Published online Mar 20, 2024. doi: 10.5493/wjem.v14.i1.91271
Peer-review started: December 26, 2023
First decision: January 11, 2024
Revised: January 24, 2024
Accepted: March 1, 2024
Article in press: March 1, 2024
Published online: March 20, 2024
Processing time: 84 Days and 8.3 Hours
Diabetes is known damage the liver and kidney, leading to hepatic dysfunction and kidney failure. Honey is believed to help in lowering the blood glucose levels of diabetic patients and reducing diabetic complications. However, the effect of stingless bee honey (SBH) administration in relieving liver and kidney damage in diabetes has not been well-studied.
To investigate the effect of SBH administration on the kidney and liver of streptozotocin-induced (STZ; 55 mg/kg) diabetic Sprague Dawley rats.
The rats were grouped as follows (n = 6 per group): non-diabetic (ND), untreated diabetic (UNT), metformin-treated (MET), and SBH+metformin-treated (SBME) groups. After successful diabetic induction, ND and UNT rats were given normal saline, whereas the treatment groups received SBH (2.0 g/kg and/or metformin (250 mg/kg) for 12 d. Serum biochemical parameters and histological changes using hematoxylin and eosin (H&E) and periodic acid–Schiff (PAS) staining were evaluated.
On H&E and PAS staining, the ND group showed normal architecture and cellularity of Bowman’s capsule and tubules, whereas the UNT and MET groups had an increased glomerular cellularity and thickened basement membrane. The SBH-treated group showed a decrease in hydropic changes and mild cellularity of the glomerulus vs the ND group based on H&E staining, but the two were similar on PAS staining. Likewise, the SBME-treated group had an increase in cellularity of the glomerulus on H&E staining, but it was comparable to the SBH and ND groups on PAS staining. UNT diabetic rats had tubular hydropic tubules, which were smaller than other groups. Reduced fatty vacuole formation and dilated blood sinusoids in liver tissue were seen in the SBH group. Conversely, the UNT group had high glucose levels, which subsequently increased MDA levels, ultimately leading to liver damage. SBH treatment reduced this damage, as evidenced by having the lowest fasting glucose, serum alanine transaminase, aspartate transaminase, and alkaline phosphatase levels compared to other groups, although the levels of liver enzymes were not statistically significant.
The cellularity of the Bowman’s capsule, as well as histological alteration of kidney tubules, glomerular membranes, and liver tissues in diabetic rats after oral SBH resembled those of ND rats. Therefore, SBH exhibited a protective hepatorenal effect in a diabetic rat model.
Core Tip: Honey products are widely recognized for their abundant vitamin content and bioactive components, which enhance their potential therapeutic benefits in the management of diabetes. This study demonstrated the hepatic and renal protective properties of stingless bee honey, which improved the architecture of the kidney and liver in diabetic rats. Thus, stingless bee honey could be useful in the treatment or prevention of liver and kidney impairment in diabetes.
- Citation: Mohd Nasir S, Ismail AF, Tuan Ismail TS, Wan Abdul Rahman WF, Wan Ahmad WAN, Tengku Din TADAA, Sirajudeen KNS. Hepatic and renal effects of oral stingless bee honey in a streptozotocin-induced diabetic rat model. World J Exp Med 2024; 14(1): 91271
- URL: https://www.wjgnet.com/2220-315x/full/v14/i1/91271.htm
- DOI: https://dx.doi.org/10.5493/wjem.v14.i1.91271
Diabetes mellitus (DM) is becoming an increasingly prevalent major health concern, characterized by hyperglycemia secondary to insulin deficiency or resistance[1]. In 2019, 9.3% (463 million) of individuals worldwide had diabetes, according to the International Diabetes Federation[2], and this is expected to increase to 10.2% (578 million) by 2030 and 10.9% (700 million) by 2045 if effective prevention strategies are not applied[3]. In particular, Malaysia has the highest rate of diabetes in the Western Pacific region and one of the highest in the world, costing around 600 million United States dollars per year[2]. Diabetics are predisposed to macrovascular problems, such as cardiovascular, cerebrovascular, and peripheral vascular illnesses, as well as microvascular consequences, such as retinopathies, nephropathies, and neuropathies[4].
One complication of uncontrolled diabetes is diabetic nephropathy, characterized by pathological quantities of urine albumin excretion, diabetic glomerular lesions, and loss of glomerular filtration rate in diabetics[5]. DM has also been associated with many liver abnormalities, such as abnormal glycogen deposition, nonalcoholic fatty liver disease, fibrosis, cirrhosis, hepatocellular carcinomas, abnormal elevated hepatic enzymes, acute liver disease, and viral hepatitis[6]. This worsens insulin resistance and leads to severe metabolic dysfunction. Moreover, it can destroy hepatocytes and contribute to increased morbidity and mortality among diabetic patients.
Regulating blood glucose levels is essential for reducing the risk of diabetic complications and improving the health of diabetic patients[7]. Until recently, conventional therapies have only attempted to manage blood glucose levels, but efforts to control diabetic complications have been unsuccessful[8]. Honey is a natural substance that consists of carbohydrates, water, organic acids, amino acids, enzymes, pigment, and pollens with antibacterial and antiinflammatory features[9,10]. Clinically speaking, honey is believed to help lower the blood glucose levels of diabetic patients and reduce diabetic complications[11]. Despite many studies on the therapeutic properties of different types of honey, it is still not regularly applied in practice, since there is still controversy regarding glycemic control in individuals with oral honey supplementation and the role of honey in diabetic complications.
Oxidative stress plays a vital role in the development of diabetic complications[12]. Honey has been studied in various ailments in animal and human models and has been discovered as a novel antioxidant agent[13]. The composition of honey depends primarily on its floral source and seasonal and environmental factors[14-16], and thus its different varieties may exhibit various health-promoting properties. In particular, SBH includes various compounds (i.e., phenolic acids, flavonoid enzymes, organic acids, and other minor compounds) that act as antioxidants, which are believed to have a synergistic effect. The phenolic and anti-inflammatory properties of SBH are believed to have a hepatorenal protective effect against diabetes. This study aimed to evaluate the effects of oral SBH on serum biochemical parameters and histological changes in the liver and kidney in a streptozotocin (STZ)-induced diabetic Sprague Dawley (SD) rat model.
