Published online Feb 20, 2017. doi: 10.5493/wjem.v7.i1.31
Peer-review started: November 28, 2016
First decision: December 15, 2016
Revised: January 5, 2017
Accepted: January 16, 2017
Article in press: January 18, 2017
Published online: February 20, 2017
Processing time: 83 Days and 16.2 Hours
To evaluate various schemes for paraquat poisoning and different variables that influence the outcome of acute paraquat poisoning.
In a cross-sectional study, the information about all cases of acute paraquat poisoning who were admitted to teaching hospitals affiliated to Shiraz University of Medical Sciences, in a five year period (September 2010 to September 2015) were evaluated. The variables included: Demographic data, medical assessment, therapeutic options, laboratory findings, and the outcomes. Data were analyzed using SPSS, version 22. Significant difference between groups was tested using t-test for continues outcomes and χ2 test for categorical. The significance level was considered to be P < 0.05.
A total of 104 patients (66.3% male) were evaluated. The mean age of the female patients was 22.81 ± 9.87 years and the male patients’ was 27.21 ± 11.06 years. Ninety seven (93.3%) of all the cases were suicide attempts with mortality rate of 43.2%. Despite the necessity for emergency hemodialysis during the first 6 h of intoxication, none of the patients had dialysis during this time. Immunosuppressive and corticosteroid medications were not administrated in adequate dosage in 31.1% and 60% of the patients, respectively. Ingestion of more than 22.5 cc of paraquat and increase in creatinine level were the most important predictors of mortality.
Treatment should start immediately for these patients. Moreover, creating a clinical guideline according to the findings can have an impact on the treatment procedure which seems to be necessary.
Core tip: In developing countries with an agriculture economy poisoning by means of herbicides is very common. Paraquat is a highly toxic compound and consumption of 30 mg/kg is lethal in humans. In this study, we have analyzed multi-center data of patients with paraquat poisoning between September 2010 and September 2015, establishing the largest series of paraquat poisoning in the Middle East. Based on the data, medical knowhow that affects its current management as well as different variables which influence the outcome were evaluated.
- Citation: Kavousi-Gharbi S, Jalli R, Rasekhi-Kazerouni A, Habibagahi Z, Marashi SM. Discernment scheme for paraquat poisoning: A five-year experience in Shiraz, Iran. World J Exp Med 2017; 7(1): 31-39
- URL: https://www.wjgnet.com/2220-315X/full/v7/i1/31.htm
- DOI: https://dx.doi.org/10.5493/wjem.v7.i1.31
Due to widespread usage of herbicides in agricultural industry reports of human poisoning has been on the rise around the world[1-3]. Paraquat (1,1′-dimethyl-4,4′ bipyridinium dichloride) is a well-known compound used in agriculture and it is a suitable due to its wide range of effects on weeds and instability in the environment[4,5].
Consumption of 30 mg/kg (equal to around 3-6 g of paraquat ion) is lethal in adults[6-8]. In the case of oral intake, it is quickly absorbed through the luminal tract, and 95% of its tissue distribution occurs within the first 6 h. Kidneys play a vital role in disposing paraquat from the body, and its maximum disposal is carried out during the first 12 to 24 h[6]. Symptoms include: Burning sensation in the mouth, throat, chest, epigaster, nausea, vomiting, abdominal pain, and diarrhea, which can be stopped after 2-3 d, if the patient is still alive[9]. Additionally if a patient has consumed more than 20 mg/kg of paraquat ion, his/her survival rate is very low[10]. I think (equal to 10 cc of 20% solution) is not a proper statement, because 20 mg/kg is more explanatory itself. The main mechanism in being poisoned with paraquat is the formation of superoxide ions, active oxygen radicals, NADPH oxidation, lipid peroxidation of cell membrane, and destruction of the cell membrane structure[4,11]. Despite progressions in critical care domain, to this point there has not been any effective treatment for paraquat poisoning. Some studies have indicated some improvement in prognosis of patients, ensuing the prescription of absorbents, treatment by immunosuppressive medications, radiotherapy and hemodialysis[12-14]. The main objective in this research was to study the clinical symptoms, laboratory abnormalities and the outcome of paraquat poisoning in a 5-year period in Fars province, Iran.
In this retrospective descriptive analytical study, a total of 104 records of paraquat poisoning patients in three main tertiary hospitals in Shiraz, Iran, from September 2010 to September 2015 were evaluated. This research was conducted following the approval of Shiraz University of Sciences Ethics Committee. The required data were manually obtained from the patients’ records. The data included; age, gender, consumed paraquat amount, occurrence of vomiting after consumption, the gap between poison consumption and treatment initiation, the treatments modalities, hospitalization duration, laboratory abnormalities, and sequela. Patients who had not signed the written informed consent for using their records, those who had taken other medications or poisons simultaneously, and those who had a history of cardiac, pulmonary, renal or hepatic diseases were excluded from the study.
