Peer-review started: May 6, 2019
First decision: September 2, 2019
Revised: November 26, 2019
Accepted: December 14, 2019
Article in press: December 14, 2019
Published online: January 8, 2020
Processing time: 250 Days and 7.5 Hours
The epiphyseal growth plate is an important anatomical segment localized on the ends of a long bone. Despite the abovementioned atractive reasons for alendronate’s use, few data on the effect of alendronate during epiphyseal growth exist.
Verify the effect of alendronate on the growth epiphyseal plate, and compare its effect with the size of the femur during the double-staining of the immunolocalization of transforming growth factor-β1 (TGF-β1) and bone morphogenetic protein-2 (BMP2) in endochondral ossifing in specimens that have received alendronate.
Forty newborn rats were randomly divided into two groups: a control group (were given applications of 1 mg/kg physiologic saline) and a group that received Alendronate (a dose of 2.5 mg/kg). These groups were then divided into two subgroups for euthanasia in two and 12 d of life. After euthanasia, the femurs were removed, and the femoral bones were measured linearly between the apex of the greater trochanter until the lower intercondylar midlle face to verify the probable bone growth between 3 and 12 d in control and alednroanto treated rats. Posteriorly, the surgical pieces were also sent to the histopathology laboratory to produce histological slides. The obtained slides were stained with hematoxylin and eosin to measure each of the cartilage zones in endochondral development. and other slides were immunohistochemically tested for anti- TGF-β1 and BMP-2 antibodies to investigate the immunolocalization of these proteins in the epiphyseal plaque area.
On the third day, some diferences between the control group and specimens treated with alendronate were verified. Macroscopiccaly, we found similarities in size between the femoral bones when we compared the control group with the specimens that received alendronate. On the 12th day, the bone size of the mice receiving the drug was significantly smaller than those of the control group. These results coincide with changes in the TGF-β1 and BMP-2 expression. In the specimens that received alendronate, the TGF-β1 was expressed in some sites of trabecular bone that was neoformed, peripherally to the bone marrow area. The BMP-2 was also positive in proliferative chondrocytes and hypertrofic chondrocytes. On the 12th day, all layers of chondrocytes exhibited positivity for BMP-2 in the specimens that received alendronate. In the interface between the trabecular bone and cartilage, an area of disorganized bone deposition was evident. Neoformed bone also appeared to be different at 12 d. In the control group, BMP-2 was positive in an intense area of bone trabeculae, whereas the alendronate-treated group showed TGF-β1 positive trabeculae and a greater bone area.
Alendronate alters the immunolocalization of TGF-β1 and BMP-2 simultaneously, a condition that changes the usual histological aspects of the cartilage zone and impairs epiphysis growth and femur growth.
Core tip: This is the first study that addresses the likely action of alendronate in appendicular bone development. Through of macroscopic, immunohistochemical and histological analysis, we revealed that alendronatemay impairs the bone growth, since alters the usual development of epiphyseal plate associated to changes in the immunolocalization of transforming growth factor-β1 and bone morphogenetic protein-2.
- Citation: Vieira JS, Cunha EJ, de Souza JF, Chaves LHK, de Souza JL, Giovanini AF. Alendronate disturbs femoral growth due to changes during immunolocalization of transforming growth factor-β1 and bone morphogenetic protein-2 in epiphyseal plate. World J Exp Med 2020; 10(1): 1-9
- URL: https://www.wjgnet.com/2220-315x/full/v10/i1/1.htm
- DOI: https://dx.doi.org/10.5493/wjem.v10.i1.1
The epiphyseal growth plate is an important anatomical segment localized on the ends of a long bone. It is responsible for the longitudinal growth of children’s long bones through coordinated sequence changes to the cartilage histomorphological aspects. The zones encountered in the growth plate include the reserve, proliferative, maturation, and hypertrophy zones. The zone of hypertrophy[1] is where the substitution of a portion of cartilage in the osteoprogenitor cells takes place. This is where bone matrix deposition appears posteriorly[2].
This well-orchestrated histomorphological transition for diverse chondroid aspects takes place until effective endochondral ossification occurs, and it seems to be regulated through autocrine and paracrine stimulation. An important growth factor that operates during stages of chondroid modification and endochondral ossification is the transforming growth factor-β1 (TGF-β1).
