Published online Aug 20, 2017. doi: 10.5493/wjem.v7.i3.84
Peer-review started: February 8, 2017
First decision: May 10, 2017
Revised: May 26, 2017
Accepted: June 30, 2017
Article in press: July 3, 2017
Published online: August 20, 2017
Processing time: 203 Days and 10.8 Hours
To investigate T-cell activation, the percentage of peripheral T regulatory cells (Tregs), Th17 cells and the circulating cytokine profile in systemic sclerosis (SSc).
We enrolled a total of 24 SSc patients and 16 healthy controls in the study and divided the patients as having diffuse cutaneous SSc (dcSSc, n = 13) or limited cutaneous SSc (lcSSc, n = 11). We performed a further subdivision of the patients regarding the stage of the disease - early, intermediate or late. Peripheral venous blood samples were collected from all subjects. We performed flow cytometric analysis of the activation capacity of T-lymphocytes upon stimulation with PHA-M and of the percentage of peripheral Tregs and Th17 cells in both patients and healthy controls. We used ELISA to quantitate serum levels of human interleukin (IL)-6, IL-10, tissue growth factor-β1 (TGF-β1), and IL-17A.
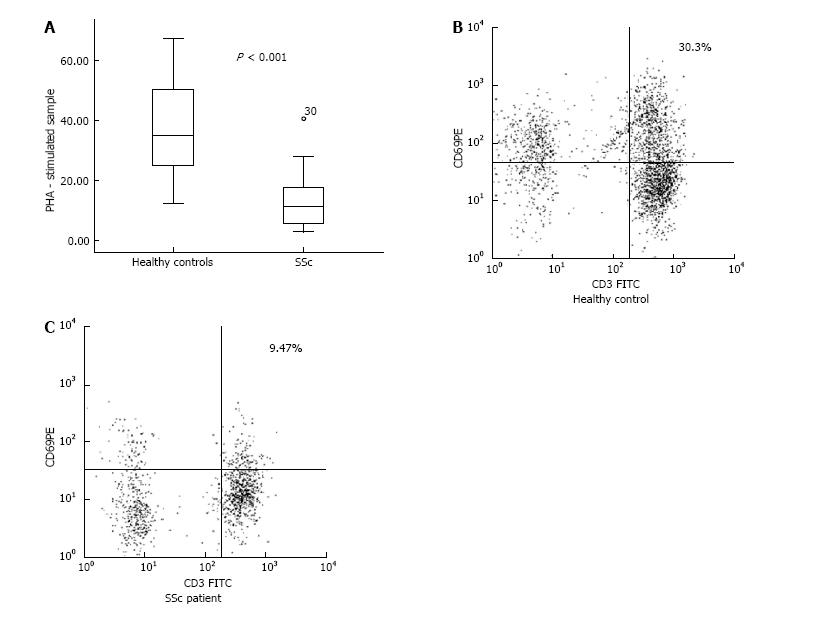
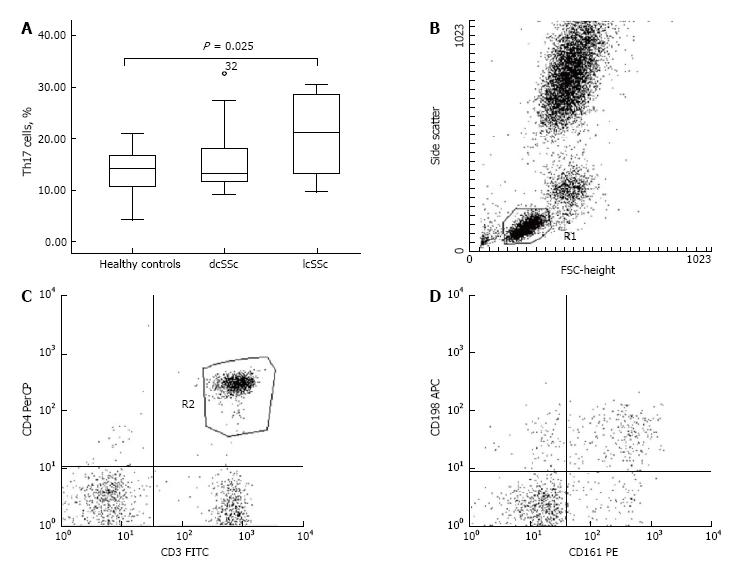
We identified a decreased percentage of CD3+CD69+ cells in PHA-stimulated samples from SSc patients in comparison with healthy controls (13.35% ± 2.90% vs 37.03% ± 2.33%, P < 0.001). However, we did not establish a correlation between the down-regulated CD3+CD69+ cells and the clinical subset, nor regarding the stage of the disease. The activated CD4+CD25+ peripheral lymphocytes were represented in decreased percentage in patients when compared to controls (6.30% ± 0.68% vs 9.36% ± 1.08%, P = 0.016). Regarding the forms of the disease, dcSSc patients demonstrated lower frequency of CD4+CD25+ T cells against healthy subjects (5.95% ± 0.89% vs 9.36% ± 1.08%, P = 0.025). With regard to Th17 cells, our patients demonstrated increased percentage in comparison with controls (18.13% ± 1.55% vs 13.73% ± 1.21%, P = 0.031). We detected up-regulated Th17 cells within the lcSSc subset against controls (20.46% ± 2.41% vs 13.73% ± 1.21%, P = 0.025), nevertheless no difference was found between dcSSc and lcSSc patients. Flow cytometric analysis revealed an increased percentage of CD4+CD25-Foxp3+ in dcSSc patients compared to controls (10.94% ± 1.65% vs 6.88% ± 0.91, P = 0.032). Regarding the peripheral cytokine profile, we detected raised levels of IL-6 [2.10 (1.05-4.60) pg/mL vs 0.00 pg/mL, P < 0.001], TGF-β1 (19.94 ± 3.35 ng/mL vs 10.03 ± 2.25 ng/mL, P = 0.02), IL-10 (2.83 ± 0.44 pg/mL vs 0.68 ± 0.51 pg/mL, P = 0.008), and IL-17A [6.30 (2.50-15.60) pg/mL vs 0 (0.00-0.05) pg/mL, P < 0.001] in patients when compared to healthy controls. Furthermore, we found increased circulating IL-10, TGF-β, IL-6 and IL-17A in the lcSSc subset vs control subjects, as it follows: IL-10 (3.32 ± 0.59 pg/mL vs 0.68 ± 0.51 pg/mL, P = 0.003), TGF-β1 (22.82 ± 4.99 ng/mL vs 10.03 ± 2.25 ng/mL, P = 0.031), IL-6 [2.08 (1.51-4.69) pg/mL vs 0.00 pg/mL, P < 0.001], and IL-17A [14.50 (8.55-41.65) pg/mL vs 0.00 (0.00-0.05) pg/mL, P < 0.001]. Furthermore, circulating IL-17A was higher in lcSSc as opposed to dcSSc subset (31.99 ± 13.29 pg/mL vs 7.14 ± 3.01 pg/mL, P = 0.008). Within the dcSSc subset, raised levels of IL-17A and IL-6 were detected vs healthy controls: IL-17A [2.60 (0.45-9.80) pg/mL vs 0.00 (0.00-0.05) pg/mL, P < 0.001], IL-6 [2.80 (1.03-7.23) pg/mL vs 0.00 pg/mL, P < 0.001]. Regarding the stages of the disease, TGF-β1 serum levels were increased in early stage against late stage, independently from the SSc phenotype (30.03 ± 4.59 ng/mL vs 13.08 ± 4.50 ng/mL, P = 0.017).