The retrieved data were analyzed using SPSS, version 22. The continuous variables were described by standard deviation ± mean and the categorical were reported in the form of frequencies. Significant difference between groups was tested using t-test for continues outcomes and χ2 test for categorical. In all cases, the significance level was considered to be P < 0.05. In order to assess the performance of laboratory changes in predicting the death occurrence, the area under receiver operating characteristic (ROC) curve and sensitivity, specificity, positive and negative predictive value were studied. The confidence interval was 95%.
In this research, a total of 104 patients poisoned by consuming paraquat were studied in a period of five years. The demographic data of the patients are presented in Table 1.
| Variable | Total = 104 |
| Gender | |
| Male (%) | 69 (66.3) |
| Female (%) | 35 (33.7) |
| Duration of hospitalization | |
| 1-6 d (%) | 61 (58.7) |
| 7-13 d (%) | 29 (27.9) |
| More than 14 d (%) | 14 (13.5) |
| Mean (d) | 6.73 ± 5.73 |
| Time interval (d) | 1-27 |
| Cause of poisoning | |
| Occupational exposure (%) | 4 (3.8) |
| Suicidal (%) | 97 (93.3) |
| Accidental (%) | 3 (2.9) |
| Habitat | |
| Rural (%) | 80 (76.9) |
| Urban (%) | 24 (23.1) |
| Type of poisoning | |
| Ingestion (%) | 101 (97.1) |
| Injection (%) | 3 (2.9) |
| Outcome | |
| Recovery (%) | 59 (56.7) |
| Death due to complications (%) | 45 (43.3) |
The duration of hospital stay for the patients was 6.73 ± 5.73 d (between 1 and 27 d) on average. Poisoning with paraquat in males was 1.9 times higher than females. The highest rate of poisoning prevalence was among females under 20 and males between the ages of 20 and 30. The mean age of the female patients was 22.81 ± 9.87 (between 1 and 61 years) and the male patients’ was 27.21 ± 11.06 (between 15 and 60 years) (P = 0.045).
Majority of the patients (76 cases; 73.1%) had vomited before being admitted to the hospital and the most common symptom during admission was nausea (74 cases; 71.1%). However, prevalence of epigasteric pain and inflammation of the oral mucosa was (29 cases; 27.9%) and (28 cases; 26.9%). No dysrhythmia was observed on the electrocardiogram at the time of presentation or during hospitalization, excluding agonal arrhythmia in dying patients.
The most common decontamination method carried out for the patients was gastric lavage in 94 cases (90.4%). Charcoal alone or along with Fuller’s earth was prescribed for gastric decontamination in 60 (57.7%) and 17 cases (16.3%), respectively. Gastric lavage was carried out in all cases that Fuller’s earth or charcoal was prescribed. Only in 17 cases, gastric lavage was the sole method carried out.
In 91 cases (87.5%), treatment was carried out by corticosteroids and in 39 (37.5%) by cyclophosphamide. In 50 cases (48.1%) N-acetyl cysteine (NAC), in 34 (32.7%) vitamin E, and in 32 (30.8%) vitamin C were prescribed as antioxidant medications.
In none of the 45 deceased patients, treatments were carried out completely. The most common type of managements were prescribing corticosteroid medications in 43 cases (95.6%), gastric lavage in 42 (93.3%), charcoal in 35 (77.78%), and NAC administration in 22 (48.9%) patients. Lack of attention in prescribing cyclophosphamide, dexamethasone and vitamin E as the most commonly ignored treatments in deceased patients, had occurred in 28 cases (62.2%), and lack of attention in prescribing NAC had occurred in 23 cases (51.1%). Methylprednisolone was not prescribed in 9 cases (20%), and in 27 cases (60% of the deceased patients), it was prescribed insufficiently.
Since, hemoperfusion was not available in any of the tertiary hospitals in Shiraz, Iran therefore; hemodialysis was carried out for extracorporeal removal of paraquat. Only 3 cases had expired due to the severity of poisoning during the early hours of admission and before performing hemodialysis. Nonetheless, initiating hemodialysis was delayed in all cases, at least for 6 h, mainly due to the delay in receiving the results of viral marker status. About 54% of the patients were hemodialyzed again due to increase in renal biomarkers after the first day.
