INTRODUCTION
Mesotheliomas are aggressive neoplasms arising from the serosal surface of the pleural cavity, peritoneal cavity, pericardium, or the tunica vaginalis of the testis. Malignant pleural mesothelioma (MPM) is the most common, contributing to 80% of all mesotheliomas. Occupational asbestos exposure is regarded as the single most important etiological factor in the development of mesothelioma. However, environmental exposure to asbestos, radiation, and other mineral exposures are beginning to emerge as important risks. In addition, MPM poses a significant treatment challenge with 5-year survival being as low as 5%-10%[1]. With an increase in awareness regarding the ill effects of asbestos and stringent laws banning the use of asbestos, there has been a decline in the incidence of MPM in the West. However, continued and unregulated use of asbestos in low-income and middle-income countries is likely to significantly increase the mesothelioma burden in the coming years.
EPIDEMIOLOGY
The incidence of MPM varies and is difficult to document in countries (like India) without registries. The age adjusted annual incidence of MPM in the United States was about 9.8 cases per million[2]. In the United States, the incidence peaked around the late 1990s to early 2000s and declined thereafter owing to the control of asbestos mining and use[2,3]. The trend is similar in other developed nations like the United Kingdom and Australia. However, due to the rampant use of asbestos in developing nations and continued mining in a few countries, the incidence of mesothelioma is predicted to increase several fold in the coming years. Although India has taken measures to curb the mining of asbestos, use of asbestos imported from Russia, Kazakhstan, China, and Canada continue[4]. Asbestos is utilized in cement pipes, plumbing, roofing of homes, insulating heavy machinery, shipbuilding and breaking, and brake lining[4]. India tops the list of countries with maximum asbestos usage of 318000 metric tons[5]. Asbestos exposure resulting in MPM has also been reported in household contacts of asbestos miners. In addition, environmental exposure also increases the risk of MPM[6]. Given the sex differences due to the occupational exposure, MPM remains more common in males when compared to females[2].
RISK FACTORS
Inhaled asbestos exposure (either occupational, para-occupational, or environmental) is the foremost cause of MPM. Asbestos is a fibrous silicate of various chemical types. It exists in two forms, amphibole and chrysotile with the latter having a lower carcinogenic potential. MPM has a long latency period of approximately 20 years to 50 years[7]. The incidence following asbestos exposures increases linearly with the intensity of the exposure and exponentially with the duration of exposure[5].
Radiation exposure in the treatment of other malignancies accounts for subsequent MPM development, again with a long latency period[8]. Exposure of nuclear radiation has also been implicated to increase MPM risk. Patients developing MPM after radiation exposure are different from those with MPM due to asbestos exposure. They are generally younger and have a significantly longer overall survival[9]. Certain germline variants have been observed in MPM. Mutations in the gene encoding BRCA1 associated protein 1 (BAP1) has been shown to accelerate development of MPM in those exposed to asbestos[5]. Variants in DNA repair genes like PALB2, BRCA1/2, and cyclin-dependent kinase inhibitor 2A (CDKN2A) also accelerate the development of MPM[5].
CLINICAL PRESENTATION
The majority of patients present with nonspecific symptoms when they are in the sixth or seventh decade, several years after the exposure to asbestos has ceased. Symptoms include progressive dyspnea, dry cough, and chest pain. The symptoms may be attributable to the pleural effusion or volume contraction associated with the disease. Chest pain may or may not be pleuritic in nature and is usually due to the local invasive nature of the tumor leading to bone erosion or nerve impingement. Fatigue, anorexia, weight loss, sweats, and malaise are noted as the disease progresses. Distant metastasis is rare, while local invasion is known. Physical findings correspond to pleural effusion early in the disease, but ipsilateral volume contraction with rib crowding and scoliosis become apparent later in the disease course. Findings such as palpable nodules (at previous thoracocentesis puncture sites) may be seen.
Radiological imaging may include a frontal chest X-ray that is done as a part of the initial evaluation of symptoms. However, contrast-enhanced computed tomography (CT) of the chest and upper abdomen is recommended as the initial method of investigation[5]. Common chest X-ray findings include varying volumes of pleural effusion with or without pleural thickening, pleural mass, plaques, and calcification. Volume loss with ipsilateral mediastinal shift may also be seen. Advanced disease may demonstrate mediastinal extension with widening. Radiological features of asbestosis (bilateral basal fibrosis) are seen in only 20% of cases of MPM.
