Published online Feb 20, 2017. doi: 10.5493/wjem.v7.i1.11
Peer-review started: September 14, 2016
First decision: October 21, 2016
Revised: November 1, 2016
Accepted: November 27, 2016
Article in press: November 29, 2016
Published online: February 20, 2017
Processing time: 156 Days and 12.9 Hours
Granulopoiesis in murine bone-marrow is regulated by both intrinsic and extrinsic factors (including hormones, drugs, inflammatory mediators and cytokines). Eosinophils, a minor subpopulation of circulating leukocytes, which remains better understood in its contributions to tissue injury in allergic disease than in its presumably beneficial actions in host defense, provide a striking example of joint regulation of granulopoiesis within murine bone-marrow by all of these classes of extrinsic factors. We first described the upregulation of eosinopoiesis in bone-marrow of allergen-sensitized mice following airway allergen challenge. Over the last decade, we were able to show a critical role for endogenous glucocorticoid hormones and cytokines in mediating this phenomenon through modification of cytokine effects, thereby supporting a positive association between stress hormones and allergic reactions. We have further shown that cysteinyl-leukotrienes (CysLT), a major proinflammatory class of lipid mediators, generated through the 5-lipoxygenase pathway, upregulate bone-marrow eosinopoiesis in vivo and in vitro. CysLT mediate the positive effects of drugs (indomethacin and aspirin) and of proallergic cytokines (eotaxin/CCL11 and interleukin-13) on in vitro eosinopoiesis. While these actions of endogenous GC and CysLT might seem unrelated and even antagonistic, we demonstrated a critical partnership of these mediators in vivo, shedding light on mechanisms linking stress to allergy: GC are required for CysLT-mediated upregulation of bone-marrow eosinopoiesis in vivo, but also attenuate subsequent ex vivo responses to CysLT. GC and CysLT therefore work together to induce eosinophilia, but through subtle regulatory mechanisms also limit the magnitude of subsequent bone-marrow responses to allergen.
Core tip: The bone-marrow is exquisitely sensitive to regulation by systemic events, which selectively increase production of different blood cell types to meet transient increases in demand following injury. An association between stress and allergy has long been known, but its mechanisms remain incompletely understood. The exploration of underlying mechanisms in a variety of murine models yielded evidence of separate but interrelated roles for adrenal glucocorticoid hormones and cysteinyl-leukotrienes in coupling systemic events to bone-marrow responses in vivo. We here discuss how these unlikely partners work together to promote eosinophilia but through subtle mechanisms also limit its magnitude.
- Citation: Xavier-Elsas P, Masid-de-Brito D, Vieira BM, Gaspar-Elsas MIC. Odd couple: The unexpected partnership of glucocorticoid hormones and cysteinyl-leukotrienes in the extrinsic regulation of murine bone-marrow eosinopoiesis. World J Exp Med 2017; 7(1): 11-24
- URL: https://www.wjgnet.com/2220-315X/full/v7/i1/11.htm
- DOI: https://dx.doi.org/10.5493/wjem.v7.i1.11
In both humans and mice, the lifelong production of blood cells (definitive hemopoiesis[1,2]; takes place in the bone-marrow of long bones, and encompasses the production, from a pool of hemopoietic stem cells (HSC), of both lymphoid (B cells, Natural Killer cells) and myeloid (erythroid, megakaryocytic, mononuclear phagocyte and granulocyte) lineages, through a series of increasingly committed (specialized, or developmentally restricted) stages, recognizable as morphologically, cytochemically and/or immunophenotypically distinct cell types[1,3-5]. Mature cells may then be exported to the circulation and remain there, until they are removed during their passage through the spleen (a typical fate for erythrocytes and platelets[6]) or emigrate to the tissues, ultimately undergoing apoptosis and clearance by resident phagocytes[7]. Emigration occurs either when inflammation follows injury (thereby allowing neutrophil granulocytes to exert short-term protective functions, in the absence or presence of infection[8]; or when chemoattractants selectively expressed in some sites attract leukocytes from a particular lineage (for instance, in the case of eosinophil granulocytes, enabling them to enter the mucosa of the digestive, respiratory and reproductive tracts to become long-term resident effector and regulatory cells[9].
Usually, peripheral clearance of senescent or apoptotic cells of bone-marrow origin is coupled to replenishment by a variety of mechanisms[10]. This is no small achievement, because specialized hemopoietic cell lineages, though ultimately derived from the same pool of pluripotent, self-renewing stem cells, differ largely in their numbers, requirements and properties[1-5]; accordingly, no single mechanism can account for the maintenance of their proportions across different compartments, nor for their lineage-selective increases or decreases often observed in immune reactions and disease[4,5,11,12].
Multiple factors intrinsic to the adult bone-marrow contribute to the maintenance of a steady output of these different cell types in very disparate proportions and rates. A major intrinsic factor is the differential expansion of hemopoietic lineages, driven by intense proliferation of lineage-committed progenitors (quantifiable in vitro as colony-forming units, or CFU), specified by unique profiles of gene expression under the control of master genes and transcription factors, in response to different hemopoietic growth factors or combinations thereof[1]. Progenitor expansion is adjusted to the turnover rate of the respective circulating forms of each lineage, so that relatively stable numbers of red cells, platelets and leukocyte subpopulations are replaced every day, enabling us to determine a range of “normal” blood cell counts, which may widely differ from one lineage to the other[1,3].
The original in vitro studies, which led to the purification and ultimately to cloning of a variety of colony-stimulating factors (CSF) of various nonlymphoid sources, endowed with selectivity for macrophage (M-CSF, or CSF-1), granulocyte (G-CSF), or granulocyte-macrophage (GM-CSF) progenitors, had suggested that hemopoiesis in steady-state conditions was driven by CSF-like molecules[3]. From this assumption one would predict that disruption of signaling by CSF-like molecules would entail profound deficiency in circulating leukocytes. This view must now be qualified, however, in view of the persistence of normal granulopoiesis in mice lacking the functions of GM-CSF and IL-3, two major CSF species[13]. Not all CSF, however, are irrelevant to steady-state granulopoiesis, as IL-5 seems necessary for normal production of eosinophils[9,13-15], G-CSF for that of neutrophils and M-CSF for that of macrophages[1]. Thrombopoietin and G-CSF, originally identified as CSF with lineage-selectivity for megakaryocytes/platelets and neutrophils, respectively, have been further characterized as multilineage regulators with complex actions, thereby overstepping the original boundaries of their function[1,3]. Therefore, while much remains to be learned about the intrinsic processes that drive definitive hemopoiesis in steady-state, it is likely that at least some CSF cytokines contribute to hemopoiesis in exceptionally demanding conditions, by mediating the actions of extrinsic factors linked to homeostatic disturbances or environmental changes on bone-marrow.
Increased demand on the bone-marrow imposed by systemic challenges, unlike regeneration of the entire hemopoietic environment[16], elicits lineage-selective responses, which may be short- or long-lived: For instance, hemorrhage and chronic hypoxemia are met with compensatory production of erythrocytes[17]; in other examples, bacterial infection elicits adaptive increases in neutrophil leukocytes[4,5,11], and helminth infection or allergic disease induce eosinophilia[9,14,18-20].
