Published online Mar 20, 2024. doi: 10.5493/wjem.v14.i1.86898
Peer-review started: July 13, 2023
First decision: September 19, 2023
Revised: September 30, 2023
Accepted: January 11, 2024
Article in press: January 11, 2024
Published online: March 20, 2024
Processing time: 250 Days and 10.9 Hours
Obesity has become more prevalent in the global population. It is associated with the development of several diseases including diabetes mellitus, coronary heart disease, and metabolic syndrome. There are a multitude of factors impacted by obesity that may contribute to poor wound healing outcomes. With millions worldwide classified as obese, it is imperative to understand wound healing in these patients. Despite advances in the understanding of wound healing in both healthy and diabetic populations, much is unknown about wound healing in obese patients. This review examines the impact of obesity on wound healing and several animal models that may be used to broaden our understanding in this area. As a growing portion of the population identifies as obese, understanding the underlying mechanisms and how to overcome poor wound healing is of the utmost importance.
Core Tip: Obesity induces a chronic low-grade inflammatory state through increased release of adipokines, cytokines, and chemokines from excess adipose tissue. The chronic low-grade inflammation is thought to contribute to a dampened immune response during the inflammatory phase of wound healing leading to delayed wound healing. While there are several animal models used to study wound healing, they have not been widely applied to studying the effects of obesity on wound healing leading to a gap in the literature on this topic.
- Citation: Cotterell A, Griffin M, Downer MA, Parker JB, Wan D, Longaker MT. Understanding wound healing in obesity. World J Exp Med 2024; 14(1): 86898
- URL: https://www.wjgnet.com/2220-315x/full/v14/i1/86898.htm
- DOI: https://dx.doi.org/10.5493/wjem.v14.i1.86898
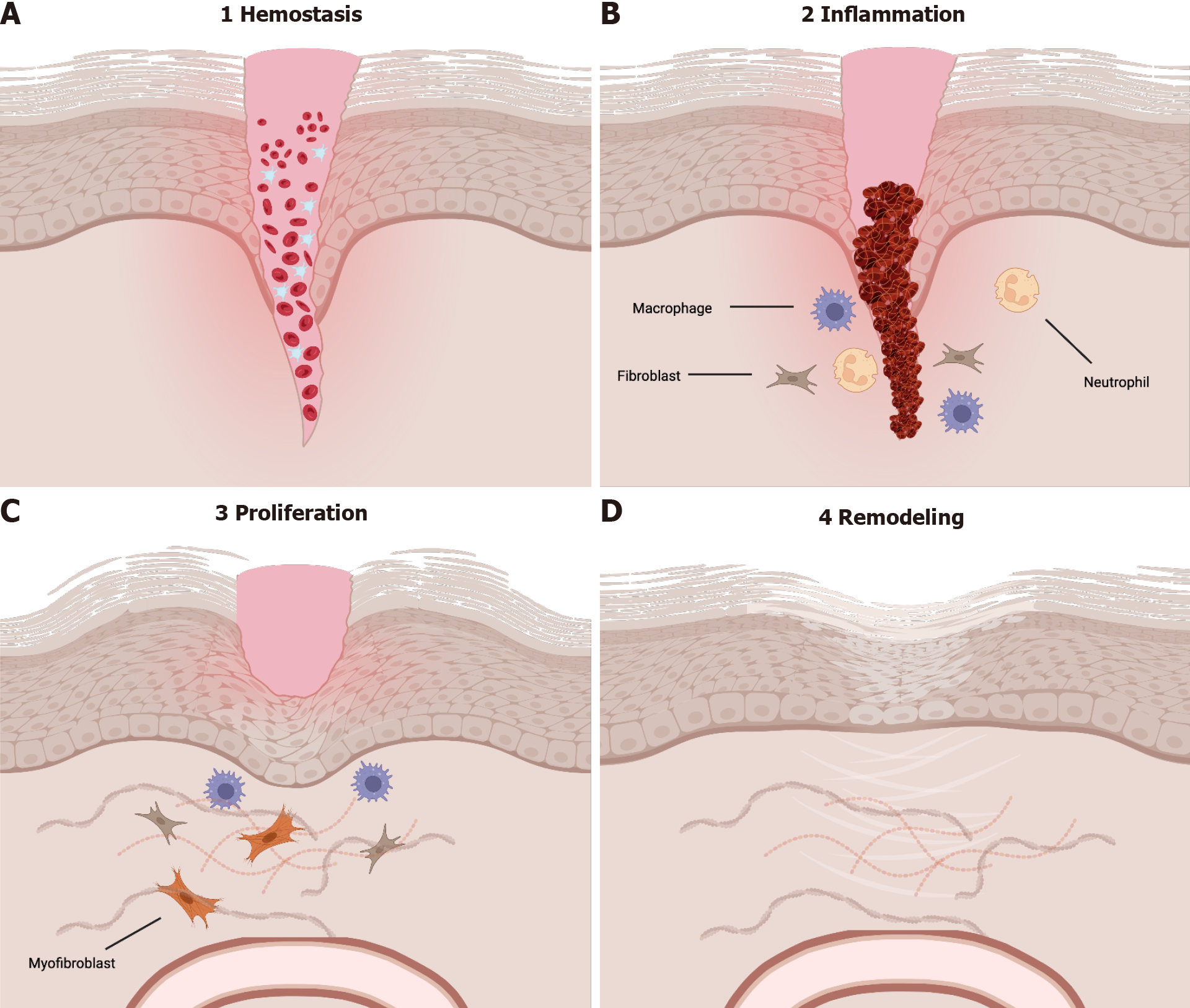
Obesity has become more common over the past 40 years, with approximately 33% of the population being classified as overweight or obese[1]. In adults, obesity is defined as a body mass index of 30.0 kg/m2 or greater[2-4]. Obesity accounts for up to 7% of total healthcare costs in developed nations, classifying it as a significant expenditure of national healthcare budgets[3]. Obesity is associated with the development of several diseases including diabetes mellitus, coronary heart disease, hypertension, and certain forms of cancer, and has been associated with a decreased lifetime expectancy[2-4]. Delayed wound healing seen in patients with diabetes mellitus, commonly associated with obesity, can be attributed to changes in the macro- and microvasculature, decreased production of growth factors, and poor quality of granulation tissue[5,6]. Impaired wound healing can be caused by changes in the four phases of wound healing – hemostasis, inflammation, proliferation, and remodeling (Figure 1).