Supporting this context, the TGF-β1 works to maintain the reserve of chondrocyte clones, inhibits premature terminal hypertrophic differentiation in pre-hypertrophic chondrocytes[3], arrests differentiation at an early stage of hypertrophy, and induces the coexistence of bone proteins that culminates in an osteoblastic appearance[4].
For osteoblast differentiation, the induction of bone morphogenetic protein-2 (BMP2) expression through TGF-β1[5] in hypertrophic chondrocytes appears to be crucial. BMP-2 directs mesenchymal cells to the osteoblast lineage, triggers the expression of major bone matrix protein genes, and orchestrates the production of phosphatase alkaline to mineralize the matrix so as to form bone[6].
Given the important roles of TGF-β1 in chondrogenesis and osteogenesis, a lack of transcription in this cytokine invariably leads to skeletal disorders and developmental defects in children-for example, fibrodysplasia ossificans progressiva[7], Camurati-Engelmann disease[8], and brachydactyly, especially type A2[9].
Thus, pharmacological agents that may stimulate a source of TGF-β1 at the same time, which may contribute to chondroprotection and modulate bone formation, are widely required for treatments for children[10-12]. It is established in the literature that the prolonged administration of alendronate, which is a nitrogenous bisphosphonate, may improve and mantain for a higher period of time the expression of TGF-β1 on an organism[12]. On other hand, the use of this drug has also been used to protect the bone tissue against the hyperactivity of osteoclasts or the bone metastasis of several neoplasms.
Despite the abovementioned atractive reasons for alendronate’s use, few data on the effect of alendronate during epiphyseal growth exist. Thus, the aim of this study was to verify the histological response of the epiphyseal growth plate under the action of alendronate in newborn rats and to compare these results with that of double-staining the immunolocalization of TGF-β1 and BMP-2. We compare these histological and immunohistochemical aspects with the size of the femur.
The principles of laboratory animal care and national laws on animal use were observed for the present study with the approval of the Ethical Committee for Animal Research of Positive University (Protocol #266).
Seven two old months female rats (Rattus norvegicus albinus, Holtzman), weighing aproximately 250 g, were used as a progenitress source. The animals were maintained in a department with controlled temperature (22 ± 2 °C), humidity (55% ± 5%), with a 12h/12h light/dark cycle and food and water available ad libitum.
In this study, the species of neonatal rats were obtained from natural generation according to the study by de Souza et al[13].
A group of newborn rats received a sterile 0.9% saline solution (n = 20 – control group), whereas other newborn rats (n = 20) received 2.5 mg/kg per day of alendronate trihydrate (Biolife, Curitiba, Brazil, Lote: 14042132C) (alendronate group). All solutions (saline or drug) were administered intraperitorially daily up to the euthanasia time period, which occurred on the third and 12th days after the rat’s birthday (n = 10 for group for each time period). All euthanasia was performed by using overdoses of isofluorane.
After euthanasia, the legs of the newborn rats were immediately removed, and the femur was measured linearly between the apex of the greater trochanter and the lower intercondylar midface using a pachymeter. The values were tabulated and subjected to statistical analysis.
Following mensuration, each surgical piece obtained was immersed in a fixative solution for 48 h at room temperature in 4% formaldehyde that was buffered to pH 7.2 with 0.1 mol/L sodium phosphate and posteriorly immersed in 7% disodium ethylenediaminetetraacetic acid for decalcification for aproximately 2 wk. After decalcification each the specimens were dehydrated in ethanol, cleared in xilene, and embedded in paraffin.
From each specimen, serial 3-µm-thick fragments were obtained and adhered to slides. The sections were carefully formed in the longitudinal plane of the femoral bone in the anterior-posterior section. Some slides containing the fragments were stained with hematoxilin and eosin to verify the histological aspects of bone, whereas other sections of fragments were submitted for double immunohistochemical detection for anti-TGF-β1 and BMP-2.
