Published online Aug 20, 2017. doi: 10.5493/wjem.v7.i3.78
Peer-review started: March 29, 2017
First decision: April 17, 2017
Revised: May 26, 2017
Accepted: June 12, 2017
Article in press: June 13, 2017
Published online: August 20, 2017
Processing time: 142 Days and 14.5 Hours
The incidence of spinal cord injury (SCI) has been gradually increasing, and the treatment has troubled the medical field all the time. Primary and secondary injuries ultimately lead to nerve impulse conduction block. Microglia and astrocytes excessively accumulate and proliferate to form the glial scar. At present, to reduce the effect of glial scar on nerve regeneration is a hot spot in the research on the treatment of SCI. According to the preliminary experiments, we would like to provide a new bionic spinal cord to reduce the negative effect of glial scar on nerve regeneration. In this hypothesis we designed a new scaffold that combine the common advantage of acellular scaffold of spinal cord and thermosensitive gel, which could continue to release exogenous basic fibroblast growth factor (BFGF) in the spinal lesion area on the basis of BFGF modified thermosensitive gel. Meanwhile, the porosity, pore size and material of the gray matter and white matter regions were distinguished by an isolation layer, so as to induce the directed differentiation of cells into the defect site and promote regeneration of spinal cord tissue.
Core tip: Traumatic spinal cord injury often leads to serious consequences and also adds great burden to families and society. Usually people believe that the regeneration of lost tissue is limited after central nervous system injury. Due to these reasons, we would like to provide a new bionic spinal cord to reduce the negative effect of glial scar on nerve regeneration. We design biomimetic spinal cord by the combination of basic fibroblast growth factor modified thermosensitive hydrogel and acellular spinal cord scaffold, which is conducive to the designation of a three-dimensional composite scaffold more suitable for cell growth, and corresponding mechanical properties and biodegradability more close to the structure of normal spinal cord.
- Citation: Liu Y, Li Q, Zhang B, Ban DX, Feng SQ. Multifunctional biomimetic spinal cord: New approach to repair spinal cord injuries. World J Exp Med 2017; 7(3): 78-83
- URL: https://www.wjgnet.com/2220-315X/full/v7/i3/78.htm
- DOI: https://dx.doi.org/10.5493/wjem.v7.i3.78
Spinal cord injury (SCI) is a central nervous system disease that is mainly manifested in sensory-motor dysfunction, incontinence and sexual dysfunction below the plane of SCI[1]. Clinically, trauma-caused SCI is common. Rehabilitation of SCI is an unsolved medical problem, in that the regeneration ability of the human central nervous system is extremely low, and a variety of pathophysiological activities and metabolites are involved in the changes in the microenvironment at the injured site, which is not conducive to axonal regeneration.
Repair of SCI with nerve tissue engineering aims at repairing the injured nerve by loading seed cells into the injured site with scaffold as carrier or implanting new tissue[2]. But it is difficult to repair SCI by tissue engineering, possible reason may be that its regenerative capacity is much lower than that of peripheral nerves, the structure of the spinal cord is complex at the same time[3]. Due to the complexity of the structure and composition of the human spinal cord, traditional single scaffold for spinal cord cannot completely simulate the macro and micro structure of the spinal cord; therefore, the development of bionic spinal cord has become a hot research topic.