Due to the significant rise in obesity worldwide, it is important to understand the role that that this disease may have on wound healing. The purpose of this review is to highlight the impact of obesity on cutaneous wound healing and discuss future studies that must be performed to advance our understanding of the subject. We also aim to review the phases of wound healing and how they are impacted in obese states.
There are several physiologic changes that occur in the body as the result of obesity. In the respiratory system, impaired diaphragmatic relaxation and chest expansion due to additional adipose tissue result in hyperventilation as well as decreased vital capacity and tidal volumes[7,8]. Fibroblasts, which require partial pressure of oxygen greater than 15 mmHg for proper function, are unable to produce collagen in the wound edges in obese patients where the pressure of arterial oxygen (PaO2) is near 0 mmHg[8]. In the cardiovascular system, there is excess workload on the heart to supply oxygen to all tissues of the body. Patients with longstanding obesity may eventually develop heart failure, resulting in decreased cardiac output, reduced blood volume, and impaired circulation. It is known that adipose tissue is not well vascularized and is more susceptible to ischemia and hypoxia when compared to the epidermis[7]. It is important to explore these changes in vascularity and asses how they may contribute to wound healing outcomes.
In addition to alterations in respiratory physiology and cardiac function, macro- and microvasulature is also impacted in the obese state. It is well documented that adipose tissue has decreased vascularity when compared to other tissues in the body[7,9]. This decrease in vascularity may contribute to the poor wound healing outcomes seen in this population. The increase in adipose tissue is also negatively correlated with angiogenesis[9]. Glucocorticoids have been well documented as inhibitors of angiogenesis. Elevated levels of 11β-hydroxysteroid dehydrogenase type 1, a glucocorticoid-amplifying enzyme, has been associated with obesity[9]. In addition to affecting angiogenesis, glucocorticoids have also been asso
It is crucial to consider changes in immune function, as alterations at baseline may have significant impact on home
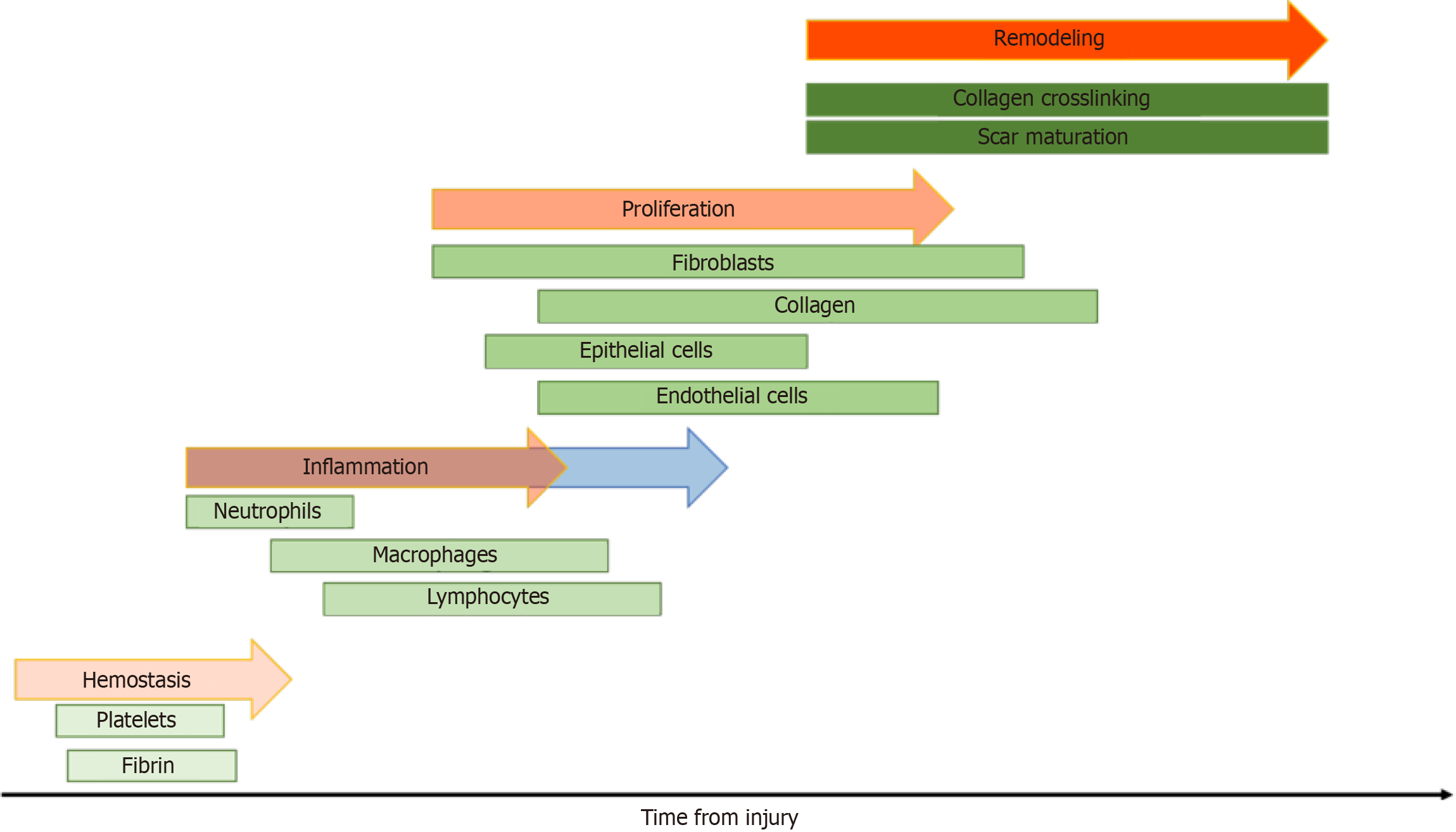
Given the physiologic changes seen in obesity, it is possible that they may have direct impact on the wound healing process. To understand possible mechanisms of interaction, one must be familiar with the wound healing process under normal physiologic conditions. Briefly, the first phase of wound healing, hemostasis, occurs when there is damage to endothelial cells. Following hemostasis, the inflammatory phase begins in which there is edema and an influx of inflammatory cells. The inflammatory phase is then followed by both proliferation and remodeling, the latter of which can take place for over two years[12].
