INTRODUCTION
Primary biliary cholangitis (PBC) is a chronic cholestatic progressive liver disease belonging to the cholangiopathies[1]. In untreated patients there is a gradual progression of the disease with the development of damage to the cholangiocytes of the small bile ducts, leading to their proliferation, fibrosis and ductulopenia, accompanied by increasing cholestasis. The second step of the disease is the progression of cholestasis, which leads to the involvement of hepatocytes in the pathological process, their damage, the development of fibrosis and, finally, cholestatic cirrhosis and hepatic cell failure. The treatment of any disease depends on the discovery of the etiologic and pathogenetic mechanisms of its development, as well as the development of appropriate drugs. Because the etiology of PBC is still unknown, there are currently no etiotropic treatments with meaningful efficacy. Therefore, the treatment of patients with PBC is predominantly symptomatic, especially in the late stages of the disease. At the same time, certain successes in the pathogenesis of cholangiocyte and hepatocyte damage development in PBC have been achieved, that allowed to use hydrophilic bile acids for the treatment of this disease. The use of hydrophilic bile acids for the treatment of PBC is associated with significant progress in expanding our understanding of the physiology of the processes of bile formation and biliary excretion, as well as the role of bile acids in the injury and death of biliary epithelial cells (BECs, cholangiocytes) in PBC[2-4]. The new knowledge has helped to better delineate the pathophysiology of cholestasis and the adaptive responses of BECs and hepatocytes to the damaging effects of bile acids. Knowledge of the physicochemical properties of various bile acids and the adaptive responses of cholangiocytes and hepatocytes to them has served as an important basis for the development of relatively effective drugs based on hydrophilic bile acids that can potentially slow down the progression of the disease. The main principle of action of preparations containing hydrophilic bile acids consists in replacement and dilution of toxic (having strong detergent properties) primary bile acids by less toxic and more hydrophilic and easily excreted from the body. Bile acid therapy in PBC aims to slow disease progression, increase life expectancy and improve quality of life. In recent years, the list of hydrophilic bile acids used for the treatment of cholestatic liver diseases, including PBC, has been expanded, and in addition to ursodeoxycholic acid (UDCA), the use of obeticholic acid (OCA), tauroursodeoxycholic acid (TUDCA), and norursodeoxycholic acid (norUDCA) as drugs is being discussed. The review considers the mechanisms explaining the beneficial therapeutic effects and the potential of each of the bile acids as a drug based on the ideas about the pathogenesis of the initial stages of PBC[4]. UDCA was the first drug approved by the United States Food and Drug Administration for the treatment of PBC[5].
UDCA AS FIRST-LINE TREATMENT FOR PBC
In 1987, the German hepatologists Leuschner and Kurtz[6] reported beneficial effects of UDCA in patients with PBC, a disease previously known as primary biliary cirrhosis[7,8]. UDCA is the 7-beta epimer of primary chenodeoxycholic bile acid (CDCA), which has a hydroxy group on the 7-carbon atom at the beta position rather than the alpha position as in CDCA (Figure 1). It is these seemingly minor structural chemical differences that lead to significant pharmacotherapeutic differences between these two bile acids: UDCA is more hydrophilic and less hepatotoxic than CDCA. Studies by many scientists have provided the basis for the accumulation of evidence supporting a positive therapeutic effect, justifying the use of UDCA as a standard of care for the treatment of patients with PBC[8-14].
Figure 1
Protonated and deprotonated forms of ursodeoxycholic acid, tauroursodeoxycholic acid, and glycoursodeoxycholic acid.
UDCA has been studied in numerous randomized, placebo-controlled trials in stage I-IV PBC with both positive and inconclusive results[11,15-18]. Clinical studies have shown that oral administration of UDCA at a dose of 13-15 mg/kg/day is well tolerated by patients and has a positive therapeutic effect in cholestatic liver diseases, including PBC[19]. Scientific publications indicate that UDCA improves biochemical markers of cholestasis (alkaline phosphatase, gamma-glutamyl transpeptidase), slows progression of PBC, and delays liver transplantation and death in most patients, with improved survival[20,21]. It has been shown that the efficacy of UDCA use depends on the stage of the disease: The earlier treatment is started (stage I and II), the more effective it is. Some authors believe that transplant-free survival in patients with early-stage PBC treated with UDCA was equivalent to that of age and sex-matched healthy controls[22-24]. The use of UDCA in PBC delays histologic progression of the disease and prolongs survival in patients without liver transplantation, therefore this drug is recommended as first-line therapy for all patients with PBC[19,25]. Despite this, the efficacy of UDCA in PBC remains a matter of debate due to the lack of evidence of efficacy on hard endpoints (e.g., survival or survival without liver transplantation), especially in patients who started UDCA drugs late in the disease[2]. The mechanism of the beneficial effect of UDCA is not fully understood. Clearly, it depends on both its physicochemical properties and its metabolism and enterohepatic circulation. In this regard, it is important to present the metabolism of UDCA in norm, on which factors it depends and how its metabolism changes in PBC.