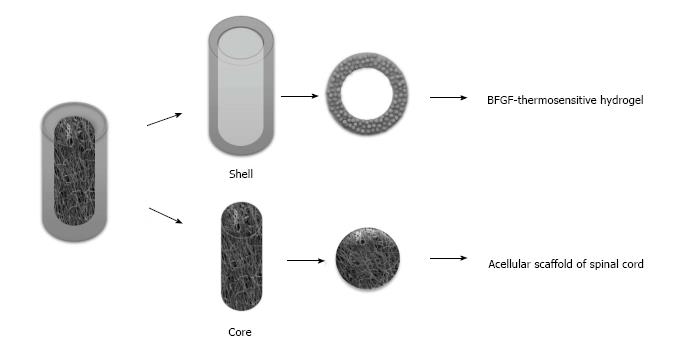
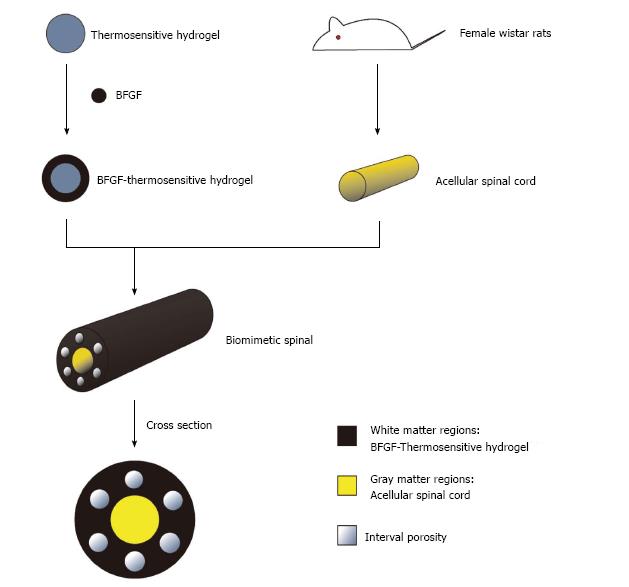
It is difficult to repair SCI by routine tissue engineering scaffolds because of the spinal cord's low regeneration ability and its complex structure, and the traditional single spinal cord cannot simulate the macro and micro structure of the spinal cord. For the above reasons and the basis of the present work, a tissue-engineered spinal cord was designed in this hypothesis by combining the common advantage of acellular scaffold of spinal cord and thermosensitive gel, which could continue to release exogenous basic fibroblast growth factor (BFGF) in the spinal lesion area on the basis of BFGF modified thermosensitive gel, meanwhile, the porosity, pore size and material of the gray matter and white matter regions were distinguished by an isolation layer (Figure 1), so as to induce the directed differentiation of cells into the defect site and promote regeneration of spinal cord tissue.
Mechanical violence in acute SCI includes traction and compression. Direct compression is caused by spinal fracture and dislocation, intervertebral disc and ligament injury, leading to vascular damage, axonal degeneration and disintegration, the apoptosis of neurons, astrocytes and oligodendrocytes, etc[4]. Slight bleeding occurs in grey matter within several minutes after injury; within a few hours, the injury rapidly spreads to the upper and lower segments of the injured spinal cord along the axial direction. Several minutes after injury, when spinal cord swelling constricts the central canal and the pressure in the spinal cord exceeds the pressure in blood vessels, local secondary ischemia occurs. Moreover, neurogenic shock after the injury aggravates spinal cord ischemia, which further causes hypoxia and leads tissues to produce and release toxic products, resulting in a series of effects of cascade and amplification damage. There are some important cellular responses after SCI. For example, astrocytes divide and proliferate to “scar-like” astrocytes; the myelin sheath splits into fragments; precursor cells of microglias and oligodendrocytes proliferate and migrate to the site of injury. Therefore, gliacytes, astrocytes, oligodendrocytes, oligodendroglia, precursor cells and microglias are detected at the site of injury. In addition, these cells have an inhibitory effect on axonal regeneration. Mature oligodendrocytes produce nogo and MAG, and the precursor cells of oligodendrocytes produce proteoglycans and NG2, which are all inhibitory molecules[5,6]. Astrocytes may promote axon growth in non-injured CNS and immediately after the injury; however, several days after the injury, they begin to produce a series of inhibitory proteoglycans. Generally, microglias play a role in the promotion of axonal regeneration, but produce various toxins to kill neurons and damaged axons after stimulation[7]. Due to considerable inhibitory molecules, the application of the therapy with all these molecules neutralized is quite difficult.