Hemostasis, the first phase of wound healing, occurs due to endothelial damage and can last from minutes to hours. Immediately upon injury, damaged blood vessels will vasoconstrict and both the intrinsic and extrinsic coagulation cascades are activated by nearby platelets and endothelial cells[13]. The thrombus that forms is rich in platelets, collagen, fibronectin, and thrombin; it serves a scaffold for neutrophils, monocytes, and other invading cells leading to the release of cytokines, growth factors, and local vasoconstrictors such as serotonin[13]. Following hemostasis, release of histamine induces migration of inflammatory cells to the site of injury, thus marking the beginning of the inflammatory phase.
The inflammatory phase generally begins shortly after initial injury and the hemostasis phase[14]. The inflammatory phase is critical to the wound healing process as it marks recruitment of the innate immune system. Within the first 48 to 96 h after injury, monocytes are recruited from the surrounding tissue and transform into macrophages[13]. These activated macrophages are necessary for the transition from the inflammatory phase to the proliferative phase. Macrophages release vascular endothelial growth factor, fibroblast growth factor, and TNF-α to stimulate angiogenesis in addition to TGF-β, epidermal growth factor, and platelet-derived growth factor for fibroplasia[13]. Neutrophils, the predominant cell type during this phase, are recruited to the site of injury via IL-8 released from platelets during degranulation, and secrete IL-1, TNF-α, and TGF-β[13]. In the skin following injury, toll-like receptors are expressed on host cells leading to the activation of two distinct pathways - the nuclear factor kappa beta and mitogen-activated protein kinase pathways. The activation of these pathways is the hallmark of the inflammatory phase[15]. As the inflammatory phase resolves, the body begins the proliferative phase. Healing may begin only after the inflammatory phase is done[16].
During the proliferative phase, the body prioritizes restoring the local vascular network and re-epithelializing the wound surface[15]. Keratinocytes begin migrating from the edges of the wound bed while epithelial stem cells begin proliferating in reactions influenced by both chemical and mechanical signals from both anti- and pro-inflammatory cells, inducing fibroplasia. During fibroplasia, fibroblasts become activated, transition to myofibroblasts and secrete components of the extracellular matrix that promote wound contraction, contributing to formation of a persistent scar[17]. These signals lead to the development of granulation tissue, which is largely comprised of collagen III, new blood vessels, and fibroblasts. Fibroblasts are the predominant cell type in granulation tissue and respond to cytokines released from macrophages to induce re-epithelialization[18]. Fibroblasts release keratinocyte growth factor 1 and 2 in addition to IL-6, and these cytokines stimulate local keratinocytes to migrate, proliferate, and differentiate in the wound bed[13]. Research has shown that wounds deficient in IL-6 have decreased collagen deposition, epithelialization, and angiogenesis[18]. Given the intricacies involved in the four phases of wound healing, it is important to note how these phases of wound healing are affected by obesity.
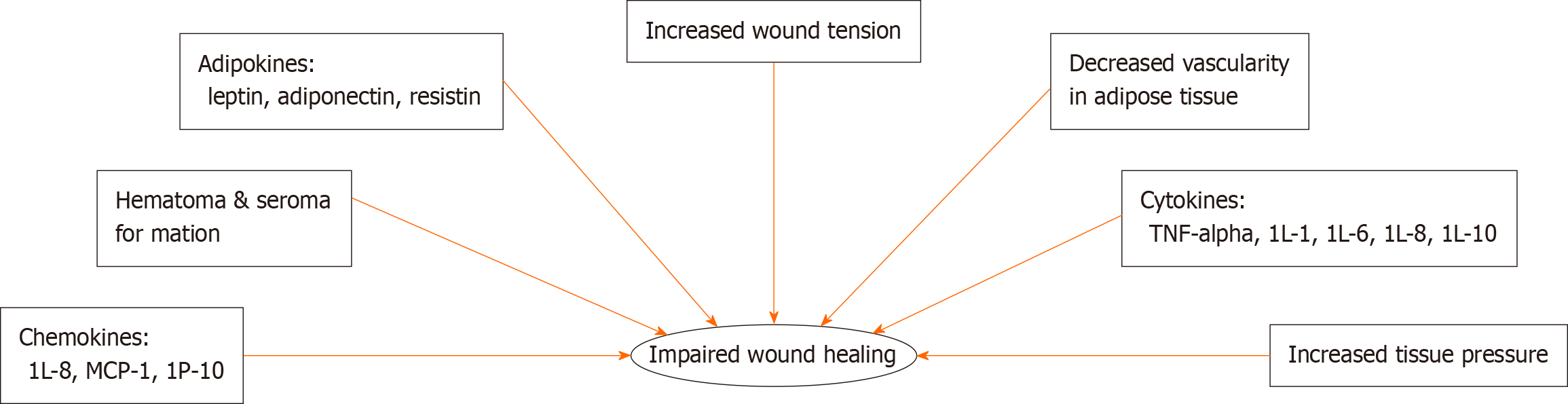
There are several factors that may contribute to poor wound healing found in obese populations (Figure 2). Patients with obesity are in a persistent inflammatory state; because of this, these patients have a prolonged inflammatory phase contributing to poor wound healing outcomes (Figure 3)[16].