It is likely that the altered percentage of Th17 and CD4+CD25-FoxP3+ cells along with the peripheral cytokine profile in patients with SSc may play a key role in the pathogenesis of the disease.
Core tip: Systemic sclerosis (SSc) is a devastating autoimmune disorder, which can be subclassified into limited cutaneous SSc (lcSSc) and diffuse cutaneous SSc (dcSSc) based on the skin manifestations. One of the original contributions of our study has demonstrated a decreased capacity for PHA-induced peripheral T-cell activation in patients with SSc. For the first time, our research group has identified an up-regulated percentage of CD4+CD25-FoxP3+ cells in the dcSSc subset. Regarding the peripheral cytokine profile in SSc, the serum levels of interleukin (IL)-17A have been increased in lcSSc as opposed to the dcSSc subset. The rest of our data, concerning the elevated circulating IL-6, IL-10, and TGF-β in SSc patients, has confirmed literature-based results.
- Citation: Krasimirova E, Velikova T, Ivanova-Todorova E, Tumangelova-Yuzeir K, Kalinova D, Boyadzhieva V, Stoilov N, Yoneva T, Rashkov R, Kyurkchiev D. Treg/Th17 cell balance and phytohaemagglutinin activation of T lymphocytes in peripheral blood of systemic sclerosis patients. World J Exp Med 2017; 7(3): 84-96
- URL: https://www.wjgnet.com/2220-315X/full/v7/i3/84.htm
- DOI: https://dx.doi.org/10.5493/wjem.v7.i3.84
Systemic sclerosis (SSc) is a generalized debilitating connective tissue disease affecting the skin and internal organs characterized by vasculopathy, fibrosis, and autoimmune alterations[1]. SSc is subclassified into two major clinical subsets, namely diffuse cutaneous (dcSSc) and limited cutaneous (lcSSc) form depending on the spread of the skin sclerosis[2]. Each of these subtypes has three stages - early, intermediate and late[2,3]. The dcSSc form distinguishes by rapidly progressive fibrosis of the skin and internal organs, which is a major cause of morbidity and mortality of the patients[4]. The lcSSc form is marked by vascular injury with milder skin and visceral fibrosis and generally, has a low progression rate[2,3].
The autoimmune dysregulation in SSc comprises lymphocyte activation that leads to the generation of autoantibodies, abnormal production of cytokines and chemokines, and impairment of the innate immunity[5-7]. Over the last decade, the accumulating data has shown the central role of T lymphocytes in the pathogenesis of SSc[8,9].
It is thought that the cytokine production by T cells influences the function of fibroblasts and endothelial cells, thereby playing a central role in vascular disease and fibrosis development[1,5]. Therefore, many efforts have been made to identify the T cell derived cytokine patterns in SSc and the subsets of T helpers involved. Most studies performed in SSc patients have examined the characteristics of T cells isolated from peripheral blood.
There is a strong evidence in literature for altered T-cell activation[10-12] and T helper cells abnormalities in SSc[8,9]. Several authors have reported higher frequency of Th17 lymphocytes in the peripheral blood of SSc patients and have pointed out the role of these cells as a factor engaged in the pathogenesis of the disease[13-15]. Th17 cells, firstly described in 2005, produce interleukin (IL)-17A, IL-17F, IL-21, IL-22, and IL-26 and play a key role in host defense against extracellular bacteria and fungi[16]. Recent data has revealed their implication in the pathogenesis of several inflammatory and autoimmune diseases, such as multiple sclerosis and rheumatoid arthritis, investigating their animal models - experimental autoimmune encephalomyelitis and collagen-induced arthritis[17]. IL-17 is an inductor of the surface expression of intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) by endothelial cells, and foreskin fibroblasts and induces the production of IL-1 and IL-6[18-20]. IL-17 also increases the production of pro-inflammatory cytokines such as chemokine (C-C motif) ligand 2 (CCL2), IL-6, IL-8 by synoviocytes and fibroblasts from both human skin and lungs[19,21]. Regarding the fibrotic process in SSc, IL-17 inhibits type I and type III collagen deposition[18,22] and reduces the connective tissue growth factor (CTGF) production via up-regulation of miR-129-5p in dermal fibroblasts[23]. Animal models of SSc have demonstrated the involvement of IL-17 in the bleomycin-induced lung and skin fibrosis[24-26]. Meanwhile, human studies have reported inverse correlation between the number of IL-17+ cells in the skin of SSc patients and the extent of skin sclerosis[27].
Not only Th17 cells, but also Tregs (CD4+FoxP3+) are involved in pathogenesis of SSc and there is a controversial data concerning their functional and numerical alterations. Some authors have found markedly up-regulated Tregs in all SSc phenotypes[10,28] particularly in active and severe disease[29]. Tregs from SSc patients demonstrated a diminished ability to control CD4 effector T cells and this defective function seemed to correlate with lower expression of CD69 and tissue growth factor-β (TGF-β) levels[10]. One study did not found Treg alterations in SSc patients compared to control groups[15]. Finally, several studies demonstrated a decreased frequency/impaired function of Tregs in SSc[30-32].
The CD4+Foxp3+ T cells produce anti-inflammatory cytokines including TGF-β and IL-10 and Tregs are mandatory to establish immune tolerance. TGF-β is a master regulator of the fibrotic process and alterations in TGF-β signaling are well described in SSc[1]. TGF-β promotes the fibrosis by both stimulating the synthesis, and suppressing the degradation of extracellular matrix[1]. TGF-β is involved in the generation of peripheral Tregs as well[33]. Accordingly, the same cytokine, TGF-β, is implicated in the generation of two functionally opposite T cell subsets, effectors - Th17 and Tregs, and the co-presence or not of pro-inflammatory cytokines, such as IL-6 and IL-1, determines the fate of TGF-β-exposed T cells[30]. Thus, the concomitance of TGF-β and IL-6 in SSc skin infiltrates could favor the generation of effector Тh17 cells at the expense of Tregs, leading to complete alteration of the homeostatic equilibrium. Regarding IL-10, it has been reported to be increased in the serum of SSc patients[34]. Moreover, one paper has revealed that the raised serum levels of IL-10, and IL-6 correlated positively with the interstitial lung disease and the modified Rodnan skin score (MRSS) of patients[35].