CT findings (in addition to pleural effusion) include pleural thickening, which may be seen in 90%-92% of the cases in some form. The pleural thickening may be circumferential, but focal thickening with nodules, loculated effusion, and mediastinal pleural thickening are more suggestive of mesothelioma[10].
Magnetic resonance imaging (MRI) is another modality that may be used in assessment. MRI has exhibited marginally higher sensitivity than CT for predicting resectability at the diaphragm and chest wall (100% vs 93%-94%, respectively) and has shown better accuracy in detecting solitary foci of chest wall invasion, endothoracic fascia involvement and brachiocephalic vessels involvement[11]. However, with the widespread use of positron emission tomography with computed tomography (PET-CT), which provides information on the metabolic activity along with anatomical extent, the use of MRI is now restricted to those cases who require additional soft tissue evaluation prior to surgery and in whom the use of iodinated contrast agents is contraindicated. Several series have suggested that PET-CT imaging is the most reliable imaging modality for initial assessment, particularly in determining whether a tumor is resectable[12].
DIAGNOSIS
The clinico-radiological presentation guides histopathological or cytological confirmation of the diagnosis. Pleural fluid cytology and immunocytochemistry is an accepted modality for diagnosing if cytopathology expertise is available. The sensitivity ranges between 30%-75% and specificity between 80%-99%[13], while an Indian study reported a sensitivity and specificity of 75% and 96%, respectively[14].
Biopsies are obtained percutaneously under image guidance during medical thoracoscopy or during video-assisted thoracoscopic surgery. Thoracoscopy is the preferred technique and allows extensive inspection of the pleura. Multiple, large biopsies that include subpleural tissue for the histological assessment of invasion can be obtained. Medical thoracoscopy can diagnose MPM with a sensitivity of 88%-100% and specificity of 100%[15]. The presence of local invasion, pleural thickening, absence of pleural effusion, and volume loss often make thoracoscopic biopsy challenging. In patients with advanced disease, bronchoscopic modalities including endobronchial ultrasound maybe used to obtain tissue[16].
SUBTYPES AND STAGING IN PLEURAL MESOTHELIOMA
Histological subtypes
Genomic analysis and prospective data from the past decade have significantly improved the understanding of pleural mesothelioma. Following these advances, the World Health Organization has updated their original classification system in 2021[17]. Overall, pleural mesothelioma has been classified based on the extent of involvement. Localized mesothelioma requires diagnosis based on imaging, surgical resection, and histology. Pathological evaluation must rule out any evidence of invasion along with confirmation of mesothelial origin using immunohistochemical (IHC) markers[18]. The previously described “well differentiated papillary mesothelial tumor” type has been renamed to well differentiated papillary mesothelioma and removed from the malignant group due to its benign course and better prognosis.
In the latest update, mesothelioma in situ has also been described as a distinct entity. It is identified based on their molecular features: Loss of BAP1 or MTAP on IHC or CDKN2A deletion on fluorescence in situ hybridization[19]. In addition to these features, invasion must be ruled out to call it mesothelioma in situ. Mesothelioma can be either localized or diffuse. The diagnosis of localized mesothelioma is often reached upon multidisciplinary discussion. Diffuse pleural mesothelioma is further classified into epithelioid, sarcomatoid, or biphasic. The World Health Organization classification stresses upon the need of good tissue for the diagnosis, achieved using medical thoracoscopy, video-assisted thoracoscopic surgery, or image-guided biopsies. Diagnosis revisions are also advised based upon resection specimens.
The epithelioid subtype is the most common subtype, representing nearly 80% of all cases. The epithelioid variant is associated with better prognosis compared to the other subtypes. Within the epithelioid morphology, it is recommended to describe the architectural patterns that might have prognostic significance (solid and micropapillary features have poorer prognosis). The report must also include the grade of differentiation (high or low) based on nuclear atypia, mitoses, and necrosis.
The sarcomatoid subtype is characterized by the presence of desmoplastic reaction with or without spindle cells arranged in sheets. Sarcomatoid tumors are associated with a distinctly poor prognosis. When compared with the epithelioid variant, sarcomatoid tumors are not commonly associated with pleural effusion.