The experimental animals in this study were male SD rats (n = 30), 8-10 wk old, weighing 200-250 g, purchased from the Animal Research and Service Centre (ARASC), University Sains Malaysia, Health Campus, Kubang Kerian. All rats were housed in plastic cages and maintained under standard laboratory conditions (21°C ± 2°C) with a 12 h light/dark cycle. Rats were given free access to normal standard rat pellet diet (10%/kg) supplied by ARASC USM and water ad libitum. The rats were acclimatized for one week before the experiment to reduce stress and familiarize them with human contacts. This study was conducted in accordance with the Institutional Guidelines for the Care and Use of Animals for Scientific Purposes (IACUC @ USM V4.Apr18).
The rats were divided into five groups based on the treatment given: non-diabetic (ND) rats as the control group (given normal saline), untreated diabetic (UNT) rats (given normal saline), SBH-treated (2.0 g/kg) diabetic rats, metformin-treated (MET) (250 mg/kg) diabetic rats, and SBH+metformin-treated (SBME) diabetic rats. In all except the ND group, Diabetes was induced by a single intraperitoneal injection of a freshly prepared solution of STZ (Sigma Aldrich, United States) 50 mg/kg BW in 0.1 M cold sodium citrate buffer (pH 4.5) after overnight fasting[17]. Diabetes was confirmed based on elevated fasting capillary plasma glucose (CPG) levels determined on days 3 and 7 after STZ injection; those persistently exhibiting CPG > 11.1 mmol/L were used for the experiment[18]. In this study, the range of CPG before treatment was 15.02-19.95 mmol/L, which was not more than 21.0 mmol/L. No mortality was observed during DM induction. The treatment was given via oral gavage by means of a tube inserted into the stomach through the mouth.
Rats were euthanized via cervical dislocation under anesthesia using ketamine plus xylazin. The kidneys and livers of all rats were harvested and preserved in 10% formalin. Samples then underwent a standard dehydration process in a series of increasing ethanol concentrations for 24 h using a processing machine. The tissue was then degreased with xylene, embedded in paraffin, and sectioned using a histological microtome. Afterward, 2.5-μm tissue sections were mounted on a glass slide, stained using hematoxylin and eosin (H&E) and periodic acid–Schiff (PAS) staining, then visualized under a light microscope at 40× magnification (Olympus BX41) and image analyzer.
CPG was measured using a URight blood glucometer (Uright, TD 4279) using the tail-prick technique to obtain tail vein blood. To collect fresh small medium of blood, an appropriate restraint device was used, and the withdrawal site was cleaned with alcohol. A finger prick lancet was used on the tail vein, and then capillary blood glucose was measured using a glucometer. After 12 d of treatment, the rat was euthanized before blood collection using cardiac puncture. The blood samples were then centrifuged at 4500 rpm for 10 min, and the serum samples were stored at 80°C until further analysis. Serum urea, creatinine, sodium (Na+), potassium (K+), calcium (Ca), magnesium (Mg), albumin, total protein, alanine transaminase (ALT), aspartate transaminase (AST), and alkaline phosphatase (ALP) were analyzed using an auto analyzer (Abbott ARCHITECT analyzer). Serum MDA was analyzed using the MDA ELISA Kit from Elabscience, USA, which uses the Sandwich-ELISA principle. All procedures were performed according to the manufacturer’s instructions.
Experimental data are presented as the mean ± SE of the mean in this study. One-way and two-way repeated measures of analysis of variance (ANOVA) were used to analyze CPG. Biochemical results were compared between groups by one-way ANOVA. All the analyses conducted were followed by Tukey’s post-test and performed using GraphPad Prism, v9.0 software (GraphPad, San Diego, CA, United States; alkaline phosphatase), with P < 0.05 denoting statistical significance.
To evaluate the antihyperglycemic effects of SBH, changes in CPG levels were measured on days 0 and 13 of treatment. Table 1 shows the effect of SBH and SBME on fasting blood glucose (FBG) concentrations in the diabetic rat groups on days 0 and 13 of treatment. The SBH, MET, and SBME groups had consistently reduced FBG levels throughout the treatment period. On day 13, the SBH- and MET-treated groups showed lower FBG levels than the SBME group. Meanwhile, UNT diabetic rats had the highest FBG compared to the other groups at all time points.
| Group | Fasting capillary plasma glucose (mmol/L) | |
| Day 0 | Day 13 | |
| Non-diabetic | 4.85 ± 0.26 | 4.48 ± 0.38 |
| Untreated diabetic | 20.00 ± 1.69 | 21.38 ± 2.94 |
| Stingless bee honey | 14.74 ± 2.25 | 7.66 ± 1.60 |
| Metformin-treated | 13.78 ± 1.18 | 7.00 ± 2.44 |
| Stingless bee honey plus metformin | 12.25 ± 2.43 | 9.73 ± 3.72 |
Serum urea and creatinine levels were measured to assess renal function and injury (Table 2). UNT diabetic rats showed the highest serum urea (14.28 + 4.92 mmol/L) and creatinine levels and (50.67 + 1.80 µmol/L) compared with ND rats. The SBH group had a lower serum urea compared with UNT diabetic rats. However, across the different treatment groups, SBME had the lowest serum urea level, followed by the SBH and MET groups. Meanwhile, for serum creatinine level, MET-treated diabetic rats had the lowest level of creatinine followed by SBME- and SBH-treated groups, but none of them were statistically significant.