Adipokines are cytokines produced by adipose tissue that affect metabolism, reproduction, and satiety. Currently known adipokines include leptin, adiponectin, and resistin[19]. Leptin, the most studied, regulates food intake and energy expenditure via the central nervous system. It has been shown to be effective in improving metabolic dysfunction in patients with either lipodystrophy or congenital leptin deficiencies[20,21]. Leptin has also been shown to be structurally similar to IL-2 and growth hormone 1, suggesting that it may have pro-inflammatory activity; it has been shown to increase the production of TNF-α and IL-6 by monocytes[22]. Interestingly, leptin levels also increase in serum in response to pro-inflammatory stimuli including TNF-α[22].
Leptin is not the only proinflammatory adipokine that has been extensively studied in recent decades. Resistin, another proinflammatory adipokine, has been shown to induce insulin resistance in mice. However, it is unclear if these effects exist in humans as well[21]. In mice, it has been shown that deficiencies of this adipokine in ob/ob mice lead to increase obesity despite improved glucose tolerance and insulin sensitivity[23].
In addition to pro-inflammatory adipokines, there are also anti-inflammatory adipokines of which adiponectin is one that has been relatively well studied. Adiponectin, almost exclusively produced and secreted by adipocytes, has become well studied for its anti-inflammatory, anti-apoptotic, and insulin-sensing properties[21]. It has been shown to protect against several disorders associated with obesity; adiponectin expression has also been found to have decreased levels in patients with obesity[18,21]. In addition to the impact of adipokines on wound healing, cytokines and chemokines have also been implicated in the pathogenesis of chronic wounds in obese and pre-diabetic populations.
Adipose tissue is also an important source of cytokines, and there are many cytokines and chemokines that influence wound healing in obesity. Proinflammatory cytokines including monocyte chemotactic protein-1, TNF-α, IL-1, IL-6, and IL-8, are notably increased in obesity (Table 1)[21,24,25]. Patients and mice with increased percentages of adipose tissue produce more of these pro-inflammatory cytokines at baseline leading to a state of chronic low-grade inflammation. Alterations in serum levels of these cytokines, chemokines, and adipokines may drive the underlying physiologic processes that contribute to poor wound healing outcomes in obese patients.
| TNF-α | Secreted by macrophages, natural killer cells, and lymphocytes[13]. Crucial in formation and maintenance of granulomas[30] |
| IL-1 | Produced by various cell types including macrophages, fibroblasts, and epithelial cells[13]. Stimulates fibroblast and keratinocyte growth and collagen synthesis by fibroblasts |
| IL-6 | Secreted by various cell types including macrophages and adipocytes[13]. An important mediator of the acute phase response and regulator of glucose homeostasis in obesity |
| IL-8 | Produced by various cell types including macrophages, epithelial cells, and endothelial cells[13]. Primarily recruits neutrophils and other granulocytes to sites of tissue injury |
| MCP-1 | Also known as CCL2. Primarily secreted by monocytes, macrophages, and dendritic cells[13]. Attracts monocytes, memory T cells, and dendritic cells to sites of inflammation produced by either tissue injury or inflammation |
In addition to chemical mediators impacting wound healing, a number of mechanical forces also impact wound healing in obesity. Due to excess adipose tissue, there is increased tension on wounds; this increased tension is frequently associated with hypertrophic scarring and stretched scars[16,26]. Increased tissue pressure is also seen due to excess adipose tissue, both in unwounded and wounded cutaneous skin[16,27]. This increased pressure is associated with reduced perfusion and vascularity[27]. Furthermore, hematoma formation is a complication frequently seen in patients due to excess tissue pressure. Given the multitude of complications associated with obesity, it is imperative to understand what impact obesity plays in the wound healing process.
As previously discussed, patients with obesity have persistently elevated levels of pro-inflammatory cytokines in serum. This elevated immune response at baseline may likely cause a dampened immune response during the inflammatory phase of wound healing resulting in the chronic, slow-healing wounds frequently seen in this population.