METABOLISM OF UDCA AND FORMATION OF CONJUGATES WITH TAURINE AND GLYCINE
Human bile normally contains about 5% UDCA. When UDCA is administered orally to treat cholestatic liver diseases, its fraction in bile increases. After oral administration, most of UDCA is absorbed by passive diffusion in the small intestine and travels with the venous blood of the portal vein to the liver, where it is taken up by hepatocytes. The side chain of UDCA is conjugated to glycine or taurine (amidation) in the liver cell. Importantly, 75% of UDCA is conjugated to glycine and 25% to taurine, like the primary bile acids[26]. The UDCA conjugates carry out an enterohepatic circulation. UDCA conjugated to glycine or taurine enters the hepatic bile in a deprotonated state (Figure 1). At neutral [pondus hydrogenii (pH) = 7.4] or slightly alkaline physiological pH of hepatic bile, glycine and taurine conjugates of UDCA are deprotonated, which prevents them from entering cholangiocytes through the biliary bicarbonate “umbrella”[27,28]. The secretion of bicarbonate (HCO3-) by BEC to create the latter. Bicarbonate, which has buffering properties, maintains neutral or slightly alkaline pH of hepatic bile, creates a negative charge in the supraepithelial layer, and maintains bile acids in a deprotonated state[29]. Since negative charges repel each other, bile acids in ionized form do not approach the apical surface of cholangiocytes, preventing their entry into BECs. Such a condition has been termed biliary bicarbonate “umbrella”. Therefore, in a normal healthy person, bile acids, including UDCA, enter the gallbladder and duodenum in ionized (deprotonated) form and conjugated with glycine or taurine. In the intestine, they take an active part in the emulsification and absorption of fats and fat-soluble vitamins thanks to their detergent properties.
RESPONSE OF UDCA CONJUGATES WITH GLYCINE AND TAURINE TO CHANGES IN THE PH OF THE HEPATIC BILE IN PBC
In recent decades, scientific evidence has emerged regarding the alterations in hepatic bile pH in PBC and its role in the development of cholangiopathy[27]. It has been shown that in PBC there is insufficient synthesis of HCO3- by cholangiocytes, which is accompanied by insufficient bicarbonate uptake into the bile ducts with simultaneous accumulation in BECs[27,30]. There is evidence that insufficient synthesis and entry of HCO3- into the bile ducts in PBC is due to decreased activity of inositol 1,4,5-trisphosphate receptor isoform 3 and chlorine/bicarbonate-anion exchanger 2 (AE2), caused by increased activity of microRNA 506 (miR-506) in cholangiocytes[30]. To date, the factors that trigger the increased expression of miR-506 gene are still unknown.
As a result of insufficient bicarbonate intake into the bile ducts in PBC there is an acidification of the hepatic bile pH and alkalinization of pH inside small BECs, which leads to impaired metabolism of glycine (but not taurine) conjugates of bile acids, including UDCA. Underlying this impairment are differences in the physicochemical properties of glycine and taurine conjugates of bile acids, which determine their protonation and deprotonation states depending on the pH of the environment. The degree of protonation and deprotonation of bile acids depends on both the pH of the hepatic bile and on the dissociation constant (pKa) of the bile acids. The pKa values for unconjugated bile acids are 5-6[31,32]. Amidation reduces the pKa to 4-5 for glycine conjugates and 1-2 for taurine conjugates[31-33] (Figure 1). Acidification of hepatic bile pH in PBC allows unconjugated bile acids and their glycine conjugates to be easily protonated due to high pKa values[31,32]. The low pKa values of the taurine conjugates of the bile acids indicate that they are stronger acids than the glycine conjugates. For this reason, taurine-conjugated bile acids will be present in a dissociated (deprotonated, ionized) form even at the highly acidic pH values of the hepatic bile. On the contrary, glycine conjugates of bile acids are weak acids and at the slightest change of bile pH in the acidic direction will quickly change to the protonated state. The latter allows them to easily overcome the biliary bicarbonate “umbrella” and penetrate into small BECs[32].
THE ROLE OF BICARBONATE AND HEPATIC BILE PH IN PROTECTING SMALL CHOLANGIOCYTES FROM BILE ACID INJURY
PBC is a chronic cholestatic progressive liver disease with destruction, apoptosis and necrosis of the epithelium of mainly small intralobular and septal bile ducts, with the development of ductulopenia and cholestasis, in the terminal stage of which liver cirrhosis develops[1]. Development of PBC already in asymptomatic stage of the disease is accompanied by damage to small cholangiocytes and development of ductulopenia leading to cholestasis. To date, there is no clear understanding of why the small cholangiocytes that line the intralobular, interlobular, and septal bile ducts are damaged in PBC. It is thought to occur due to an imbalance between aggression factors “bile acids” of the hepatic bile and the defense factors (biliary bicarbonate “umbrella”) of the cholangiocytes[4].
Bile is an aggressive medium for cholangiocytes. The presence of bile acids in bile, which have strong detergent properties, can cause damage to the cell membranes of cholangiocytes. Hydrophobic bile acids are cytotoxic to many cell types[27]. Cholangiocytes are exposed to very high (millimolar) concentrations of hydrophobic bile acids. However, they show no evidence of cytotoxicity[34]. This resistance suggests the presence of mechanisms that protect cholangiocytes from the toxic effects of bile acids in the normal state. Known defense factors that enter the bile during its passage through the bile ducts include the production and secretion of mucin and HCO3- by cholangiocytes[35]. In physiological conditions the main function of BECs is biliary secretion of bicarbonate necessary for maintaining neutral or slightly alkaline pH of liver bile[29]. This pH maintains the bile acids in a deprotonated state. Bicarbonate is produced by cholangiocytes throughout the biliary tree.
