INTRODUCTION
Aortic aneurysm (AA) and aortic dissection (AD) pose significant health risks due to demographic trends and contemporary lifestyles. Mortality from ruptured AA reaches as high as 80%[1]. Current opinion suggests that AD results from rupture of the intima, leading to splitting of the aortic wall layers, hemorrhage, and the risk of aortic rupture. Degenerative processes that contribute to the progressive increase in aortic diameter and, ultimately, a significant tear in the inner vascular layer are undoubtedly among the elements driving the pathogenesis of aortic disorders. However, clinical observations reveal that cardiothoracic surgery patients can exhibit aortic diameters exceeding 5.0 cm without signs of dissection, displaying instead only degenerative tissue changes. Understanding the development of dissections requires insight into the remodeling processes of the extracellular matrix (ECM). Matrix metalloproteinases (MMPs) function in the extracellular environment of cells and degrade both matrix and non-matrix proteins. They are involved in morphogenesis, wound healing, tissue repair, and remodeling in response to injury, such as after myocardial infarction, as well as in the progression of diseases including atheroma, arthritis, cancer, and chronic tissue ulcers. Inspired by Barkhordarian et al[2], the authors present MMPs and their inhibitors in immunohistological analyses as contributing factors in the pathophysiology of AA. MMPs are multi-domain proteins whose activities are regulated by tissue inhibitors of metalloproteinases (TIMPs)[3]. Upregulation of MMPs, particularly MMP-2 and MMP-9, has been identified as a key event in aneurysmal growth. The therapeutic potential of suppressing MMP-2 and MMP-9 activity, as well as modulating other MMPs and TIMPs involved in pathology and aging, has been explored[4,5], although the extent of mechanical load also appears to be pivotal[6,7]. In an experimental abdominal AA model, Longo et al[8] found that macrophage-derived MMP-9 and mesenchymal cell-derived MMP-2 are both required and work in concert to produce aneurysms.
CD40 ligand (CD40L), myeloperoxidase (MPO), MMPs including (MMP-1, MMP-2, and MPP-9), and TIMPs (TIMP-1 and TIMP-2) are biologically related molecules that integrate inflammation, tissue injury, and remodeling—all processes associated with AD[9]. Vianello et al[10] evaluated whether circulating levels of these molecules could serve as potential biomarkers for the diagnosis of AD and highlighted the simultaneous evaluation of CD40L, MPO, MMP-1, and TIMP-1. This combination may contribute to structural changes in aortic tissue in AD patients and appears to represent a promising novel diagnostic panel[10-12]. To date, no specific biomarkers are available to accelerate diagnosis. This gap motivated our group to perform a histological examination of diseased aortas[12]. In our recently published histological study, we examined the extent of MMP-1 and MMP-9 expression, as well as the detection of TIMP-1 and TIMP-2, to gain insights into potential distinctions between aneurysm formation and the conditions leading to AD[11,12]. It is well established that inflammatory and necrotic mechanisms drive degenerative and structural remodeling of aortic tissue. Tumor necrosis factor (TNF)-α and interleukin (IL)-1 stimulate MMP-1 production, while transforming growth factor-beta (TGF-β) enhances the production of type I and type III collagen, increases the expression of protease inhibitors, and upregulates plasminogen activator inhibitor-1 and TIMP-1. Based on the evaluation of our findings, we propose the following hypothesis.
Hypothesis
The formation of AA is not solely driven by endoluminal pressure loading of the aortic wall but is instead caused by degenerative processes in the ECM[11]. Importantly, AA do not necessarily imply dissection. Reduced oxygen supply to the tissue, particularly the media, due to incomplete capillarization or neocapillarization, leads to tissue destruction, facilitating blood entry. Tissue remodeling is a dynamic process, as we understand it, occurring in response to mechanical action or biochemical processes, such as ischemia. The widely accepted view posits that “the pathognomonic lesion in AD is a tear in the intima which allows pulsatile surging of blood into the intimo-medial plane of the aorta”. Typically, the entry site is transverse but does not involve the entire circumference of the aorta[13,14]. In tissue sections of the ascending aorta, we used antibodies against MMP-1, MMP-9, and TIMP-1 and TIMP-2[11]. We studied patients with AA and AD to address the following questions: (1) Are the target antigens equally distributed in the sections; (2) If not, what differences exist among the intima, media, and adventitia in individual sections; and (3) Furthermore, what differences can be observed between aneurysmal and dissected tissue. We hypothesize that MMPs function primarily to degrade various components of the ECM, but they also regulate extracellular tissue signaling networks. MMP expression may therefore reflect the pathophysiological impact on aortic tissue. MMPs contribute to the homeostasis of many tissues and participate in myocardial remodeling, angiogenesis, immunity, and wound healing. In summary, we found the highest MMP-1 and MMP-9 expressions in the media and adventitia layers of dissected aortic vessels (Figure 1).