Importantly, the critical elements in these adaptations of bone-marrow to a transient stress are lineage-committed progenitors, rather than the HSC endowed with self-renewing and long-term repopulating potential. This makes biological sense, since progenitors are closer than stem cells to terminally differentiated, functional blood cells, and the physiologically relevant increase in circulating blood cells will be faster, because it will require less rounds of cell division. By contrast, HSC, as a rule, are protected from such transient challenges for a good reason, since infection at least may severely impair their function[11].
GM-CSF and interleukin (IL)-3 may be more relevant to the stress (or emergency) myelopoiesis in systemic microbial infection[4,5,15], and, in the more restricted context of helminth infection and allergic disease, IL-5 plays an important role for its selectivity to the eosinophil lineage[9,14,19].
Importantly, however, in the case of neutrophil or eosinophil granulocytes, proliferative and maturation-promoting effects of these CSF on production are only part of their contribution to the adaptive hematological responses, since they also have important mobilizing effects on the reserve pool associated with bone-marrow and other sites, and they further extend the lifespan of selected hemopoietic lineages outside bone-marrow, thereby increasing the total number of cells belonging to these lineages in the periphery, and decreasing their turnover by a lineage-selective reduction in cell death rates[7]. The consistently positive action of the same CSF at multiple steps in the life cycle of granulocytes highlights the integration of these proliferative and nonproliferative cytokine effects, which translates in physiologically meaningful outcomes.
It is important that these granulopoietic/mobilizing/antiapoptotic cytokines are not restricted to the bone-marrow compartment, but are often produced by multiple cell types in the context of specific adaptive (specific) as well as innate (nonspecific) immune responses at distant sites. Nevertheless, cytokines acting on bone-marrow targets act early in this sequence, and due to the amplification of their effects through multiple rounds of cell division, they have long-lasting effects.
In the context of allergic disease or helminth infection, IL-5, the lineage-specific cytokine required for both constitutive and stress eosinopoiesis, is secreted in different contexts by different cells, especially by activated, allergen-specific, Th2 lymphocytes[1,9,14,15], and could contribute in various ways to the effects of allergen challenge. Recognition of allergen at the challenge site by TH2 lymphocytes, which subsequently secrete IL-5, is one way to couple allergen recognition in the peripheral sites to generation of a stimulus within the bone-marrow. Other possibilities include production of IL-5 by mast cells following recognition of allergen by specific IgE bound to FcεRI in the mast cell surfaces[15,20]. Secretion of IL-5 inside bone-marrow by lymphocyte populations[21] might also contribute, although it is unclear at present whether these would necessarily be conventional T lymphocytes, requiring MHC restricted allergen presentation by dendritic or B cells.
Cytokines can transduce the effects of immune reactions on the bone-marrow, by one of two ways: (1) systemic diffusion of the cytokine itself, from the inflammatory site to bone-marrow through the circulation; and (2) selective homing of cytokine-producing cells to the bone-marrow, followed by local cytokine production.
In the first case, the cytokine stimulus is widespread, but the response remains restricted because the relevant target cells are concentrated in bone-marrow or, if present elsewhere, are presumably absent or dormant. In the second case, such a systemically diffusible stimulus is not necessary or even relevant, since the effect of immunity on granulopoiesis is elicited through a local accumulation of cytokine-producing leukocytes inside the bone-marrow, which only activate the relevant target cells in their neighborhood. Both mechanisms depend on the stimulus not being constitutively present, or effective, but becoming so in the wake of allergen challenge.
These alternatives have clearly distinct counterparts in an experimental setting: In the first case, bone-marrow effects can be elicited by intravenous transfer of plasma from the appropriate donors to naive recipients[22], and effects of this transfer will be restricted to the bone-marrow as long as there are no responsive targets elsewhere; in the second, transfer of leukocyte populations capable of homing to the bone-marrow compartment will be sufficient, and responses will both be limited to the bone-marrow compartment, and associated with the physical presence of the transferred leukocytes in this compartment[23].
It should be noted that these various possibilities are not mutually exclusive, but may better describe events at different time points. This is probably relevant to the sequence of events elicited by allergen exposure of sensitized mice (“challenge”), thought to represent chronic processes that underlie allergic diseases, especially asthma[12,24], as discussed below in the context of eosinophil production, which is the prime target of IL-5 actions.
It is also important that CSF-like molecules are just a small fraction of the cytokines that might be influencing bone-marrow responses, which is defined by its ability to directly stimulate hemopoiesis. A much larger number of cytokines may be unable to act as primary hemopoietic stimulus, but remain quite effective in modifying the actions of primary stimuli to achieve particular effects. In the case of the eosinophil lineage, IL-5 is the best characterized (and highly selective) primary stimulus[9,15,20]; however, a number of cytokines discussed below, including eotaxin/CCL11, IL-13 and IL-17, do not stimulate directly eosinopoiesis, but interact with IL-5 to achieve quite different outcomes[25,26].
Another important feature of cytokine-coordinated processes is the potential for interactions involving multiple partners. These are, in some cases, other cytokines as mentioned above; however, they may also include noncytokine mediators of inflammation such as proinflammatory or antiinflammatory lipid mediators, hormones, or vitamins. In the context of therapy, drugs or immunoregulatory leukocyte subpopulations may become partners for novel interactions. Generally speaking, the actions of a given cytokine may be only understood in context, which encompasses immunoendocrine and immunopharmacological interactions, in addition to cytokine network interactions.
Eosinophils, a minor subset of circulating leukocytes, remain better understood in their contributions to tissue injury in allergic disease such as asthma[27,28], than in their presumably beneficial actions in host defense and tissue repair[9,14,15,29,30]. Nevertheless, eosinophils are very interesting cells, which produce a large number of specialized (mostly cationic, or “basic”) proteins, a complex mixture of lipid mediators, and an impressive number of cytokines, which overstep the boundaries of the conventional TH1 and TH2 “profiles”. In addition, eosinophils aided by antibody are capable of killing some tumor cell targets, and, at high effector/target ratios, some larval stages of worms[19]. It is both biologically puzzling and therapeutically serendipitous that eosinophil-depleting interventions in experimental models as well as in chronic treatments have no consistent adverse effects. Hence, the damage they could cause in people with a variety of diseases is prevented by such treatments, but no obvious function for eosinophils in an otherwise healthy subject is unveiled through eosinophil depletion[29]. This does not necessarily prove that eosinophils have become obsolete in the course of evolution; it nevertheless suggests that beneficial functions for eosinophils might be relevant in very specific conditions which have not yet been addressed in these previous studies. Two such examples in the recent literature are a beneficial role for eosinophils in liver regeneration[31] and a role for eosinophils in the recruitment of other leukocyte classes in response to CCL11[32].
Eosinophils are very suitable for the experimental analysis of stress granulopoiesis in mice, for a number of reasons.