Mucin glycoproteins are produced by the peribiliary glands (PBG)[36]. The latter are located in the wall of only large intrahepatic and extrahepatic bile ducts and are directly connected with their lumen. Mucin produced by PBG protects cholangiocytes from the damaging effects of bile acids only in large bile ducts[35]. The cholangiocytes of the large intra, and extrahepatic bile ducts have a double defense: The mucin produced by PBG and the bicarbonate. The intralobular, interlobular and septal bile ducts, which are affected in PBC, do not contain PBG, which is accompanied by the absence of mucin in these ducts[36]. As a result, only bicarbonate serves as a defense factor of small BECs at the level of intralobular, interlobular, and septal ducts.
EFFECT OF A DEFECTIVE BILIARY BICARBONATE “UMBRELLA” ON UDCA METABOLISM IN PBС
The primary mechanism responsible for the damage to small BECs and the development of cholestasis in PBC is the entry and accumulation of hydrophobic bile acids within cholangiocytes[4]. The mechanism of uncontrolled entry and accumulation of endogenous bile acids in small BECs is associated with a decrease in the protective role of bicarbonate in PBC. The hypothesis of a malfunctioning biliary bicarbonate “umbrella” is currently a topic of active debate[27,28,37]. This hypothesis is based on a number of clinical and experimental studies demonstrating a reduction in the synthesis of HCO3- by cholangiocytes, an insufficient influx it into the bile ducts, and a concurrent accumulation in BECs in PBC[27,30]. Insufficient production and supply of HCO3- in the lumen of the bile ducts results in the formation of a so-called defective biliary bicarbonate “umbrella”. A shift in the pH of the intraductal “hepatic” bile occurs, moving towards a slightly acidic range, while the pH within the cholangiocytes increases, approaching a slightly alkaline range[30].
The acidification of hepatic bile pH and alkalinization of pH within small BECs in PBC results in the impaired metabolism of glycine (but not taurine) conjugates of primary bile acids and UDCA, which subsequently enter and accumulate in BECs. This results in the apoptosis of small cholangiocytes, the development of ductulopenia, and subsequent cholestasis and toxic “detergent” effects of bile acids on not only cholangiocytes but also hepatocytes as cholestasis progresses[4]. The presence of a mucin-containing glycocalyx layer on the apical surface of large cholangiocytes serves to protect them from the penetration and damaging effects of protonated conjugated and unconjugated bile acids. Therefore, they are not implicated in the pathogenesis of PBC development. The prolonged oral administration of UDCA preparations at a dose of 13-15 mg/kg/day has been demonstrated to result in a significant replacement of hydrophobic primary bile acids by less toxic and more hydrophilic UDCA. However, the ratio of glycine (75%) to taurine (25%) UDCA conjugates remains in favor of the former. This allows for the possibility of protonation and penetration of glycine (but not taurine) UDCA conjugates into small BECs through the defective biliary bicarbonate “umbrella” to the same extent as primary bile acids during the acidification of hepatic bile in patients with PBC[4,27]. The moderately alkaline pH within small BECs results in the deprotonation of bile acids. An accumulation of glycine conjugates of bile acids, including UDCA, is observed in cholangiocytes. This is a prerequisite for the cytotoxic (detergent) effects that they exert[27]. However, due to the hydrophilic properties of UDCA, its detergent (toxic, damaging) properties are less than those of primary bile acids, which results in a positive therapeutic effect.
Concurrently, taurine conjugates of UDCA remain in a deprotonated state and are unable to overcome the biliary bicarbonate “umbrella”[4]. Given that taurine conjugates of UDCA, which have low pKa, are in hepatic bile in a deprotonated state, it can be concluded that even at an acidic pH of hepatic bile in PBC, they will not penetrate inside BECs and will not have a damaging effect on cholangiocytes. However, the concentration of taurine conjugates of UDCA in hepatic bile is approximately four times lower than that of glycine conjugates. Consequently, to halt the progression of PBC more effectively, it is essential to enhance the supply of taurine conjugates of UDCA into hepatic bile. This can be achieved through the use of TUDCA, which will lead to the replacement of glycine conjugates of UDCA with taurine conjugates in hepatic bile. One possible avenue for future research is the alkalinization of hepatic bile. However, this is currently impossible due to the lack of appropriate drugs.
THE USE OF TUDCA AND ITS METABOLISM IN PBC
The aforementioned fundamental and clinical studies demonstrate that ionized (deprotonated, negatively charged) taurine conjugates of bile acids are unable to cross the biliary bicarbonate “umbrella” and penetrate into cholangiocytes[34,38-40]. These findings have suggested that TUDCA may have potential as a treatment for cholestatic liver diseases[41,42]. Despite the fact that UDCA has become a recognized drug in the treatment of cholestatic liver disease a number of studies have been conducted with TUDCA, a natural component of human bile albeit in minute quantities. In patients with PBC, TUDCA was administered at doses of 500 mg, 1000 mg, and 1500 mg per day[43]. The analysis revealed no statistically significant clinical difference between the three doses[43]. The administration of TUDCA has been demonstrated to result in a notable improvement in serum parameter of hepatic enzymes associated with cholestasis and cytolysis. Additionally, favorable alterations in the composition of bile acids in bile have been observed. During drug administration, hepatic bile is enriched in TUDCA, indicating the replacement of primary bile acids. It is shown that a low dose (500 mg) of TUDCA was sufficient to achieve satisfactory enrichment of bile with taurine conjugates of UDCA and to improve biochemical indices[43]. Studies with similar results suggest that a daily dose of about 10 mg/kg body weight per day is appropriate[43,44]. TUDCA administration has also been demonstrated to contribute to the preservation of clinical and functional stability during the waiting period preceding terminal liver transplantation in patients with PBC[44]. Conversely, administration of unconjugated UDCA at the terminal stage of PBC does not exhibit such properties.