Based on all the aforementioned data, we decided to evaluate the activation capacity of T cells in the peripheral blood of SSc patients and healthy controls, using phytochaemagglutinin (PHA-M). Our next aim was to determine both the percentage of the effector (Th17) and regulatory (Treg) cell subsets in the peripheral blood of the patients and the controls. We also investigated the serum levels of the peripheral cytokine milieu in both SSc patients and controls, scilicet, IL-6, IL-10, TGF-β1 and IL-17A.
It is laborious to obtain reliable incidence and prevalence estimates of SSc since the disease rarely occurs. Up to now, no studies have been carried out on the SSc incidence and prevalence in Bulgarian population. However, several epidemiological studies have been performed in Southeastern Europe. For instance, the incidence of SSc in Greece (North West) was 11 cases/million per year and the prevalence - 7.7 cases/million (1981-2002[36]. Respectively, the estimated prevalence of SSc in Croatia (Split-Dalmatia) based on 2008 data was 15 cases/million[37]. Although our study included a relatively small cohort of SSc patients, it could be assessed quite representative for our population if compared to the existing epidemiological data.
Informed written consent was obtained from all the subjects, enrolled in our study after approval of the Ethics Committee at the University Hospital St. Ivan Rilski, Sofia. All experiments carried out complied with the Declaration of Helsinki.
Twenty-four patients, who attended the Clinic of Rheumatology of Department of Internal Medicine, Medical University of Sofia, were enrolled in this study. The mean age of the patients (male - 1, female - 23) was 47.1 ± 13.2 years. All the patients fulfilled the 2013 ACR/EULAR Criteria for the classification of SSc[38] and were divided as having dcSSc or lcSSc depending on the extent of skin sclerosis[2]. A further subdivision of the patients was performed in the groups based on the years from diagnosis[3]. Patients with dcSSc were divided in three groups: Early dcSSc (< 3 years’ duration), intermediate (3-6 years) and late (6+ years). In the lcSSc group, the following subdivision was performed: Early lcSSc (< 5 years’ duration), intermediate (5-10 years) and late (10+ years) stages. The disease activity was assessed according to the Preliminarily Revised EUROSTAR Activity Index[39]. Sixteen age and gender-matched healthy individuals served as controls. Patients’ clinical data as well as treatment regimens are shown in Table 1.
| Patient No. | Gender | Age | Form | Stage | Active SSc | Visceral damage | Autoantibodies1 | Treatment regimen |
| 1 | M | 50 | dcSSc | Intermediate | Yes | No | Speckled | PMP |
| 2 | F | 49 | dcSSc | Late | No | E | Anti-Scl70 | MTX |
| 3 | F | 55 | dcSSc | Intermediate | No | E | Speckled | MP |
| 4 | F | 58 | dcSSc | Late | Yes | PF | Anti-Scl70 | PMP, PCYP |
| 5 | F | 44 | dcSSc | Early | Yes | PF | Anti-Scl70 | DPA, MP |
| 6 | F | 27 | lcSSc | Early | Yes | No | Anti-Scl70 | DPA, MP, TCZ |
| 7 | F | 48 | dcSSc | Early | Yes | PF | Anti-Scl70 | DPA, MP |
| 8 | F | 37 | lcSSc | Early | Yes | No | Anti-Ro52 | CHQ |
| 9 | F | 65 | dcSSc | Late | No | No | Anti-CENP-A, Anti-CENP-B | MP, CHQ |
| 10 | F | 36 | lcSSc | Intermediate | Yes | No | Speckled | PMP, PCYP, DPA |
| 11 | F | 47 | dcSSc | Early | Yes | SRC | Anti-Scl70 | PMP, PCYP |
| 12 | F | 32 | lcSSc | Early | Yes | PF | Speckled | MP, TCZ |
| 13 | F | 62 | dcSSc | Early | Yes | No | Speckled | PMP, PCYP |
| 14 | F | 27 | lcSSc | Late | Yes | No | Anti-PM/Scl-100 | MTX |
| 15 | F | 73 | lcSSc | Intermediate | No | PF | Speckled | MP, MTX |
| 16 | F | 32 | dcSSc | Late | Yes | PF | Anti-Scl70, Anti-PM/Scl-75 | PMP, PCYP |
| 17 | F | 60 | dcSSc | Late | Yes | No | Speckled | PMP, PCYP |
| 18 | F | 34 | dcSSc | Early | Yes | No | Anti-Scl70 | MP, MTX |
| 19 | F | 56 | lcSSc | Late | No | E | Anti-CENP-B | MP |
| 20 | F | 53 | lcSSc | Early | Yes | No | Anti-PM/Scl-75 | MP, AZA |
| 21 | F | 30 | lcSSc | Late | Yes | No | Speckled | MP, DPA |
| 22 | F | 61 | dcSSc | Late | No | E, PF, PH | Anti-Scl70 | MP |
| 23 | F | 39 | lcSSc | Early | No | No | Anti-CENP-B | MTX |
| 24 | F | 56 | lcSSc | Intermediate | No | E | Speckled | MP, MTX |
Heparinized whole venous blood, 2 mL was collected (LH 68 IU BD-Plymouth, United Kingdom, 5 mL) from each subject and was separated equally into two tubes - control tube and a PHA-M stimulated sample. To the stimulated test tube 20 μg/mL PHA-M (Roche Diagnostics GmbH, Germany) was added and the two samples were incubated for 4 h at 37 °C, 5% CO2. The samples were gently shacked, at regular intervals, on a multispeed vortex (MSV-3500 BioSan LV). Afterwards, 100 μL blood from each tube was labeled with monoclonal anti-CD3 FITC (for determination of the T lymphocytes) and anti-CD69 PE, an early activation marker for T cells (BD Biosciences, United States) and incubated for 30 min, at room temperature (RT) in the dark. Followed a lysis of erythrocytes (BD FACS Lysing Solution, BD Biosciences, United States), then centrifuging at 1300 rpm for 10 min and double washing (CellWash, BD Biosciences, United States). Subsequently, cells were fixed with 200 μL CellFIX (BD Biosciences, United States) and were analyzed with BD FACSCalibur flowcytometer using the Cell Quest software for data acquisition and analysis. Then 20000 lymphocytes were counted and analyzed for expression of CD69. The results obtained for each patient and healthy subject were analyzed for PHA-stimulated and unstimulated lymphocytes.
Peripheral whole venous blood, 1 mL was collected (K2E BD-Plymouth, United Kingdom, 5 mL) from each subject. Monoclonal anti-CD3 FITC, anti-CD161-PE, anti-CD4-PerCP and anti-CD196-Alexa Flour 647 antibodies (BD Biosciences, United States) were added to the blood samples and incubated for 30 min, at RT in the dark. Followed a lysis of erythrocytes with a lysing solution (BD FACS Lysing Solution, BD Biosciences, United States) and after double washing in a CellWash solution (BD Biosciences, United States) the cells were fixed (CellFIX, BD Biosciences, United States). The specific fluorescent labeling was analyzed with BD FACSCalibur flowcytometer and 10000 lymphocytes were counted and analyzed using the Cell Quest software program of the same company.