From each specimen, sections 3 micrometers thick were submitted to the immunohistochemical detection of all proteins. For antigen retrieval, deparaffinized sections were immersed in 10 mmol/L sodium citrate buffer (pH 6.0) and subjected to a microwave three times for 5-min cycles. The sections were then incubated overnight at 4 ºC with primary BMP-2 (0.5 mg/mL, ab6285; Abcam, Cambridge, United Kingdom) with a dilution factor of 1:150. The labeled akaline-phosphatasis antibody-binding detection system (Universal HRP immunostaining kit – Diagnostic Byosystem, Foster City, CA, United States) was employed to detect the primary antibodies for 30 min, whereas the revelation of the immunohistochemical reaction was performed with Fast Red substrate, producing a red color in positive protein sites. The specimens were washed in phosphate-buffeed saline (pH 7.2), the sections were incubated overnight at 4 ºC with primary TGF-β1 (200 mg/mL, sc-146; Santa Cruz Biotechnology, Santa Cruz, CA, United States), with a dilution factor of 1:200. The labeled streptavidin biotin antibody-binding detection system (Universal HRP immunostaining kit – Diagnostic Byosystem, Foster City, CA, United States) was employed to detect the TGF-β1. Following washing in 0.05 mol/L Tris-HCl buffer (pH 7.2), the sections were incubated for 30 min at room temperature in biotinylated anti-rabbit/mouse/goat immunoglobulin (LSAB-plus Kit, Dako, CA, United States) and were revealed with diamine benzidine, producing a brownish color in the sites positive for protein. All of the sections were counterstained with Harris hematoxylin. For the negative control, the primary antibodies were omitted.
Images of immunohistochemistry sections were taken with a digital camera (DP-71, Olympus, Tokyo, Japan) connected to a microscope (BX-51, Olympus, Tokyo, Japan), and DP Controller software (Version 2.1.1.183, Olympus, Tóquio, Japan) with an original magnification of × 100. The digital images were collected and saved with a resolution of 300 dpi (image size 113.66 cm × 75.95 cm). All histomorphometric measurements were made with Image J software. The data were counted manually. The perimeter of each morphological zone of endochondral ossifying was carefully drawn and measured, and they are presented in mm2. The immunohistochemistry protein identified as a brown color for TGF-β1 or a red color for BMP-2 were manually counted and tagged. An image of a 1-mm slide micrometer was used to calibrate all measurements for areas, whereas positive cells for both proteins were transformed into a percentage. The slides were analyzed for each of the above parameters. An average of the three measurements for each parameter was then calculated for each specimen.
The linear measurement of the femur, the areas of histological chondroid zones, and percentages of immunohistochemical data for each zone were evaluated within the monitoring period. An analysis of variance and Shapiro-Wilk analysis were used to determine the normality, followed by the Kruskal-Wallis non-parametric test to verify significant differences among groups. A P value < 0.05 was considered to be statistically significant.
On the third day of analysis, the median values of the specimens that received alendronate were slightly larger than the median values of the linear measures of the femurs of the control species. In contrast to the control species, the growth between three to 12 d of analysis was scarce in the group that received alendronate. Thus, on the 12th day of analysis, the measures of the femurs of the specimens that received the drug were significantly lower when compared with the alendronate-free specimens. All data are present in Table 1.
| Day | Linear Size of Femur (cm) | P value | |
| Control | Alendronate | ||
| 3 | 5.93 (5.67 – 6.04) | 6.85 (5.99 – 7.21) | P = 0.062 |
| 12 | 12.72 (11.67-13.52) | 8.84 (8.43 – 9.08) | P = 0.027 |
A brief description of the histologic features found among groups is described bellow while the histomorphometric parameters are summarized in Table 2 (P < 0.05).