| Analytes | ND | UNT | SBH | MET | SBME |
| Urea (mmol/L) | 8.28 ± 0.59 | 14.28 ± 4.92 | 7.95 ± 1.85 | 9.70 ± 0.30 | 7.30 ± 3.20 |
| Creatinine (µmol/L) | 46.00 ± 1.73 | 50.67 ± 1.80 | 46.17 ± 2.48 | 37.82 ± 8.62 | 40.80 ± 11.91 |
| Na+ (mmol/L) | 135.30 ± 1.93 | 138.40 ± 0.68 | 136.30 ± 0.88 | 134.40 ± 1.12 | 135.00 ± 1.23 |
| K+ (mmol/L) | 4.68 ± 0.12 | 4.45 ± 0.10 | 4.56 ± 0.32 | 4.52 ± 0.17 | 4.48 ± 0.11 |
| Ca2+ (mmol/L) | 2.36 ± 0.06 | 2.28 ± 0.04 | 2.35 ± 0.05 | 2.32 ± 0.04 | 2.36 ± 0.03 |
| Mg2+ (mmol/L) | 0.95 ± 0.03 | 0.88 ± 0.03 | 1.07 ± 0.05 | 0.93 ± 0.05 | 1.01 ± 0.14 |
Na+ levels in all diabetic rats were not significantly different compared to ND rats, but this was highest in UNT diabetic rats. The SBH group had a lower Na+ level compared to the UNT group. However, the MET groups had the lowest Na+ levels compared to all the treatment groups. The UNT group had a lower level of K+ (4.45 + 0.10 mmol/L) vs controls. Compared to the UNT group, the highest level of K+ was seen in the MET group (4.56 + 0.32 mmol/L), followed by the MET (4.52 + 0.17 mmol/L) and SBME (4.48 + 0.11 mmol/L) groups. The serum Ca2+ level of the UNT group was also lower than controls. The SBH, SBME, and MET groups had higher Ca2+ levels than that the UNT group, but this was not statistically significant. Meanwhile, serum Mg2+ levels were reduced in UNT rats vs ND rats. Serum Mg2+ was highest in the SBG group, followed by the SBME and MET groups.
Serum albumin in the SBH and SBME groups was higher than that of the UNT group, but lower than that of the ND group. The MET group had the lowest albumin level (28.40 + 0.51 g/L) out of all the groups of rats, but this was not significantly different. UNT rats had lower serum total protein than the ND rats. The SBME group, as well as the SBH and MET groups, had a higher total protein level than UNT diabetic rats, but this was not statistically significant.
Serum ALT was higher in the UNT group (75.50 + 33.18 U/L) vs ND rats. The SBH group had a lower serum ALT vs the UNT group. Conversely, serum AST (121.00 + 7.44 U/L) and ALP (162.00 + 25.65 U/L) were highest in the UNT group, compared to the ND and treatment groups. Meanwhile, the lowest serum ALP was seen in the MET group (126.70 + 14.84 U/L), followed by the SBH (131.70 + 23.92 U/L) and SBME (149.30 + 7.75 U/L) groups. The biochemical evaluation of liver function tests in rats treated with different treatments for 12 d are shown in Table 3.
| Group | Analytes | ||||
| Albumin (g/L) | Total | ALT (U/L) | AST (U/L) | ALP (U/L) | |
| Protein (g/L) | |||||
| Non-diabetic | 32.00 ± 1.10 | 54.25 ± 0.75 | 58.00 ± 5.40 | 110.40 ± 12.14 | 134.60 ± 5.59 |
| Untreated diabetic | 28.50 ± 0.65 | 49.67 ± 2.19 | 75.50 ± 33.18 | 121.00 ± 7.44 | 162.00 ± 25.65 |
| Stingless bee honey | 29.33 ± 0.71 | 52.67 ± 2.32 | 64.20 ± 0.80 | 108.30 ± 11.46 | 131.70 ± 23.92 |
| Metformin | 28.40 ± 0.51a | 50.33 ± 0.33 | 69.00 ± 7.41 | 110.30 ± 6.921 | 126.70 ± 14.84 |
| Stingless bee honey plus metformin | 29.50 ± 0.87 | 53.67 ± 1.20 | 71.50 ± 15.50 | 116.00 ± 11.41 | 149.30 ± 7.75 |
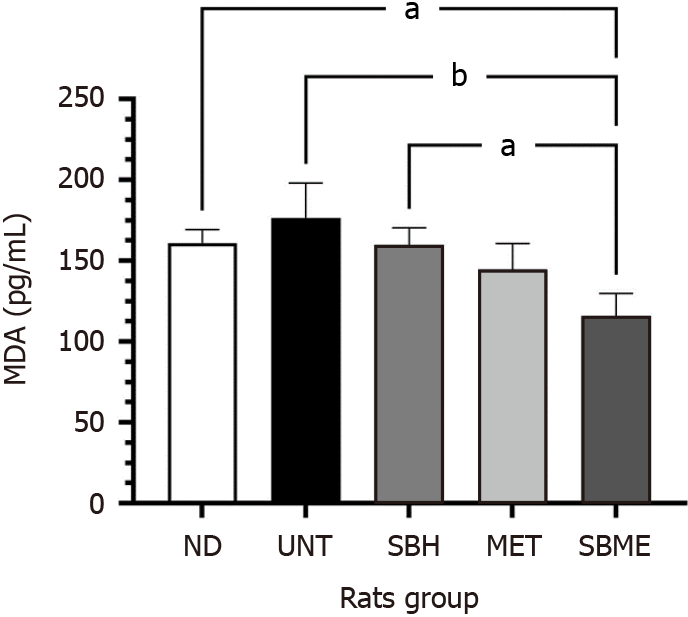
An MDA assay was performed to determine the role of SBH supplementation in the lipid peroxidation process. Figure 1 shows the MDA levels after 12 d of treatment. The MDA level was significantly higher in the UNT group vs ND rats (P < 0.05), whereas it was significantly lower in the treatment groups than in the UNT group (P < 0.01). The SBME group had significantly lower MDA levels compared to the SBH group (P < 0.05).