The biphasic subtype is characterized by the presence of both types of tumor cells. In resected specimens, it is recommended to report the individual component of cells with epithelioid and sarcomatoid characteristics. Biphasic tumors must have greater than 10% of either feature. However, in smaller biopsies such qualification has been found to be difficult and is not considered mandatory. However, the prognosis is driven mainly by the proportions of the sarcomatoid component in such cases[20].
IHC features
Reporting of mesothelial tumors must include IHC workup to confirm the mesothelial origin of such tumors. IHC markers of common cancers with frequent pleural metastasis must be done based on suspicion, including lung (TTF1 and napsin for adenocarcinoma, p63 for squamous cell carcinoma), breast, ovarian, and colorectal cancers. In addition to the diagnosis and differentiation of such (especially epithelioid) tumors from other metastatic cancers, these markers are also useful in predicting prognosis[21]. However, none of the makers have absolute sensitivity or specificity, which mandates the use of an array of such markers. In a 2021 update, claudin 4 has been highlighted as a negative IHC marker with high diagnostic accuracy to differentiate mesothelioma from other metastatic carcinomas. Additionally, the European Respiratory Society/European Society of Thoracic Surgeons/European Association for Cardio-Thoracic Surgery/European Society for Radiotherapy and Oncology guidelines have recommended the use of CDKN2A (p16) deletion (using fluorescence in situ hybridization, commonly found in sarcomatoid variants) and BAP1 (using IHC, commonly demonstrated in epithelioid variants) for further characterization of the tumors[22]. In a 2021 update, loss of MTAP expression using IHC was found to correlate well with CDKN2A deletion and can be used as a surrogate. These markers have been demonstrated to have acceptable accuracy in cytological specimens as well.
Evaluation of tumor immune microenvironment has also been analyzed and shown to have a linear relationship between PDL1 expression and sarcomatoid differentiation leading to an overall poor prognosis in tumors with increased PDL1 expression. Non-epithelial tumors are also characterized by higher cytotoxic T cell and macrophage infiltration[23]. However, such features have not been shown to predict response to immune checkpoint inhibitors.
Common genomic alterations
Molecular characteristics of mesothelioma have been found to influence overall prognosis and have been recently proposed to be integrated with histopathological reporting. Common among them are CDKN2A (p16) deletion (associated with poorer prognosis), BAP1 loss (associated with favorable prognosis), LATS2 mutation (associated with poorer prognosis), and YAP1 overexpression[24]. Other genetic biomarkers like p53, NF2, W1F1, and DNA methylation have been associated with predicting prognosis but have limited clinical implications[25,26]. In a recent study, BAP1 loss by IHC has been demonstrated to predict superior overall survival following first-line platinum-pemetrexed combination.
Staging of mesothelioma
The rarity of the tumor with finite prospective data has limited the development of accurate staging systems. However, using prospective data from over 3700 cases, the International Association for The Study of Lung Cancer has updated their Tumor-Node-Metastasis (8th) staging for pleural mesothelioma[27]. Involvement of ipsilateral parietal or visceral pleural has been included in T1, whereas invasion into diaphragmatic muscle or lung has been categorized as T2. Involvement of chest wall soft tissue (solitary focus), mediastinal fat, or non-transmural pericardial involvement has been classified as T3. T1, T2, and T3 tumors are considered as potentially resectable. Pericardial involvement with or without effusion is classified as T4, which also includes involvement of contralateral pleura and peritoneal involvement. Involvement of any ipsilateral regional lymph node (intrathoracic, internal mammary, or scalene nodes) is classified as N1, whereas contralateral involvement or any-side supraclavicular involvement is classified as N2. The metastasis descriptor has been left unchanged[28].