Lung radiography was done for 45 cases. Table 2 presents the frequency of the positive lung radiography findings in the studied patients.
| Radiographic findings | Time interval of radiographic study (d) | No. of patients (percent in the survivors/non-survivors groups) |
| Non-survivors (n = 18) | ||
| Pneumothorax (%) | 2-5 | 2 (11.1) |
| Pneumomediastinum/emphysema (%) | 1-2 | 2 (11.1) |
| ARDS (%) | 1-10 | 4 (22.2) |
| Lung fibrosis (%) | 4-14 | 10 (55.6) |
| Survivors (n = 27) | ||
| Pneumothorax (%) | 1-5 | 2 (7.4) |
| Lung fibrosis (%) | 4-32 | 4 (14.8) |
| Normal (%) | 1-8 | 21 (77.8) |
The amount of consumed poison in patients was 34.61± 55.36 mL (between 1.5 and 300 mL) on average. While the deceased patients had consumed 66.63 ± 72.61 mL of poison on average, this amount was 10.18 ± 5.77 mL on average for the patients who survived (P = 0.001); that is, 85.5% of the patients who had consumed less than 10 cc and 12.7% of the patients who had consumed between 10 and 20 cc of poison were discharged after recovery. On the other hand, 91.1% of the patients who had consumed more than 20 cc of poison ultimately expired (P = 0.000).
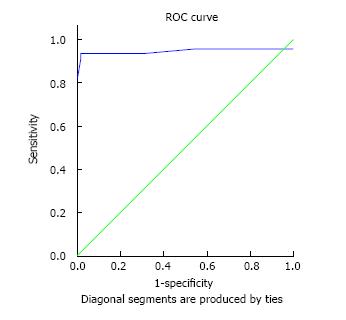
Figure 1, illustrates the ROC curve related to poison consumption and mortality rate in patients. Table 3 shows the cutoff point for the amount of poison consumed and patients’ mortality, by considering the minimum positive predictive value of 90%. Based on poison consumption rate and ROC curve, the best cutoff point was calculated at 22.5 cc or higher (considering the minimum positive predictive value of 90%).
| Variable | Area under the ROC (the minimum positive predictive value of 90%) | Cutoff point | Positive predictive value | Negative predictive value | Sensitivity | Specificity |
| Amount of consumed poison | 0.945 (0.87-1.0) | 22.5 | 93.3 | 98.3 | 93.3 | 98.3 |
On average patients’ were deceased after 4.8 ± 4.62 d of hospitalization, (between the first and 21st days). Total of 9 cases expired during the first day of hospitalization who had consumed about 35 and 300 cc of poison.
This study indicates that maximum average levels of serum creatinine was 2.50 ± 1.80 (between 0.6 and 9.5), maximum average of blood urea nitrogen 16.42 ± 29.18 (between 6 and 66), maximum average of AST levels 114.52 ± 246.85 (between 8 and 1509), and maximum average of ALT 301.43 ± 145.31 (between 8 and 1803) were observed after the third day of admission.
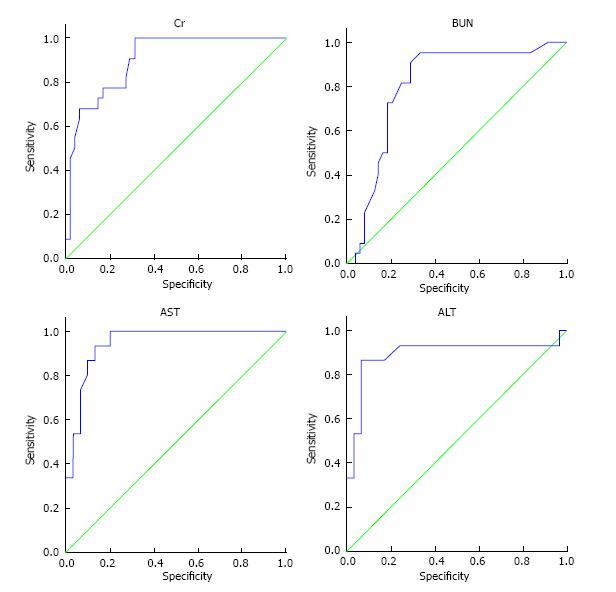
Figure 2, illustrates the ROC curve related to Levels of serum creatinine, blood urea nitrogen (BUN), aspartate aminotransferase (AST), and alanine transaminase (ALT) after the third day of admission and mortality rate in patients. Table 4 shows the cutoff point of serum creatinine level, BUN, AST, and ALT on the third day in relation with patients’ mortality by considering the minimum positive predictive value of 90%. The best cutoff point (considering the minimum positive predictive value of 90%) was calculated based on serum creatinine level, BUN, AST, and ALT on the third day and ROC curve. This cutoff point was calculated to be 1.95 or higher for creatinine level, 25 or higher for BUN level, 24.5 or higher for AST level, and 12 or higher for ALT level on the third day.