| Cartilage zone | Area accounted (mm2) | |||||
| 3rd day | 12th day | |||||
| Control | Alendronate | P value | Control | Alendronate | P value | |
| Reserve | 6.12 (5.52-6.51) | 5.63 (5.07-5.71) | P = 0.061 | 3.33 (3.22-3.51) | 2.26 (2.11-2.29) | P = 0.056 |
| Proliferative | 4.02 (3.82-4.44) | 4.12 (3.98-4.58) | P = 0.071 | 3.38 (3.35-3.43) | 3.11 (3.05-3.26) | P = 0.061 |
| Maturation | 0.4 (0.32-0.46) | 0.36 (0.29-0.40) | P = 0.073 | 0.27 (0.16-0.29) | 0.04 (0.01-0.06) | P = 0.002 |
| Hipertrophic | 4.12 (3.66-4.31) | 7.59 (7.31-7.77) | P = 0.032 | 4.02 (3.96-4.26) | 2.17 (2.11-2.32) | P < 0.0001 |
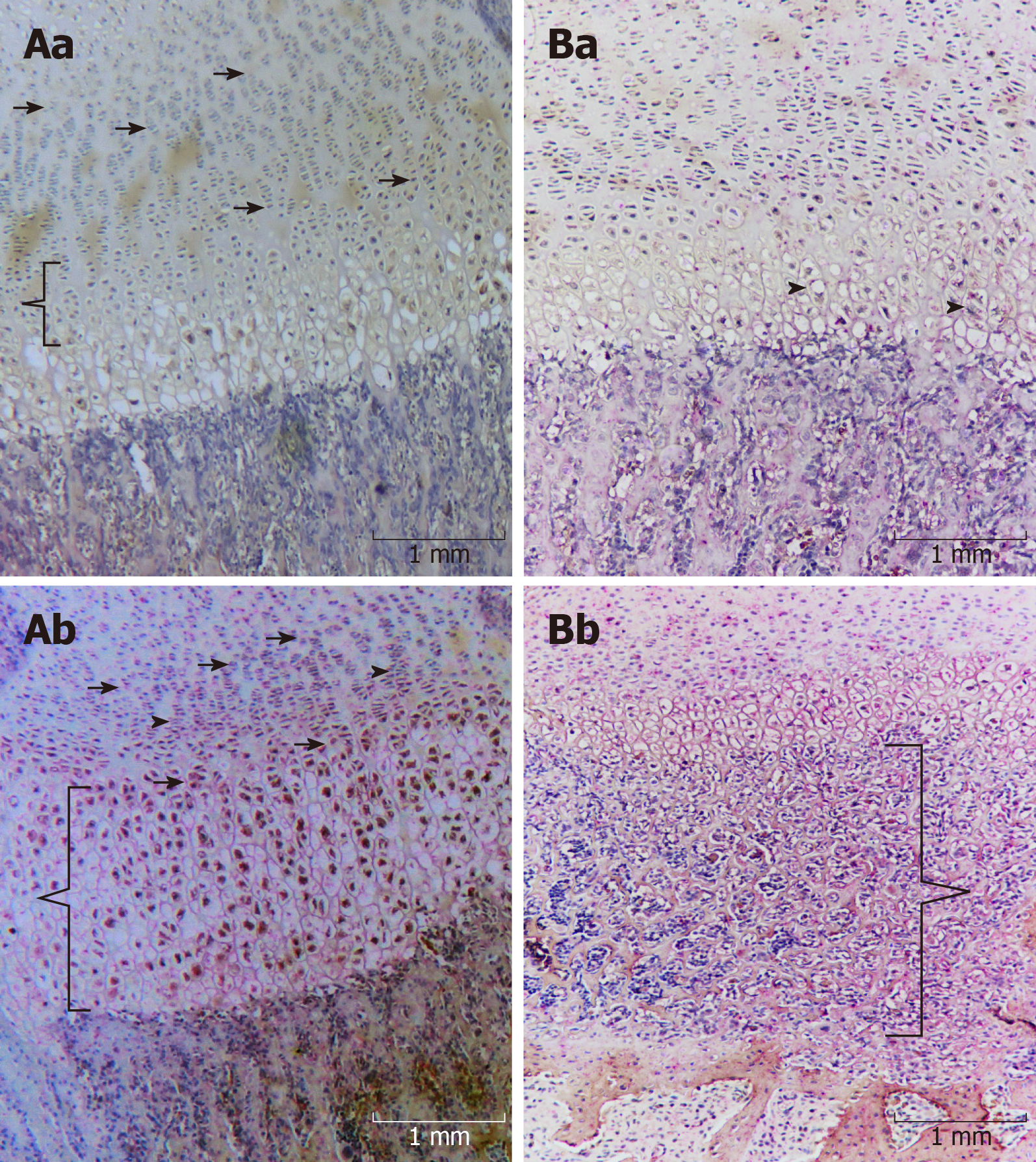
On the third day (Figure 1A), it was possible to verify that the areas of the reserve, proliferative, and maturation zones were similar (Figure 1Aa and b), whereas the hypertrophic zone was significantly larger in terms of the number of specimens that received alendronate (Figure 1Ab). On the 12th day, the control group exhibited similar characteristics when compared with the third day (Figure 1Ba). In contrast, the specimens that received alendronate demonstrated significantly fewer areas of maturation and hypertrophic zones. However, an unorganized bone matrix deposition was observed between the hypertrophic zone and cortical bone (Figure 1Bb).