TREATMENT
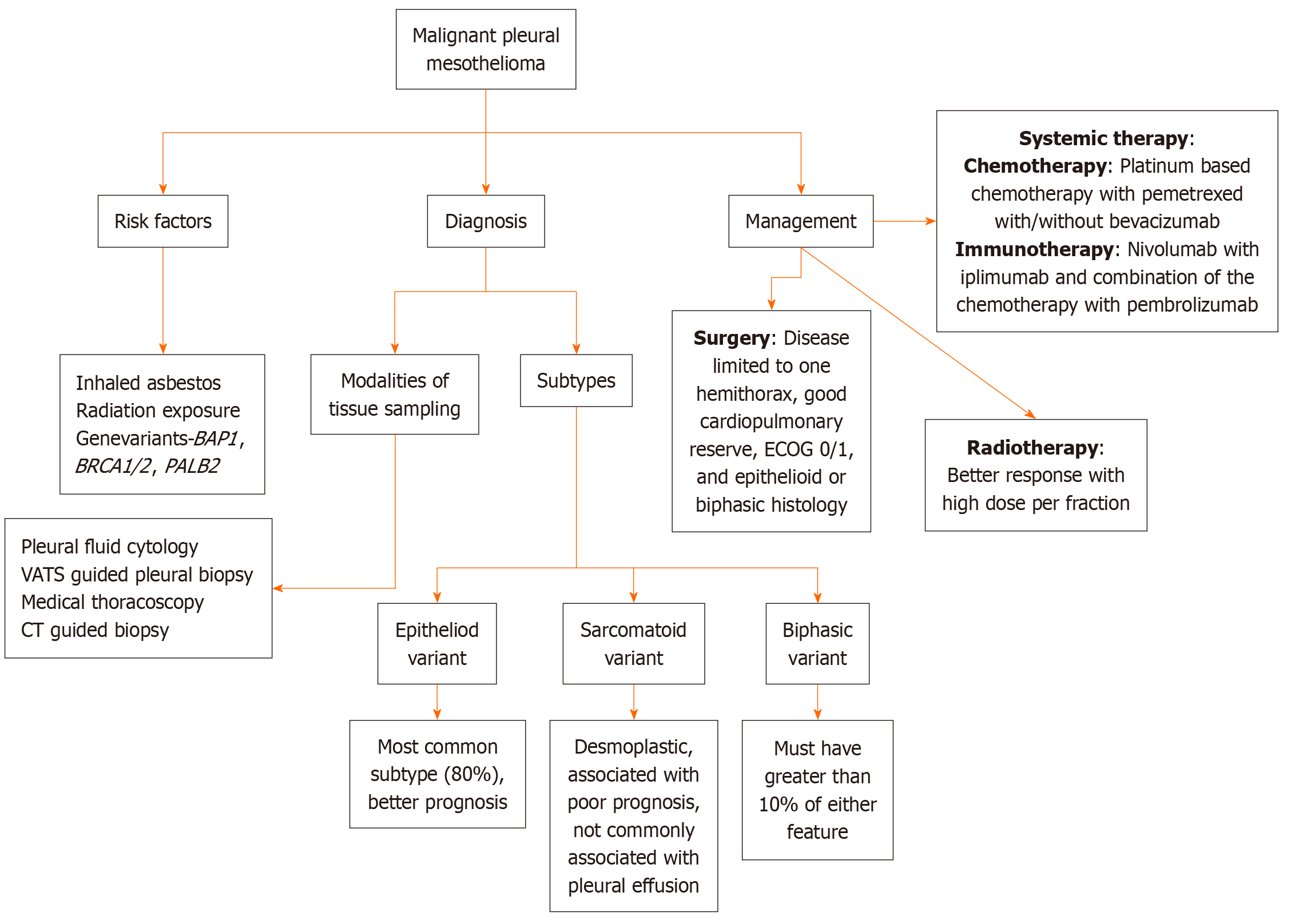
MPM is insidious at the onset and slowly progressive. The patient usually presents with extensive intrathoracic disease. The prognosis is poor with a median survival of 10 months to 22 months. The optimal treatment is still not established; however, early diagnosis with a multimodal treatment approach is imperative to improve outcomes in MPM. The treatment strategies include surgery, radiation therapy, chemotherapy, targeted treatment, and immunotherapy. Treatment depends on the extent of the disease, histological subtype, age, comorbidities, and performance status. Palliative treatment plays a crucial role in management, and it should be incorporated in the practice right from the diagnosis as patients with MPM have a significant symptom burden and palliative care needs, which if addressed can improve the quality of life. Figure 1 illustrates the general approach towards the management of MPM, encompassing diagnostic procedures, therapeutic strategies, and multidisciplinary care pathways.
Figure 1 General approach towards management of malignant pleural mesothelioma.