The internal structure of the spinal cord is composed of gray matter and white matter. Located in the center of the spinal cord, the gray matter is shaped as a symmetrical butterfly seen in cross-section, composed of various neural cells. The gray matter can be divided into anterior, lateral and posterior horns. There are a large number of motor neurons in the anterior horn. The lateral horn contains sympathetic nerve cells and the posterior horn contains sensory nerve cells. Composed of longitudinal nerve fibers for conduction, the white matter is located around the gray matter. These nerve fibers are mainly composed of corticospinal tracts, namely the motor nerve fibers for the conduction from the brain to the spinal cord, and thalamic tracts, namely the sensory nerve fibers for the conduction from the spinal cord to the brain. The design idea of the partition-type artificial spinal cord is to correctly guide the regeneration and extending of the main descending fiber tracts according to the original position of the spinal cord[8-10]. And the idea also intends to adjust the deacetylation degrees of chitosan in the outer wall of the catheter and of partition chitosan between the partitions in the catheter in the chitosan production, in order to make the partition chitosan degrade in a short time after the beginning of spinal cord regeneration and facilitate the regenerated spinal cord to horizontally form a neural network. And the outer wall of the catheter should degrade after the spinal cord regeneration to block the invasion of foreign non-nerve tissues.
Hydrogel materials are characterized by high water content and similar mechanical properties to collagen in the spinal cord, which is the major structural protein of human. As an important component of extracellular matrix, collagen in the spinal cord has a gene sequence of arginine-glycine-aspartic acid with cell adhesion signal, which promotes the adhesion of seed cells to scaffold, and the differentiation and migration of seed cells. The axons of the organism are favorable for the attachment to the collagen scaffold, and thereby promoting the regeneration of axons[11]. In the site of SCI, collagen can also carry growth factors to regulate the local microenvironment and reduce scar formation, which is conducive to the recovery of the injury. So hydrogel materials are often used in the implantation of scaffold into the spinal cord. However, these regenerated nerve fibers are disorganized, and collagen scaffold cannot lead regenerated nerve fibers to caudal tissue through the injured site to form complete neural pathway[12]. It has also been reported that an overly high collagen concentration in the injured site inhibits the growth of axons.
As a neuropeptide substance, BFGF plays an important role in embryonic development, angiogenesis, wound healing, and the growth and development of nervous system in the organism, and is a novel neurotrophic factor that has been frequently studied in recent years[13]. Moreover, BFGF not only has a nutritional effect on a variety of neurons cultured in vitro, but also can promote the regeneration of injured peripheral nerve in vivo, which has been evidenced by studies. Research has demonstrated that the expression of c-fos mRNA in spinal cord neurons increases, while BFGF inhibits the expression of c-fos gene after SCI, suggesting that BFGF may have a protective effect on nerve in SCI. Haenzi et al[14] have found that after SCI, early continuous administration of exogenous BFGF may play an important role in the protection of the area of SCI, promoting the recovery of spinal cord function. Furthermore, research has demonstrated that after SCI, early continuous administration of exogenous BFGF may significantly protect the area of SCI, significantly decrease calcium accumulation and edema in the injured area, decrease magnesium ion loss and its degeneration, obviously alleviate SCI, and enhance the recovery of spinal cord function.
Acellular allogenic grafts is a tissue scaffolds produced by artificial extraction and decellularization, etc. It is widely used to substitute natural biomaterial scaffold in the studies of tissue repair[15]. The protein and other substances in the tissue were removed by chemical method. Then the antigen-free acellular tissue scaffold was obtained. This scaffold has the advantages of good biocompatibility, low immunogenicity, and it is convenient to manufacture. When implanted into the body, it can provide seed cells with the growth space similar to their in vivo niche. Fresh sciatic nerve was removed, then the cells and other parts of the sciatic nerve tissue was taken off by Triton X-100 and sodium deoxycholate through chemical extraction, and the fibrous skeleton as well as the basement membrane have been left. The loose three-dimensional porous structure left by the nerve cells can be viewed under the electron microscopy. This scaffold was transplanted into the body, and 20 d later, compared with the control group without extraction, the extracted groups contained more microvessels and nerve axons through the injury area. The motor function has been greatly improved in the extracted groups[16]. Hudson et al[17] and Rovak et al[18] subsequently demonstrated this scaffold causes little immunological rejection after transplantation in a large number of acellular nerve allografts in rodent. Hu et al[19] used the bone marrow stromal cells of acellular allogenic nerve grafts to repair long-segment ulnar nerve defects of a primate. The repair effect is similar to autologous transplantation in 6 mo after surgery[19]. Ban et al[20] frozen and thawed the spinal cord tissue, then prepared acellular spinal tissue scaffold by modified chemical extraction. The appearance of the scaffold is comparable to that of the normal spinal cord. It is in a translucent villous shape, and the axons of the tissue scaffold and the auxiliary cells are successfully removed, leaving the loose three-dimensional porous structure. Its flat structure is constituted by the different sized gaps which are longitudinally parallel or irregularly arranged in a channel-like way and are connected to each other with a high degree of emulation. These structures can provide a natural guide for the regeneration of the axon. Regenerated axons can effectively pass through the lesion area, so to provide the conditions for the coupling of regenerated nerve and terminal nerve tissue. Moreover, co-culture with neuronal cells has proved its excellent biocompatibility[20].