The mechanism by which TUDCA exerts a beneficial effect in patients with PBC is attributed to her low dissociation constant (pKa is 1.5-2). As a result, TUDCA exists in an ionized “deprotonated” state in acidified hepatic bile in PBC. The deprotonated state enables her incorporation into the enterohepatic circulation, thereby replacing the majority of glycine-conjugated primary bile acids with tauroursodeoxycholate. This results in a notable reduction in glycine conjugates of primary bile acids, accompanied by a considerable elevation in TUDCA within the hepatic bile. However, complete replacement of glycine conjugates of bile acids by taurine conjugates is not achievable, even with TUDCA administration. Nevertheless, a notable replacement of glycine conjugates of bile acids by taurine conjugates markedly reduces the influx and deleterious impact of the former on cholangiocytes, as well as in the advanced stage of cholestasis and on hepatocytes. Based on the pathogenetic mechanisms, TUDCA should contribute to a more significant suspension of the progression of PBC than when taking unconjugated UDCA. The deceleration of the rate of progression of PBC is depending on the quantity of substituted glycine conjugates of bile acids and the stage of the disease. The reduction in the glycine/taurine ratio observed in patients with PBC may be considered as a compensatory response of the body, aimed at maintaining bile acids in a deprotonated state[45-48]. Further multicenter long-term studies are required to ascertain the efficacy of TUDCA administration. The use of TUDCA in asymptomatic and early-stage disease is likely to be particularly efficacious. In case of positive response of patients to oral administration of UDCA, TUDCA and good tolerability of the drugs therapy should be continued for life.
ADMINISTRATION OF OCA IN PBC
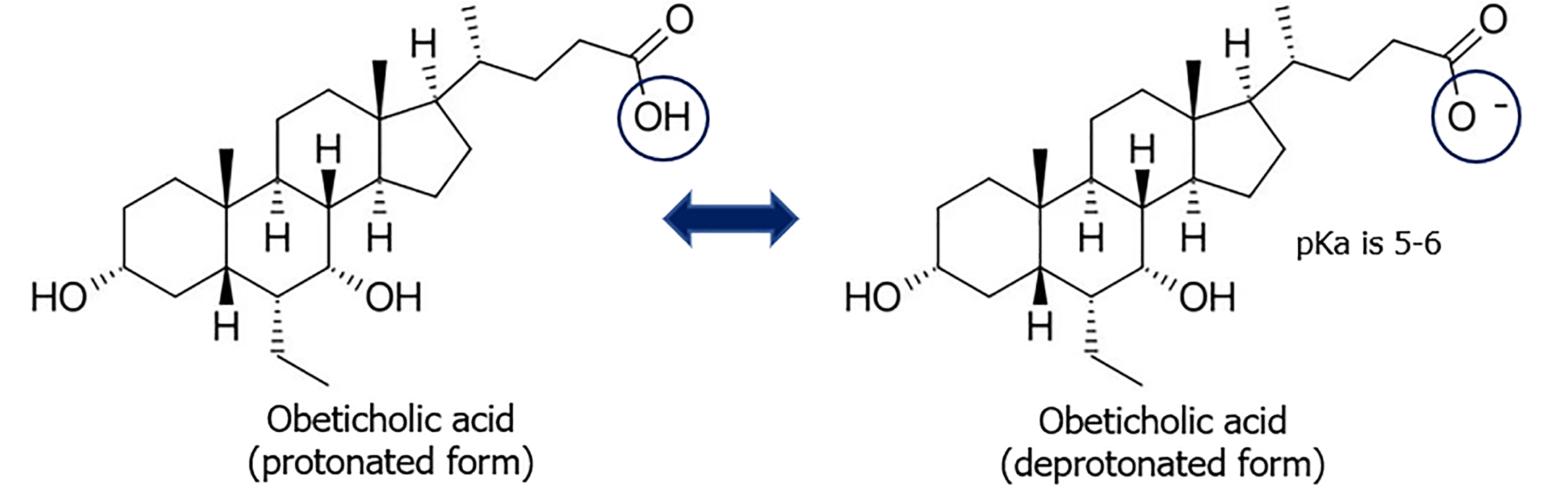
While UDCA treatment has been demonstrated to yield favorable clinical outcomes in the majority of patients, approximately 30%-40% of patients with PBC do not respond adequately to therapy, resulting in an elevated risk of disease progression and significant complications[49]. Agonists of farnesoid X receptors (FXR) and peroxisome proliferator-activated receptors are considered as an efficacious treatment option for patients with cholestatic liver diseases who do not respond adequately to UDCA[25]. OCA is currently being investigated as a potential therapeutic option for patients with PBС who have not responded to UDCA treatment[50]. In such instances, the treatment regimen is maintained through the concurrent administration of UDCA and OCA[49]. OCA has been approved for use as a second-line therapy for patients with PBC who have not responded adequately to UDCA, or as monotherapy in adults who are intolerant of UDCA[49,51,52]. The rationale for approval OCA was based on the reduction in alkaline phosphatase levels in patients with PBC, which is one of the biomarkers of PBC, and thus indicative of clinical improvement[52]. OCA is a semi-synthetic derivative of CDCA, also known as 6α-ethyl chenodeoxycholic acid, which has a strong affinity for the nuclear FXR (Figure 2)[53]. As a potent selective FXR agonist, OCA has been demonstrated to possess pronounced properties of suppressing bile acid synthesis in cholestatic liver diseases through the transcription of the CYP7A1[54].