| Laboratory variables in the third day of admission | Area under the ROC (the minimum positive predictive value of 90%) | Cutoff point | Positive predictive value | Negative predictive value | Sensitivity | Specificity |
| Serum creatinine | 0.90 (0.83-0.97) | 1.95 | 90.9 | 70.8 | 90.9 | 70.8 |
| BUN | 0.80 (0.69-0.91) | 25 | 95.4 | 66.7 | 95.5 | 66.7 |
| AST | 0.85 (0.58-1.000) | 24.5 | 100 | 66.78 | 100 | 72.7 |
| ALT | 0.79 (0.57-1.000) | 12 | 93.3 | 10.34 | 88.9 | 9.1 |
Being poisoned with herbicides in developing countries of South, East and Southeast Asia with an agriculture economy is very common[15]. In this study, a total of 104 paraquat poisoning cases in Fars province, which is one of the agriculture hubs in Iran, were studied in a 5-year interval. More than 65% of all the cases were male. The gender ratio of the paraquat poisoning in other studies was reported to be between 55% and 70% in males[5,6,10]. However, the mean age of female patients which was around 23 years, was 4 years lower than the male patients; this was in accordance with Kim’s study[16]. The highest prevalence was observed in teenage girls and males between the ages of 20 and 30, which are considered as the active population. Around 77% of the patients were from rural areas. Other studies, also showed that poisoning was more common among the rural population, from 56% to 73%[6,13]. Around 93% of the cases were suicide attempts, which is in accordance with the results from other studies[5,6,13].
The most common clinical symptoms in patients included nausea, epigasteric pain and inflammation of the oral mucosa, which were in line with results from Cherukuri et al[9]. However, Sandhu et al[12] had reported that all patients diagnosed with paraquat poisoning experienced nausea and vomiting, but oral mucosal ulcers were reported in only 59% of the patients.
Gastric lavage was carried out as the most common type of emergency procedure in about 90% of all the cases, prescribing charcoal in about 58% and prescribing charcoal along with Fuller’s earth was carried out in 16% of the all the patients in the present study. In Senara-thna’s study[17], it was shown that Fuller’s earth was prescribed in about 75%, and charcoal prescription was carried out in about 22% of the cases; however, in 16% of their patients, both were prescribed. Nevertheless, it is worth mentioning that Fuller’s earth (magnesium citrate) and charcoal have similar effects[18], and there is no need for their simultaneous prescription. Although some of the old studies had proposed the necessity for gastric lavage in paraquat poisoning[19,20], Wilks et al[21] showed that gastric lavage can lead to increase in mortality rate in cases where the patient has consumed lesser than 30 cc of poison. It seems that gastric cleansing was inappropriate in the studied patients.
Research has suggested that paraquat can reach plasma concentration peak in one hour due to rapid absorption[22,23], and subsequently accumulate in targeted tissues. However, there is a chance of re-distribution from tissues to plasma, as well. Paraquat distribution half-life is around five hours in human, and around 6 h after its consumption, it reaches the maximum of tissue concentration in the lungs[24,25]. Considering the pathology of free radicals in paraquat poisoning, some older studies have proposed the use of antioxidant medications such as vitamin E and vitamin C in order to reduce tissue injuries. However, the impact of such treatments has not been proven[26,27]. Also, NAC, as a proper source of sulphydryl groups, could play a great role in scavenging free radicals[28]. In this research, 33% of the patients were treated with vitamin E, while vitamin C was prescribed for about 30% and NAC for about 48% of the patients. It seems that other researchers in various studies did not choose similar antioxidant medications; Cherukuri et al[9] in their study showed that treatment with vitamin E was done in about 18%, while vitamin C was prescribed in 25% and NAC in 50% of their patients. About 15% of the patients in Delirrad’s[6] study were treated with NAC. However, in Sabzghabaee[10] and Sandhu et al[12]’s studies, all patients were treated with antioxidant medications. In their study on 9 cases, Yasaka et al[30] showed that mortality rate in patients who were treated with vitamin E reached 78%, however, in Hong et al[31], study on 5 cases they showed that all treated patients survived. Some previous studies have proposed the impact of treatment by pulse corticosteroids and cyclophosphamide in preventing pulmonary fibrosis[31,32]. In this study, 87% of the patients were treated with corticosteroids and 37% with cyclophosphamide. Other researches have shown to use similar antioxidant medications, but they did not use similar immunosuppressive and corticosteroid medications. As Cherukuri et al[9] showed in their study, treating with pulse methylprednisolone was carried out in 38% of patients, while treating with cyclophosphamide was carried out in 22% of the cases. In Delirrad’s[6] study, 54% were treated with corticosteroids, and 22% of the cases were treated with cyclophosphamide. On the other hand, all patients were treated with corticosteroids in Sabzghabaee[10] and Banday’s studies[4].
Our study suggests that consuming more than 22.5 cc of 20% paraquat can lead to poor prognosis in the patients, and this is in accordance with the results of Hosseinian Amiri et al[5] and Delirrad et al[6], studies. In addition, Buckley et al[33] showed that consuming around 10 to 20 cc of this poison could lead to fatal complications.