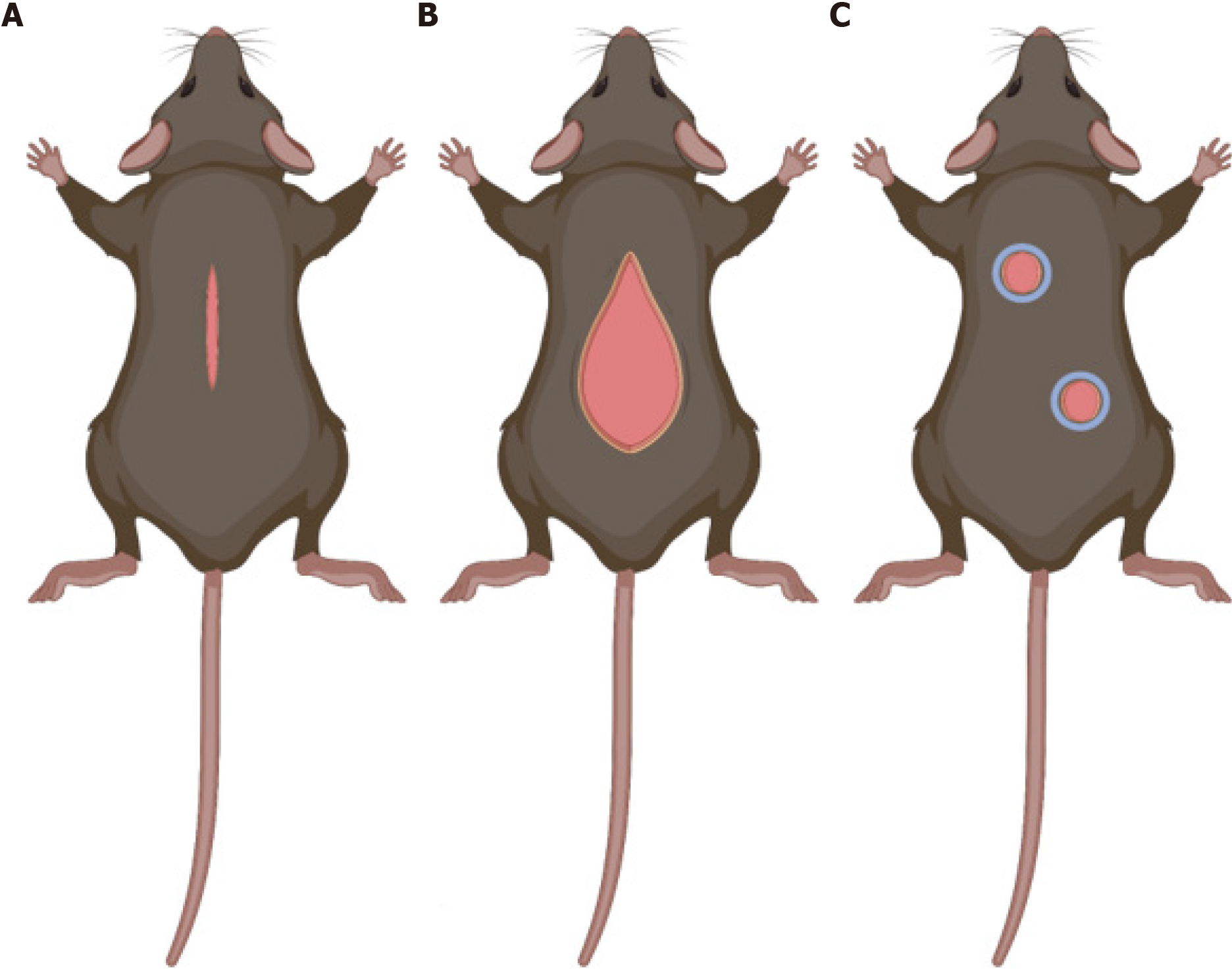
To study wound healing, it is essential to create animal models that mimic wound healing in human skin. There are three main models to study this process – the hypertrophic wound model, the wound-induced hair follicle neogenesis (WIHN) model, and the excisional wound model (Figure 4). Although many animal species are used to study healing, this review will only discuss advantages and disadvantages of murine models. Mice and rats are popular for these animal models as they are widely available and relatively inexpensive. However, skin in these animals contain myofibroblasts, which allow their wounds to heal via contraction rather than through re-epithelialization and granulation as seen in human skin.
Cutaneous incisional mouse wounds rapidly heal with minimal fibrosis and will not result in hypertrophic scarring during the normal wound healing process; thus, strategies have been developed to induce hypertrophic scar formation. Hypertrophic scars are associated with excessive scarring due to severe trauma and delayed wound healing. These scars may result in skin disfiguration and restriction of joint mobility[28,29]. In humans, hypertrophic scars develop within 4-8 wk following wound closure and growth may persist for 6 months before gradual recession[29,30]. Understanding the pathophysiology of these scars is imperative for the development of restorative treatments. Therefore, the Incisional wound model in mice has been adapted to generate pathologic scarring with mechanical tension devices, and termed the Hypertrophic skin model. Advantages of this model include low cost and reproducible production of a hypertrophic scar. Disadvantages include generation of a hypertrophic scar thinner than humans and requirement of a specially designed device to generate mechanical tension.
In this model, a 2-cm full thickness incisional wound is made on the mouse dorsal surface, and a loading device is sutured on either side of the wound bed to create tension[29,31]. Due to this external mechanical tension, a hypertrophic scar that is more similar in both histology and morphology to human cutaneous scars is created in mouse skin. Scars studied using this model demonstrate epidermal thickening with loss of adnexal structures and hair follicles, mast cell infiltrate (similar to what is seen in human HTS), hypervascularity, collagen whorls, and increased cellularity[31]. The increased cellularity observed is accompanied by a significant decrease in cellular apoptosis mediated by upregulation of Akt, a pro-survival marker[31].