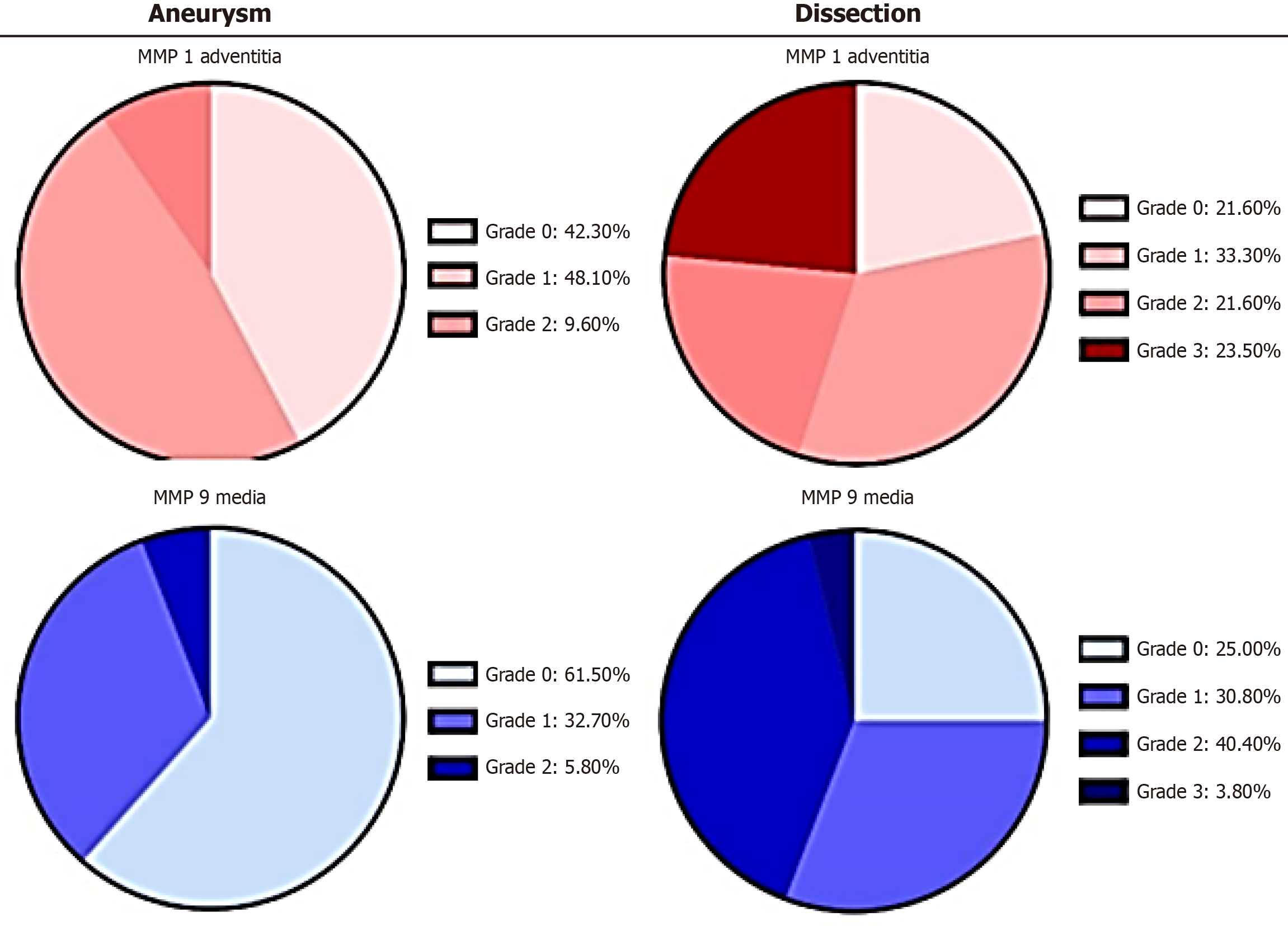
Figure 1 An example of the distribution differences of matrix metalloproteinas-1 and matrix metalloproteinas-9 between aneurysm and dissection.
The charts illustrate the absolute mean values (%) of immunohistological detection of the corresponding target antigens in the adventitia and media for the groups “aneurysm” and “dissection” (n = 52 each). Minimal staining was observed in the intima. Grading evaluations were conducted independently by two observers/pathologists. MMPs: Matrix metalloproteinases.
These proteases were expressed at significantly lower levels in aneurysmal tissue. TIMP-1 expression was elevated in the adventitia of dissected aortic vessels, whereas TIMP-1 and TIMP-2 staining, as well as MMP-1 and MMP-9 staining, were much lower in the intima. This suggests that the mechanical impact on the aorta occurs primarily at the adventitia and media rather than the intima, as previously proposed.
Arguments for the hypothesis
Rupture of the intima due to an increase in intraluminal pressure is considered to be the cause of AD[15], assuming a progressive increase in diameter causes thinning of the aortic wall, all tissue layers should show metabolic changes, from the intima to the adventitia. Additionally, this view would suggest that increased intraluminal pressure initially causes dilation of the aortic tube, and mechanical shear, MMPs and their inhibitors differs significantly within the vascular segments. Second, preoperative and intraoperative echocardiography revealed that the native diameters of the aortas in dissection are significantly smaller than in aneurysms[12]. Hence, intraluminal pressure alone cannot account for the tearing of a damaged intima and the subsequent intimo-medial blood flow. Instead, intrinsic changes in the aortic wall—material-dependent causes—must underlie AD. ECM remodeling occurs in both cases (aneurysm vs dissection) in response to increased mechanical forces such as elevated blood pressure and flow. Mechanical load may directly influence mesenchymal cell activity or indirectly stimulate the release of profibrotic growth factors. These growth factors may act in response to mechanical load through paracrine or autocrine signaling or arise as a consequence of the underlying pathology driving increased blood pressure[6].