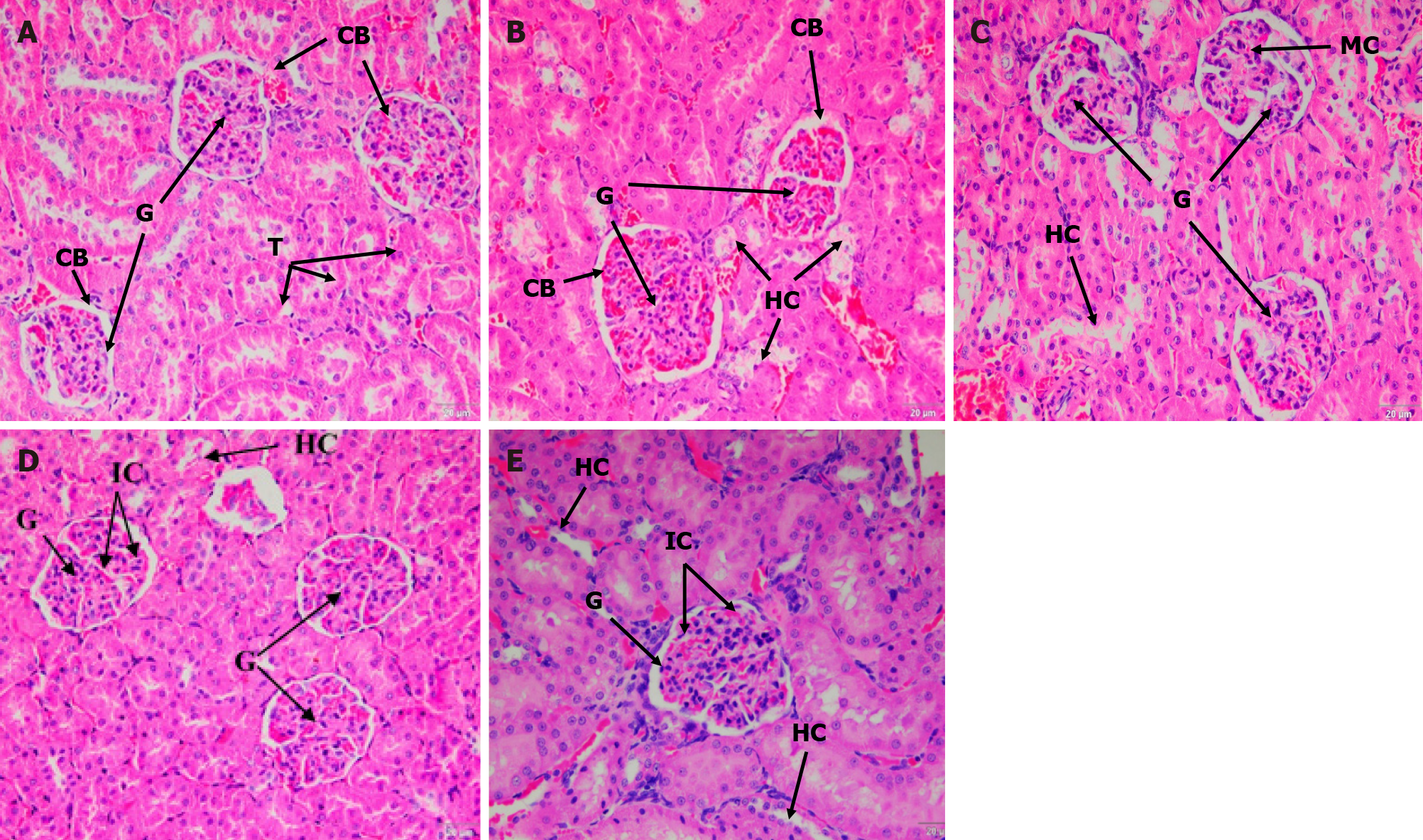
ND rats showed a normal architecture of Bowman’s capsule and tubules; the glomeruli had normal cellularity and no hydropic changes of the tubules (Figure 2A). Contrarily, the UNT group had increased glomerular cellularity and tubular hydropic changes was also observed (Figure 2B). In the SBH-treated groups, the kidney architecture showed a decrease in hydropic changes and mild cellularity of the glomerulus compared with the ND group (Figure 2C). The MET and SBME groups had higher cellularity than the SBH group as well as tubular hydropic changes were seen (Figure 2D and E). Mesangial matrix expansion was observed in the kidneys of both MET and SBME rats.
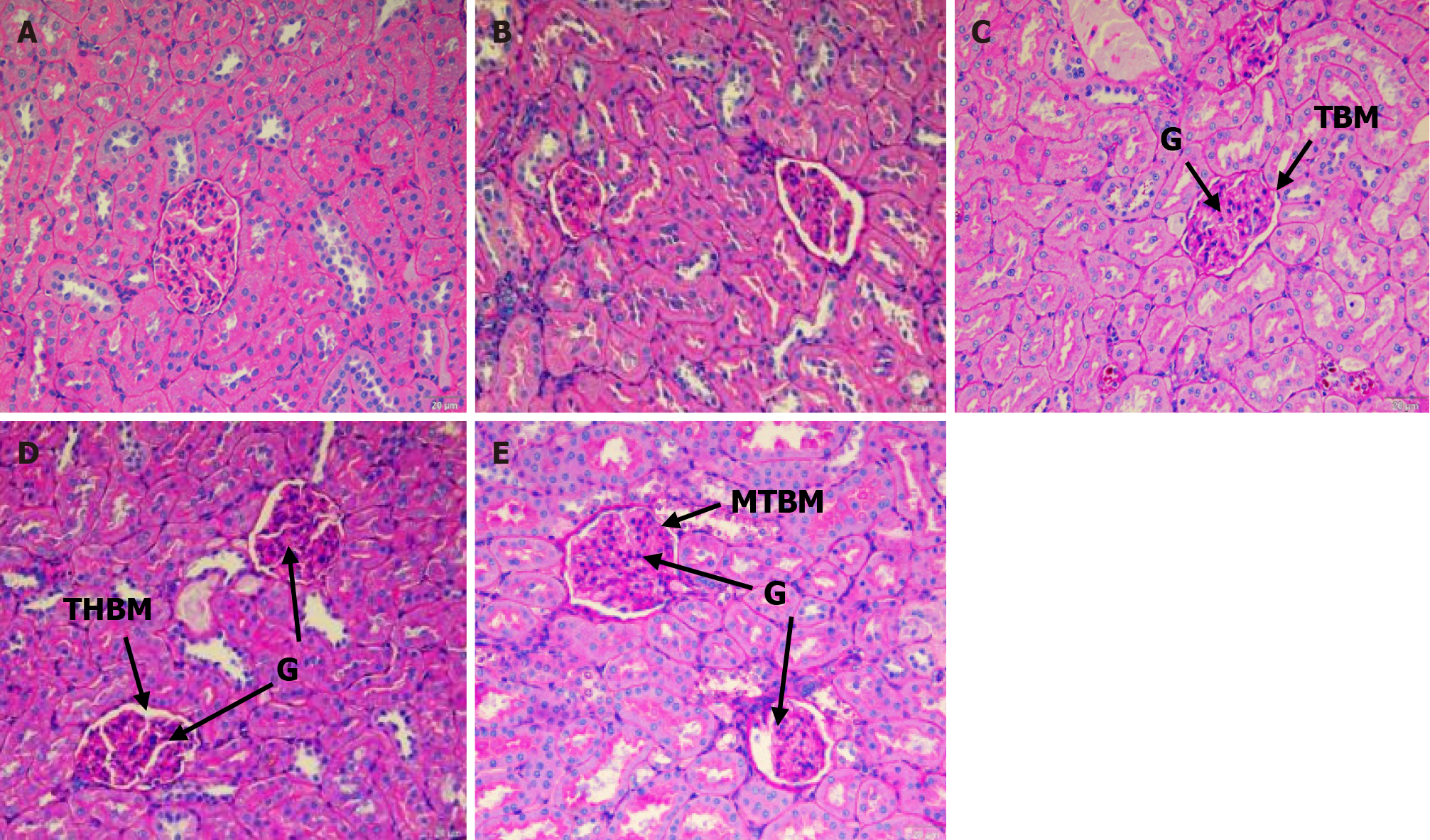
The ND rats showed a thin layer of glomeruli basement membrane when stained with PAS (Figure 3A). UNT diabetic rats showed tubular hydropic tubules, which appeared to be smaller than that of other groups. Figure 3B also indicates thickening of the basement membrane of glomeruli, tubules, and blood capillaries as the intensity of PAS stain increased. The basement membrane of the SBH-treated rat kidney (Figure 3C) appears to resemble the ND rat kidney. The MET-treated rat kidney (Figure 3D) showed mild thickening of the glomeruli basement membrane. The basement membrane of the rat kidney in the SBME-treated group (Figure 3E) was also comparable to that of the SBH and ND groups.