Figure 2
Formulation of the protonated and deprotonated form of obeticholic acid.
Concurrently with the inhibition of bile acid synthesis, OCA via FXR induces the expression of the bile salt export pump protein, which is responsible for bile acid efflux from the hepatocyte[3,54]. As a result, hepatocytes are protected from the accumulation and toxic “detergent” effects of bile acids[55]. Good tolerability and significant improvement in liver biochemical parameters associated with cholestasis demonstrated in clinical trials of OCA in PBC[49,52]. Results of a randomized, double-blind, phase 3 study comparing OCA to placebo showed that approximately 50% of patients achieved a significant reduction in serum alkaline phosphatase levels, a marker that predicts disease progression in PBC[49]. Cutaneous pruritus was most common adverse event in PBC patients and OCA dose-dependent[51]. According to Li et al[51] skin itching was more frequent in the combination therapy group (61.3%) than in the monotherapy group (42.3%). In patients with PBC, OCA is recommended at a dose of 5 mg daily, which is more effective than doses of 10 mg (P = 0.001), 25 mg (P = 0.06), and 50 mg (P = 0.04) OCA, with the lowest risk of side effects[52].
METABOLISM OF OCA IN PBC PATIENTS AFTER ORAL ADMINISTRATION
In the small intestine, OCA is taken up by enterocytes and transported with the venous blood of the portal vein to hepatocytes, where it is conjugated with glycine (75%) and taurine (25%) in the same ratio as the primary bile acids. The mechanism of action of OCA is the inhibition of primary bile acid synthesis via FXR, accompanied by a decrease in their levels in hepatocytes and hepatic bile, and the substitution of primary bile acids by OCA. Induction of expression of bile acid transporter proteins on hepatocyte membrane leads to decrease of bile acid content in hepatocytes, which is very important for prevention of their damaging effect on membrane structures of liver cells. And on the other hand, against the background of OCA administration and increased intake of bile acids into hepatic bile, there is an increase of bile acid excretion into the systemic blood flow due to the presence of ductulopenia and intrahepatic cholestasis in PBC. And the more pronounced the cholestasis, the greater is the efflux of OCA into the general bloodstream and the greater is the probability of development of an undesirable side effect, skin itching. The entry of increased amounts of OCA into the general bloodstream leads to the involvement of the kidneys and skin in the process of her excretion from the body. Getting into the skin and having strong detergent (on lipid components of myelin sheath of nerve fibers) and irritating properties on nerve endings OCA causes intensification of skin itching, which has a dose-dependent effect[51,52].
In spite of decrease in accumulation and damaging effect of bile acids on hepatocytes, OCA administration does not reduce toxic, detergent effect on cholangiocytes, which is due to OCA metabolism. The predominant glycine conjugates of primary bile acids and OCA in acidified hepatic bile of PBC patients undergo protonation (Figure 2). This promotes overcoming of biliary bicarbonate “umbrella” by glycine conjugates of OCA and her entry into the cholangiocytes. There is an accumulation and damaging effect of glycine conjugates of primary bile acids and OCA on cholangiocytes, due to the change in pH of hepatic bile to acidic region and alkalinization of BECs cytosol in patients with PBC. OCA is not recommended in patients with advanced stage PBC[51,52]. Given the differing mechanisms of action of UDCA and OCA, combined therapy using both drugs are recommended for refractory PBC. The combined use of UDCA and OCA has been shown to result in a positive therapeutic effect due to the inhibition of primary bile acid synthesis by OCA and the replacement of toxic, hydrophobic primary bile acids with more hydrophilic, less toxic UDCA for cholangiocytes and hepatocytes[51]. Given the mechanisms of action of bile acid preparations as above described, it can be postulated that the combination of TUDCA and OCA will prove to be a more efficacious treatment. The use of OCA in combination with tauroursodeoxycholate may become a vital means of pharmacotherapy for patients with refractory PBC. The therapeutic efficacy of OCA can likely be enhanced by developing a novel drug formulation, specifically a taurine conjugate of OCA that is sulfated at the 3rd carbon atom of the cyclopentane-perhydrophenanthrene ring. It is anticipated that the latter will result in a reduction of the compound’s toxic properties, an increase in her water solubility, and enhanced excretion from the body via urine[56,57].