In mice and humans, eosinophils are easy to recognize and to detect in tissues, by a combination of surface marker expression and morphological criteria, including cytochemical reactions. Although nuclear morphology is not identical between human and mouse eosinophils, they are polymorphonuclear leukocytes presenting segmented, thick bands of chromatin when mature[9,14,15,33]. In both species, they contain numerous cytoplasmic granules of various sizes, and in the mouse a coarse type of granule, displaying the characteristic affinity for the acidic dye eosin, also stains positive in the cytochemical reaction for eosinophil peroxidase (EPO), which yields a brown color because of precipitated diaminobenzidine product formed in the presence of exogenous H2O2[34]. Murine EPO, unlike its homologues in other species, including humans[35], is resistant to cyanide, which makes this reaction a very useful marker for mouse eosinophils, as distinct from mouse neutrophils. Experimentally, the expression of a characteristic array of surface markers, including the receptor for the lineage-selective chemokine, CCL11 (or eotaxin-1), CCR3[36], as well as the cell surface lectin Siglec-F or Siglec-8[9], makes it easier to monitor the presence of eosinophils in cell suspensions by flow cytometry.
The numbers and even the presence of eosinophils in individual animals can be manipulated conveniently in various murine models. Allergic sensitization and challenge, helminth infection, IL-5 overexpression and/or administration, IL-9[37] and more recently IL-33 infusion[38], have all been reported to induce eosinophilia in mice[9,14,22,39]. Eosinopenia, the opposite state, can be induced in variable degrees by a variety of neutralizing antibodies to IL-5[22,40] or by induced mutations, including selective deletion of an internal autoamplification site in the promoter of the GATA-1 transcription factor, through which the coding regions of the GATA-1 gene remain functionally intact and sufficient for differentiation of erythrocytes and platelets, while eosinophil production, which requires an early autoamplification step, fails on a permanent basis[41]. Even more conveniently for the experimenter, this model is suitable for reconstitution, since mature eosinophils can be transferred from wild type (BALB/c) control donors in high purity[32]. Alternative models, based on selective and conditional elimination of eosinophils by genetic engineering, have provided important insights in other experimental models[27,28,42]. It is reassuring that no obvious damage to the organism is associated with eosinophilia or eosinopenia per se, as documented even in IL-5 transgenic models. By contrast, damage to heart and nervous tissue, and extremely high eosinophilia, not induced by an external agent, coexist in humans with the so-called hypereosinophilic syndromes; there is, however, extensive evidence that, in these conditions, eosinophils are functionally activated and possibly abnormal[9,20]. In murine models[39], by contrast, no damage secondary to the induction of eosinophilia is likely to confound the interpretation of results.
Usually, marked changes in eosinophil counts occur in bone-marrow, spleen or blood without significant changes in total cell counts. This apparent selectivity is due to eosinophils being a minority[9,14], amounting to 3% or less of circulating leukocytes in noninfected, nonallergic humans, for instance, while most other leukocyte populations are much larger (compare with up to 70% neutrophils in human blood). To reach significance, a much larger change in other leukocyte populations would be needed, because random fluctuation is larger in this case. Eosinophils have a specialized growth factor (IL-5), and differ from other leukocyte types in responses to other cytokines and mediators of inflammation, including rather selective chemoattractants, such as CCL11[33] and the cysteinyl-leukotrienes (CysLT)[43]. All of these differences contribute to the relative selectivity of the effects observed. Nevertheless, some stimuli, such as GM-CSF, elicit major responses in several hemopoietic lineages at once, including eosinophils and neutrophils[44]. It is relevant, in this context, that GM-CSF and IL-5 receptors, although distinct in their composition, share an essential signaling component, the common β chain (βc[13,44]). This subunit is also found in IL-3 receptors, although in mice there is evidence for a separate IL-3 receptor lacking βc[13]. Eosinophils and neutrophils present GM-CSF and IL-3 receptors bearing βc, while eosinophils (and basophils[42]) have IL-5 receptors, unlike neutrophils[8,13]. This implies that even though GM-CSF, IL-3 and IL-5 can stimulate eosinophil production through similar signaling pathways (mediated by βc), IL-5, unlike GM-CSF and IL-3, should not directly induce neutrophil production.
We have first described the upregulation of eosinophil production in murine bone-marrow in mice sensitized and challenged with allergen in the airways. In this murine model of allergic eosinophilia, the critical stimulus is specific allergen challenge in the airways[22] or in alternative sites[45], and the major outcome is an increase in bone-marrow eosinophil-lineage cells (eosinophil peroxidase positive, or EPO+, cells) in bone-marrow harvested 24 h after challenge, which is taken as direct evidence of allergen-induced eosinophilia of the bone-marrow in vivo. To keep a focus on bone-marrow events, we will not discuss here extramedullary effects of allergen challenge, such as the accumulation of eosinophil progenitors in the lungs[46] and the large increase in eosinophils in the spleen[47]. Nevertheless, these are interesting in themselves and share important mechanisms with events in bone-marrow[47].
Challenge-induced bone-marrow eosinophilia can be fully prevented in mice made specifically tolerant to the allergen before sensitization and challenge, as well in recipients of splenic T cells from these tolerized donors[48]. It is important, however, that the tolerance induction oversteps the boundaries of the original phenomenon, as tolerance-induced changes also affect neutropoiesis and therefore extends to another lineage[48].
This sensitization/challenge experimental setting provides a wealth of experimental opportunities, which have been explored in recent years. Because of the rapidity with which events in the airways translate into distant consequences in the bone-marrow, we hypothesized that a mediator released in the circulation would account for communication between the sites of challenge and of eosinopoiesis. Plasma transfer experiments from sensitized/challenged donors to naive recipients did support this view[22].
While in vivo observations suggest the relevance of the phenomenon, important information was provided by ex vivo protocols, defined as the in vitro analysis of changes induced by previous interventions in vivo. The outcome of in vivo interventions and the associated ex vivo observations is summarized in Table 1. In addition to rapidly inducing eosinophilia of bone-marrow in vivo (24 h), challenge also increases the magnitude of the responses of bone-marrow cells to IL-5. This effect was described as “priming” because it takes place in vivo, during the 24 h that follow challenge, but it remains undetected until cells are exposed to IL-5 in culture for several days - hence it corresponds to a silent change in developmental potential that is unveiled by subsequent IL-5 exposure[22,45]. It is analogous, but not identical, to priming for other cellular responses by exposure to IL-5 itself[49], since it distinguishes between in vivo allergen-challenged and -unchallenged sensitized mice, which do not necessarily differ in their circulating IL-5 levels[22]. The endpoint that defines priming is in vitro differentiation of precursors that had been exposed in vivo to allergen and presumably to newly released IL-5 as well. Nevertheless, these precursors are not IL-5-autonomous, since they do not complete differentiation in culture if exogenous IL-5 is not added[22]. This apparent paradox suggests that even though endogenous IL-5 has been shown be present in vivo in the bone-marrow after challenge[21], it is not sufficient to sustain eosinophil production over a week’s culture.