Thus, results from our study indicates that on average, patients’ mortality occurred on the fifth day of hospitalization, which is in accordance with Afzali’s[34] study. Also, in 9 cases who had consumed around 35 to 300 cc of the poison, death occurred on the first day of admission. In fact, consuming higher doses of poison can lead to death during the first few hours through acute multi organ failure[1].
Even though hemoperfusion has been introduced as an effective treatment in washing off the poison from plasma in the first 6 h[35], but for the patients in this study hemodialysis was performed, due to lack of hemoperfusion facilities. However, this measure was not performed for any of the patients during the first 6 h of admission. It seems that the main reason for this delay was that they had to wait for receiving the results for viral markers for hemodialysis. Nevertheless, based on Marashi et al[36], considering the high mortality rate due to poisoning and the low probability of viral infections, there is no need to study the viral markers, and in such cases hemodialysis should be carried out by the device allocated for patients diagnosed with hepatitis B.
Pulmonary fibrosis is among the known complications in paraquat poisoning which occurs approximately 7 to 14 d after poisoning along with acute respiratory failure[37,38]. This complication transpires due to body’s inability to repel the free radicals that leads to the destruction of cell membrane and lipid peroxidation[39]. In this study, among the expired patients, pulmonary fibrosis was the most common radiography finding, which was observed in almost 22% of the patients (more than 55% of the expired patients), which was initially observed on the fourth day. Also, Hsu et al[40] showed that pulmonary fibrosis had led to death in about 25% of patients. Our radiography findings in deceased patients were acute respiratory distress syndrome (ARDS), pneumothorax, and pneumomediastinum, which is in accordance with the results of Weng et al[41].
Unfortunately, despite various studies, limited variables have been identified for predicting prognosis. Although one of the best prognostic criteria is to determine paraquat serum concentration and to use nomogram[18], but this factor could not be studied due to lack of serum paraquat concentration measurement as a common laboratory test in Iran.
This research showed that the maximum average of serum creatinine levels increased on the third day (up to around 2.5 mg per deciliter), and that the serum creatinine average decreased, subsequently. Serum creatinine level on the third day higher than 1.95 mg per deciliter was accompanied with a poor prognosis in our patients. Ragoucy-Sengler and Pilerire[42] showed that an increase in serum creatinine lower than 0.03 mg during five hours accompanies an acceptable prognosis. On the other hand, Roberts et al[43] showed that an increase more than 0.05 mg during 12 h could lead to poor prognosis. However, according to Levey et al[44], creatinine level has insignificant value, even for assessing the kidney damages.
According to other studies, other biomarkers for renal function were not appropriate factors in predicting patients’ sequela[23]. This study showed that BUN level higher than 25 on the third day was accompanied with a poor prognosis.
Studying the changes in liver enzymes showed that the maximum average of AST and ALT levels on the third day were 114 and 145, respectively. AST level higher than 24.5 or ALT level higher than 12 on the third day was accompanied with poor prognosis. Considering the fact that these levels are in the normal range for AST and ALT, it seems that they are not appropriate factors for predicting severe poisoning. Furthermore, in their study, Almasi et al[45] showed that exposure to paraquat could lead to liver cell damage and increase in AST and ALT levels in rats, which could be treated by prescribing ginger extract. It seems that routine treatment by NAC played a role in improving liver cells’ performance and decreased AST and ALT in a number of patients in this study. Since prescribing NAC, as an antioxidant, is an approved treatment for paraquat poisoning, hence it seems that liver biomarkers are not appropriate factors in predicting the patients’ sequela.
This research showed that cardiac dysrhythmia is not a common finding in paraquat poisoning, which was in line with the results from Noguchiet et al[46]. In contrast, some other herbicides such as glyphosate, glufosinate and chloracetanilide herbicides (e.g., alachlor, metachlor, butachlor, and propanil) appeared to have significant cardiotoxicity[47-51]. Even though other types of herbicides such as; glyphosate, glufosinate and chloracetanilide herbicides are available in Iran, by reviewing the published literature, we could find only one case report of butachlor dermal exposure[52]. It seems that, paraquat as a highly toxic compound is recognized by those who are seeking to commit suicide.
This research showed that a standardized treatment protocol was not used in all paraquat poisoning cases and in some cases, unnecessary or improper measures were carried out for the patients and in contrast or in some cases, a patient was deprived of necessary treatments.
Since there is no charcoal hemoperfusion available in our hospitals, but hemodialysis, which is the alternative choice was not used for extracorporeal elimination in the right time. Therefore, it is necessary to immediately carry out hemodialysis in these patients, along with training physicians and assistants working in teaching hospitals.
It seems that due to lack of paraquat poisoning treatment guidelines, patients are deprived of proper treatment by antioxidant and immunosuppressive medications. Providing treatment guidelines for this type of poisoning could assist in choosing a suitable treatment method. Finally, to better identify this type of poisoning systematic and meta-analysis reviews must be performed.