In recent years, research has focused on new key players in the remodeling process of the ECM. MMPs are a family of zinc-dependent ECM remodeling endopeptidases with the capacity to degrade nearly every component of the ECM. The degradation of the ECM is significant because it is associated with embryonic development, angiogenesis, cell repair, and tissue remodeling. An imbalance between MMPs and TIMPs has been implicated in the pathophysiology and progression of several diseases. This perspective emphasizes the roles of both MMPs and TIMPs in physiological processes and highlights how their dysregulation is linked to human diseases[16], such as mitral valve insufficiency[12].
SHEAR STRESS INTERACTION
Blood vessels are exposed to various forms of mechanical force, including shear stress, pressure, and tensile stress. Tensile stress itself has several components, including circumferential stress caused by the expansion or dilation of the vessel wall, as well as internal stresses generated by cells in response to external forces. Endothelial cells, which line blood vessel walls, are exposed to all these forces. As the biological interface between the blood and underlying vascular tissue, endothelial cells may transmit information about changes in these forces to the underlying cells. This transmission is mediated through the production and release of vasoactive substances and growth factors by endothelial cells in response to changes in shear and tensile stresses. Shear stress, for instance, stimulates the production of polypeptide growth factors by endothelial cells, such as TGF-β, which enhances mesenchymal cell proliferation and matrix production. These factors may therefore promote vascular remodeling to compensate for increased tensile wall stress. MMPs, according to current knowledge, represent a branched network of specific proteases with both physiological and pathophysiological significance[16]. Collagenases (e.g., MMP-1, MMP-8, MMP-13, and MMP-18) are hydrolytic enzymes that act on collagen in the ECM. Their catabolic activity results in the production of denatured collagen or gelatin. In vertebrates, collagenases are produced by various cells, including fibroblasts, macrophages, and epithelial cells. These enzymes also act on other ECM molecules. Gelatinases (e.g., MMP-2 and MMP-9) assist in the catabolism of type I, II, and III collagens, as well as denatured collagen or gelatin produced by the action of collagenases. TIMP-1 and TIMP-2 serve as counterparts to these proteases, regulating their activity. Together, all proteases and their inhibitors are key players in the ECM remodeling.
MMP AND TIMP AS MEDIATORS FOR ECM REMODELING
Interstitial collagenase, also known as fibroblast collagenase or MMP-1, is involved in the breakdown of the ECM during normal physiological processes such as embryonic development, reproduction, and tissue remodeling, as well as pathological processes, including arthritis and metastasis. Higher rates of aneurysm rupture with a reduced survival prognosis are associated with increased MMP-1 expression. The protease is stimulated by TNF-α and IL-1[4]. MMP-1 activation occurs through pathways involving p38 kinase, c-jun N-terminal kinases, and extacellular signal-regulated kinases. Under normal conditions, MMP-1 is expressed in the kidneys, liver, colon, and various other organs. However, in tissues from the heart, skin, lungs, and pancreas, MMP-1 overexpression is observed only in diseased stages[16]. TNF-α, a stimulating factor for MMP-1 expression, is both an adipokine and a cytokine. It is a member of the TNF superfamily, which includes various transmembrane proteins characterized by a homologous TNF domain. TNF-α plays a dual role: It can cause tissue destruction or contributes to the repair of existing defects. TNF receptor 2 signaling, for instance, promotes wound healing. TNF-α can also prevent apoptosis through a mechanism involving the activation of the transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), which induces the expression of antioxidative enzymes and B-cell lymphoma 2. Additionally, TNF-α-triggered intracellular signaling cascades mediate cytochrome c oxidase inhibition, affect mitochondrial respiration, and influence reactive oxygen species (ROS) production[17]. Further downstream epigenetic modifications are triggered for an enhancement of pro-inflammatory response in cells. TNF-α serves as a potent chemoattractant for neutrophils and enhances the expression of adhesion molecules on endothelial cells, facilitating neutrophil migration. In our study, the highest levels of MMP-1 expression were observed in the adventitia of patients with AD (Figure 1). This suggests that perivascular inflammatory processes favor the formation of AD as opposed to aneurysms. This finding aligns with observations by LeMaire et al[18], whose experimental work demonstrated that mice treated with ciprofloxacin exhibited less expression of lysyl oxidase (LOX), an essential enzyme for elastic fibers and collagen. In a mouse model of moderate, sporadic AD, LeMaire et al[18] found that ciprofloxacin increased vulnerability to AD and rupture[19]. Ciprofloxacin contributes to the breakdown of the ECM in during inflammatory processes by inducing MMP-1, which facilitates the dissolution of interstitial collagens[20].