| In vivo treatment | Bone-marrow effects | Systemic factors | Ref. | ||||||
| In vivo bone-marrow Eosinophilia | Ex vivo effects on response to GM-CSF (CFU counts) in bone-marrow culture | Ex vivo effects of challenge on responses to IL-5 or CysLT in bone-marrow culture | GC measurements and interventions targeting GC | ||||||
| Total | Eosinophil | Priming | Maturation of eosinophils in culture | Regulation of CysLT responses | Blockade by GC targeting | Plasma corticosterone | |||
| None | Baseline | NA | NA | NA | Complete | NA | No effect | Baseline | [36,52] |
| Dexamethasone (5-20 mg/kg) | Baseline (BALB/c) | Increase | Increase | Primed by 24 h of injection | Incomplete, rescued by PGE2, anti-α4 integrin antibody | NE | RU486 | NA | [51,36] |
| Increased (C57BL/6) | Increase | NE | NE | NE | NE | RU486 | N. A. | [53] | |
| Surgical trauma | Increased (BALB/c) by 24 h of trauma | NE | NE | Primed by 24 h of trauma | Complete | NE | RU486 Metirapone | Stress level by 24 h of trauma | [52] |
| Sensitization and challenge | Increased (BALB/c, B6, BP-2) by 24 h of challenge | No significant increase | Increased by 24 h of challenge | Primed by 24 h of challenge | Complete | Attenuated by 24 h of challenge | RU486 Metirapone (eosinophilia, priming) | Stress level by 24 h of challenge (BALB/c), TNF- induced | [22,25,45, 47,50,54,70] |
| Oral tolerance induction, sensitization and challenge | Increase prevented (BALB/c, BP-2) by 24 h of challenge | Increased by 24 h of challenge | Increased by 24 of challenge | Priming prevented (BP-2) by 24 h of challenge | Complete | NE | NA | NE | [48] |
Importantly, priming is a positive phenomenon: It rapidly increases the magnitude of the responses to IL-5 as well as to other eosinopoietic stimuli, such as IL-3[22]. Therefore, it is assumed to contribute to the eosinophilia of allergic disease, rather than to oppose it. It is detectable as early as 24 h and as long as one week after challenge of sensitized mice.
Priming, however, is paralleled by a distinct ex vivo effect[50], which we call immunoregulation of pharmacological responses, because it reduces the magnitude of the subsequent responses of cultured bone-marrow to some drugs, as well as to exogenously provided mediators and cytokines. In contrast to priming, which changes the response to the primary stimulus (IL-5), immunoregulation of pharmacological responses attenuates the response to secondary stimuli, such as nonsteroidal antiinflammatory drugs (NSAID) and proallergic cytokines, all of which require IL-5 to be effective. Hence, priming upregulates a core response to IL-5; by contrast, immunoregulation restricts a peripheral response to modifiers of IL-5 activity. While priming is assumed to promote an eosinophilic response, immunoregulation should, in principle, restrict further expansion of eosinophil numbers by exposure to other stimuli. These two effects do not cancel each other, but may interact, depending on the precise stimulation context and on their relative timing.
Indomethacin and aspirin, two NSAID with biochemically distinct mechanisms of action, proved stimulatory not only for IL-5-driven eosinopoiesis in bone-marrow culture, but for colony formation by myeloid progenitors of several lineages as well[50]. Unexpectedly, however, when bone-marrow from sensitized and challenged mice was studied, the ability of both NSAID to enhance eosinopoiesis was lost[50], and, depending on the experimental conditions, NSAID can even become inhibitory in this assay (manuscript in preparation). Therefore, immunoregulation of pharmacological responses comprises two aspects: (1) attenuation of the effectiveness of NSAID as enhancers of eosinopoiesis; and (2) inversion of its effects, leading to active suppression of eosinopoiesis when NSAID are present. Because pharmacological responses are usually not assumed to depend on the immune status of the organism, this apparently paradoxical observation has theoretical interest in itself. In its original description[50], no physiological relevance was ascribed to it. More recent results, as detailed below, substantially increased the scope of this effect and highlighted its potential to modulate eosinophilia in a biologically more relevant context.
The twin phenomena of priming and pharmacological immunoregulation highlight two important features of extrinsic control of bone-marrow: (1) changes can be both silent and durable, as in priming, thereby accounting for effects that may become visible only in the long-term; and (2) the apparent paradox of a drug response that depends on the immune status of the organism - challenged vs unchallenged mice - reflects the mechanism of action of the drug as well as the inflammatory events elicited by challenge. Both priming and immunoregulation remain silent effects, until they are unveiled by the appropriate stimuli (exposure to IL-5, in the first case; or to NSAID in the presence of IL-5, in the second). In both cases, challenge changes the properties of the target cell.
A central contribution of glucocorticoids (GC) to extrinsic bone-marrow regulation was first reported in a pharmacological setting, following exposure to dexamethasone, both in vivo and in vitro[51]. Subsequent experiments in a sterile trauma model indicated that stress-induced GC (corticosterone, in mice) selectively induced bone-marrow eosinophilia in the absence of specific immune responses[52]. Finally, an essential role for corticosterone in allergen-induced bone-marrow eosinophilia was also demonstrated in a sensitization/challenge model[45], which necessarily involves specific immunity. Hence, evidence of a link between GC and bone-marrow eosinophilia was consistently provided by experiments ranging from pharmacological through physiological to immunological settings, which are also summarized in Table 1. This coherence of effects is to be expected from the well-established fact that GCs, synthetic or natural, act through the same receptor, which is blocked by RU486 (mifepristone[45,51-53]).
Dexamethasone did not induce bone-marrow eosinophilia in vivo in our original study, which used mice of the BALB/c background; however, it did prime bone-marrow for strongly enhanced responses to IL-5 ex vivo over a period of from 24 h[51] up to 4 wk after injection (manuscript in preparation); more recently, however, an important difference between strains of distinct backgrounds was observed for this drug effect, since bone-marrow eosinophilia was observed in C57BL/6 mice injected with dexamethasone, 24 h after injection, unlike BALB/c controls[53]. In both BALB/c and C57BL/6 mice, dexamethasone primed bone-marrow for increased eosinophil production in IL-5-stimulated cultures; dexamethasone did not replace IL-5 as a primary eosinopoietic stimulus, but greatly enhanced its effectiveness. However, dexamethasone significantly modified IL-5 effects, since a large fraction of the eosinophils produced in dexamethasone-exposed BALB/c cultures were cytologically immature and formed extensive homotypic aggregates[36,51], none of which had been observed in preceding studies of BALB/c[22,45] or C57BL/6[54] sensitized/challenged mice. Further studies[36] demonstrated the ability of PGE2 to synergize with dexamethasone in promoting terminal cytological maturation of these eosinophils in BALB/c bone-marrow cultures. Because neutralizing antibodies to VLA-4 (CD49; α4β1 integrin) were able to dissociate the homotypic aggregates formed in dexamethasone-exposed cultures, leading to an increased recovery of fully mature eosinophils, we hypothesized that homotypic aggregation interfered with terminal maturation, and that release from aggregates allowed terminal maturation to proceed. Accordingly, PGE2 was shown to dissociate the same aggregates, through an effect on α4β1 integrin expression[36].