On the third day, only TGF-β1 was observed in the maturation and hpertrophic zones in the control group. In these specimens, the BMP-2 was positive in bone matrix deposition. All zones of cartilage revealed positivity for BMP-2, whereas the TGF-β1 was present in some proliferative cells and in the majority of cells that compounded both the maturation and the hypertrophic zone. In addition, intense double positivity for TGF-β1 and BMP-2 was observed in the bone matrix. On the 12th day, the control group exhibited a similar immunohistochemical pattern; however, it was possible to verify positivity for BMP-2 in hypertrophic cartilage and the medullary bone matrix. The alednronate group displayed the presence of BMP-2 in cartilage and in the bone matrix. In the area of the unorganized matrix, the BMP-2 was prevalent, whereas in the larger medular bone matrix, both proteins were detected (Table 3).
| Chondroid layer | Day | TGF-b1 | P value | BMP-2 | P value | ||
| Control | Alendronate | Control | Alendronate | ||||
| Reserve | 3 | - | - | - | - | 12 (10-14) | P < 0.001 |
| 12 | - | - | - | - | 34 (30-36) | P < 0.001 | |
| Proliferative | 3 | 23.01 (19-26) | 16.27 (14-19) | P = 0.047 | - | 72.21 (70-75) | P < 0.001 |
| 12 | 19.12 (17-23) | 2.33 (0-3) | P < 0.001 | - | 67.43 (66-69) | P < 0.001 | |
| Maturation | 3 | 89.64 (85-93) | 93.07 (91-94) | P = 0.673 | - | 88.61 (86-81) | P < 0.001 |
| 12 | 83 (80-84) | 1.06 (0-1) | P < 0.001 | 13 (12-15) | 94.05 (92-98) | P < 0.001 | |
| Hipertrophic | 3 | 96.35 (94-99) | 97.66 (95-98) | P = 0.772 | 2 (2-3) | 82.17 (80-85) | P < 0.001 |
| 12 | 93.52 (92-95) | 92.31 (90-93) | P = 0.694 | 42 (41-44) | 93.00 (90-95) | P = 0.027 | |
The study demonstrated that alendronate decreased the longitudinal femur size from three to 12 d post the prolonged administration of alendronate, a situation that coincided with changes in the usual histological aspects of chondroid zones, especially in the maturation and hypertrophic strata, as well as changes in co-immunoexpression between TGF-β1 and BMP-2.
To understand the changes that occurred, one must comprehend the importance of the usual immunolocalization of each protein and the likely effect that each protein exerted in usual conditions of endochondral growth.
In hyaline cartilage, TGF-β1 presence is required for the homeostasis of this anatomical area, and this cytokine seems to play a fundamental role in the production and remodelation of the chondroid extracellular matrix.
However, the action of TGF-β1 for each stratum of cartilage of the methaphisyae of long bones seems to be distinct[14]. In this pathway, D’Angelo et al[15] (2001) indicated that maturing and hypertrophic chondrocytes produce and express active TGF-β1, and this cytokine operates in paracrine form in another layer of cartilage. In this way, TGF-β1 influences maturation, proliferation, and hypertrophy, whereas its presence inhibits the terminal differentiation of mature chondrocytes. This effect may help chondrocytes to remain in the pre-hypertrophic stage, thus inhibiting their terminal differentiation to hypertrophic chondrocytes.
It is noteworthy that TGF-β1 positively regulates the expression of specific cartilage proteins during re-differentiation; conversely, substantial evidence shows that chondrocyte exposure to TGF-β1 during the proliferative phase obstructs these cells’ re-differentiation potential[14,16]. This condition may explain the positivity seen in the TGF-β1 prevalent in the prehypertrophic and hypertrophic zones of cartilage as seen in our control group on the third and 12th days of analysis, despite the fact that some hypertrophic chondrocytes during this period exhibited positivity for BMP-2, whereas in the precocious time period, BMP-2 was positive only in the bone matrix.
In contrast, for specimens that received alendronate, we observed TGF-β1 in all strata of chondrocytes, a fact that coincided with a significantly larger area of the hypertrophic zone on the 3rd day. Furthermore, the cells of all chondroid strata also exhibited concomitant expression with BMP-2. This peculiar situation coincided with an unorganized bone matrix formation between a thinner layer of hypertrophic chondrocytes and trabecular bone on the 12th day of analysis. These results suggest that alendronate may promote the cartilage expansion of the maturated and hypertrophic chondroid zones only during the early stages of the administration of the drug. However, the concomitant expression between TGF-β1 and BMP-2 in areas of the chondroid zones featuring endochondral ossification also indicates the likely premature transdifferentiation from cells of chondroid lineage to osteoprogenitor cells, especially when these specimens were exposed to the drug for a prolonged period of time.