Peripheral venous blood, 1 mL was collected (K2E BD Vacutainer, BD-Plymouth, United Kingdom, 5 mL) from each individual. Monoclonal anti-CD25 FITC and anti-CD4-PE (BD Biosciences, United States) were added to the blood samples and incubated for 30 min, at RT in the dark. Followed a lysis of erythrocytes with a lysing solution (BD FACS Lysing Solution, BD Biosciences, United States) and after double washing in a CellWash solution (BD Biosciences, United States) a Human FoxP3 Buffer set (BD Biosciences, United States) was used for permeabilization of the cell membranes, as described by the manufacturer’s instructions. Afterwards, a monoclonal antibody against intracellular expression of FoxP3 was used (anti-FoxP3 PE). After double washing, the cells were re-suspended in a wash buffer and analyzed immediately with BD FACSCalibur flowcytometer. At least 20000 CD4 positive lymphocytes were acquired using the Cell Quest software program.
Serum from each subject, 5 mL was collected using serum separator tubes (Vacutainer BD-Plymouth, United Kingdom, 5 mL). Circulating cytokine levels (serum IL-6, IL-10, IL-17A, TGF-β1) were measured using Diaclone Human ELISA kits (Diaclone SAS, France) according to the manufacturer’s instructions and every sample was tested in duplicates.
For the analysis of the data’s distribution, the Kolmogorov-Smirnov test was used. In cases of normal distribution, we determined mean ± SE, minimum, and maximum values and used a two-sample t-test and ANOVA for further statistical evaluation of the experimental data. In cases of non-normal distribution, median, interquartile range (IQR), minimum, and maximum values, were calculated and the Mann-Whitney test was applied. The strength of linear relationship between two continuous variables was examined using Pearson’s correlation coefficient. Differences were considered as significant at P < 0.05. All statistical analyses were performed using IBM SPSS Statistics (IBM® SPSS® Statistics, Version 19).
We found no significant differences in the frequency of early activated T cells (CD3+CD69+) in unstimulated peripheral blood samples (control test tube) between healthy control subjects and SSc patients. However CD4+CD25+ lymphocytes, which are considered to be activated cells, were represented in decreased percentage in patients when compared to controls (P = 0.016, Table 2). Regarding the disease phenotype, dcSSc patients demonstrated lower frequency of CD4+CD25+ T cells against healthy subjects (5.95% ± 0.89% vs 9.36% ± 1.08%, respectively, P = 0.025).
| T cell subpopulation (%) | SSc patients | Healthy controls | P value |
| CD4+Foxp3+ | 14.24 ± 1.39 (5.68-28.73) | 11.04 ± 1.22 (3.55-20.84) | |
| CD4+CD25-Foxp3+ | 10.22 ± 1.21 (2.09-23.09) | 6.88 ± 0.91 (1.42-12.79) | 0.052 |
| CD4+CD25+Foxp3+ | 4.02 ± 0.52 (0.71-10.77) | 4.16 ± 0.53 (2.08-8.05) | |
| CD4+CD25+ | 6.30 ± 0.68 (1.40-13.36) | 9.36 ± 1.08 (2.84-19.60) | 0.016 |
| Th17 | 18.13 ± 1.55 (9.18-32.64) | 13.73 ± 1.21 (4.30-20.99) | 0.031 |
In the PHA-stimulated samples, CD3+CD69+ cells were represented in decreased percentage in patients when compared to controls (13.35% ± 2.90% vs 37.03% ± 2.33%, respectively, P < 0.001) (Figure 1). As regards the lcSSc and dcSSc, there was no difference between the two phenotypes and in comparison with the healthy subjects.
With regard to the Th17 cells, we found an up-regulated percentage in patients as opposed to controls (P = 0.031; Table 2). Accordingly, an increased percentage of Th17 cells was detected within the lcSSc subset vs controls (20.46% ± 2.41% vs 13.73% ± 1.21%, respectively, P = 0.025) (Figure 2). We detected no difference regarding the percentage of Th17 cells between the dcSSc and lcSSc phenotypes nor, when compared to controls.
There was no difference between patients and healthy individuals regarding CD4+Foxp3+ cells. We detected a certain trend toward increased percentage of these cells within the dcSSc subgroup as opposed to controls (14.73% ± 1.71% vs 11.04% ± 1.22%, respectively, P = 0.083). There was also no difference between patients and healthy individuals, regarding CD4+CD25+Foxp3+ T cells, nor within the distinct subtypes of SSc (Table 2). The percentage of CD4+CD25-Foxp3+ was marginally higher in patients (P = 0.052; Table 2) compared to controls. Although their percentage was increased in dcSSc vs controls (10.94% ± 1.65% vs 6.88% ± 0.91%, respectively, P = 0.032) (Figure 3). Still, we did not found differences between the dcSSc and lcSSc subsets.
Regarding the peripheral cytokine profile, we detected increased levels of IL-6 [2.10 (1.05-4.60) pg/mL vs 0.00 pg/mL, P < 0.001], TGF-β1 (19.94 ± 3.35 ng/mL vs 10.03 ± 2.25 ng/mL, P = 0.02), IL-10 (2.83 ± 0.44 pg/mL vs 0.68 ± 0.51 pg/mL, P = 0.008), and IL-17A [6.30 (2.50-15.60) pg/mL vs 0 (0.00-0.05) pg/mL, P < 0.001] in patients when compared to healthy controls (Table 3). Furthermore, we found increased circulating IL-10, TGF-β, IL-6 and IL-17A in the lcSSc subset vs control subjects, as it follows: IL-10 (3.32 ± 0.59 pg/mL vs 0.68 ± 0.51 pg/mL, P = 0.003), TGF-β1 (22.82 ± 4.99 ng/mL vs 10.03 ± 2.25 ng/mL, P = 0.031), IL-6 [2.08 (1.51-4.69) pg/mL vs 0.00 pg/mL, P < 0.001], and IL-17A [14.50 (8.55-41.65) pg/mL vs 0.00 (0.00-0.05) pg/mL, P < 0.001]. Furthermore, circulating IL-17A was higher in lcSSc as opposed to dcSSc subset (31.99 ± 13.29 pg/mL vs 7.14 ± 3.01 pg/mL, P = 0.008). Within the dcSSc subset, raised levels of IL-17A and IL-6 were detected vs healthy controls: IL-17A [2.60 (0.45-9.80) pg/mL vs 0.00 (0.00-0.05) pg/mL, P < 0.001], IL-6 [2.80 (1.03-7.23) pg/mL vs 0.00 pg/mL, P < 0.001].
| Cytokine | SSc patients | Healthy controls | P value |
| IL-10, pg/mL | 2.83 ± 0.44 (0.10-6.90) | 0.68 ± 0.51 (0.00-5.20) | 0.008 |
| IL-17A, pg/mL | 6.30 [2.50-15.60] (0.20-124.90) | 0.00 [0.00-0.05] (0.00-1.36) | < 0.001 |
| TGF-β1, ng/mL | 19.94 ± 3.35 (0-52.80) | 10.03 ± 2.25 (1.16-21.80) | 0.02 |
| IL-6, pg/mL | 2.10 [1.05-4.60] (0.45-198.10) | 0 (0.00-0.27) | < 0.001 |
The findings on circulating cytokines regarding the comparison of the two SSc phenotypes and vs healthy controls are depicted in Figure 4.