By contrast, in the trauma model[52], the physiologically relevant GC, corticosterone, was elevated to stress levels 24 h after surgery and selectively induced eosinophilia in the bone-marrow, as well as primed for increased IL-5-dependent eosinopoiesis. These effects of trauma-induced GC were long-lasting, and significant at least for two weeks after surgery[52]. The direct contribution of glucocorticoids in this model was documented by blocking with RU486, as had been previously done with dexamethasone[51], and confirmed by two other independent approaches (metirapone treatment and adrenalectomy followed by trauma after a recovery period).
Unlike the response to dexamethasone injection, it is likely that the response to trauma adds to the GC surge other variables related to cell injury and innate immunity. One important such variable, is tumor necrosis factor-α (TNF-α), which may interact with corticosterone so as to modify its actions, in a way consistent with the differences observed between the pharmacological (dexamethasone) and the physiological (trauma) models, especially in the induction of bone-marrow eosinophilia and on cytological maturity of the eosinophils.
Finally, we recently demonstrated that the eosinophilia induced by challenge in sensitized mice involves endogenous GC[45], which are induced by a product of immune cell activation, TNF-α, because: (1) eosinophilia is abolished with equal effectiveness by RU486 or by anti-TNF-α neutralizing antibody; and (2) a corticosterone surge, reaching stress levels, is observed in wild-type controls, with or without RU486-pretreatment, but not in TNF-α type I receptor-deficient (TNFRI-KO) mice.
Challenge-induced eosinophilia is sustained by IL-5 acting in vivo[21,22]; by contrast, priming requires endogenous GC to act in vivo to prime for an increased ex vivo response to IL-5 upon subsequent culture[45]. Although IL-5 has usually been considered the target of changes initiated by challenge, there is evidence that responses to IL-5 are self-limiting themselves, since exposure to IL-5, IL-3 or GM-CSF, presumably acting through βc, was shown to down-regulate IL-5Rα chain expression[55], thereby reducing IL-5 binding and strength of stimulation. Similar observations were reported with other extrinsic regulators, such as all-trans retinoic acid, which suppresses expression of IL-5Rα in culture of human hemopoietic cells[56]; in murine eosinopoiesis, the effects of all trans retinoic acid are effectively blocked by GC (Xavier-Elsas et al, submitted).
Together, these observations, summarized in Table 1, are consistent with the reported ability of TNF-α as well as IL-1β, both major inflammatory cytokines, to mediate, in animal models, an immunoendocrine response to tissue damage with stress-levels of adrenal GC[57]. They are equally consistent with the reported link between elevated levels of cortisol in humans subjected to stress and an increase in the frequency and severity of allergic reactions[58,59]. Against this background information, the extrinsic upregulation of bone-marrow eosinophilia by dexamethasone, trauma and allergy in our own studies is better explained as a paradoxical stimulatory effect of GC on progenitors and precursors of the eosinophil lineage. While this may go against the predominant view of GC effects in allergy[57,60-62], it is a reproducible effect with pathophysiological implications[58,59,63-65], which may appear less paradoxical as a result of its interaction with the 5-lipoxygenase (5-LO) pathway of arachidonate metabolism, as detailed below.
The 5-LO pathway, which produces leukotrienes (LT), has been intensively studied over three decades in the context of allergic disease[66,67]. While its involvement in the functional abnormalities of the airways in asthma is well-established, its roles in extrinsic regulation of bone-marrow remain incompletely explored, even though bone-marrow is believed to be central to chronic inflammation in asthma[12,24]. LT, especially CysLT (a class comprising LTC4, LTD4 and LTE4), besides making important contributions to asthma pathophysiology, have significant pharmacological effects on hemopoietic cells[68,69]. Such effects are of special interest in the case of eosinophils, which both produce and respond to LT[43], and play important roles in allergic disease. Type 1 CysLT receptors (CysLT1R) play important roles in the pathophysiology of human and experimental asthma, and CysLT1R antagonists, such as pranlukast, zafirlukast and montelukast, are currently approved for the treatment of asthma[47,66,67]. CysLT1R are expressed in several cell populations in the bone-marrow[68], and CysLT were shown to enhance colony formation by progenitors of different myeloid lineages in addition to eosinophils[69].
The stimulatory effect of NSAID on eosinopoiesis is of special interest not only because it is subject to immunoregulation of pharmacological responses, but because of what it tells us about the roles of COX and 5-LO in bone-marrow regulation.
NSAID, which block the cyclooxygenase (COX) pathway, were originally tested in the context of the effects of prostaglandin E2 (PGE2), a COX product, in murine bone-marrow culture. Eosinopoiesis is significantly suppressed by exogenously added PGE2 in bone-marrow cultures established from allergen-challenged mice, as well as from unchallenged controls. This suppressive effect of PGE2, which is unaffected by allergen challenge, is not surprising in itself, because nonselective inhibitory effects of PGE2 on hemopoiesis in vitro have long been known[36], and suppression of eosinopoiesis would seem to be just one particular example of this general phenomenon. On the other hand, if NSAID were working solely as COX inhibitors, they should be ineffective against exogenously added PGE2, which bypasses the COX pathway to act directly on PGE2 receptors. However, we showed that NSAID prevent the suppressive effects of PGE2 on eosinopoiesis, and further stimulate eosinophil production strongly (in this case, the eosinophils are cytologically mature)[70]. This rules out a mechanism involving only COX inhibition, which cannot protect against preformed COX products. Furthermore, blockade of the 5-LO pathway by genetical or pharmacological approaches abolishes the effectiveness of NSAID in promoting eosinopoiesis, which implies a 5-LO-mediated mechanism, quite distinct from simple COX inhibition. This alternative mechanism was shown to depend on CysLT, endogenously produced in bone-marrow culture, as evidenced by its absence in LTC4 synthase-deficient mice, and by its blockade by CysLT1R antagonists and CysLT1R deletion. In support of this view, exogenously added CysLT strongly stimulate eosinopoiesis, even in the absence of functional 5-LO or LTC4 synthase[70].
Importantly, this same CysLT-mediated mechanism was subsequently shown to underlie the stimulatory effects of eotaxin/CCL11 and IL-13, two cytokines central to allergic inflammation, on eosinopoiesis in naive bone-marrow culture[25]. Again, both depend strictly on functional 5-LO and CysLT1R to enhance eosinophil production, and both lose effectiveness when bone-marrow from sensitized-challenged mice is used.
It is clear, therefore, that extrinsic regulators of bone-marrow eosinopoiesis may be subject to immunoregulation (NSAID, proallergic cytokines) or not (PGE2), depending on their mechanism of action.
Since IL-13 and eotaxin are produced during allergic episodes and present systemic effects[15,33,40], this suggests that CysLT in bone-marrow are proximal elements in a chain of events started by a distant allergic reaction, and therefore might play a role in the strong upregulation of eosinophil production following challenge. Consequently, one might predict that targeting CysLT production or signaling with drugs currently in use (respectively zileuton for production and montelukast and its analogues for signaling), would not only be beneficial in attenuating local allergic symptoms, but also in preventing increased eosinophil production. Such an effect of pranlukast has been previously reported in humans[71].