MMP-9 is associated with the development of AA, as its suppression prevents their formation. Interestingly, doxycycline, in contrast to ciprofloxacin, suppresses the growth of AA in animal models by inhibiting MMP-9 and reducing aortic inflammation in humans[20-23]. MMP-9, also known as gelatinase B, is a matrixin—a class of enzymes within the zinc-metalloproteinase family—that is involved in the degradation of the ECM during various physiological processes, including angiogenesis, bone development, and wound healing. Alongside MMP-2, MMP-9 is a key effector of ECM remodeling and is upregulated during wound repair. Unlike MMP-1, MMP-9 functions as a regulatory factor in neutrophil migration. MMP-9 also plays a significant role in angiogenesis and neovascularization. Moreover, its expression increases with the progressive onset of idiopathic atrial fibrillation, suggesting a potential common pathophysiological mechanism involving both the heart and aorta[24,25]. Notably, the increased expression of MMP-9 is not part of the normal expression pattern observed in healthy tissues of the colon, bone marrow, or lungs[16]. MMP-2 and MMP-9 collaborate in ECM remodeling, vascular smooth muscle cell (VSMC) apoptosis, ROS balance, and the initiation of inflammation. Maguire et al[4] suggest that concerted activation of TGF–β may be one mechanism through which MMP-2 and MMP-9 interact. Although TGF-β signaling—activated by pH changes, ROS, thrombospondin-1, and integrins—generally provides protection against aneurysm development by enhancing type I and III collagen production, it can also damage tissue under certain conditions[4,26-28]. The TGF-β complex is bound to CD36 via its ligand, thrombospondin-1[29]. This molecular complex represents an intriguing link between mechanical deformation and molecular change, as the activation of TGF-β by integrins depends on conformational changes in a latency-associated peptide (LAP) protein induced by mechanical pulling. Molecular simulations based on the αVβ6 integrin-LAP/TGF-β1 structure illustrate how mechanical traction leads to these conformational changes. Experimentally, it has been demonstrated that free, active TGF-β1 is released from its binding to ECM proteins through this process, establishing a direct link to the shear stress loading of the vascular wall[30]. As expected, TGF-β signaling likely plays a major role in the pathogenesis of Marfan syndrome. In a study by Irqsusi et al[11], the highest levels of MMP-9 expression were observed in cases of AD (Figure 1). Notably, no differences were found in the intima; however, the media and adventitia exhibited significant increases in MMP-9 expression. During all phases of tissue repair, MMP-9 is secreted by infiltrating leukocytes, cardiomyocytes, fibroblasts or endothelial cells. It increases the inflammatory reaction, supports the transition to a reparative cell status and promotes neovascularization[31]. Additionally, nitric oxide (NO) and MMP-9 levels are found to increase during inflammatory states[32].
In cases of dissection, we observed an increase in TIMP-1 and TIMP-2 expression from the "inside to outside" of the aortic wall[11], with the highest levels detected in the adventitia. In addition to its inhibitory role against most known MMPs, TIMP-1 promotes cell proliferation across various cell types and may also have an anti-apoptotic function. TIMP-1 expression is stimulated by TNF-α, and its highest levels were observed in the media and adventitia during dissection, but not in the intima. TIMP-2, on the other hand, appears critical for maintaining tissue homeostasis. In ECM remodeling protease activities are inhibited and in quiescent tissues the response to angiogenic factors is suppressed[33,34]. Conversely, we found higher TIMP-2 expression in the adventitia during dissection and, to a lesser extent, in aneurysms, with no differences observed in the media[11]. These findings suggest that a clear proteolytic transformation occurs in the investigated aortic tissue, favoring the progressive destruction of the ECM.
FOCUS ON NEOVASCULARIZATION
A stimulation of angiogenesis is evident when significant increases in vascular endothelial growth factor (VEGF) A concentrations, alongside elevated MMP-9, TIMP-1, and TIMP-2 levels, are observed in cases of ischemia[29]. MMPs and TIMPs play a critical role in regulating angiogenesis during new blood vessel formation. MMP-1 and 92 kDa gelatinase B/type IV collagenase (MMP-9) dissolve ECM, initiating and promoting angiogenesis. In contrast, TIMP-1 and TIMP-2 inhibit neovascularization. MMP-9 may also block angiogenesis by converting plasminogen to angiostatin, a potent angiogenesis antagonist[35,36]. MMPs modulate the balance between pro-angiogenic and anti-angiogenic factors, thereby mediating angiogenesis either directly or indirectly[37]. The contribution of vasa vasorum (VV) to atherosclerosis is not yet fully understood, but experimental and clinical data suggest a possible involvement in vascular proliferative disorders[38]. VV, a network of very small arteries, contributes to hypoxia and/or ischemia in the intima or media cells of the arterial wall[39]. Recruitment and accumulation of inflammatory cells, particularly resident macrophages, promote the expansion of the existing VV network and facilitate the formation of new microvessels that exhibit high permeability. This angiogenesis of the VV appears to play a pivotal role in the initiation and progression of atherosclerosis[40,41]. The pathogenesis is initiated by an activation of the vascular endothelium, an accumulation of lipids, followed by fibrosis and calcification, as well as vessel narrowing and activation of inflammatory pathways[42]. The VV network represents a microvasculature predominantly originating from the adventitial layer of large arteries. These vessels provide oxygen and nutrients to the outer layers of the arterial wall. Expansion of the VV is associated with neovascularization, which contributes to the progression of atherosclerosis. Immunohistological analysis of human plaques from autopsied aortas demonstrates plaque progression significantly correlates with VV neovascularization (Figure 2). There is growing evidence that factors collaborate with the VV to enhance the disease process[43]. In our study, increased TIMP-1 and TIMP-2 expression was observed in the adventitia of dissected aortic tissue[11]. These markers indicate inhibition of neoangiogenesis[35]. We suggest that the trigger for this process is hypoxia. An imbalance between oxygen demand and supply in the arterial wall is a key factor in the development of atherosclerosis and is dependent on hypoxia-inducible factor 1 (HIF-1) regulation. HIF-1 expression is localized in perivascular tissues, inflammatory macrophages, and smooth muscle cells adjacent to the necrotic core of atherosclerotic lesions. It regulates several genes essential to vascular function, including VEGF, NO synthase, endothelin-1, and erythropoietin[44].