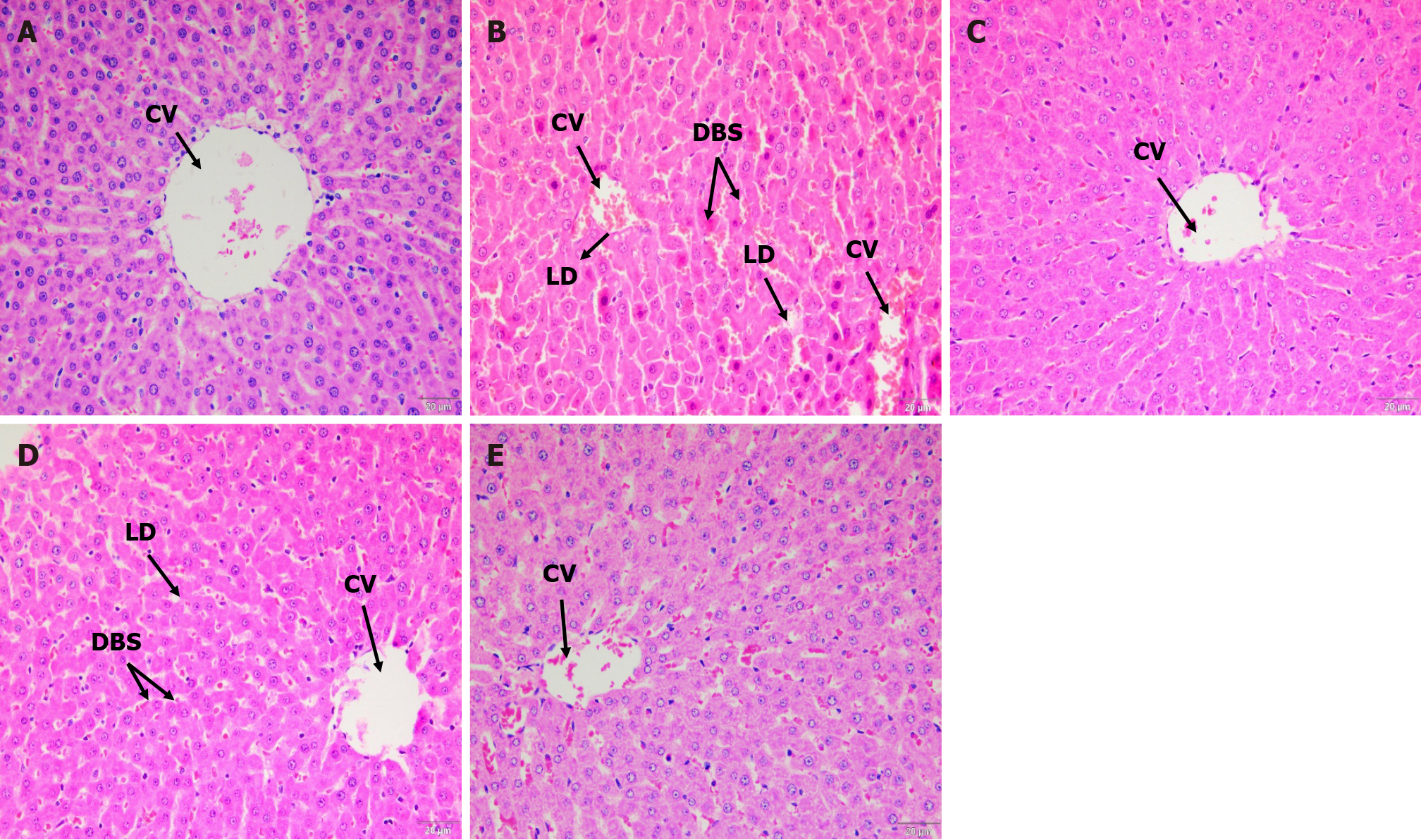
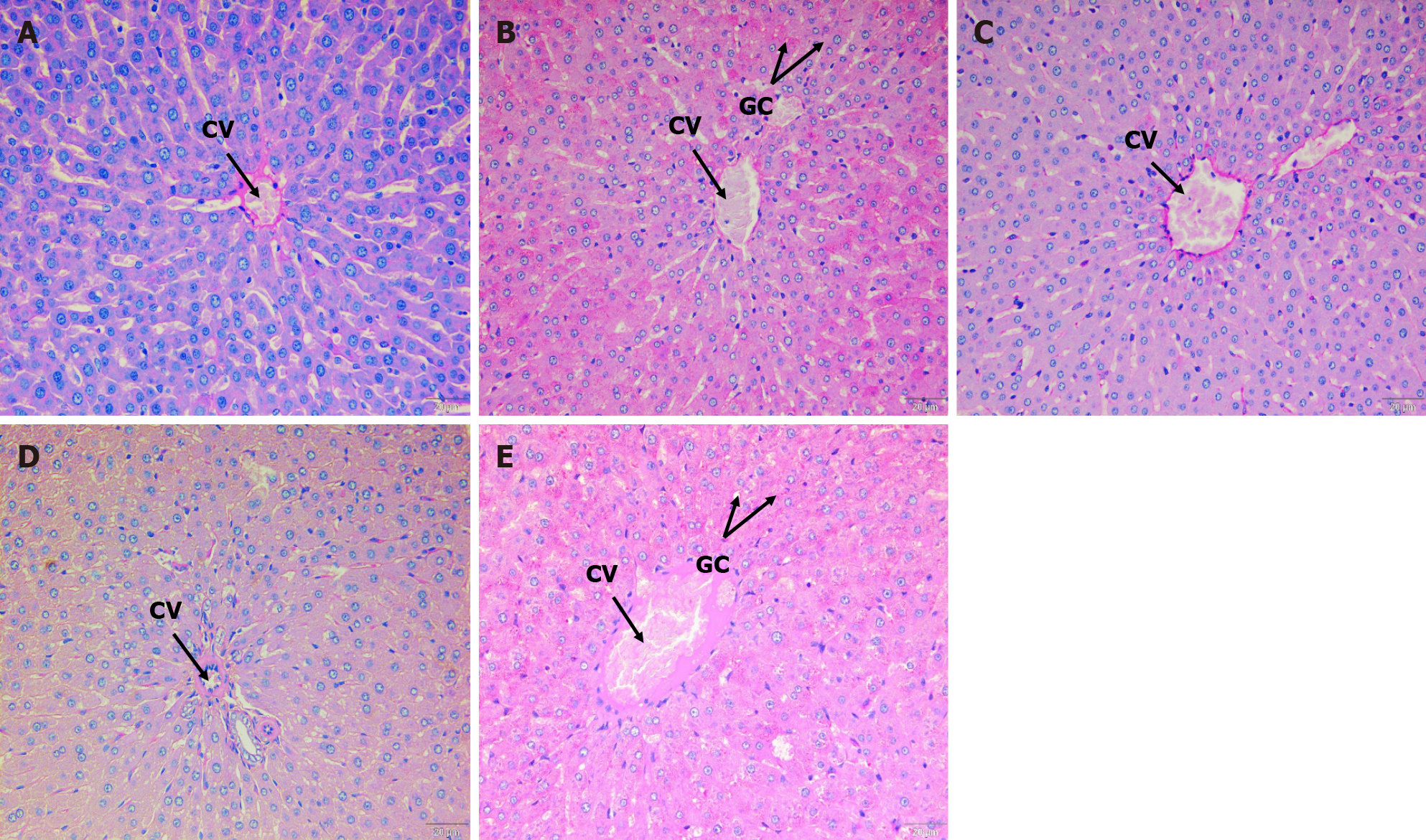
Hematoxylin and eosin staining: Compared to controls (Figure 4A), histological alterations were detected in the liver tissue of UNT diabetic rats, which revealed significant steatosis harboring lipid droplet formation in hepatocytes with dilated blood sinusoids and congestion surrounding the central veins (Figure 4B). This was in accordance with an increase in the serum ALT and AST, which are indicators of hepatocyte damage. However, these pathological alterations were reduced after treatment with SBH (Figure 4C), which showed a normal structure of the hepatic cells, specifically, reduced steatosis and congestion of sinusoids. MET-treated rats still showed dilation of sinusoids but had reduced formation of lipid droplets compared to the UNT group (Figure 4D). The SBME group had similar liver structure to the SBH group, wherein the fatty vacuoles and dilation of sinusoids were decreased vs UNT diabetic rats (Figure 4E). Thus, SBH administration ameliorated STZ-induced diabetic hepatic toxicity and hyperlipidemia.
Periodic acid–Schiff staining: The ND rat liver showed normal staining of glycogen content on PAS (Figure 5A). In the UNT group, the glycogen content in the liver structure of rats can be observed clearly (Figure 5B). Interestingly, after treatment with SBH for 12 consecutive days, the glycogen content was decreased, as only some cells were maintained (Figure 5C). Similarly, in the SBME group, only some cells had a positive PAS stain effect (Figure 5D). Treatment with MET (Figure 5E) did not change the rat liver structure, since it was still similar to that of the UNT group, wherein the intensity of glycogen stain increases in almost all hepatocytes.
The International Diabetes Federation estimates that 537 million people were living with diabetes in 2021, with an expected increase to 784 million by the year 2045[19]. The association between liver illness and diabetes mellitus (DM) is well established[20]. Diabetes is the most common cause of kidney failure requiring kidney transplantation or dialysis worldwide[21].