Humans regenerate neither terminal nor vellus hair following large full-thickness wounds, presenting a significant clinical issue. Hair follicle regeneration is a complex process that requires coordination between multiple tissues including epidermis, dermis, muscles, and nerves. WIHN is a powerful tool used to study de novo skin regeneration following large full-thickness trauma[32]. Murine primary hair follicles are analogous to human terminal hair, while secondary hair follicles are analogous to human vellus hair, making mice a prime animal to study changes in hair growth following large full-thickness trauma. Advantages of this model include low cost, the ability to study regeneration following adult skin wounding, and development of a model to test therapeutics to activate regenerative wound healing[33]. Disadvantages of this model include variability between mouse strains, environmental conditions, age of mice, and need for creation of large wound size for the animal[33].
This model has shown that wound stiffness modulates hair follicle neogenesis and is partially regulated through mechanotransduction pathways[32]. WIHN observed using this model shows decreased focal adhesion kinase, α-smooth muscle actin, extracellular matrix expression, and cytoskeletal signaling with increased cell survival and increased levels of phospho-signal transducer and activator of transcription 3 and ephrin tyrosine kinase A3 in the central wound area[32]. These characteristics provide a wound environment that is optimal for promoting tissue regeneration following injury occurs only in the central wound area[32-34].
Through the use of splints, excisional wound models have been used in mice to create an animal model that more closely mimics the wound healing process in human skin[34]. The full-thickness excisional wound model is the most commonly used model to study wound healing. These wounds extend through the panniculosus carnosus, and a silicone splint is fixed around the wound to minimize contraction. Splinting increases time to complete wound closure and the amount of granulation tissue produced with no significant changes to the capacity for epithelialization[35]. The use of splinting in mice to study the wound healing process has been widely used and has contributed significantly to what we know about the wound healing process[35-37]. Advantages of this model include ease of access to the wound bed to study the effects of pharmaceuticals, biomaterials, and other agents to augment the wound healing process and assess cellular populations in granulation tissue at various stages of healing using histological and immunofluorescence techniques[35-37].
All of these models have their own unique set of advantages and disadvantages (Table 2). Nevertheless, each has contributed greatly to what we know about wound healing in healthy animals, yet there is a lack of studies using these models in understanding the effects of obesity on wound healing. Before applying these tools, we must first understand existing models to study obesity.
| Animal model | Advantages | Disadvantages |
| Hypertrophic wound model | Low cost | Hypertrophic scar different from human[27,29] |
| Allows for production of hypertrophic scar[28,29] | Requires special device[28,29] | |
| Labor intensive: Frequent care to maintain tension and device placement[31] | ||
| Wound-induced hair follicle neogenesis (WIHN) model | Low cost | |
| Regeneration following adult wounding with minimal recovery of hair follicles at the scar center[32,48] | High variability: Mouse strains, environmental conditions, age of mice, and wound size)[33] | |
| Test therapeutics to activate regenerative wound healing[33] | May not translate to human injury[48] | |
| Excisional wound model | Low cost | Labor intensive: Frequent dressing changes to maintain tension[35] |
| Wound healing similar to human[35] | ||
| Allows for fibroblast lineage tracing[49] |
Several models have been developed in mice to study the impact of obesity on varying physiological processes including the use of genetic mutations and diet-induced obesity models. As described above, these animal models can be used in conjunction with existing wound healing models to examine the influence obesity has on wound healing under varying conditions. First, we will take a look at diet-induced obesity models.
Human studies have also shown that high-fat diet diets with over 30% kilocalories (kcal) from fat can easily induce obesity[38]. Therefore, it is important to study obesity in this context as the dramatic rise in worldwide obesity over such a short period of time cannot be attributed to genetics[38]. In mice, a positive relationship has been found between dietary fat consumption and both body weight and fat gain[39,40]. To produce dietary-induced obesity in mice, diets contain between 40% and 60% kcal from fat. Similarly to human studies, the amount of dietary fat in the diet is associated with reduced glucose tolerance and increased white adipose tissue, blood glucose levels, and body weight over a 12-wk study period[39]. As previously mentioned, an advantage of diet-induced obesity models is that they may mimic development of obesity similarly to what is observed in humans. In addition to the low costs associated with this model, potential disadvantages of this model include length of time required to induce obesity. Diet-induced obesity models also display leptin insensitivity, which may be caused by persistently elevated leptin levels[41]. To further determine the effects of leptin on obesity, models have been developed to study changes in leptin metabolism.
The use of ob/ob mice (leptin-deficient due to obese gene mutation) and db/db mice (leptin-resistant due to diabetes gene mutation in the leptin receptor) have allowed researchers to investigate the pathogenesis of both obesity and type 2 diabetes mellitus[41]. Although the mechanisms by which these mutations cause obesity are different, mice display similar phenotypes including hyperphagia, hyperglycemia, obesity, and decreased metabolism[42,43]. Ob/ob mice display altered lipid metabolism in the liver associated with higher incidence of inflammatory infiltrate and hepatic steatosis, while db/db mice display lower hepatic inflammation with increased inflammatory tone in adipose tissue[43]. These genetic mouse models of obesity are easily replicated as the strains are readily available for purchase and are useful to study genetic impact on obesity, however they are limited in their ability to study obesity that arises due to environmental factors.
While studies using the hypertrophic skin, WIHN, and excisional splinted wound models in ob/ob, db/db, or diet-induced obesity mice have not been widely published to date, there have been studies in both mice and rats investigating the effects of obesity on cutaneous wound repair[44-47]. These data have shown significant changes in inflammatory cell infiltrate, tissue organization, and gene expression in cutaneous skin wounds[44-47]. While these studies give insight on the wound healing process in obesity, one limitation is the lack of splinting in excisional wounds. Without splinting, there is reduced mechanical tension on wounds, which reduces granulation tissue in the wound similar to what is seen in human wounds[35-37]. In an analysis between genetic and diet-induced obesity, researchers found that genetic murine models displayed wound healing capacity similar to that of diabetes mellitus in humans with reduced tissue responses in diet-induced mice with reduced serum levels of TGF-β and elevated TNF-α[47]. These results suggest that diet-induced obesity models may be better suited to study the effects of obesity on wound healing.