In ovalbumin-sensitized mice, we have observed the complete blockade of challenge-induced eosinophilia of the bone-marrow using both the leukotriene synthesis inhibitor diethylcarbamazine (DEC)[54] and 5-LO-activating protein inhibitor MK-886[47], which prevent production of CysLT; the same effect was observed with montelukast, which blocks CysLT1R[47]. Consistently with this hypothesis, DEC had no effect in mice lacking functional 5-LO. Together, this evidence supports an essential role for CysLT in challenge-induced eosinophilia, similar but distinct from that previously attributed to endogenous GC.
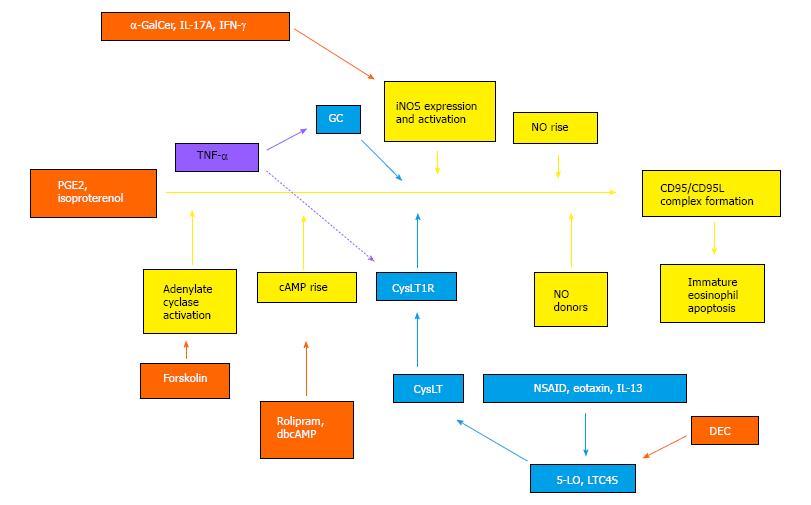
Further insight on the underlying cellular mechanisms is provided by the effects of DEC. Interestingly, DEC requires not only 5-LO to be effective[47] but inducible NO synthase (iNOS) as well[54]. This enzyme, which produces large amounts of NO in the course of cellular immune responses to a number of intracellular pathogens, had already been shown to be required for the suppressive effects of PGE2 on bone-marrow eosinopoiesis[72]; more recently, it was shown to account for similar effects of α-galactosylceramide (α-GalCer; an anticancer agent and immunomodulator)[23] and IL-17 (a powerful proinflammatory cytokine)[26] on bone-marrow. These observations, therefore, establish DEC as a pharmacological link between 5-LO and iNOS in bone-marrow, as discussed below. It should be noted that GC powerfully suppress iNOS expression[72], and this underlies their ability to block the suppressive effect of PGE2: In vitro, when iNOS expression is blocked by dexamethasone, this GC interacts with PGE2 to increase the production of mature eosinophils in culture[36], a somewhat unexpected interaction between an anti-inflammatory drug and a proinflammatory mediator.
It is therefore important that CysLT can counteract the effects of both IL-17[26] and α-GalCer[23] on eosinopoiesis, just as CysLT counteract those of PGE2[70]. Like GC, therefore, CysLT target the iNOS-CD95-dependent proapoptotic mechanism that suppresses eosinopoiesis, as part of their eosinophilia-promoting actions.
Together, the available evidence suggests that challenge-induced eosinophilia in the bone-marrow is associated with both iNOS suppression (by GC) and 5-LO-mediated mechanisms; by contrast, its prevention is associated with iNOS-mediated mechanisms and blockade of 5-LO. It is therefore important to understand how these regulatory and effector elements (GC, 5-LO, iNOS) relate to each other.
If immunoregulation of responses to NSAID involved modulation of the COX pathway as opposed to the 5-LO pathway, responses to exogenously added CysLT in the bone-marrow would not depend on the immune status of the mouse. However, just like for NSAID, responses to LTD4 are strongly immunoregulated in murine bone-marrow (manuscript in preparation). This suggests that challenge not only requires CysLT to increase eosinophil production, it also profoundly attenuates the effectiveness of CysLT, thereby limiting the magnitude of responses to drugs and cytokines which are mediated by endogenous CysLT.
Because the effects of challenge on bone-marrow are counteracted with similar effectiveness by blockade of endogenous GC signaling, and by blockade of CysLT1R signaling, this raises the issue of the relationship of endogenous GC to CysLT in the context of sensitization and challenge.
While the similar actions of endogenous GC and CysLT might seem unrelated or even incompatible, recent studies point to a critical partnership of these mediators in vivo. This prompted us to address in the following section how these quite dissimilar classes of extrinsic regulators might work together to induce eosinophilia in murine bone-marrow, and to further limit the magnitude of this response to challenge through subtle regulatory mechanisms.
The observations summarized above may appear paradoxical in several respects: (1) GC are usually thought of as antiallergic agents, not as promoting allergy; (2) GC are believed to suppress the generation of lipid mediators from arachidonate metabolism (eicosanoids) and should accordingly prevent the generation of CysLT; (3) even though GC are essential to the effects of challenge on the bone-marrow, dexamethasone alone cannot reproduce all of these effects; (4) GC (anti-allergic agents) and CysLT (proallergic agents) seem to elicit the same outcome - increased eosinophil production - and therefore constitute a highly unlikely couple; and (5) the effects of CysLT, furthermore, are attenuated after challenge, and may even become suppressive, in a clear inversion of the original signal provided by these mediators.
GC and CysLT form indeed an odd couple: GC are widely used as anti-inflammatory agents and for the long-term maintenance in asthma control; by contrast, CysLT account for some of the most visible manifestations of asthma and allergy, and CysLT antagonists are useful for asthma control. So GC and CysLT would be expected to be natural antagonists, not partners. However, the eosinopoiesis-enhancing effects of dexamethasone are observed at lower concentrations that its anti-inflammatory and anti-allergic effects[51], and are compatible, in terms of glucocorticoid activity, with the GC surges associated with acute stress[45,52]. So, GC promotion of eosinopoiesis by dexamethasone might be dose-dependent and self-limiting over time.
This priming effect of GC is reproduced by surgical trauma in the absence of allergen sensitization[52], and is therefore independent of underlying allergic processes. When the relevant GC, corticosterone, is released by challenge of sensitized mice, this release depends on TNF-α type 1 receptors, and is therefore part of the nonspecific host response to aggression, mediated by proinflammatory cytokines[45]. The effect of challenge seems to last about one week[54], although surgical trauma has a longer-lasting impact on bone-marrow[52], and dexamethasone may have a priming effect demonstrable in bone-marrow culture as long as one month after a single injection (manuscript in preparation). Our interpretation is that the duration of GC effects is significantly curtailed by factors operating in vivo during trauma or allergic challenge, and, in a sense, this makes the extrinsic regulation of bone-marrow eosinopoiesis by allergen challenge a self-limiting process.