Although there are many advantages, acellular scaffold of spinal cord is difficult to undertake the second modification process. A variety of measures have been taken to try to regenerate the spinal cord nerve fibers, however, the result is that this kind of regeneration is a disordered growth or extension, and the repair effect is not ideal. Therefore, it is necessary to correctly guide the orderly extension of the regenerated nerve fibers in the specific division of the original fiber bundle so as to achieve better repair purposes[21]. Different configurations of scaffolds for tissue engineering affect the effect of nerve regeneration to a great extent, including the upstream and downstream fiber bundles on the macroscopic and microscopic axonal growth. Nevertheless, the scaffold material has pores, even single or multiple conduits at present, the location of these holes or catheters is random relative to the structure of the spinal cord, and not consistent with the histological structure of the gray and white matter of spinal cord, not to mention the correspondence with major fiber tracts in white matter[22]. In this regard, upstream and downstream bundles, which are distributed in the white matter of the spinal cord, are regenerated in the scaffold material, they can only grow in mismatched or even misplaced pipes or micropores, in this way, the regenerated nerve fibers can still not grow and extend regularly in the corresponding region, but grow in disorder, the upstream and downstream regenerated fibers are hence twisted into a group to affect the extension of other fibers or the migration of neurons, greatly affecting the recovery effect. Thus, the design and construction of configuration of the artificial scaffold material consistent with the gray and white matter of the spinal cord, as well as upstream and downstream fiber bundles of the white matter is one of the prerequisites for tissue engineering to repair SCI and also a key problem to be solved urgently, which may improve the repair effect of SCI significantly.
A single scaffold material is often difficult to have the ideal characteristics of spinal tissue scaffold material at the same time, the study of composite biomaterials made of two or more than two kinds of materials has hence become a hot topic in the research of spinal cord tissue engineering. Composite biomaterials can make up for the deficiency of single material and retain the characteristics of raw materials, which is conducive to the designation of a three-dimensional composite scaffold more suitable for cell growth, and corresponding mechanical properties and biodegradability more close to the structure of normal spinal cord (Figure 2). This method will provide new ideas for clinical treatment of SCI.