It is noteworthy that our result is not an isolated finding in the literature and that some evidence shows that alendronate possesses an important osteogenic capacity. Supporting this perspective, Wang et al[17] (2010) demonstrated that the administration of alendronate in animal models was responsible for the transcription of osteoprogenitor genes in medullar mesenchymal cells. They also added that osteogenic potential occurs because the alendronate stimulates the MAP kinases’ pathways, which, in turn, may excite the expression of osteoproteins. These effects may indicate and explain the previous radiological results published by Silva et al[18] (2010), who demonstrated sclerotic metaphyseal lines in the long bones of young patients treated with alendronate. They may also indicate that the extended use of alendronate compromises the usual growth of children and its bone development.
Thus, alendronate impairs the epiphysis growth plate at the same time as the growth of the femur, as it simultaneously alters the immunolocalization of TGF-β1 and BMP-2, and it may produces a precocious transdifferentiation from chondroid cells to osteogenic cells-a condition that impairs the usual development and epiphyseal plate, thus impairing femoral growth.
However, it should be highlighted that this study has some limitations. Herein, we evaluated the macroscopic, histological and immunohistochemical aspects on 3rd and 12th day of development and none data may be stipulated in the intermediated stages of development of epiphyseal plate. The immunohistochemistry staining identifies proteins present either chondroid matrix or bone matrix, regardless of the time when they were expressed.
In addition, this study focused only on epiphyseal plate area and the present results may not be extrapolated to total appendicular as well as craniofacial bones[19]. However, this study may give an important information, and it may contribute to the discussion over the clinical use of alendronate in earlier stages of bone development.
Endochondral ossifying constitutes the fundamental model for the development of appendicular skeletal formation. This process requires well-organized changes on the cartilage morphological aspects that includes the resting, proliferative, prehypertrophic, hypertrophic segments which it is followed by replacement of mineralized hypertrophic cartilage with osteoprogenitor cells, and the calcified cartilage is subsequently replaced by bone. Under pathological situation this usual development does not occur, promoting growth deficiencies. Since alendronate may contribute either for chondrogenic or osteogenic development, herein we evaluated the likely effect of alendronate in epiphyseal plate of newborn rats, in order to verify the real effect of alendronate during earlier development of appendiclar bones.
The fundamental topics adressesd in the present study were: (1) Would be alendronate a drug that should be able to improve the chondrogenic and osteogenic in eralier stage of development; and (2) Alendronate alters the correlation between transforming growth factor-β1 (TGF-β1) and bone morphogenetic protein-2 (BMP2) during endochondral ossification? The answer of this question, could give us a direction between for a likely therapy apporach in child that possess congenital deficies of growth.
Thus, the main objective of this study was to verify how alendornate acts on the epifeaseal developmental plaque. In addition, we evaluated which region of endochondral development is most stimulated or inhibited by alendronate, as well as whether the drug may cause early ossification.
Thus, we analyzed the evaluation of femoral bone growth of neonatal rats by measuring the distance between the apex of the greater trochanter until the lower intercondylar midlle face. We also measured the area of each stratification of the endochondral histological zones, as well as where the ossifications occurred - late or early. We also evaluated by immunohistochemistry, the immunopresence of TGF-β1 and BMP-2 in each of these endochondral zones.
Here we reveal that specimens given alednroanto developed greater cartilage hypertrophy while the reserve zone was decreasing. These results also coincide with changes in co-immunolocation between TGF-β1 and BMP-2. Here we demonstrate a greater presence of osteoprotein in areas where endochondral proliferation and endochondral diphonication should occur, triggering early ossification. These results also coincide with lower bone growth.
In this study we may conclude that: (1) Alendronate may compromise the usual development and growth of epiphyseal plaque. (2) This pathological event may occur as alednronate provides prior expression of BMP-2, while TGF-1 immunolocation decreases. And (3) the fundamental result found here indicates that alendronate does not induce appendicular bone growth. Thus, it may indicate that the use of this drug is not a plausible conduct for children suffering from endochondral growth disorders.