THE USE OF NORUDCA IN CHOLESTATIC DISEASES
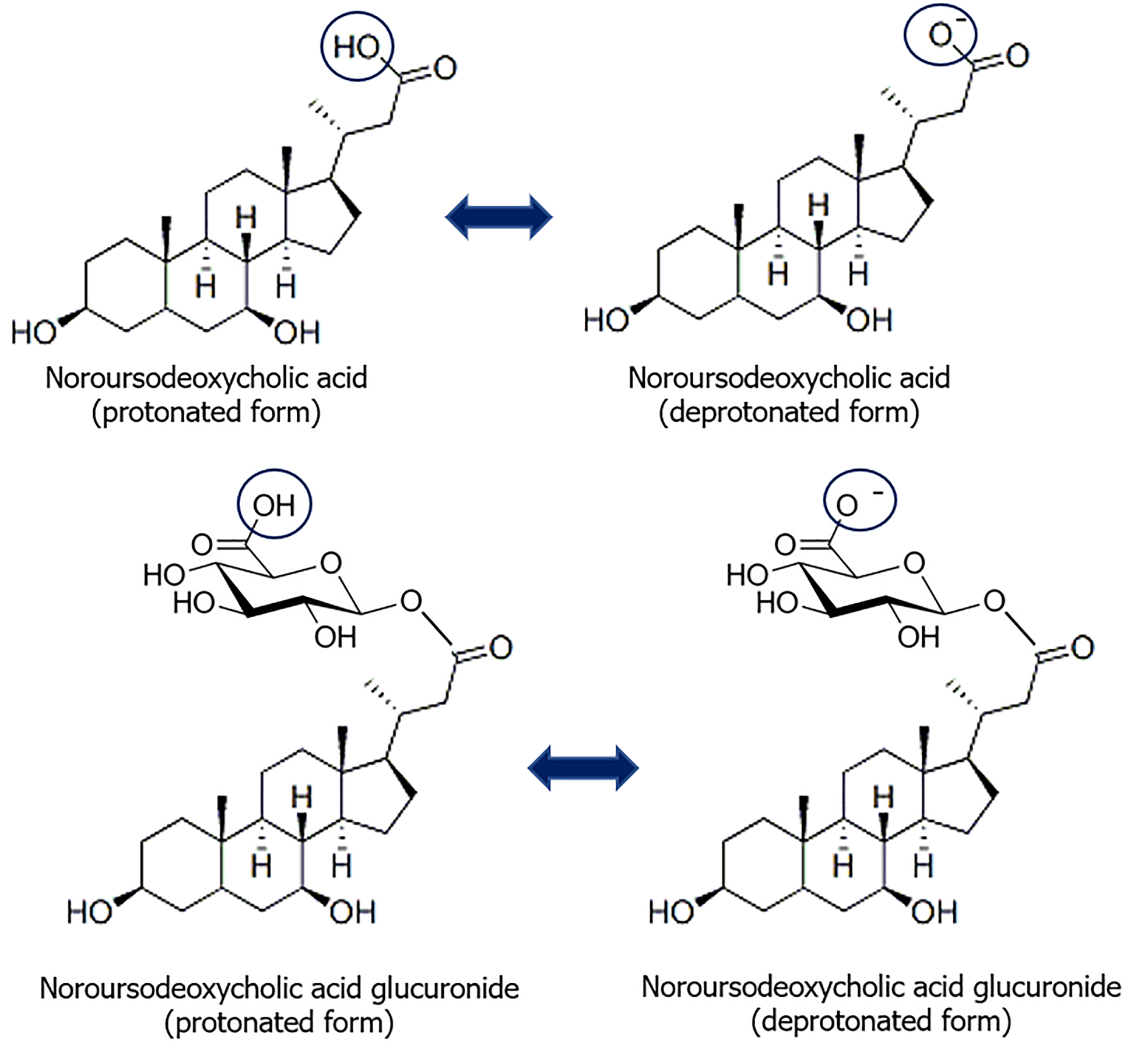
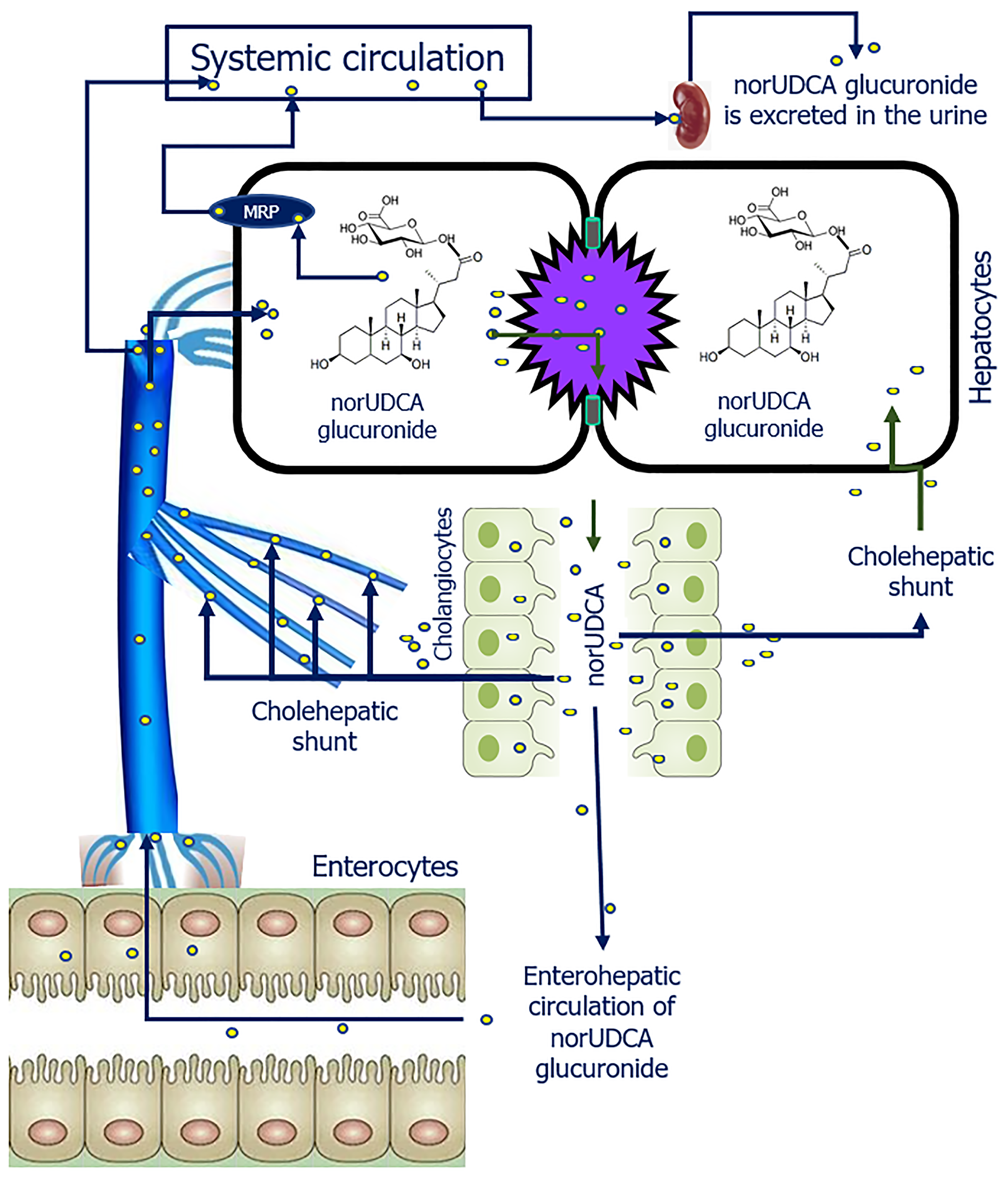
The administration of norUDCA has been described as an alternative to UDCA or for concomitant use with it for the treatment of a number of cholestatic liver and biliary tract diseases[58]. NorUDCA is a derivative of UDCA with a shortened side chain, in which one methyl group is absent. This structural modification confers relative resistance to the side-chain amidation (Figure 3)[59]. Nordihydroxy bile acids, such as norUDCA, are excreted into the bile partly unchanged and partly as glucuronide or sulfate conjugate[59,60]. In humans, the majority of norUDCA is metabolized via side-chain glucuronidation in hepatocytes, rather than through amidation with glycine or taurine[61]. The glucuronidation of the side chain, as opposed to amidation, endows norUDCA with distinctive physiological and pharmacological attributes. Instead of undergoing a complete enterohepatic circulation, it is capable of undergoing cholehepatic shunting (Figure 4)[61-63]. As a result of side chain glucuronidation, there is significant renal elimination of the glucuronide C-23 ester of norUDCA[61]. It seems probable that renal excretion occurs as a consequence of the efflux of norUDCA glucuronide from the hepatocyte to blood plasma via the basolateral membrane, with the involvement of a transporter belonging to the multiple drug resistance family (Figure 4)[64].
Figure 3
Formulas of norursodeoxycholic acid and its glucuronide in protonated and deprotonated forms.
