Onoda N, Sawada T, Nishiguchi Y, Hasuma T, Sowa M. Establishment and characterization of a new human gallbladder carcinoma cell line (OCUG-1) producing TA-4. Int J Oncol. 1997;10:1251-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Sekine S, Shimada Y, Nagata T, Moriyama M, Omura T, Yoshioka I, Hori R, Matsui K, Sawada S, Okumura T, Yoshida T, Tsukada K. Establishment and characterization of a new human gallbladder carcinoma cell line. Anticancer Res. 2012;32:3211-3218. [PubMed] |
| 8. | Liu ZY, Xu GL, Tao HH, Yang YQ, Fan YZ. Establishment and characterization of a novel highly aggressive gallbladder cancer cell line, TJ-GBC2. Cancer Cell Int. 2017;17:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Wang JH, Li LF, Yu Y, Li B, Jin HJ, Shen DH, Li J, Jiang XQ, Qian QJ. Establishment and characterization of a cell line, EH-GB2, derived from hepatic metastasis of gallbladder cancer. Oncol Rep. 2012;27:775-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Kumar AS, Rayala SK, Venkatraman G. Targeting IGF1R pathway in cancer with microRNAs: How close are we? RNA Biol. 2018;15:320-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Osher E, Macaulay VM. Therapeutic Targeting of the IGF Axis. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 12. | Kornprat P, Rehak P, Rüschoff J, Langner C. Expression of IGF-I, IGF-II, and IGF-IR in gallbladder carcinoma. A systematic analysis including primary and corresponding metastatic tumours. J Clin Pathol. 2006;59:202-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Santos FR, Pandya A, Tyler-Smith C. Reliability of DNA-based sex tests. Nat Genet. 1998;18:103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 137] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Chang YM, Burgoyne LA, Both K. Higher failures of amelogenin sex test in an Indian population group. J Forensic Sci. 2003;48:1309-1313. [PubMed] |
| 15. | Mitchell RJ, Kreskas M, Baxter E, Buffalino L, Van Oorschot RA. An investigation of sequence deletions of amelogenin (AMELY), a Y-chromosome locus commonly used for gender determination. Ann Hum Biol. 2006;33:227-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Kumagai R, Sasaki Y, Tokuta T, Biwasaka H, Aoki Y. DNA analysis of family members with deletion in Yp11.2 region containing amelogenin locus. Leg Med (Tokyo). 2008;10:39-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Bianchi NO. Y chromosome structural and functional changes in human malignant diseases. Mutat Res. 2009;682:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Schiffman M, Doorbar J, Wentzensen N, de Sanjosé S, Fakhry C, Monk BJ, Stanley MA, Franceschi S. Carcinogenic human papillomavirus infection. Nat Rev Dis Primers. 2016;2:16086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 644] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 19. | Kirkegård J, Farkas DK, Søgaard M, Schmidt SA, Ostenfeld EB, Cronin-Fenton D. Conization as a marker of persistent cervical human papillomavirus (HPV) infection and risk of gastrointestinal cancer: a Danish 34-year nationwide cohort study. Cancer Causes Control. 2014;25:1677-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Rajendra S, Pavey D, McKay O, Merrett N, Gautam SD. Human papillomavirus infection in esophageal squamous cell carcinoma and esophageal adenocarcinoma: a concise review. Ann N Y Acad Sci. 2020;1482:36-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Zhou XB, Guo M, Quan LP, Zhang W, Lu ZM, Wang QH, Ke Y, Xu NZ. Detection of human papillomavirus in Chinese esophageal squamous cell carcinoma and its adjacent normal epithelium. World J Gastroenterol. 2003;9:1170-1173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Koshiol J, Wozniak A, Cook P, Adaniel C, Acevedo J, Azócar L, Hsing AW, Roa JC, Pasetti MF, Miquel JF, Levine MM, Ferreccio C; Gallbladder Cancer Chile Working Group. Salmonella enterica serovar Typhi and gallbladder cancer: a case-control study and meta-analysis. Cancer Med. 2016;5:3310-3235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 23. | Shukla R, Shukla P, Behari A, Khetan D, Chaudhary RK, Tsuchiya Y, Ikoma T, Asai T, Nakamura K, Kapoor VK. Roles of Salmonella typhi and Salmonella paratyphi in Gallbladder Cancer Development. Asian Pac J Cancer Prev. 2021;22:509-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Khan AA, Bano Y. Salmonella enterica subsp. enterica host-pathogen interactions and their implications in gallbladder cancer. Microb Pathog. 2021;157:105011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/