| 1. | Cao HQ, Dong ED. An update on spinal cord injury research. Neurosci Bull. 2013;29:94-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Sakiyama-Elbert S, Johnson PJ, Hodgetts SI, Plant GW, Harvey AR. Scaffolds to promote spinal cord regeneration. Handb Clin Neurol. 2012;109:575-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Prang P, Müller R, Eljaouhari A, Heckmann K, Kunz W, Weber T, Faber C, Vroemen M, Bogdahn U, Weidner N. The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials. 2006;27:3560-3569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Trofimenko V, Hotaling JM. Fertility treatment in spinal cord injury and other neurologic disease. Transl Androl Urol. 2016;5:102-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 5. | Kawano H, Kimura-Kuroda J, Komuta Y, Yoshioka N, Li HP, Kawamura K, Li Y, Raisman G. Role of the lesion scar in the response to damage and repair of the central nervous system. Cell Tissue Res. 2012;349:169-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 222] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 6. | Liu Y, Ban DX, Ma C, Zhang ZG, Zhang JY, Gao SJ, Feng SQ. Photodynamic therapy mediated by upconversion nanoparticles to reduce glial scar formation and promote hindlimb functional recovery after spinal cord injury in rats. J Biomed Nanotechnol. 2016;12:2063-2075. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Meletis K, Barnabé-Heider F, Carlén M, Evergren E, Tomilin N, Shupliakov O, Frisén J. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008;6:e182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 436] [Cited by in RCA: 511] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 8. | de Ramon Francàs G, Zuñiga NR, Stoeckli ET. The spinal cord shows the way - How axons navigate intermediate targets. Dev Biol. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Vagnoni A, Rodriguez L, Manser C, De Vos KJ, Miller CC. Phosphorylation of kinesin light chain 1 at serine 460 modulates binding and trafficking of calsyntenin-1. J Cell Sci. 2011;124:1032-1042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Ban DX, Kong XH, Feng SQ, Ning GZ, Chen JT, Guo SF. Intraspinal cord graft of autologous activated Schwann cells efficiently promotes axonal regeneration and functional recovery after rat’s spinal cord injury. Brain Res. 2009;1256:149-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Wang YH, Chen J, Zhou J, Nong F, Lv JH, Liu J. Reduced inflammatory cell recruitment and tissue damage in spinal cord injury by acellular spinal cord scaffold seeded with mesenchymal stem cells. Exp Ther Med. 2017;13:203-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Chen J, Zhang Z, Liu J, Zhou R, Zheng X, Chen T, Wang L, Huang M, Yang C, Li Z. Acellular spinal cord scaffold seeded with bone marrow stromal cells protects tissue and promotes functional recovery in spinal cord-injured rats. J Neurosci Res. 2014;92:307-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | van De Rijke F, Zijlmans H, Li S, Vail T, Raap AK, Niedbala RS, Tanke HJ. Up-converting phosphor reporters for nucleic acid microarrays. Nat Biotechnol. 2001;19:273-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 257] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 14. | Haenzi B, Gers-Barlag K, Akhoundzadeh H, Hutson TH, Menezes SC, Bunge MB, Moon LD. Overexpression of the Fibroblast Growth Factor Receptor 1 (FGFR1) in a Model of Spinal Cord Injury in Rats. PLoS One. 2016;11:e0150541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Guo SZ, Ren XJ, Wu B, Jiang T. Preparation of the acellular scaffold of the spinal cord and the study of biocompatibility. Spinal Cord. 2010;48:576-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Sondell M, Lundborg G, Kanje M. Regeneration of the rat sciatic nerve into allografts made acellular through chemical extraction. Brain Res. 1998;795:44-54. [PubMed] |
| 17. | Hudson TW, Zawko S, Deister C, Lundy S, Hu CY, Lee K, Schmidt CE. Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng. 2004;10:1641-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 277] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 18. | Rovak JM, Bishop DK, Boxer LK, Wood SC, Mungara AK, Cederna PS. Peripheral nerve transplantation: the role of chemical acellularization in eliminating allograft antigenicity. J Reconstr Microsurg. 2005;21:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Hu J, Zhu QT, Liu XL, Xu YB, Zhu JK. Repair of extended peripheral nerve lesions in rhesus monkeys using acellular allogenic nerve grafts implanted with autologous mesenchymal stem cells. Exp Neurol. 2007;204:658-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Ban DX, Liu Y, Cao TW, Gao SJ, Feng SQ. The preparation of rat’s acellular spinal cord scaffold and co-culture with rat’s spinal cord neuron in vitro. Spinal Cord. 2017;55:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Liu J, Chen J, Liu B, Yang C, Xie D, Zheng X, Xu S, Chen T, Wang L, Zhang Z. Acellular spinal cord scaffold seeded with mesenchymal stem cells promotes long-distance axon regeneration and functional recovery in spinal cord injured rats. J Neurol Sci. 2013;325:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8:235-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1242] [Cited by in RCA: 1515] [Article Influence: 89.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Langdon S, Radenovic L S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