The wound healing process in healthy patients is well documented and is conserved between both humans and animal models used to study wound healing[12-14,17,48,49]. The effects of diabetes mellitus on wound healing have been well documented in both human and animal studies. Despite the growing body of literature surrounding wound healing, there is a gap in the literature investigating the effects of obesity on wound healing and how best to augment it in this population. Understanding the wound healing process in obesity is imperative as care requires knowledge of the phy
While there are a number of models used to study wound healing, these powerful tools have not yet been applied to study wound healing in the context of obesity. Studies using either the WIHN, excisional wound, or hypertrophic wound models should be applied to existing animal models of obesity to deepen our knowledge of the effects of obesity on wound healing. Obesity induces a chronic low-grade inflammatory response with increased production of pro-inflammatory adipokines, cytokines, and chemokines that contribute to poor wound healing phenomena seen in this population[8,11,21,44,47]. Much is known about these important proteins in the context of both obesity and normal wound healing. With an increasing number of studies assessing alterations in the both inflammatory responses and wound healing in obese mice and patients, it is imperative to continue these investigations. Future experimentation must center on altering levels of these proteins to determine if it is possible to augment wound healing outcomes in obese and overweight populations.
Approximately one third of the global population is classified as obese or overweight. With millions of patients sustaining cutaneous wounds annually, it is imperative to investigate the impact of obesity on wound healing and how to augment cutaneous wound healing in this growing population. Dysregulation of inflammatory responses due to production of pro-inflammatory mediators at baseline may contribute to poor wound healing outcomes.
The authors thank the Hagey Laboratory for Pediatric Regenerative Medicine for its continued support of our research.
| 1. | Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1143] [Cited by in RCA: 1861] [Article Influence: 265.9] [Reference Citation Analysis (0)] |
| 2. | Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087-2102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1006] [Cited by in RCA: 974] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 3. | Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3230] [Cited by in RCA: 3169] [Article Influence: 121.9] [Reference Citation Analysis (0)] |
| 4. | Pi-Sunyer X. The medical risks of obesity. Postgrad Med. 2009;121:21-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 825] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 5. | Greenhalgh DG. Wound healing and diabetes mellitus. Clin Plast Surg. 2003;30:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 312] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 6. | Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117:1219-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1013] [Cited by in RCA: 1228] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 7. | Wilson JA, Clark JJ. Obesity: impediment to wound healing. Crit Care Nurs Q. 2003;26:119-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Groszek DM. Promoting wound healing in the obese patient. AORN J. 1982;35:1132-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Pierpont YN, Dinh TP, Salas RE, Johnson EL, Wright TG, Robson MC, Payne WG. Obesity and surgical wound healing: a current review. ISRN Obes. 2014;2014:638936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 185] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 10. | Kalupahana NS, Moustaid-Moussa N, Claycombe KJ. Immunity as a link between obesity and insulin resistance. Mol Aspects Med. 2012;33:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 188] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 11. | Dallman MF, Pecoraro NC, La Fleur SE, Warne JP, Ginsberg AB, Akana SF, Laugero KC, Houshyar H, Strack AM, Bhatnagar S, Bell ME. Glucocorticoids, chronic stress, and obesity. Prog Brain Res. 2006;153:75-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 142] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3609] [Cited by in RCA: 4561] [Article Influence: 253.4] [Reference Citation Analysis (0)] |
| 13. | Broughton G 2nd, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006;117:12S-34S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 854] [Cited by in RCA: 963] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 14. | Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6:265sr6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2114] [Cited by in RCA: 2348] [Article Influence: 195.7] [Reference Citation Analysis (0)] |
| 15. | Landén NX, Li D, Ståhle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci. 2016;73:3861-3885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 613] [Cited by in RCA: 1214] [Article Influence: 121.4] [Reference Citation Analysis (4)] |
| 16. | Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3887] [Cited by in RCA: 3415] [Article Influence: 213.4] [Reference Citation Analysis (0)] |
| 17. | Zangooei MH, Jalili S. Protein fold recognition with a two-layer method based on SVM-SA, WP-NN and C4.5 (TLM-SNC). Int J Data Min Bioinform. 2013;8:203-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Lin ZQ, Kondo T, Ishida Y, Takayasu T, Mukaida N. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J Leukoc Biol. 2003;73:713-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 451] [Article Influence: 19.6] [Reference Citation Analysis (14)] |
| 19. | Fasshauer M, Blüher M. Adipokines in health and disease. Trends Pharmacol Sci. 2015;36:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 779] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 20. | Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, Gorden P, Garg A. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 873] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 21. | Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3103] [Cited by in RCA: 3317] [Article Influence: 221.1] [Reference Citation Analysis (1)] |