Patients were divided in two groups depending on the disease activity. Sixteen patients had active disease, while eight patients were with stable/inactive SSc (Table 1). We identified no differences between the two groups, regarding Tregs, Th17 cells and levels of the serum soluble cytokines.
The distribution of the patients according to the stage of SSc was as follows: Early SSc, n
Twelve patients enrolled in the study had visceral organ involvement, the distribution was as follows: Pulmonary arterial hypertension (PAH), n
For the purposes of our study, we used PHA-M to activate resting T cells. PHA is a classical mitogen leading to selective nonspecific T-cell activation and proliferation[40]. In the mid-1970s, it was found that T-cell proliferation induced by PHA requires the presence of monocytes. Ceuppens et al[41] confirmed this statement and identified that the addition of purified human IL-6, along with monocytic supernatant, to PHA-stimulated cell cultures has led to effective T-cell activation and proliferation.
Aiming to approach our study to the conditions in vivo, we used heparinized venous blood samples. Moreover, we identified increased serum levels of IL-6 in our SSc patients, which as previously mentioned, is a factor involved in T-cell activation. In the PHA - stimulated samples, we detected a decreased percentage of CD3+CD69+ cells in patients when compared to healthy controls.
The circulating cytokine profile in our SSc patients might relate to the decreased ability of T cells to be activated. Our data has revealed increased levels of IL-10, TGF-β, and IL-6 in peripheral blood of SSc patients and all these cytokines are engaged directly or not in the process of suppression of T-cell activation.
IL-10 is a pleiotropic cytokine with important anti-inflammatory and immunoregulatory functions, which inhibits the activity of Th1 cells[42,43]. Along with the tolerogenic dendritic cells and Treg subsets, other immunocompetent cells secreting IL-10 has been studied, including B cells, NK cells, neutrophils, and macrophages. The role of Th2 cells that produce IL-10 is also well-established[44]. However, recent data have paradoxically demonstrated that Th1 and Th17 cells are also able to secrete IL-10. It is thought that these “double-natured” T cell subsets use the secretion of IL-10 to suppress their own proinflammatory activity, directly, or in concert with tolerogenic antigen-presenting cells[43]. Some studies suggest that IL-10 (alone or in combination with IFN-γ) also has an inhibitory function regarding the fibrotic process in SSc[45]. Based on the literature, IL-6 inhibits the differentiation of monocytes in dendritic cells alone or through induced autocrine secretion of IL-10[46,47]. Likewise, both IL-6 and IL-10 restrain the antigen-presenting function of dendritic cells, which ultimately results in a formation of immature tolerogenic myeloid cells secreting IL-10 and their antigen-presenting capacity results in T lymphocytes’ anergy[48]. Along with IL-10, TGF-β also exerts an inhibitory action on T cells. TGF-β inhibits the IL-2 promoter/enhancer activity, which results in a block of IL-2 gene expression in T cells[49].
TGF-β inhibitory effect on T cells may be mediated through up-regulation of cyclin-dependent kinase inhibitors p15, p21, and p27 expression[50] and down-regulation of C-myc, cyclin D2, and cyclin E expression, too[51]. The concept for the suppressive role of the circulating cytokine milieu in SSc, regarding the T-cell activation, is in agreement with data reporting inhibited activation of Tregs from healthy donors or SSc by SSc plasma[10].
On the other hand, the peripheral T cell anergy upon PHA-stimulation in our SSc patients may be due to the immunosuppressive therapy administered. Most of the patients enrolled in the study were under treatment with glucocorticoids (GCs) (Table 1).
Normally, stimulation of T cells by cross-linking of both T-cell receptor TCR/CD3 and CD28 up-regulates both the nuclear factor of activated T cells (NFAT) and activating protein 1 (AP-1) transcription factors, resulting in increased transcription of the interleukin-2 (IL-2) gene and activation[52]. One of the important genomic mechanisms of GC action includes the interaction of activated cytosolic GC receptor (cGCR) monomers with transcription factors. The GC/cGCR complex modulates the activity of AP-1, NFAT, and NF-κB (nuclear factor-kB)[53]. The inhibition of their nuclear translocation and function leads to blockage of the expression of many proinflammatory cytokines, e.g., IL-2, IL-6, TNF-α[54]. This genomic mechanism of GC action may explain the decreased percentage of peripheral CD4+CD25+ cells in our SSc patients compared to healthy subjects, bearing in mind that CD25 along with a marker for T cell activation is an IL-2 receptor alpha chain as well. Moreover, we found decreased peripheral CD4+CD25+ cells in dcSSc patients, all of which had been under treatment with methylprednisolone.
Based on our results, we are not able to answer unconditionally to the question who exactly is responsible for the decreased activation capacity of T cells in SSc patients - the therapeutic regiment, cytokines, both of them or perhaps, additional factors get involved.
One of the most considerable findings of our study is the increased percentage of Th17 cells and the elevated serum levels of their respective cytokine, IL-17.
Many papers have reported a higher frequency of Th17 cells in the peripheral blood of SSc patients as opposed to healthy controls[13-15,30] which corresponds to our results.
Overproduction of IL-17 by T cells in the peripheral blood and in both skin and lung Kurasawa et al[18] have described overproduction of IL-17 by T cells in the peripheral blood and in both skin and lung lesions from SSc patients. These results suggest the central role that IL-17 overproduction plays in the pathogenesis of SSc, especially in the early stages of the disease, by enhancing the fibroblast proliferation and the production of IL-1 and the expression of adhesion molecules on endothelial cells[18]. Our data have not revealed any difference in the level of serum IL-17, regarding the stage of SSc. However, we describe for the very first time elevated serum levels of IL-17 in patients with lcSSc when compared to the dcSSc phenotype.
Even though IL-17 enhances the fibroblast proliferation, this cytokine does not induce collagen production in dermal fibroblasts, but rather decreases the ability of TGF-β to activate them. Furthermore, the number of IL-17 positive cells in SSc skin has been reported to correlate inversely with the extent of global skin thickness[27]. Thus, in humans IL-17 may instead act as an antifibrotic inflammatory mediator. It is worth mentioning that prostanoids currently used to treat SSc vasculopathy, including prostaglandin I2, increase in vivo the number of Th17 cells[55]. Therefore they could be beneficial to the vascular compartment, particularly to endothelial cells, and might be crucial for the modulation of the inflammatory response.
Whether Th17 cells and IL-17 might have indirect pro-fibrotic effects via interaction with endothelial/epithelial cells or via the enhanced production of pro-angiogenic factors, such as IL-8, CCL-2, remains to be investigated. Similarly, the role of Th17 cells in autoantibody generation in SSc has not been investigated so far. However, in animal studies IL-17 has been shown to promote autoantibody generation in BXD2 mice by orchestrating the spontaneous formation of autoreactive germinal centers[56].