In this study, after 12 d of treatment, the concentrations of serum urea and creatinine for all groups of rats were within a normal reported range. UNT rats had the highest level of serum urea and creatinine compared with ND rats, suggesting that STZ-induced toxic effects on the kidneys[22]. Tavafi[23] reported significantly high levels of serum urea and creatinine in diabetic rats (induced by alloxan) compared with normal control rats after 8 wk, which might be due to a longer period of treatment taken for treating DM rats than in our current study of only 12 d.
Histological findings revealed tubular hydropic alterations and an increase in glomerular cellularity of kidney sections in UNT diabetic rats vs ND rats. Structural changes in the kidney could be related to diabetic metabolic changes. After staining with H&E and PAS, kidney sections from STZ-induced diabetic rats showed significant vacuolar degeneration of tubules, increased glomerular cellularity, mesangial matrix enlargement, and basement membrane thickening, likely caused by the extreme hyperglycemia from STZ induction. In line with this, Obi-Ezeani et al[24] described tubular epithelial alterations, increased capsular space, and glomerular degeneration in diabetic rats. Treatment with SBH had a favorable effect in mitigating renal injury by reducing hydropic alterations and improving basement membrane integrity. Compared to SBH-treated diabetic rats, high glomerular cellularity and mesangial matrix growth can still be detected with MET and SBME treatment. Furthermore, after staining with PAS, MET-treated rat kidneys had a slightly thickened glomeruli basement membrane. SBME-treated animals had similar basement membranes to those of the SBH and ND groups. Thus, therapy with SBH alone considerably reduced the changes identified on H&E and PAS staining, indicating its preventive role in renal injury.