Figure 4 Cholehepatic bypass and metabolism of norursodeoxycholic acid glucuronide.
norUDCA: Norursodeoxycholic acid glucuronide; MRP: Multidrug-resistance protein.
NorUDCA is considered as a bile acid with choleretic properties, what is associated with her cholehepatic shunting[61,65]. Based on animal studies, it has been shown that the physicochemical properties of norUDCA glucuronide promote its constant flow through the BECs of the bile ducts “cholehepatic circulation”, which may be of therapeutic importance[61]. Due to its hydrophilic properties and glucuronidation of side-chain, norUDCA is considered a promising pharmacological agent for the treatment of a variety of cholestatic liver and biliary diseases. NorUDCA has been successfully tested clinically in patients with primary sclerosing cholangitis[58]. A double-blind, randomized, multicenter, placebo-controlled, comparative phase III study on oral administration of norUDCA at a dose of 1500 mg/day for the treatment of primary sclerosing cholangitis was conducted. NorUDCA administration resulted in a dose-dependent decrease in serum levels of alkaline phosphate and other liver enzymes after 12 weeks of treatment[66]. NorUDCA was effective both in patients who had previously taken UDCA (whether or not they responded to UDCA therapy) and in patients who had not previously taken UDCA[66,67]. According to the authors, the drug was well tolerated. The number of treatment-related adverse events was similar in all groups[58,66].