| 22. | Santos-Alvarez J, Goberna R, Sánchez-Margalet V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell Immunol. 1999;194:6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 433] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 23. | Qi Y, Nie Z, Lee YS, Singhal NS, Scherer PE, Lazar MA, Ahima RS. Loss of resistin improves glucose homeostasis in leptin deficiency. Diabetes. 2006;55:3083-3090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Wang T, He C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 699] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 25. | Vendrell J, Broch M, Vilarrasa N, Molina A, Gómez JM, Gutiérrez C, Simón I, Soler J, Richart C. Resistin, adiponectin, ghrelin, leptin, and proinflammatory cytokines: relationships in obesity. Obes Res. 2004;12:962-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 384] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 26. | Meyer M, McGrouther DA. A study relating wound tension to scar morphology in the pre-sternal scar using Langers technique. Br J Plast Surg. 1991;44:291-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Fleischmann E, Kurz A, Niedermayr M, Schebesta K, Kimberger O, Sessler DI, Kabon B, Prager G. Tissue oxygenation in obese and non-obese patients during laparoscopy. Obes Surg. 2005;15:813-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Kloeters O, Tandara A, Mustoe TA. Hypertrophic scar model in the rabbit ear: a reproducible model for studying scar tissue behavior with new observations on silicone gel sheeting for scar reduction. Wound Repair Regen. 2007;15 Suppl 1:S40-S45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 103] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Li J, Wang J, Wang Z, Xia Y, Zhou M, Zhong A, Sun J. Experimental models for cutaneous hypertrophic scar research. Wound Repair Regen. 2020;28:126-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 30. | Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17:113-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 986] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 31. | Aarabi S, Bhatt KA, Shi Y, Paterno J, Chang EI, Loh SA, Holmes JW, Longaker MT, Yee H, Gurtner GC. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 2007;21:3250-3261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 384] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 32. | Harn HI, Chiu PY, Lin CH, Chen HY, Lai YC, Yang FS, Wu CC, Tang MJ, Chuong CM, Hughes MW. Topological Distribution of Wound Stiffness Modulates Wound-Induced Hair Follicle Neogenesis. Pharmaceutics. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 33. | Xue Y, Lim CH, Plikus MV, Ito M, Cotsarelis G, Garza LA. Wound-Induced Hair Neogenesis Model. J Invest Dermatol. 2022;142:2565-2569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | Harn HI, Wang SP, Lai YC, Van Handel B, Liang YC, Tsai S, Schiessl IM, Sarkar A, Xi H, Hughes M, Kaemmer S, Tang MJ, Peti-Peterdi J, Pyle AD, Woolley TE, Evseenko D, Jiang TX, Chuong CM. Symmetry breaking of tissue mechanics in wound induced hair follicle regeneration of laboratory and spiny mice. Nat Commun. 2021;12:2595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 35. | Wong VW, Sorkin M, Glotzbach JP, Longaker MT, Gurtner GC. Surgical approaches to create murine models of human wound healing. J Biomed Biotechnol. 2011;2011:969618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 245] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 36. | Lintel H, Abbas DB, Lavin CV, Griffin M, Guo JL, Guardino N, Churukian A, Gurtner GC, Momeni A, Longaker MT, Wan DC. Transdermal deferoxamine administration improves excisional wound healing in chronically irradiated murine skin. J Transl Med. 2022;20:274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 37. | Mascharak S, desJardins-Park HE, Davitt MF, Griffin M, Borrelli MR, Moore AL, Chen K, Duoto B, Chinta M, Foster DS, Shen AH, Januszyk M, Kwon SH, Wernig G, Wan DC, Lorenz HP, Gurtner GC, Longaker MT. Preventing Engrailed-1 activation in fibroblasts yields wound regeneration without scarring. Science. 2021;372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 421] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 38. | Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutr Res Rev. 2010;23:270-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 721] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 39. | Takahashi M, Ikemoto S, Ezaki O. Effect of the fat/carbohydrate ratio in the diet on obesity and oral glucose tolerance in C57BL/6J mice. J Nutr Sci Vitaminol (Tokyo). 1999;45:583-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Bourgeois F, Alexiu A, Lemonnier D. Dietary-induced obesity: effect of dietary fats on adipose tissue cellularity in mice. Br J Nutr. 1983;49:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Lin S, Thomas TC, Storlien LH, Huang XF. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int J Obes Relat Metab Disord. 2000;24:639-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 440] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 42. | Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 912] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 43. | Suriano F, Vieira-Silva S, Falony G, Roumain M, Paquot A, Pelicaen R, Régnier M, Delzenne NM, Raes J, Muccioli GG, Van Hul M, Cani PD. Novel insights into the genetically obese (ob/ob) and diabetic (db/db) mice: two sides of the same coin. Microbiome. 2021;9:147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 179] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 44. | Min KK, Neupane S, Adhikari N, Sohn WJ, An SY, Kim JY, An CH, Lee Y, Kim YG, Park JW, Lee JM, Suh JY. Effects of resveratrol on bone-healing capacity in the mouse tooth extraction socket. J Periodontal Res. 2020;55:247-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Slavkovsky R, Kohlerova R, Tkacova V, Jiroutova A, Tahmazoglu B, Velebny V, Rezačová M, Sobotka L, Kanta J. Zucker diabetic fatty rat: a new model of impaired cutaneous wound repair with type II diabetes mellitus and obesity. Wound Repair Regen. 2011;19:515-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 46. | Nascimento AP, Costa AM. Overweight induced by high-fat diet delays rat cutaneous wound healing. Br J Nutr. 2006;96:1069-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Seitz O, Schürmann C, Hermes N, Müller E, Pfeilschifter J, Frank S, Goren I. Wound healing in mice with high-fat diet- or ob gene-induced diabetes-obesity syndromes: a comparative study. Exp Diabetes Res. 2010;2010:476969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 48. | Talbott HE, Mascharak S, Griffin M, Wan DC, Longaker MT. Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell. 2022;29:1161-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 492] [Article Influence: 123.0] [Reference Citation Analysis (0)] |
| 49. | Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1313] [Cited by in RCA: 1388] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mostafavinia A, Iran S-Editor: Qu XL L-Editor: A P-Editor: Zhao YQ