CT: Computed tomography; ECOG: Eastern Cooperative Oncology Group; VATS: Video-assisted thoracoscopic surgery.
SURGERY
The goal of surgery in MPM is macroscopic complete resection. It should be considered in selected patients with disease limited to one hemithorax, appropriate cardiopulmonary reserve, good performance status, epithelioid or biphasic histology, and if complete cytoreduction can be achieved. It should be performed in specialized centers where appropriate expertise is available. As there are no randomized trials indicating survival benefit with surgery, the role of surgery remains uncertain. The main surgical approaches are extrapleural pneumonectomy (EPP), extended pleurectomy/decortication, and pleurectomy/decortication (P/D). The choice of surgical approach (EPP vs P/D) depends on expertise or preferences of the surgeon, the patient’s medical condition, and availability of multimodal treatment strategies within that center.
The more radical EPP approach consists of en bloc resection of the ipsilateral lung with parietal and visceral pleura, pericardium, and diaphragm. The pericardium and diaphragm may be left intact if they are not involved with the tumor. In extended pleurectomy/decortication, parietal and visceral pleurectomy is performed with resection of the pericardium and diaphragm. The other less radical approach, P/D, consists of removal of parietal and visceral pleura without resection of the pericardium or diaphragm[29].
There are no randomized trials that compare these surgical approaches. A recently published meta-analysis of EPP vs P/D, which included 18 studies with total of 4852 patients, showed better median survival and reduced 30-day mortality and complications in the P/D group[30]. The morbidity and mortality were higher after EPP leading to adoption of P/D by various thoracic surgeons[31,32]. The common complications following EPP are cardiac arrhythmias, pulmonary embolism, pneumonia, broncho-pleural fistula, and respiratory failure. The most common complication after P/D is prolonged air leak[33].
The Mesothelioma and Radical Surgery trial evaluated the efficacy of EPP in the context of trimodality therapy. All patients received three initial cycles of platinum-based chemotherapy followed by clinical assessment. Patients were then randomized (1:1) to EPP (n = 24) followed by postoperative hemithoracic radiotherapy or to no EPP (n = 26). The trimodality therapy did not result in any survival benefit but was associated with high morbidity[34]. In contrast to the Mesothelioma and Radical Surgery trial, the Surgery for Mesothelioma After Radiotherapy (SMART) trial[35] demonstrated the benefit of surgical resection using EPP in resectable patients with clinical stage T1-3N0M0. The treatment protocol consisted of a short course of accelerated hemithoracic radiotherapy of 25 Gy in five fractions over 1 week followed by extrapleural pneumonectomy followed by adjuvant chemotherapy in patients with pathological mediastinal lymph node involvement. The target volume includes the entire hemithorax including diaphragmatic attachments, ipsilateral mediastinal and upper retroperitoneal nodes, and any chest tube or biopsy tracts. The entire irradiated lung was removed, thus reducing the radiation pneumonitis risk. Patients with ypN0 epithelial histology had better overall survival and disease-free survival. The SMART trial has shown that EPP after radiotherapy resulted in improvements in median overall survival but is associated with morbidity. The SMART protocol should be carried out in centers with surgical and radiation expertise.
RADIOTHERAPY
A traditional treatment approach for pleural mesothelioma includes surgery and systemic therapy followed by radiotherapy as adjuvant treatment with radical or palliative intent. Recent advances in radiotherapy techniques have improved treatment accuracy and allows delivery of radical doses with fewer toxicities. The former perception is that mesothelioma is intrinsically radioresistant, but certain studies have shown that it responds better to a high dose per fraction. Various studies where radiotherapy has been used in palliative setting have shown better response with a high dose per fraction (4 Gy/# compared to 3 Gy/#). This is explained by a low α/β ratio, low proliferation index, and non-squamous histology[36].
The role of radiotherapy in pleural mesothelioma can be: (1) Hemithorax radiation prior to or after EPP; (2) Procedure tract radiation; (3) Hemithorax radiation after P/D; and (4) Palliation of local symptoms caused by disease[37].
For simulation for radiotherapy, after proper immobilization a free breathing or four-dimensional CT scan is recommended for patients undergoing hemithoracic radiation. Image guided target delineation using fluorodeoxyglucose PET has shown significant alterations in target volumes and thus improves treatment accuracy. MRI is also considered a preferred imaging modality for T3 or T4 disease for delineating pleural gross tumor volume[38].