MDA is a highly toxic aldehyde produced as a result of polyunsaturated fatty acid peroxidation, and it is a common indicator of lipid peroxidation[24]. DM can cause tissue damage through lipid peroxidation. Our findings revealed that the UNT group had the highest serum MDA level, likely from an increase in reactive oxygen species production caused by persistent hyperglycemia in UNT diabetic rats. Hyperglycemia reduces antioxidant levels in rats while increasing free radicals. However, treatment with SBME in our study significantly suppressed MDA levels in STZ-induced diabetic rats; this could help reduce oxidative stress. Our findings were consistent with those of other studies that indicated that SBH improved the MDA level in diabetic rats[25].
Elevated serum aminotransferase activity is a common indicator of liver illness. ALT and AST are common serum biochemical indicators that are regularly examined to diagnose liver injury and abnormalities[24,26]. The biochemical data in our study agreed with our histological observations using H&E and PAS staining. In UNT diabetic rats, lipid droplets accumulated in the cytoplasm of hepatocytes (Figure 4B and D). STZ caused acute liver injury in the current investigation, as evidenced by an increase in liver enzymes and histological abnormalities in the liver tissue of UNT diabetic rats (Figure 5B) after 12 d compared with ND rats, but these differences were not statistically significant. Nevertheless, after 12 d of SBH and MET treatment, ALT, AST, and ALP activity were all reduced, possibly because of the hepatoprotective effects of SBH and MET. Thus, even if hyperglycemia increased the MDA levels, causing oxidative liver damage, treatment with SBH was able to protect the liver by lowering blood ALT, AST, and ALP levels, possibly due to its hypoglycemic and antioxidant properties. Therefore, SBH can help improve impaired liver function in STZ-induced diabetic rats.
The current study has some limitations. The short treatment period may have contributed to the non-significant changes in biochemical results, despite clear histological changes seen in the liver and kidneys of diabetic rats. Moreover, during the 12-d treatment period, the ND rats did not receive SBH treatment. Future studies should explore the potential benefits and effects of oral SBH delivery in ND rats.
Although there were no significant variations in serum creatinine and urea levels between the groups, kidneys from the SBH group showed a normal thin layer of glomeruli basement membrane, equivalent to that of ND rats, and less hydropic alteration than the other groups. Although the decrease serum ALT, AST, and ALP levels were not significant, SBH treatment caused a histologically evident improvement in hepatocytes and reduced the formation of fatty vacuoles and dilatations of blood sinusoids in diabetic rat livers.
Studies have demonstrated an inverse relationship between honey consumption and the risk of diabetes and its complications. The argument about using honey to lower blood glucose and prevent hepatorenal diabetic complications however, continued.
Recent research has focused on exploring the biochemical and histological effects on kidney and liver of diabetic rats given oral stingless bee honey (SBH) and compared with rats given metformin (MET), combination SBH+MET, untreated and non-diabetic rats.
We investigated the effect of SBH administration on the serum biochemical parameters and the histological changes in streptozotocin induced diabetic rats’ kidney and liver using hematoxylin and eosin (H&E), Periodic Acid Schiff (PAS) staining.
Sprague Dawley rats (n = 30) grouped into 5, i.e. SBH-treated, metformin-treated (MET) and SBH+metformin-treated (SBME) non-diabetic (ND) and untreated diabetic (UNT) groups. The rats were sacrificed after 12 d of treatment, and serum, kidney and liver collected from the rats were used for biochemical parameters assessment.
SBH-treated group showed reduce in hydropic changes and mild cellularity of glomerulus as compared to ND group on H&E staining, but resembled ND group on PAS staining. Liver tissues of SBH-treated groups revealed lower fatty vacuoles formation and dilatation of blood sinusoids as compared to other groups. Biochemically, glucose reduction and lowest serum MDA, AST, ALT and ALP observed in SBH-treated compared to MET, SBME and UNT groups.
The oral SBH prevent diabetic complications development indicated by liver and kidney histology of SBH-treated diabetic rats. This finding offers a good perspective of honey role in managing diabetes.
Longer duration of treatment might improve the biochemical parameters. Future study on honey role in diabetic patients and metabolomic analysis to profile the serum of human treated with SBH and MET is necessary to uncover the underlying mechanisms.
We extend our heartfelt gratitude and appreciation to the personnel and staff of ARASC and Endocrine laboratory and Chemical Pathology laboratory staff, Universiti Sains Malaysia for their invaluable support throughout this research endeavor.