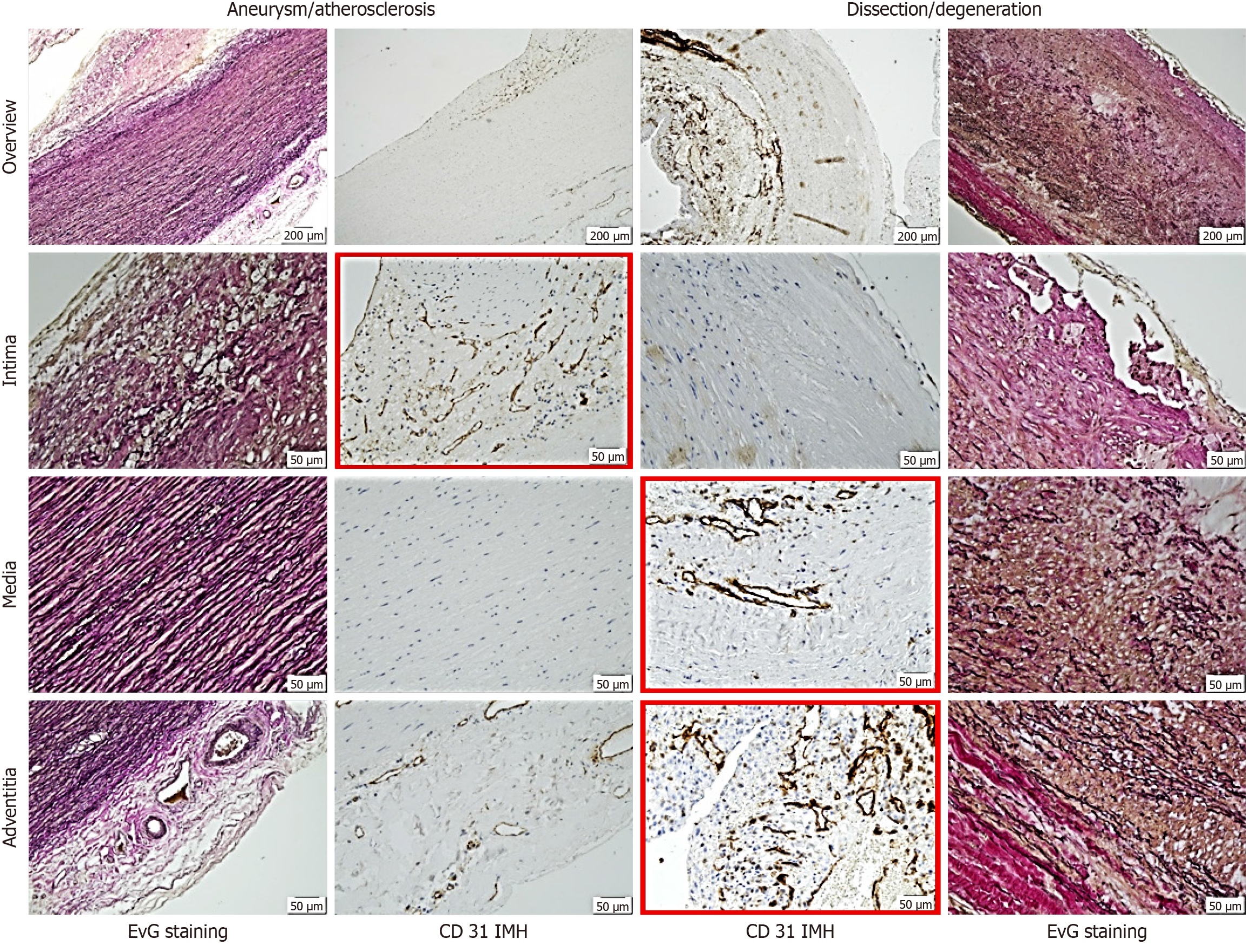
Figure 2 Representative histological and immunohistological findings in aneurysm and dissection.
Note the differing staining patterns of vessels: In the intima for aneurysmal changes and in the media and adventitia for dissection (red frames). The distinct pattern of small vessels reflects vascularization or neovascularization, suggesting the primarily degenerative nature of dissection pathogenesis. Staining methods included hematoxylin (S3301, Dako), Elastica van Gieson, and immunohistochemistry using a primary antibody (monoclonal Mouse Anti-human CD31, Endothelial Cell Clone JC70A, Dako). EvG: Elastica van Gieson.