Hemithoracic radiation is the standard of care post EPP for medically operable stage I to III patients, consistent with the National Comprehensive Cancer Center guidelines (version 2.2018) and American Society of Clinical Oncology 2018 recommendations. The radiotherapy technique has evolved over the years from conventional anteroposterior/posteroanterior fields to three-dimensional conformal radiotherapy to intensity modulated radiotherapy, which is currently the standard of care. The main concern for patients undergoing intensity modulated radiotherapy for mesothelioma is the associated risk of pneumonitis due to large volumes of treatment after a pneumonectomy[39]. The recommended constraints after pneumonectomy is to keep the opposite lung V5 < 60%, V20 < 4 - 7%, and mean lung dose to < 8 Gy[39,40]. The postoperative field encompasses the entire pleural bed. The clinical target volume includes ribs in the lateral aspect along with a margin at the pleural/mediastinal interface followed by a planning target volume expansion of 0.5 cm. A dose of 45-54 Gy in 1.8-2.0 Gy/ fraction and a boost to 54 to 60 Gy for R1 or R2 resection is recommended according to the International Association for The Study of Lung Cancer guidelines. Prophylactic irradiation of mediastinal nodes is not recommended.
With recent progress towards more conservative lung-sparing procedures and the availability of advanced radiation techniques, the use of EPP has declined. Multimodality approach using hemithoracic intensity-modulated pleural radiation therapy technique along with P/D and chemotherapy with a dose of 50.4 Gy in 28 fractions is feasible and could be delivered safely with no reported grade 4 or grade 5 pneumonitis[41]. Additional advanced modalities such as proton therapy are emerging. Stereotactic radiotherapy for recurrent pleural mesothelioma has been studied with promising results. Palliative radiotherapy is used for symptom control.
SYSTEMIC TREATMENT
The standard systemic treatment for mesothelioma was a combination of pemetrexed and cisplatin with carboplatin being used if cisplatin was contraindicated[42,43]. In the phase 3 EMPHACIS trial by Vogelzang et al[42], a combination of pemetrexed/cisplatin chemotherapy (pemetrexed 500 mg/m2 and cisplatin 75 mg/m2 three times weekly) resulted in a response rate of 41.3% compared to 16.7% with cisplatin alone with a median overall survival of 12.1 months vs 9.3 months for cisplatin alone. Supplementation with folic acid and vitamin B12 resulted in a significant reduction in toxicities and greater improvement in all efficacy parameters in the pemetrexed/cisplatin chemotherapy arm. There were no adverse effects of supplements. Patients who received vitamin supplementation were able to receive more cycles of chemotherapy, which might have also contributed to the results. This trial established a combination of pemetrexed and cisplatin as a new standard of care in systemic treatment for mesothelioma. Since then, this combination of platinum and pemetrexed has been used as the backbone of combination therapies and the control arm in various trials.
The role of maintenance pemetrexed in mesothelioma is still not defined. In a phase 2 trial (CALGB 30901) of maintenance pemetrexed vs observation, patients with unresectable disease were randomized to maintenance pemetrexed after four to six cycles of pemetrexed and platinum. A total of 49 patients with 27 in the pemetrexed arm and 22 in the observation arm were included. The median overall survival was 11.8 months for the observation arm and 16.3 months for the maintenance arm (P = 0.67). The progression free survival (PFS) was 3.4 months in the maintenance arm and 3 months in the observation arm (P = 0.97). Thus, maintenance pemetrexed did not result in improvement of PFS and did not provide any additional benefit[44].
The addition of bevacizumab to the pemetrexed/cisplatin doublet was tested in the phase 3 Mesothelioma Avastin Cisplatin Pemetrexed Study trial[45]. Patients received pemetrexed/cisplatin combination or 15 mg/kg bevacizumab on day 1 in addition to pemetrexed/cisplatin combination (PCB) in three weekly cycles for up to six cycles. The median overall survival was significantly longer with PCB (18.8 months vs 16.1 months, P = 0.0167). Similarly, PFS was better with PCB (median PFS 9.2 months vs doublet 7.3 months, P < 0.0001). The grade 3-4 adverse effects were more in the PCB group resulting in more patients stopping the treatment, but there was no worsening of quality of life.