Recent data has revealed that IL-6 plays an important role in the regulation of the balance between IL-17-producing Th17 cells and Tregs[30,35]. Our results demonstrate increased serum levels of IL-6 in both of lcSSc and dcSSc patients compared to controls with no difference between the two clinical subsets. IL-6 in concert with TGF-β induces the expression of RORγt in naïve T cells, transforming them in Th17 cells; in contrast, IL-6 inhibits TGF-β-induced Treg differentiation[57].
Even though Th17 cells are crucial in the modulation of the inflammatory response, the Treg subset might also play a central role in the pathogenesis of SSc. Our results demonstrate nonsignificant increase in CD4+Foxp3+ Tregs in SSc patients when compared to controls and no difference between patients and healthy individuals regarding the percentage of CD4+CD25+FoxP3+ Tregs. There is controversial data in literature concerning the Treg numerical and functional alterations in SSc. Some of the papers have announced elevated circulating CD4+CD25+Foxp3+ Treg cells[10,28] particularly in active and severe disease[29]. Besides the up-regulation, Tregs from SSc patients demonstrate a defective suppressive capacity, which has been reported to correlate with a diminished CD69 expression and TGF-β levels[10]. One study has not detected any differences between SSc patients and control groups[15]. Finally, several studies have demonstrated a decreased frequency/impaired function of Tregs in SSc[30-32].
However, our data reveals an increased percentage of CD4+CD25-Foxp3+ cells in our dcSSc patients in comparison with the healthy controls. Recent studies have reported up-regulated CD25 negative CD4+Foxp3+ cells in the peripheral blood of patients with systemic lupus erythematosus (SLE)[58-60]. Both CD4+CD25-Foxp3+ T cells and CD4+CD25+ Foxp3+ Treg cells from SLE patients have demonstrated a similar pattern regarding the expression of CD62L, CD95, GITR, CD127, and CTLA-4, which are typical markers for the Treg phenotype[61]. A considerable suppressive activity of CD4+CD25-Foxp3+ cells, comparable to the suppressive capacity exerted by the classical Tregs (CD4+CD25+Foxp3+ cells) has been reported[62]. According to another hypothesis, CD4+CD25-Foxp3+ T cells subset could represent a peripheral reservoir of the CD4+CD25+ Foxp3+ Treg cell subset[61]. In case of autoimmune reactivation, such as in SLE patients, CD25 negative Foxp3+ T cells could regain the expression of CD25, trying to reverse a homeostatic imbalance shift to more aggressive expansion of autoreactive T cells and B cells[61]. However, another paper have considered CD4+CD25-Foxp3+ cells as functionally incompetent in SLE[63].
The GC treatment of our dcSSc patients could also unravel the up-regulated peripheral CD4+CD25-Foxp3+ cells that we have found. The CD4+CD25-Foxp3+cell subset has been reported increased in patients with rheumatoid arthritis treated with GCs and have correlated inversely with the disease parameters[64]. GC-treated patient carriers of the high IL-10 genotype demonstrated higher levels of CD4+CD25-Foxp3+ cells, which finding corresponds to our results.
In conclusion, our study demonstrates a decreased capacity for PHA-induced peripheral T-cells activation in patients with SSc. We also describe for the first time an up-regulated percentage of CD4+CD25-FoxP3+ cells in patients with dcSSc. Regarding the circulating cytokine profile in SSc, we originally identify increased serum levels of IL-17 in lcSSc as opposed to patients with dcSSc phenotype. The rest of our data, concerning the elevated circulating IL-6, IL-10, and TGF in SSc, confirms literature-based results.
Systemic sclerosis (SSc) is a generalized debilitating connective tissue disease affecting the skin and internal organs characterized by vasculopathy, fibrosis, and autoimmune alterations. The autoimmune dysregulation in SSc comprises lymphocyte activation that leads to the generation of autoantibodies, abnormal production of cytokines and chemokines, and impairment of the innate immunity. Over the last decade, the accumulating data has shown the central role of T lymphocytes in the pathogenesis of SSc. There is strong evidence in literature for altered T-cell activation and T helper cells abnormalities in SSc.
There is accumulating data for numerical and functional alterations of Tregs and Th17 cells in patients with SSc. However, a functional heterogeneity exists between the T lymphocytes in the peripheral blood of patients with SSc and the corresponding T cell subsets in skin lesions or internal organs. The cytokine production by T cells affects the function of fibroblasts and endothelial cells, thereby influencing the vascular disease progression and the fibrosis development. Many efforts have been made to identify the cytokine patterns in SSc. Nevertheless, important issues remain unresolved, among them, identification of the trigger of the autoimmune response in SSc and the immunological differences between the dcSSc and lcSSc.
This is the first study demonstrating an up-regulated percentage of CD4+CD25-FoxP3+ cells in patients with dcSSc as compared to healthy subjects. Another of the original contributions of this research demonstrates a decreased capacity for PHA-induced peripheral T-cells activation in patients with SSc. Regarding the peripheral cytokine profile in SSc, this research group describes for the first time elevated serum levels of IL-17A in the lcSSc as opposed to the dcSSc subset of the disease.
It is likely that the altered percentage of Th17 and CD4+CD25-FoxP3+ cells may play a key role in the disease progression along with the peripheral cytokine profile in SSc patients.
SSc is an abbreviation for systemic sclerosis as well as lcSSc and dcSSc are abbreviations for the limited cutaneous and the diffuse cutaneous subsets of the disease. Tregs represent the T regulatory lymphocytes (CD4+FoxP3+ cells), a T helper cell subset which is crucial for the establishment of immunological self-tolerance and for the prevention of autoimmunity.
The study represents an interesting continuum to the research series towards unveiling the immunological profile in SSc.