On the other hand, the results of complete prevention of challenge-induced eosinophilia by a variety of interventions that target CysLT production or signaling (DEC, MK886, 5-LO deficiency; montelukast) can only be understood if one assumes that despite the elevation in endogenous GC levels induced by challenge[45], the 5-LO pathway is operative and CysLT are produced. Resistance of CysLT to GC even at therapeutic levels has been reported in human studies[67] and underlies the rationale for using antileukotrienes as complementary to GCin asthma control[62,66,67].
If both endogenous GC and CysLT are present in vivo after challenge, what is their relationship? They might be present simultaneously, but at different, unconnected sites and therefore act independently of each other; alternatively, they might be coupled. Prevention of challenge-induced eosinophilia by GC or by CysLT blockade to the same extent might seem to argue against either having an independent effect on bone-marrow, since one would logically expect an additive effect of blocking both targets, which is not observed. However, bone-marrow of naive mice[70], or from sensitized mice pretreated with RU486 before challenge (manuscript in preparation), does respond to CysLT in culture in the absence of exogenously added GC. Also, bone-marrow from unsensitized C57BL/6 mice shows eosinophilia in vivo after dexamethasone administration[53], in the absence of any known CysLT inducer, just as dexamethasone enhances eosinopoiesis in culture without addition of CysLT. The effectiveness of both partners in the absence of the other is thus established, showing that they have independent pharmacological effects outside the framework of sensitization/challenge. Nevertheless, blocking either inside this framework achieves full prevention of the effects of challenge. This suggests that during challenge they become functionally coupled in vivo, which does not necessarily occur following dexamethasone administration. Coupling is here characterized by continuity in time (one event follows the other) and by dependence of the latter event on the former (blockade of the former event prevents the latter). However, it is not synonimous with causality in the usual sense: The first event might be just permissive for the second, not necessarily its immediate cause. Coupling is a term applicable to events that take place at separate moments in separate sites, just as it is to events that take place at separate moments in very close sites or even at exactly the same site, provided the second event is reproducibly prevented by blockade of the first. These distinct possibilities are illustrated by the two modes of cytokine action discussed in section 2.
Our hypothesis (coupling of GC and 5-LO in response to challenge) is consistent with the observed difference between the effects of challenge and of in vivo exposure to dexamethasone in the BALB/c strain. Challenge induces eosinophilia and primes for better responses to IL-5. Dexamethasone does not induce eosinophilia, but does good priming. Challenge effects are GC-dependent, with both eosinophilia and priming being abolished by RU486. Hence, even though GC are central to challenge, there is a hitherto unidentified factor present in vivo during challenge in addition to elevated GC, which modifies the ultimate effects of GC on bone-marrow, by coupling GC to CysLT. Below we develop the hypothesis that this unidentified factor is TNF-α, also produced in the course of challenge, and capable of inducing both GC[45] and CysLT[73,74].
Further insight can be provided by a comparison of dexamethasone and challenge: Dexamethasone-exposed eosinophils are cytologically immature and show no resemblance to circulating eosinophils[36,51]; challenge-induced eosinophils[22], as well as those induced by CysLT in vitro[70], are fully mature. In a sense, dexamethasone is an incomplete enhancer of eosinopoiesis, for it increases the number of eosinophil-lineage cells but prevents their full maturation. The maturation sequence, which involves downregulation of α4 integrin in dexamethasone-exposed immature eosinophils, can be completed in the presence of PGE2[36] or IL-17[26] in vitro, just as it is completed in the presence of CysLT in vivo[47].
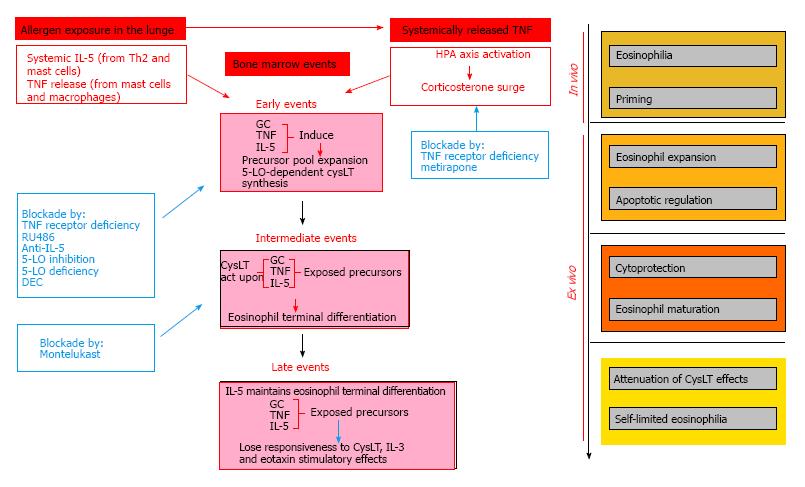
To reconcile all of these apparent paradoxes, we propose a model in flow chart format (Figure 1) which has the following essential tenets: (1) GC and CysLT become functionally coupled in vivo as a consequence of allergen challenge, so that TNF-α-dependent GC signaling makes it possible for CysLT generated locally in the context of allergic challenge to induce bone-marrow eosinophilia; (2) because the upstream permissive element (GC) and the downstream effector element (CysLT) are coupled, blocking the former will prevent the actions of the latter; (3) as a result of this coupling, full maturation of the eosinophils produced can be achieved in vivo after challenge; and (4) nevertheless, this mechanism is not operating unchecked in vivo, for its operation makes it less effective in subsequent rounds of allergen challenge, as shown by the attenuation of the proallergic effects of CysLT ex vivo, and by the observation that the positive effects of GC on the eosinophil lineage tend to less marked following chronic (repeated challenge) than acute (single challenge) exposures. Attenuation of CysLT effects ex vivo in murine bone-marrow would be consistent with observations that allergen challenge leads to a large increase in CysLT production in humans, and that a mechanism of desensitization to CysLT effects may limit the untoward effects of these potent bronchoconstrictors[75], although at present it is unclear whether and how this applies to hemopoietic effects.
In our model, we propose a role for TNF-α, not only in inducing GC production through HPA axis activation, but in coupling GC surge to CysLT production. The hypothesis of a complex relationship of TNF-α produced after challenge to actions of GC and CysLT is illustrated in Figure 2, which provides a graphical abstract of the essential biochemical events identified so far in the extrinsic regulation of bone-marrow eosinopoiesis by allergen challenge, drugs and cytokines. Coupling of GC to CysLT is portrayed, based on the available evidence, as a transient relationship between events taking place within a single cell target (eosinophil precursor inside the bone-marrow), since these events all impinge upon a signaling sequence that begins at surface receptors and ends at apoptotic cell death[72,73] which is limited to immature eosinophils and their immediate precursos[36]. In this continuous sequence of signaling events, GC and CysLT act as suppressors of apoptosis (indicated in light blue colored boxes and arrows) by blocking distinct signaling steps upstream from iNOS; conversely, inhibitors of CysLT production or action, including DEC, promote apoptosis (indicated in orange-colored boxes and arrows, for DEC as well as a wide panel of pharmacological agents which act at CysLT-unrelated steps) by acting on targets upstream from iNOS[23,26,45,47,54,70,72,73]. In addition to its systemic effects on adrenal release of GC during allergen challenge, which are not shown in the picture, TNF-α is hypothesized to have separate effects on GC and CysLT-mediated responses: A constitutive effect permissive for GC action on eosinophil precursors (solid lavender line), and an adaptive (coupling) effect permissive for GC control of CysLT responses in the same cell target (discontinuous lavender line). TNF-α has been reported by others to induce CysLT production and the expression of critical enzymes in CysLT biosynthesis; in addition, LTD4 duplicates these effects, providing a mechanism for amplification of TNF-α actions[74]. It at present is unclear whether these observations from other groups apply to bone-marrow, and, if so, how TNF-α induction of CysLT might be dependent on GC signaling.