The United States Food and Drug Administration approved the use of tumor treating fields (TTF) in combination with systemic therapy with pemetrexed and platinum chemotherapy for first-line of treatment of unresectable mesothelioma following the publication of the STELLAR trial in 2019[46]. Patients were treated with continuous TTF (≥ 18 h a day with 150 kHz) to the thorax with concomitant chemotherapy with pemetrexed and platinum combination every 3 weeks for up to six cycles. TTF were given as maintenance treatment to patients whose disease did not progress following completion of chemotherapy. The combination of TTF and chemotherapy resulted in a median overall survival of 18.2 months.
Immunotherapy (IO) has restructured the therapeutic armamentarium in mesothelioma. The combination of nivolumab plus ipilimumab was approved as first-line systemic treatment for mesothelioma based on results of the CheckMate 743 trial[47]. In this multicentric randomized phase 3 study, patients were randomized to nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks for up to 2 years or platinum plus pemetrexed chemotherapy (cisplatin or carboplatin) once every 3 weeks for up to six cycles. The IO combination significantly improved median overall survival compared to chemotherapy (18.1 months vs 14.1 months, P = 0.002). The 2-year overall survival was 41% in the dual IO combination vs 27% in the chemotherapy group. Due to toxicity, 15% of patients discontinued treatment in the dual IO group compared to 7% in the chemotherapy group. The median overall survival with dual IO was similar between epithelioid (18.7 months) and non-epithelioid types (18.1 months). However, the median overall survival with chemotherapy in non-epithelioid type was 8.8 months and 16.5 months in epithelioid histology. The magnitude of benefit with dual IO was thus greater in the non-epithelioid histology. On subgroup analysis based on PD-L1 expression, survival outcomes with dual IO were similar in the patients with less than 1% and with 1% or higher PD-L1 expression. The 2-year outcomes were better with nivolumab plus ipilimumab than with chemotherapy in both the subgroups. The updated results of CheckMate 743[48] with a median follow-up of 43 months published in 2022 showed that the combination of nivolumab and ipilimumab continued to prolong overall survival over chemotherapy with a tolerable side effect profile. Twenty-eight percent of the patients continued to have an ongoing response at 3 years.
Based on results of this trial, nivolumab and ipilimumab became the standard of care for patients with non-epithelioid pleural mesothelioma. For patients with epithelioid histology, chemotherapy remains the treatment of choice, and immunotherapy can be kept as an alternative.
The combination of pembrolizumab with pemetrexed/platinum was tested against pemetrexed/platinum in a phase 3 randomized trial conducted at 51 hospitals[49]. Chemotherapy was given in standard doses with or without intravenous pembrolizumab 200 mg every 3 weeks for up to 2 years. Median survival was 17.3 months with pembrolizumab compared to 16.1 months with chemotherapy alone (P = 0·0324). The combination of pembrolizumab with chemotherapy improved the survival and was tolerable.
There are currently no approved second-line treatment options for patients progressing on first-line treatment. The treatment approach depends on the initial regimen used for first-line treatment. Patients treated initially with IO can be treated with chemotherapy with pemetrexed/platinum combination or singlet depending on performance status. For patients who have been treated initially with chemotherapy, the second-line treatment depends on the treatment-free period after the first-line chemotherapy. The options available are re-challenging patients with the initial regimen if the gap is ≥ 6 months or IO or single agent chemotherapy.
CONCLUSION
MPM is a rare and aggressive malignancy with a dismal prognosis. Management of MPM is challenging and requires a multimodal and holistic approach. Before the positive trial of dual IO, chemotherapy with pemetrexed and platinum has been the standard of care for nearly two decades. There has been recent significant progress in the management with nivolumab and ipilimumab becoming the new standard of care for non-epithelioid type mesothelioma. Palliative care is an integral component of the treatment paradigm to reduce the symptom burden and maintain or improve quality of life. There is a need of translational biomarker studies to identify the patients who will benefit from IO or chemotherapy and bridge the gap between the current scenario and desired future. There is still a long road ahead to improve the overall management of MPM.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country of origin: India
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Sun GH S-Editor: Liu JH L-Editor: Filipodia P-Editor: Wang WB