| 1. | Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117:557-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 792] [Cited by in RCA: 884] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 2. | Mayes M, Assassi S. Classification and epidemiology of scleroderma. Rheumatology. Philadelphia: Mosby, ELSEVIER 2015; 1153-1158. |
| 3. | Medsger TA, Bombardieri S, Czirjak L, Scorza R, Della Rossa A, Bencivelli W. Assessment of disease severity and prognosis. Clin Exp Rheumatol. 2003;21:S42-S46. [PubMed] |
| 4. | Domsic RT, Rodriguez-Reyna T, Lucas M, Fertig N, Medsger TA. Skin thickness progression rate: a predictor of mortality and early internal organ involvement in diffuse scleroderma. Ann Rheum Dis. 2011;70:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 5. | Chizzolini C, Brembilla NC, Montanari E, Truchetet ME. Fibrosis and immune dysregulation in systemic sclerosis. Autoimmun Rev. 2011;10:276-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | van Bon L, Cossu M, Radstake TR. An update on an immune system that goes awry in systemic sclerosis. Curr Opin Rheumatol. 2011;23:505-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Lafyatis R, York M. Innate immunity and inflammation in systemic sclerosis. Curr Opin Rheumatol. 2009;21:617-622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | O’Reilly S, Hügle T, van Laar JM. T cells in systemic sclerosis: a reappraisal. Rheumatology (Oxford). 2012;51:1540-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 9. | Brembilla NC, Chizzolini C. T cell abnormalities in systemic sclerosis with a focus on Th17 cells. Eur Cytokine Netw. 2012;23:128-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Radstake TR, van Bon L, Broen J, Wenink M, Santegoets K, Deng Y, Hussaini A, Simms R, Cruikshank WW, Lafyatis R. Increased frequency and compromised function of T regulatory cells in systemic sclerosis (SSc) is related to a diminished CD69 and TGFbeta expression. PLoS One. 2009;4:e5981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Mathian A, Parizot C, Dorgham K, Trad S, Arnaud L, Larsen M, Miyara M, Hié M, Piette JC, Frances C. Activated and resting regulatory T cell exhaustion concurs with high levels of interleukin-22 expression in systemic sclerosis lesions. Ann Rheum Dis. 2012;71:1227-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Kalogerou A, Gelou E, Mountantonakis S, Settas L, Zafiriou E, Sakkas L. Early T cell activation in the skin from patients with systemic sclerosis. Ann Rheum Dis. 2005;64:1233-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Truchetet ME, Brembilla NC, Montanari E, Allanore Y, Chizzolini C. Increased frequency of circulating Th22 in addition to Th17 and Th2 lymphocytes in systemic sclerosis: association with interstitial lung disease. Arthritis Res Ther. 2011;13:R166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 14. | Radstake TR, van Bon L, Broen J, Hussiani A, Hesselstrand R, Wuttge DM, Deng Y, Simms R, Lubberts E, Lafyatis R. The pronounced Th17 profile in systemic sclerosis (SSc) together with intracellular expression of TGFbeta and IFNgamma distinguishes SSc phenotypes. PLoS One. 2009;4:e5903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Rodríguez-Reyna TS, Furuzawa-Carballeda J, Cabiedes J, Fajardo-Hermosillo LD, Martínez-Reyes C, Díaz-Zamudio M, Llorente L. Th17 peripheral cells are increased in diffuse cutaneous systemic sclerosis compared with limited illness: a cross-sectional study. Rheumatol Int. 2012;32:2653-2660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3754] [Cited by in RCA: 3881] [Article Influence: 228.3] [Reference Citation Analysis (0)] |
| 17. | Hemdan NY, Birkenmeier G, Wichmann G, Abu El-Saad AM, Krieger T, Conrad K, Sack U. Interleukin-17-producing T helper cells in autoimmunity. Autoimmun Rev. 2010;9:785-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Kurasawa K, Hirose K, Sano H, Endo H, Shinkai H, Nawata Y, Takabayashi K, Iwamoto I. Increased interleukin-17 production in patients with systemic sclerosis. Arthritis Rheum. 2000;43:2455-2463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593-2603. [PubMed] |
| 20. | Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483-5486. [PubMed] |
| 21. | Yamamoto T, Eckes B, Hartmann K, Krieg T. Expression of monocyte chemoattractant protein-1 in the lesional skin of systemic sclerosis. J Dermatol Sci. 2001;26:133-139. [PubMed] |
| 22. | Brembilla NC, Montanari E, Truchetet ME, Raschi E, Meroni P, Chizzolini C. Th17 cells favor inflammatory responses while inhibiting type I collagen deposition by dermal fibroblasts: differential effects in healthy and systemic sclerosis fibroblasts. Arthritis Res Ther. 2013;15:R151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Nakashima T, Jinnin M, Yamane K, Honda N, Kajihara I, Makino T, Masuguchi S, Fukushima S, Okamoto Y, Hasegawa M. Impaired IL-17 signaling pathway contributes to the increased collagen expression in scleroderma fibroblasts. J Immunol. 2012;188:3573-3583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 24. | Gasse P, Riteau N, Vacher R, Michel ML, Fautrel A, di Padova F, Fick L, Charron S, Lagente V, Eberl G. IL-1 and IL-23 mediate early IL-17A production in pulmonary inflammation leading to late fibrosis. PLoS One. 2011;6:e23185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 174] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 25. | Okamoto Y, Hasegawa M, Matsushita T, Hamaguchi Y, Huu DL, Iwakura Y, Fujimoto M, Takehara K. Potential roles of interleukin-17A in the development of skin fibrosis in mice. Arthritis Rheum. 2012;64:3726-3735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 26. | Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, Cheever AW, Wynn TA. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med. 2010;207:535-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 476] [Cited by in RCA: 622] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 27. | Truchetet ME, Brembilla NC, Montanari E, Lonati P, Raschi E, Zeni S, Fontao L, Meroni PL, Chizzolini C. Interleukin-17A+ cell counts are increased in systemic sclerosis skin and their number is inversely correlated with the extent of skin involvement. Arthritis Rheum. 2013;65:1347-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 28. | Giovannetti A, Rosato E, Renzi C, Maselli A, Gambardella L, Giammarioli AM, Palange P, Paoletti P, Pisarri S, Salsano F. Analyses of T cell phenotype and function reveal an altered T cell homeostasis in systemic sclerosis. Correlations with disease severity and phenotypes. Clin Immunol. 2010;137:122-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Slobodin G, Ahmad MS, Rosner I, Peri R, Rozenbaum M, Kessel A, Toubi E, Odeh M. Regulatory T cells (CD4(+)CD25(bright)FoxP3(+)) expansion in systemic sclerosis correlates with disease activity and severity. Cell Immunol. 2010;261:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Fenoglio D, Battaglia F, Parodi A, Stringara S, Negrini S, Panico N, Rizzi M, Kalli F, Conteduca G, Ghio M. Alteration of Th17 and Treg cell subpopulations co-exist in patients affected with systemic sclerosis. Clin Immunol. 2011;139:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 31. | Papp G, Horvath IF, Barath S, Gyimesi E, Sipka S, Szodoray P, Zeher M. Altered T-cell and regulatory cell repertoire in patients with diffuse cutaneous systemic sclerosis. Scand J Rheumatol. 2011;40:205-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Antiga E, Quaglino P, Bellandi S, Volpi W, Del Bianco E, Comessatti A, Osella-Abate S, De Simone C, Marzano A, Bernengo MG. Regulatory T cells in the skin lesions and blood of patients with systemic sclerosis and morphoea. Br J Dermatol. 2010;162:1056-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 33. | Yamagiwa S, Gray JD, Hashimoto S, Horwitz DA. A role for TGF-beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J Immunol. 2001;166:7282-7289. [PubMed] |