The authors would like to thank the Research Consultation Center (RCC) of Shiraz University of Medical Sciences in their invaluable assistance in English editing of this manuscript.
Poisoning with herbicides in developing countries of South, East and Southeast Asia with an agriculture economy is highly common. Paraquat poisoning, is a highly mortal toxicity and rapid management is required to increase patient survival. However, without a standard guideline, there are no agreement on therapeutic strategies conducted in different healthcare facilities. The use of hemoperfusion or hemodialysis during the first hours of admission, followed by administration of immunosuppressive and corticosteroid medications, as well as antioxidants, is currently accepted to be the conventional treatment protocol for these cases. However, in practice, negligence is responsible for low survival rate of patients with paraquat poisoning.
Reviewing the published literature, it seems that paraquat poisoning has the most prevalence rate in Fars province Iran, amongst different parts of Middle-Eastern countries. The research purpose was to evaluate the accuracy of treatment strategies, conducted to treat this fatal poisoning, as well as to demonstrate prognostic factors regarding long-term survival outcome.
Medical treatment for paraquat poisoning is improving in developing countries. This study represents the largest series of paraquat poisoning cases in the Middle-East ever reported. The current data suggests that therapeutic inaccuracies are common amongst healthcare providers. On the other hand, it was determined that there hasn’t been any particular protocol used to treat patients diagnosed with paraquat poisoning. This indicates the necessity to develop a guideline for treating paraquat poisoning in order to provide better healthcare services to these patients. In case there is no access to charcoal hemoperfusion, hemodialysis should be used as an alternative choice in extracorporeal elimination; however, due to delay in reaching the results from viral marker study, hemodialysis was not carried out in most of the patients in the six-hour golden time. The importance of immediate use of extracorporeal removal techniques and omitting the viral markers results, also accurate use of immunosuppressive, corticosteroid and antioxidant medications should be considered as the main treatment protocols.
Results from this research indicated that the increase in renal biomarkers in the third day could be used in prognosis of the patients; hence, by identifying the patients who are at risk, it could be used as a guideline in intensive treatment measures.
Paraquat poisoning is a lethal toxicity in patients who have consumed this herbicide, which is characterized by a rapidly progressive multi-organ failure in severe cases and progressive lung fibrosis in moderate cases. The most important treatment for paraquat poisoning is extracorporeal elimination within the first 6-h of toxicity. By using extracorporeal elimination technics, paraquat ion removal takes place through a machine performing blood circulation outside the body. By using charcoal hemoperfusion, paraquat cleansing will exceed that of hemodialysis. During hemoperfusion, blood is pumped through a cartridge containing activated charcoal.
Kavousi-Gharbi et al from Shiraz University of Medical Sciences, Shiraz, Iran investigated cross-sectionally the information about all cases of acute poisoning by the herbicide paraquat (1,1′-dimethyl-4,4′ bipyridinium dichloride) admitted to 3 main teaching hospitals of Shiraz University in a 5-year period (September 2010 to September 2015). A total of 104 patients (66% male) with a mean age 26 ± 11 years were evaluated. The mortality rate was 43%. Despite the necessity of emergency hemodialysis in first 6 h of intoxication, none of the patients had dialysis during this time.
| 1. | Bertsias GK, Katonis P, Tzanakakis G, Tsatsakis AM. Review of clinical and toxicological features of acute pesticide poisonings in Crete (Greece) during the period 1991-2001. Med Sci Monit. 2004;10:CR622-CR627. [PubMed] |
| 2. | Eddleston M, Karalliedde L, Buckley N, Fernando R, Hutchinson G, Isbister G, Konradsen F, Murray D, Piola JC, Senanayake N. Pesticide poisoning in the developing world--a minimum pesticides list. Lancet. 2002;360:1163-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 240] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 3. | Peter JV, Cherian AM. Organic insecticides. Anaesth Intensive Care. 2000;28:11-21. [PubMed] |
| 4. | Banday TH, Bashir Bhat S, Bashir Bhat S. Manifestation, complications and clinical outcome in paraquat poison? A hospital based study in a rural area of Karnataka. J Environ Occup Sci. 2014;3:21-24. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Hosseinian Amiri A, Delfan B, Jaferian S. Paraquat poisoning cases treated at Shohada Ashayer hospital of Khorramabad in 2001-2006. Res J Biol Sci. 2008;3:525-529. |