MODIFICATION OF THE ECM BY HIF
HIF-1 is a dimeric protein complex that plays a crucial role in the cellular response to low oxygen concentrations, or hypoxia. HIF-1 is one of the primary regulators involved in homeostatic processes that increase vascularization in hypoxic areas, such as regions of localized ischemia and tumors. Hypoxia-related acidosis can modulate the composition and architecture of the ECM, impacting three-dimensional cellular organization and promoting metastasis. Through HIF, hypoxia modulates LOX, an enzyme that catalyzes the crosslinking of collagen and elastin. LOX activity results in peroxide production, which damages tissue. Membrane type 1-MMP-1 expression has been shown to respond to HIF-1 and HIF-2 signaling[44]. Additionally, metabolic changes and HIF action influence adhesion, particularly in interactions with vascular endothelial cells via selectin-mediated and integrin-mediated pathways. Angiogenesis requires the differentiated action of collagenases (MMP-1, MMP-8, and MMP-13). A loss of these proteins results in irreversible matrix rupture. TIMP-1 and TIMP-2 regulate type IV collagen production and participate in endothelial cell migration. They are potentially key players in regulation of angiogenesis by inhibiting neovascularization[37]. Supporting this, Billaud et al[45] reported VV remodeling in the aneurysmal ascending aorta induced through medial hypoxia. Their findings indicate that aneurysmal tissue is characterized by a lower density of small VV. These findings highlight the differences in vascular remodeling and associated markers of chronic medial hypoxia among aneurysms with varying etiologies. Such abnormalities may contribute to the malnourishment of the aortic media, potentially playing a significant role in the pathogenesis of thoracic AA. Similarly, Son et al[46] histologically demonstrated a complete regional absence of VV in the ascending aorta. In adults, angiogenesis is initiated only under inflammatory or hypoxic conditions. During the early proliferative stage, vascular repair predominates to control bleeding through vasoconstriction and coagulation. HIF is one of the first growth factors to initiate the abnormal process of vascular growth in response to low oxygen tension. Subsequently, VEGF promotes the formation of a vascular network that ensures the exchange of oxygen and nutrients. MMP-9 dynamically modulates ECM remodeling by activating and deactivating proteolytic cleavages, which release biological activities that regulate cellular processes. Specifically, MMP-1 promotes the expression of VEGF receptor 2 and endothelial cell proliferation by stimulating serine/threonine-protein kinase microtubule affinity-regulating kinase 2 (protease activated receptor 1) and activating the transcription factor NF-κB. This suggests the existence of a mechanism through which MMP-1 stimulates vascular remodeling and angiogenesis. Pro-angiogenic factors such as TNF-α and IL-8 stimulate MMP-9 production in endothelial cells, regulating the angiogenesis process[36]. In our study, we observed higher MMP-1 and MMP-9 expression in the adventitia, as well as increased MMP-9 expression in the media, consistent with the previously discussed remodeling processes in the aortic wall (Figure 1). Additionally, increasing TIMP-1 and TIMP-2 expression from "inside to outside" (intima, media, and adventitia) suggests affected neoangiogenesis of VV in cases of dissection. This finding may represent a potential histological marker for dissection (Figure 2). Tonar et al[47], in an experimental study on normal anatomically designed aortic vessels in pigs, found that the tunica media of the thoracic aorta had a greater VV density, with these vessels penetrating deeper into the aortic wall toward the lumen compared to the abdominal aorta. The centrally medial course of the VV observed by Tonar et al[47], combined with the increased MMP-9 medial expression and additional TIMP-2 expression in the adventitia for suppressed neoangiogenesis, highlights an important biomechanical factor contributing to dissection. Interestingly, the study conducted by Irqsusi et al[11] demonstrated that the diameters of dissected aortas were smaller than those observed in the aneurysm group. According to Laplace's law, this finding suggests that the surface tension of the aortic wall was likely lower in the dissection group compared to that in the aneurysm group[11].