Here we suggest the perspective that alendronate compromises the development of long bones. There is a need for research using other drugs to try to promote greater growth of endochondral zones, ossification in suitable areas, conditions that are important for appendicular bone growth.
| 1. | Emons J, Chagin AS, Sävendahl L, Karperien M, Wit JM. Mechanisms of growth plate maturation and epiphyseal fusion. Horm Res Paediatr. 2011;75:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS, Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol. 2008;40:46-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 694] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 3. | Derynck R, Akhurst RJ. Differentiation plasticity regulated by TGF-beta family proteins in development and disease. Nat Cell Biol. 2007;9:1000-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 315] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 4. | Tekari A, Luginbuehl R, Hofstetter W, Egli RJ. Transforming growth factor beta signaling is essential for the autonomous formation of cartilage-like tissue by expanded chondrocytes. PLoS One. 2015;10:e0120857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Kang JS, Alliston T, Delston R, Derynck R. Repression of Runx2 function by TGF-beta through recruitment of class II histone deacetylases by Smad3. EMBO J. 2005;24:2543-2555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 290] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 6. | Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1040] [Cited by in RCA: 1010] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 7. | Katagiri T, Tsukamoto S, Nakachi Y, Kuratani M. Recent Topics in Fibrodysplasia Ossificans Progressiva. Endocrinol Metab (Seoul). 2018;33:331-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Wang C, Zhang BH, Liu YJ, Hu YQ, He JW, Zhang ZL. Transforming growth factor-β1 gene mutations and phenotypes in pediatric patients with CamuratiEngelmann disease. Mol Med Rep. 2013;7:1695-1699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Lehmann K, Seemann P, Stricker S, Sammar M, Meyer B, Süring K, Majewski F, Tinschert S, Grzeschik KH, Müller D, Knaus P, Nürnberg P, Mundlos S. Mutations in bone morphogenetic protein receptor 1B cause brachydactyly type A2. Proc Natl Acad Sci USA. 2003;100:12277-12282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 125] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Rahman MS, Akhtar N, Jamil HM, Banik RS, Asaduzzaman SM. TGF-β/BMP signaling and other molecular events: regulation of osteoblastogenesis and bone formation. Bone Res. 2015;3:15005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 342] [Cited by in RCA: 461] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 11. | Cheung MS. Drugs Used in Paediatric Bone and Calcium Disorders. Endocr Dev. 2015;28:277-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Feehan AG, Zacharin MR, Lim AS, Simm PJ. A comparative study of quality of life, functional and bone outcomes in osteogenesis imperfecta with bisphosphonate therapy initiated in childhood or adulthood. Bone. 2018;113:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | de Souza JF, Gramasco M, Jeremias F, Santos-Pinto L, Giovanini AF, Cerri PS, Cordeiro Rde C. Amoxicillin diminishes the thickness of the enamel matrix that is deposited during the secretory stage in rats. Int J Paediatr Dent. 2016;26:199-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Li TF, O'Keefe RJ, Chen D. TGF-beta signaling in chondrocytes. Front Biosci. 2005;10:681-688. [PubMed] |
| 15. | Dangelo M, Sarment DP, Billings PC, Pacifici M. Activation of transforming growth factor beta in chondrocytes undergoing endochondral ossification. J Bone Miner Res. 2001;16:2339-2347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Narcisi R, Quarto R, Ulivi V, Muraglia A, Molfetta L, Giannoni P. TGF β-1 administration during ex vivo expansion of human articular chondrocytes in a serum-free medium redirects the cell phenotype toward hypertrophy. J Cell Physiol. 2012;227:3282-3290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Wang CZ, Chen SM, Chen CH, Wang CK, Wang GJ, Chang JK, Ho ML. The effect of the local delivery of alendronate on human adipose-derived stem cell-based bone regeneration. Biomaterials. 2010;31:8674-8683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Silva EC, Terreri MT, de Castro TC, Barbosa CP, Fernandes AR, Hilário MO. Sclerotic metaphyseal lines in children and adolescents treated with alendronate. Rev Bras Reumatol. 2010;50:283-290. [PubMed] |
| 19. | Giovanini AF, Deliberador TM, Gonzaga CC, de Oliveira Filho MA, Göhringer I, Kuczera J, Zielak JC, de Andrade Urban C. Platelet-rich plasma diminishes calvarial bone repair associated with alterations in collagen matrix composition and elevated CD34+ cell prevalence. Bone. 2010;46:1597-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Stavroulopoulos A S-Editor: Ma RY L-Editor: A E-Editor: Wu YXJ