METABOLISM OF NORUDCA DURING ORAL ADMINISTRATION
The mechanism of action that mediates the beneficial effects of norUDCA continues to be the subject of ongoing research[68,69]. Beuers et al[70] suggest that it is likely that norUDCA passing through cholangiocytes stimulates bicarbonate secretion by BECs to maintain a protective biliary bicarbonate “umbrella”. However, this statement is not supported by experimental studies[71]. Denk et al[71] showed that norUDCA administration has a choleretic effect only in normal isolated perfused rat liver and has no anticholestatic effect in an experimental model of induced cholestasis. But norUDCA taurine conjugate (TnorUDCA) was effective, although inferior to UDCA taurine conjugate[71]. Indirectly, these data indicate another mechanism of action of norUDCA. The physicochemical properties of norUDCA suggest the following metabolic mechanism. After being absorbed in the intestine, norUDCA is transported through the portal vein system to the liver, where it is taken up by the hepatocytes. In liver cells, the side chain of norUDCA is glucuronidated (glucuronides of norUDCA are formed), giving her the properties of a very weak acid. The latter, entering hepatic bile and having properties of very weak acid, is protonated and easily overcomes biliary bicarbonate “umbrella” enters cholangiocytes. At normal pH of the BECs cytosol, it is excreted into the peribiliary space and, once in the blood, is transported to hepatocytes and the systemic circulation (Figure 4). The portion of norUDCA glucuronides that enters the liver cells is included into the cholehepatic (and only partially into the enterohepatic) circulation and partially enters the systemic circulation by eflux[64]. There is a partial replacement of primary bile acids by the glucuronide norUDCA, which has a less toxic effect on hepatocytes and BECs due to the formation of esters with glucuronic acid. The portion of norUDCA glucuronides that enters the systemic circulation due to the presence of glucuronic acid in the molecule is easily excreted from the body by the kidneys[61]. This mechanism is most likely the basis for the beneficial effect of norUDCA when used in patients with primary sclerosing cholangitis[58].
However, in PBC, bicarbonate supply to the bile ducts is disturbed, hepatic bile acidification and pH alkalinization within the cholangiocytes occur. Glucuronides of norUDCA are very easily protonated (due to acidification of hepatic bile pH) and overcoming the biliary bicarbonate “umbrella” enter cholangiocytes. Since inside BECs at PBC there is alkalinization of cytosol, glucuronides of norUDCA can be deprotonated and their exit to peribiliary space will be worsened. This will lead to retention and accumulation of norUDCA glucuronides in cholangiocytes, with subsequent damaging effect, although to a lesser extent than primary bile acids due to their hydrophilicity and glucuronidation of the side chain. Probably, this mechanism can explain the absence of anticholestatic effect in the experimental model of induced cholestasis[71]. Denk et al[71] showed that conjugation of norUDCA with taurine is necessary to achieve the anticholestatic effect. Taurine conjugates of norUDCA are a strong acid. And they will not be protonated in hepatic bile, will not be in the BECs, and will not be part of the cholehepatic shunt. Meanwhile, TnorUDCA will be part of the enterohepatic circulation and will be a substitute for primary bile acids. This may explain the Denk et al[71] efficacy result of TnorUDCA similar to the taurine conjugate of UDCA. It may be possible to increase the therapeutic efficacy of TnorUDCA by sulfation or glucuronidation of the third carbon atom of the cyclopentane-perhydrophenanthrene ring. The latter should reduce her toxic properties, increase her water solubility and her excretion from the body with urine[56,57,61].
CONCLUSION
Treatment of PBC remains a challenge, as the cause causing this chronic, slowly progressive cholestatic disease has not been identified. Advances in the use of hydrophilic bile acids for the treatment of PBC are associated with progress in the study of the physicochemical properties of bile acids and the disclosure of the pathogenetic mechanisms of cholangiocyte damage development and appearance of the first signs of this disease. The use of bile acid preparations (UDCA, TUDCA, OCA, norUDCA) in the treatment of PBC has resulted in slowing the progression of the disease and improving the quality of life in these patients. Unfortunately, the treatment of PBC with bile acid preparations is not associated with a complete cure of the disease. The revelation of the mechanisms underlying the positive therapeutic effect of these drugs, described in this review, demonstrates the limited efficacy of bile acid drugs.
Therefore, there is an urgent need for the development of new, more effective drugs and treatment methods for this cholestatic disease. At the same time, new drugs should take into account the data on the mechanism of development of initial signs of PBC, on metabolism of various forms and conjugates of hydrophilic bile acids used for treatment of this disease, and also new targets revealed by deeper study of the pathophysiology of the disease[4,72]. New drugs based on UDCA and her derivatives should contain in their structure taurine in the side chain, as well as glucuronic acid or sulfogroup at the 3-carbon atom of the cyclopentane-perhydrophenanthrene ring in order to increase the efficacy, improve urinary excretion and reduce the number of side effects. The taurine conjugates will maintain the deprotonated form of the bile acids in the acidified hepatic bile of PBC patients, and the sulfogroup or glucuronic acid will reduce toxicities and increase aqueous solubility and renal excretion. At the same time, intestinal absorption of such drugs will be reduced, which will need to be taken into account when selecting the dosage of the drug. Such drugs are designed to better stop the symptoms and further inhibit PBC from progressing. The development of such drugs and the conduct of experimental and multicenter clinical trials can be expected in the near future. After 35 years of using UDCA as a unique drug of choice for patients with PBC, a number of targets have been identified based on a deeper understanding of the pathophysiology of the disease. These include the discovery of impaired mechanisms of bicarbonate formation by cholangiocytes in PBC, through decreased activity of inositol-1,4,5-trisphosphate receptor isoform 3 and chlorine/bicarbonate AE2, caused by increased miR-506 activity. This may provide a rationale for the future development of new drugs aimed at locally reducing miR-506 activity or activating the AE2 in cholangiocytes. It is likely to be one of the new therapeutic approaches in the treatment PBC or will complement existing methods that use hydrophilic bile acids.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country of origin: Russia
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Li YC S-Editor: Bai Y L-Editor: A P-Editor: Yu HG