ASPECT OF BIOMECHANICS
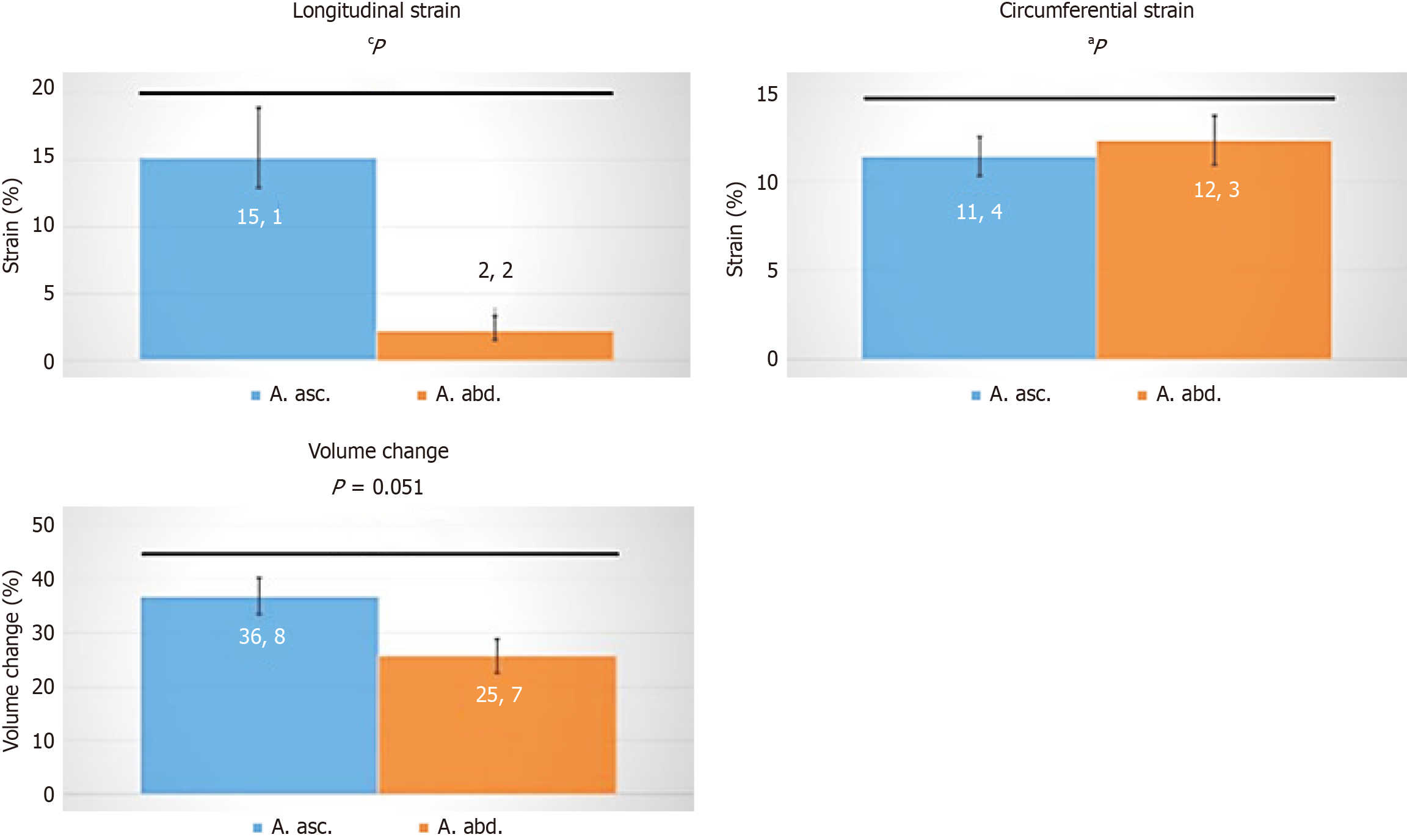
The inflammatory response, including TNF-α activity, MMP-1, and TIMP-1 expression in the adventitia, along with increased MMP-9 expression in the media (indicative of ECM remodeling and VSMC apoptosis), suggests potential VV wall remodeling in cases of thoracic AA (TAA). Medial hypoxia and inflammatory remodeling of adventitial VV are likely contributors. Reduced expression of pro-angiogenic and hypoxia gene targets in the adventitia of aneurysmal patients leads to a decreased density of VV in TAA, resulting in further biomechanical consequences. Langheinrich et al[37] characterized the dynamic forces affecting arterial wall perfusion by the VV[48]. Using a finite element method to determine the anisotropic hyperelastic characteristics of healthy and diseased aortic walls, our group discovered that the ascending aorta had higher cyclic rotation amplitudes and cyclic longitudinal strain than the abdominal aorta (Figure 3)[49,50]. The ascending aorta exhibits complex deformations, with alternating clockwise and counterclockwise twists. These deformations, including longitudinal strain and its phase shift relative to circumferential strain, contribute to the proximal aorta's Windkessel function. However, complex cyclic deformations are known to be highly fatiguing, potentially accelerating the degradation of aortic wall components and promoting AD or aneurysm formation. Furthermore, a strong negative correlation between flow-induced vascular stress and media thickness has been observed, indicating that structural stress mediates degeneration of the aortic media[39].
Figure 3 Differences in strain between the ascending (blue) and descending (orange) aortic root, representing varying volume charges.
Statistical analysis was performed using Wolfram MATHEMATICA 9. Data were tested for normal distribution with the function Distribution Fit test. Normal distribution was rejected for P < 0.05. For normally distributed data, values are presented as mean ± SEM, and an unpaired two-tailed t-test was conducted. For non-normally distributed data, values are given as median, and a Mann-Whitney U-test was performed. aP < 0.01; cP < 0.005.
CONCLUSION
Our perspective was prompted by the article by Barkhordarian et al[2] in this journal. Their insightful review presents new contributing factors involved in the pathophysiology of AA. From our standpoint, the ECM holds center stage. A rupture or hemorrhage of the VV represents the initial trigger for dissection. Our view remains may be discussed. Higher distress between longitudinal and circumferential strain results in deformation[49,50], and enlarged ascending aortic diameters. This is likely caused by hypoxia or HIF-mediated ECM remodeling, compromises the mechanical support of the aortic wall. This cyclic deformation, which facilitates the aspiration and expulsion of medial blood to perfuse the vasculature, is consequently lost. Under hypoxic conditions, small and malformed vessels are induced through interactions between HIF-1α and VEGF[45]. Intercellular leakage from these vessels leads to lipoprotein exudation by immunocompetent cells, perpetuating inflammation[51]. Liu et al[51] suggest a link between abnormal flow patterns and PCSK9 expression in inflammatory states, identifying helical flow and pro-inflammatory cytokines as potential therapeutic targets. Knowledge of metalloproteases and their inhibitors and better understanding of metabolism under hypoxic conditions supports the hypothesis that intrinsic processes within the aortic wall drive dissection[52]. This "outside-in mechanism" (Figure 4) characterizes AD as a disease of the VV. Moving forward, it seems prudent to perform biopsies during cardiac surgical interventions to assess the risk of potential aortic diameter increases.