| 34. | Hasegawa M, Fujimoto M, Kikuchi K, Takehara K. Elevated serum levels of interleukin 4 (IL-4), IL-10, and IL-13 in patients with systemic sclerosis. J Rheumatol. 1997;24:328-332. [PubMed] |
| 35. | Sato S, Hasegawa M, Takehara K. Serum levels of interleukin-6 and interleukin-10 correlate with total skin thickness score in patients with systemic sclerosis. J Dermatol Sci. 2001;27:140-146. [PubMed] |
| 36. | Alamanos Y, Tsifetaki N, Voulgari PV, Siozos C, Tsamandouraki K, Alexiou GA, Drosos AA. Epidemiology of systemic sclerosis in northwest Greece 1981 to 2002. Semin Arthritis Rheum. 2005;34:714-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Radić M, Martinović Kaliterna D, Fabijanić D, Radić J. Prevalence of systemic sclerosis in Split-Dalmatia county in Southern Croatia. Clin Rheumatol. 2010;29:419-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, Matucci-Cerinic M, Naden RP, Medsger TA, Carreira PE. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013;65:2737-2747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1745] [Cited by in RCA: 2382] [Article Influence: 183.2] [Reference Citation Analysis (0)] |
| 39. | Valentini G, Iudici M, Walker UA, Jaeger VK, Baron M, Carreira P, Czirják L, Denton CP, Distler O, Hachulla E. The European Scleroderma Trials and Research group (EUSTAR) task force for the development of revised activity criteria for systemic sclerosis: derivation and validation of a preliminarily revised EUSTAR activity index. Ann Rheum Dis. 2017;76:270-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 165] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 40. | Potter MR, Moore M. PHA stimulation of separated human lymphocyte populations. Clin Exp Immunol. 1975;21:456-467. [PubMed] |
| 41. | Ceuppens JL, Baroja ML, Lorre K, Van Damme J, Billiau A. Human T cell activation with phytohemagglutinin. The function of IL-6 as an accessory signal. J Immunol. 1988;141:3868-3874. [PubMed] |
| 42. | Ng TH, Britton GJ, Hill EV, Verhagen J, Burton BR, Wraith DC. Regulation of adaptive immunity; the role of interleukin-10. Front Immunol. 2013;4:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 259] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 43. | O’Garra A, Vieira P. T(H)1 cells control themselves by producing interleukin-10. Nat Rev Immunol. 2007;7:425-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 468] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 44. | Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, Jack RS, Wunderlich FT, Brüning JC, Müller W. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34:566-578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 763] [Cited by in RCA: 774] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 45. | Sziksz E, Pap D, Lippai R, Béres NJ, Fekete A, Szabó AJ, Vannay Á. Fibrosis Related Inflammatory Mediators: Role of the IL-10 Cytokine Family. Mediators Inflamm. 2015;2015:764641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 211] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 46. | Ivanova-Todorova E, Bochev I, Mourdjeva M, Dimitrov R, Bukarev D, Kyurkchiev S, Tivchev P, Altunkova I, Kyurkchiev DS. Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunol Lett. 2009;126:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 47. | Blanco P, Palucka AK, Pascual V, Banchereau J. Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev. 2008;19:41-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 406] [Cited by in RCA: 390] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 48. | Kyurkchiev D, Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K, Kyurkchiev S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells. 2014;6:552-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 408] [Cited by in RCA: 481] [Article Influence: 40.1] [Reference Citation Analysis (4)] |
| 49. | Brabletz T, Pfeuffer I, Schorr E, Siebelt F, Wirth T, Serfling E. Transforming growth factor beta and cyclosporin A inhibit the inducible activity of the interleukin-2 gene in T cells through a noncanonical octamer-binding site. Mol Cell Biol. 1993;13:1155-1162. [PubMed] |
| 50. | Voss M, Wolff B, Savitskaia N, Ungefroren H, Deppert W, Schmiegel W, Kalthoff H, Naumann M. TGFbeta-induced growth inhibition involves cell cycle inhibitor p21 and pRb independent from p15 expression. Int J Oncol. 1999;14:93-101. [PubMed] |
| 51. | Warner BJ, Blain SW, Seoane J, Massagué J. Myc downregulation by transforming growth factor beta required for activation of the p15(Ink4b) G(1) arrest pathway. Mol Cell Biol. 1999;19:5913-5922. [PubMed] |
| 52. | Peterson EJ, Maltzman JS, Koretzky GA. T-cell activation and tolerance. Clinical Immunology: principles and practice. Saunders: ELSEVIER 2012; 160-171. |
| 53. | Buttgereit F, Seibel M JH, Bijlsma J WJ. In: Rich RR, Fleisher TA, Shearer WT, Schroeder H, Frew AJ, Weyand CM. Clinical Immunology: principles and practice. Saunders: ELSEVIER 2012; 1066-1076. |
| 54. | Buttgereit F, Saag KG, Cutolo M, da Silva JA, Bijlsma JW. The molecular basis for the effectiveness, toxicity, and resistance to glucocorticoids: focus on the treatment of rheumatoid arthritis. Scand J Rheumatol. 2005;34:14-21. [PubMed] |
| 55. | Truchetet ME, Allanore Y, Montanari E, Chizzolini C, Brembilla NC. Prostaglandin I(2) analogues enhance already exuberant Th17 cell responses in systemic sclerosis. Ann Rheum Dis. 2012;71:2044-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 56. | Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, Yi J, Guentert T, Tousson A, Stanus AL. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9:166-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 583] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 57. | Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641-649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1075] [Cited by in RCA: 1073] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 58. | Lin SC, Chen KH, Lin CH, Kuo CC, Ling QD, Chan CH. The quantitative analysis of peripheral blood FOXP3-expressing T cells in systemic lupus erythematosus and rheumatoid arthritis patients. Eur J Clin Invest. 2007;37:987-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 59. | Zhang B, Zhang X, Tang FL, Zhu LP, Liu Y, Lipsky PE. Clinical significance of increased CD4+CD25-Foxp3+ T cells in patients with new-onset systemic lupus erythematosus. Ann Rheum Dis. 2008;67:1037-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 60. | Suen JL, Li HT, Jong YJ, Chiang BL, Yen JH. Altered homeostasis of CD4(+) FoxP3(+) regulatory T-cell subpopulations in systemic lupus erythematosus. Immunology. 2009;127:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 61. | Yan B, Liu Y. The Nature of Increased Circulating CD4CD25Foxp3 T Cells in Patients with Systemic Lupus Erythematosus: A Novel Hypothesis. Open Rheumatol J. 2009;3:22-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 62. | Curotto de Lafaille MA, Lafaille JJ. CD4(+) regulatory T cells in autoimmunity and allergy. Curr Opin Immunol. 2002;14:771-778. [PubMed] |
| 63. | Walker LS. Regulatory T cells overturned: the effectors fight back. Immunology. 2009;126:466-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 64. | de Paz B, Prado C, Alperi-López M, Ballina-García FJ, Rodriguez-Carrio J, López P, Suárez A. Effects of glucocorticoid treatment on CD25+FOXP3+ population and cytokine-producing cells in rheumatoid arthritis. Rheumatology (Oxford). 2012;51:1198-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Bulgaria
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Guo ZS, Mohammed RHA S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