| 6. | Delirrad M, Majidi M, Boushehri B. Clinical features and prognosis of paraquat poisoning: a review of 41 cases. Int J Clin Exp Med. 2015;8:8122-8128. [PubMed] |
| 7. | Pavan M. Acute kidney injury following Paraquat poisoning in India. Iran J Kidney Dis. 2013;7:64-66. [PubMed] |
| 8. | Kolilekas L, Ghizopoulou E, Retsou S, Kourelea S, Hadjistavrou C. Severe paraquat poisoning. A long-term survivor. Respiratory Medicine Extra. 2006;2:67-70. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Cherukuri H, Pramoda K, Rohini D, Thunga G, Vijaynarayana K, Sreedharan N, Varma M, Pandit V. Demographics, clinical characteristics and management of herbicide poisoning in tertiary care hospital. Toxicol Int. 2014;21:209-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Sabzghabaee AM, Eizadi-Mood N, Montazeri K, Yaraghi A, Golabi M. Fatality in paraquat poisoning. Singapore Med J. 2010;51:496-500. [PubMed] |
| 11. | Marashi SM, Raji H, Nasri-Nasrabadi Z, Majidi M, Vasheghani-Farahani M, Abbaspour A, Ghorbani A, Vasigh S. One lung circumvention, an interventional strategy for pulmonary salvage in acute paraquat poisoning: an evidence based review. Tzu Chi Med J. 2015;27:99-101. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Sandhu JS, Dhiman A, Mahajan R, Sandhu P. Outcome of paraquat poisoning - a five year study. Indian J Nephrol. 2003;13:64-68. |
| 13. | Harshavardhan L, Rajanna B, Shashikanth YS. A study on epidemiological and clinical profile of acute paraquat poisoning and its consequences in tertiary care centre. Int J Bioassays. 2014;3:3577-3580. |
| 14. | Fock KM. Clinical features and prognosis of paraquat poisoning: a review of 27 cases. Singapore Med J. 1987;28:53-56. [PubMed] |
| 15. | Eddleston M, Wilks MF, Buckley NA. Prospects for treatment of paraquat-induced lung fibrosis with immunosuppressive drugs and the need for better prediction of outcome: a systematic review. QJM. 2003;96:809-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Kim SJ, Gil HW, Yang JO, Lee EY, Hong SY. The clinical features of acute kidney injury in patients with acute paraquat intoxication. Nephrol Dial Transplant. 2009;24:1226-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Senarathna L, Eddleston M, Wilks MF, Woollen BH, Tomenson JA, Roberts DM, Buckley NA. Prediction of outcome after paraquat poisoning by measurement of the plasma paraquat concentration. QJM. 2009;102:251-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 18. | Gaudreault P, Friedman PA, Lovejoy FH. Efficacy of activated charcoal and magnesium citrate in the treatment of oral paraquat intoxication. Ann Emerg Med. 1985;14:123-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Beswick E, Millo J. Fatal poisoning with glyphosate-surfactant herbicide. J Iran Chem Soc. 2011;12:37-39. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Vale JA, Kulig K. Position paper: gastric lavage. J Toxicol Clin Toxicol. 2004;42:933-943. [PubMed] |
| 21. | Wilks MF, Tomenson JA, Buckley NA, Dawson A. Influence of gastric decontamination on patient outcome after paraquat ingestion. J Med Toxicol. 2008;4:212-213. |
| 22. | Conning DM, Fletcher K, Swan AA. Paraquat and related bipyridyls. Br Med Bull. 1969;25:245-249. [PubMed] |
| 23. | Hoffman RS, Nelson LS, Howland MA, Levin NA, Flomenbaum NE, Goldfrank LR. Goldfrank’s manual of toxicologic emergencies. 1st edition. New York: McGraw Hill Publishing 2007; 856-859. |
| 24. | Murray RE, Gibson JE. Paraquat disposition in rats, guinea pigs and monkeys. Toxicol Appl Pharmacol. 1974;27:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 62] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Bismuth C, Scherrmann JM, Garnier R, Baud FJ, Pontal PG. Elimination of paraquat. Hum Toxicol. 1987;6:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Gil HW, Hong JR, Jang SH, Hong SY. Diagnostic and therapeutic approach for acute paraquat intoxication. J Korean Med Sci. 2014;29:1441-1449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Bismuth C, Garnier R, Baud FJ, Muszynski J, Keyes C. Paraquat poisoning. An overview of the current status. Drug Saf. 1990;5:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Bateman DN. Pharmacological treatments of paraquat poisoning. Hum Toxicol. 1987;6:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Moldéus P, Cotgreave IA, Berggren M. Lung protection by a thiol-containing antioxidant: N-acetylcysteine. Respiration. 