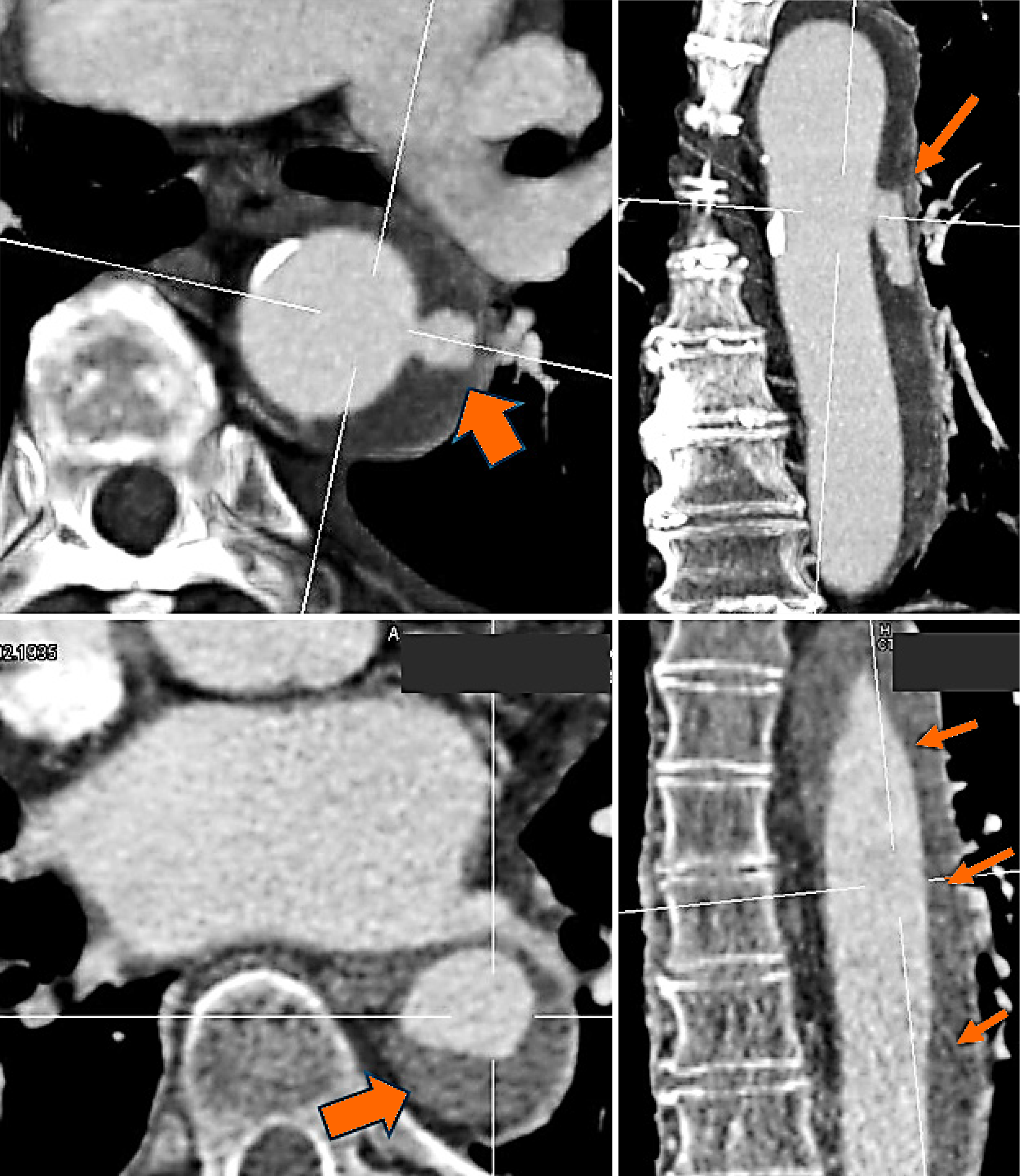
Figure 4 A clinical radiologic image (angio-computed tomography) of a current dissection leading to the urgent admission of a patient to the emergency unit at our hospital.
A small lesion in the inner layer of the aorta is clearly visible. Penetrating blood spreads into the already damaged medial areas (arrows), resulting in hemorrhage throughout the vascular tube.
ACKNOWLEDGEMENTS
The authors are very grateful to Kanwal N and Ramzan R for their support, discussion and suggestions. The authors are also very grateful to Dr. Wittek and Dr. Bubelko for providing data support.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country of origin: Germany
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade C
Creativity or Innovation: Grade C
Scientific Significance: Grade B
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
P-Reviewer: Kehinde SA S-Editor: Luo ML L-Editor: A P-Editor: Wang WB