| 1. | Thangavel P, Ramachandran B, Chakraborty S, Kannan R, Lonchin S, Muthuvijayan V. Accelerated Healing of Diabetic Wounds Treated with L-Glutamic acid Loaded Hydrogels Through Enhanced Collagen Deposition and Angiogenesis: An In Vivo Study. Sci Rep. 2017;7:10701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 2. | Akhtar S, Nasir JA, Ali A, Asghar M, Majeed R, Sarwar A. Prevalence of type-2 diabetes and prediabetes in Malaysia: A systematic review and meta-analysis. PLoS One. 2022;17:e0263139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 3. | Wang H, Li N, Chivese T, Werfalli M, Sun H, Yuen L, Hoegfeldt CA, Elise Powe C, Immanuel J, Karuranga S, Divakar H, Levitt N, Li C, Simmons D, Yang X; IDF Diabetes Atlas Committee Hyperglycaemia in Pregnancy Special Interest Group. IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group's Criteria. Diabetes Res Clin Pract. 2022;183:109050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 703] [Article Influence: 175.8] [Reference Citation Analysis (0)] |
| 4. | Ganasegeran K, Hor CP, Jamil MFA, Loh HC, Noor JM, Hamid NA, Suppiah PD, Abdul Manaf MR, Ch'ng ASH, Looi I. A Systematic Review of the Economic Burden of Type 2 Diabetes in Malaysia. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Lim AKh. Diabetic nephropathy - complications and treatment. Int J Nephrol Renovasc Dis. 2014;7:361-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 414] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 6. | Mohamed J, Nazratun Nafizah AH, Zariyantey AH, Budin SB. Mechanisms of diabetes-induced liver damage: The role of oxidative stress and inflammation. Sultan Qaboos Univ Med J. 2016;16:el32-el41. |
| 7. | Yang DK, Kang HS. Anti-Diabetic Effect of Cotreatment with Quercetin and Resveratrol in Streptozotocin-Induced Diabetic Rats. Biomol Ther (Seoul). 2018;26:130-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 8. | Du M, Hu X, Kou L, Zhang B, Zhang C. Lycium barbarum Polysaccharide Mediated the Antidiabetic and Antinephritic Effects in Diet-Streptozotocin-Induced Diabetic Sprague Dawley Rats via Regulation of NF-κB. Biomed Res Int. 2016;2016:3140290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Nikpour M, Shirvani MA, Azadbakht M, Zanjani R, Mousavi E. The effect of honey gel on abdominal wound healing in cesarean section: a triple blind randomized clinical trial. Oman Med J. 2014;29:255-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Mohd Rafie AZ, Syahir A, Wan Ahmad WAN, Mustafa MZ, Mariatulqabtiah AR. Supplementation of Stingless Bee Honey from Heterotrigona itama Improves Antiobesity Parameters in High-Fat Diet Induced Obese Rat Model. Evid Based Complement Alternat Med. 2018;2018:6371582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Biluca FC, Braghini F, Gonzaga LV, Costa ACO, Fett R. Physicochemical profiles, minerals and bioactive compounds of stingless bee honey (Meliponinae). J Food Compos Anal. 2016;50:61-69. [RCA] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Ali H, Abu Bakar MF, Majid M, Muhammad N, Lim SY. In vitro anti-diabetic activity of stingless bee honey from different botanical origins. Food Res. 2020;4:1421-1426. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Ahmed S, Sulaiman SA, Baig AA, Ibrahim M, Liaqat S, Fatima S, Jabeen S, Shamim N, Othman NH. Honey as a Potential Natural Antioxidant Medicine: An Insight into Its Molecular Mechanisms of Action. Oxid Med Cell Longev. 2018;2018:8367846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 153] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 14. | Erejuwa OO, Nwobodo NN, Akpan JL, Okorie UA, Ezeonu CT, Ezeokpo BC, Nwadike KI, Erhiano E, Abdul Wahab MS, Sulaiman SA. Nigerian Honey Ameliorates Hyperglycemia and Dyslipidemia in Alloxan-Induced Diabetic Rats. Nutrients. 2016;8:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Alvarez-Suarez JM, Gasparrini M, Forbes-Hernández TY, Mazzoni L, Giampieri F. The Composition and Biological Activity of Honey: A Focus on Manuka Honey. Foods. 2014;3:420-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 213] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 16. | Zakaria F, Rosli W, Ishak W, Sze N, Shazwan M. Assessment of Glycaemic Effect of Benincasa hispida Aqueous Extract in Streptozotocin Diabetic Rats 2016; 1-12. Available from:https://www.researchgate.net/publication/307974568_Assessment_of_Glycaemic_Effect_of_Benincasa_hispida_Aqueous_Extract_in_Streptozotocin_Diabetic_Rats. |
| 17. | Nain P, Saini V, Sharma S, Nain J. Antidiabetic and antioxidant potential of Emblica officinalis Gaertn. leaves extract in streptozotocin-induced type-2 diabetes mellitus (T2DM) rats. J Ethnopharmacol. 2012;142:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 18. | Shamsudin S, Selamat J, Sanny M, A R SB, Jambari NN, Khatib A. A Comparative Characterization of Physicochemical and Antioxidants Properties of Processed Heterotrigona itama Honey from Different Origins and Classification by Chemometrics Analysis. Molecules. 2019;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Weng J, Zhou J, Liang L, Li L. UHPLC/QTOF-MS-based metabolomics reveal the effect of Melastoma dodecandrum extract in type 2 diabetic rats. Pharm Biol. 2019;57:807-815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | de Boer IH, Khunti K, Sadusky T, Tuttle KR, Neumiller JJ, Rhee CM, Rosas SE, Rossing P, Bakris G. Diabetes Management in Chronic Kidney Disease: A Consensus Report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care. 2022;45:3075-3090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 488] [Article Influence: 122.0] [Reference Citation Analysis (0)] |
| 21. | Hamed AE, Elsahar M, Elwan NM, El-Nakeep S, Naguib M, Soliman HH, Ahmed Aboubakr A, AbdelMaqsod A, Sedrak H, Assaad SN, Elwakil R, Esmat G, Salh S, Mostafa T, Mogawer S, Sadek SE, Saber MM, Ezelarab H, Mahmoud AA, Sultan S, El Kassas M, Kamal E, ElSayed NM, Moussa S. Managing diabetes and liver disease association. Arab J Gastroenterol. 2018;19:166-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Nørgaard SA, Søndergaard H, Sørensen DB, Galsgaard ED, Hess C, Sand FW. Optimising streptozotocin dosing to minimise renal toxicity and impairment of stomach emptying in male 129/Sv mice. Lab Anim. 2020;54:341-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Tavafi M. Diabetic nephropathy and antioxidants. J Nephropathol. 2013;2:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 24. | Obi-Ezeani NC. Evaluation of oxidative stress-induced diabetic complications on alloxan-treated hyperglycaemic rats, using some biochemical parameters and histological profiles of three major organs. MOJ Toxicol. 2018;4:59-67. [DOI] [Full Text] |
| 25. | Touzani S, Al-Waili N, Imtara H, Aboulghazi A, Hammas N, Falcão S, Vilas-Boas M, Arabi IE, Al-Waili W, Lyoussi B. Arbutus Unedo Honey and Propolis Ameliorate Acute Kidney Injury, Acute Liver Injury, and Proteinuria via Hypoglycemic and Antioxidant Activity in Streptozotocin-Treated Rats. Cell Physiol Biochem. 2022;56:66-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Ahmed Z, Ahmed U, Walayat S, Ren J, Martin DK, Moole H, Koppe S, Yong S, Dhillon S. Liver function tests in identifying patients with liver disease. Clin Exp Gastroenterol. 2018;11:301-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Health care sciences and services
Country/Territory of origin: Malaysia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Roomi AB, Iraq S-Editor: Liu JH L-Editor: A P-Editor: Yuan YY