1986;50 Suppl 1:31-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 178] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Yasaka T, Okudaira K, Fujito H, Matsumoto J, Ohya I, Miyamoto Y. Further studies of lipid peroxidation in human paraquat poisoning. Arch Intern Med. 1986;146:681-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Hong SY, Hwang KY, Lee EY, Eun SW, Cho SR, Han CS, Park YH, Chang SK. Effect of vitamin C on plasma total antioxidant status in patients with paraquat intoxication. Toxicol Lett. 2002;126:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Lin JL, Leu ML, Liu YC, Chen GH. A prospective clinical trial of pulse therapy with glucocorticoid and cyclophosphamide in moderate to severe paraquat-poisoned patients. Am J Respir Crit Care Med. 1999;159:357-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 89] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Buckley NA. Pulse corticosteroids and cyclophosphamide in paraquat poisoning. Am J Respir Crit Care Med. 2001;163:585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Afzali S, Gholyaf M. The effectiveness of combined treatment with methylprednisolone and cyclophosphamide in oral paraquat poisoning. Arch Iran Med. 2008;11:387-391. [PubMed] |
| 35. | Wu WP, Lai MN, Lin CH, Li YF, Lin CY, Wu MJ. Addition of immunosuppressive treatment to hemoperfusion is associated with improved survival after paraquat poisoning: a nationwide study. PLoS One. 2014;9:e87568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Marashi SM, Raji H, Nasri-Nasrabadi Z, Majidi M. Use of extracorporeal removal techniques in patients with paraquat toxicity and unknown hepatitis viral marker status. Tzu Chi Med J. 2016;28:39. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 37. | Jo YH, Kim K, Rhee JE, Suh GJ, Kwon WY, Na SH, Alam HB. Therapeutic hypothermia attenuates acute lung injury in paraquat intoxication in rats. Resuscitation. 2011;82:487-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Lalloo UG, Ambaram A. Survival after massive intentional overdose of paraquat. S Afr Med J. 2008;98:370-372. [PubMed] |
| 39. | Yao R, Zhou Y, He Y, Jiang Y, Liu P, Ye L, Zheng Z, Lau WB, Cao Y, Zeng Z. Adiponectin protects against paraquat-induced lung injury by attenuating oxidative/nitrative stress. Exp Ther Med. 2015;9:131-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Hsu CW, Lin JL, Lin-Tan DT, Chen KH, Yen TH, Wu MS, Lin SC. Early hemoperfusion may improve survival of severely paraquat-poisoned patients. PLoS One. 2012;7:e48397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Weng CH, Hu CC, Lin JL, Lin-Tan DT, Hsu CW, Yen TH. Predictors of acute respiratory distress syndrome in patients with paraquat intoxication. PLoS One. 2013;8:e82695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Ragoucy-Sengler C, Pileire B. A biological index to predict patient outcome in paraquat poisoning. Hum Exp Toxicol. 1996;15:265-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Roberts DM, Wilks MF, Roberts MS, Swaminathan R, Mohamed F, Dawson AH, Buckley NA. Changes in the concentrations of creatinine, cystatin C and NGAL in patients with acute paraquat self-poisoning. Toxicol Lett. 2011;202:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Levey AS, Perrone RD, Madias NE. Serum creatinine and renal function. Annu Rev Med. 1988;39:465-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 410] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 45. | Almasi H, Habibian R, Kamali M. Effect of Zingiber officinale on liver oxidative status and biochemical parameters in rats exposed to paraquat. Comp Clin Pathol. 2013;22:1165-1171. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 46. | Noguchi N, Misawa S, Tsuchiya S, Yamamoto H, Naito H. Cardio-respiratory effects of paraquat with and without emetics on Wistar rats. Vet Hum Toxicol. 1985;27:508-510. [PubMed] |
| 47. | Gress S, Lemoine S, Séralini GE, Puddu PE. Glyphosate-based herbicides potently affect cardiovascular system in mammals: review of the literature. Cardiovasc Toxicol. 2015;15:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | Gress S, Lemoine S, Puddu PE, Séralini GE, Rouet R. Cardiotoxic Electrophysiological Effects of the Herbicide Roundup(®) in Rat and Rabbit Ventricular Myocardium In Vitro. Cardiovasc Toxicol. 2015;15:324-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 49. | Moon JM, Chun BJ. Predicting acute complicated glyphosate intoxication in the emergency department. Clin Toxicol (Phila). 2010;48:718-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Mao YC, Hung DZ, Wu ML, Tsai WJ, Wang LM, Ger J, Deng JF, Yang CC. Acute human glufosinate-containing herbicide poisoning. Clin Toxicol (Phila). 2012;50:396-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 51. | Seok SJ, Choi SC, Gil HW, Yang JO, Lee EY, Song HY, Hong SY. Acute oral poisoning due to chloracetanilide herbicides. J Korean Med Sci. 2012;27:111-114. [PubMed] [DOI] [Full Text] |
| 52. | Daryani NE, Hosseini P, Bashashati M, Haidarali M, Sayyah A. Butachlor-induced acute toxic hepatitis. Indian J Gastroenterol. 2007;26:135-136. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Iran
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kim ST, Puddu PE S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