To test the validity of these models, some points are critical, foremost the definition of the site, timing and mechanism of coupling of GC to CysLT, and of the role played by TNF-α therein. To define the mechanisms of attenuation of CysLT-dependent response is equally essential, including the roles played by GC hormones themselves and by changes in CysLT receptor types, expression or intracellular signaling. These steps should help us put in proper perspective the paradoxical enhancement of eosinophil production by GC, which, despite being at odds with the prevailing views of the contributions of GC and eosinophils to immune responses, is likely to shed some light on the puzzle of stress-related mechanisms in allergic disease.
| 1. | Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2046] [Cited by in RCA: 1897] [Article Influence: 105.4] [Reference Citation Analysis (0)] |
| 2. | Kaimakis P, Crisan M, Dzierzak E. The biochemistry of hematopoietic stem cell development. Biochim Biophys Acta. 2013;1830:2395-2403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Metcalf D. Some general aspects of hemtopoietic cell development. In Zon L (Ed.). Hematopoiesis. A developmental approach. Oxford University Press. New York: Oxford 2001; 3-14. |
| 4. | Kobayashi H, Suda T, Takubo K. How hematopoietic stem/progenitors and their niche sense and respond to infectious stress. Exp Hematol. 2016;44:92-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Takizawa H, Boettcher S, Manz MG. Demand-adapted regulation of early hematopoiesis in infection and inflammation. Blood. 2012;119:2991-3002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 332] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 6. | Bronte V, Pittet MJ. The spleen in local and systemic regulation of immunity. Immunity. 2013;39:806-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 800] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 7. | Simon HU. Regulation of eosinophil and neutrophil apoptosis--similarities and differences. Immunol Rev. 2001;179:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol. 2014;9:181-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 979] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 9. | Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1070] [Cited by in RCA: 1141] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 10. | Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 756] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 11. | Rodriguez S, Chora A, Goumnerov B, Mumaw C, Goebel WS, Fernandez L, Baydoun H, HogenEsch H, Dombkowski DM, Karlewicz CA. Dysfunctional expansion of hematopoietic stem cells and block of myeloid differentiation in lethal sepsis. Blood. 2009;114:4064-4076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 12. | Denburg JA, Keith PK. Eosinophil progenitors in airway diseases: clinical implications. Chest. 2008;134:1037-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Nishinakamura R, Miyajima A, Mee PJ, Tybulewicz VL, Murray R. Hematopoiesis in mice lacking the entire granulocyte-macrophage colony-stimulating factor/interleukin-3/interleukin-5 functions. Blood. 1996;88:2458-2464. [PubMed] |
| 14. | Kita H. Eosinophils: multifaceted biological properties and roles in health and disease. Immunol Rev. 2011;242:161-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 237] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 15. | Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125:S73-S80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 977] [Cited by in RCA: 905] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 16. | Pietras EM, Reynaud D, Kang YA, Carlin D, Calero-Nieto FJ, Leavitt AD, Stuart JM, Göttgens B, Passegué E. Functionally Distinct Subsets of Lineage-Biased Multipotent Progenitors Control Blood Production in Normal and Regenerative Conditions. Cell Stem Cell. 2015;17:35-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 561] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 17. | Barminko J, Reinholt B, Baron MH. Development and differentiation of the erythroid lineage in mammals. Dev Comp Immunol. 2016;58:18-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Makepeace BL, Martin C, Turner JD, Specht S. Granulocytes in helminth infection -- who is calling the shots? Curr Med Chem. 2012;19:1567-1586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Klion AD, Nutman TB. The role of eosinophils in host defense against helminth parasites. J Allergy Clin Immunol. 2004;113:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 321] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 20. | Takatsu K, Nakajima H. IL-5 and eosinophilia. Curr Opin Immunol. 2008;20:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 260] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 21. | Tomaki M, Zhao LL, Lundahl J, Sjöstrand M, Jordana M, Lindén A, O’Byrne P, Lötvall J. Eosinophilopoiesis in a murine model of allergic airway eosinophilia: involvement of bone marrow IL-5 and IL-5 receptor alpha. J Immunol. 2000;165:4040-4050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Gaspar Elsas MI, Joseph D, Elsas PX, Vargaftig BB. Rapid increase in bone-marrow eosinophil production and responses to eosinopoietic interleukins triggered by intranasal allergen challenge. Am J Respir Cell Mol Biol. 1997;17:404-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 109] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Gaspar-Elsas MI, Queto T, Masid-de-Brito D, Vieira BM, de Luca B, Cunha FQ, Xavier-Elsas P. α-Galactosylceramide suppresses murine eosinophil production through interferon-γ-dependent induction of NO synthase and CD95. Br J Pharmacol. 2015;172:3313-3325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Baatjes AJ, Sehmi R, Saito H, Cyr MM, Dorman SC, Inman MD, O’Byrne PM, Denburg JA. Anti-allergic therapies: effects on eosinophil progenitors. Pharmacol Ther. 2002;95:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Queto T, Gaspar-Elsas MI, Masid-de-Brito D, Vasconcelos ZF, Ferraris FK, Penido C, Cunha FQ, Kanaoka Y, Lam BK, Xavier-Elsas P. Cysteinyl-leukotriene type 1 receptors transduce a critical signal for the up-regulation of eosinophilopoiesis by interleukin-13 and eotaxin in murine bone marrow. J Leukoc Biol. 2010;87:885-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Xavier-Elsas P, de Luca B, Queto T, Vieira BM, Masid-de-Brito D, Dahab EC, Alves Filho JC, Cunha FQ, Gaspar-Elsas MI. Blockage of Eosinopoiesis by IL-17A Is Prevented by Cytokine and Lipid Mediators of Allergic Inflammation. Mediators Inflamm. 2015;2015:968932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776-1779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 656] [Cited by in RCA: 661] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 28. | Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O’Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 550] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 29. | Gleich GJ, Klion AD, Lee JJ, Weller PF. The consequences of not having eosinophils. Allergy. 2013;68:829-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 30. | Hogan SP, Waddell A, Fulkerson PC. Eosinophils in infection and intestinal immunity. Curr Opin Gastroenterol. 2013;29:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Goh YP, Henderson NC, Heredia JE, Red Eagle A, Odegaard JI, Lehwald N, Nguyen KD, Sheppard D, Mukundan L, Locksley RM. Eosinophils secrete IL-4 to facilitate liver regeneration. Proc Natl Acad Sci USA. 2013;110:9914-9919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 224] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 32. | Luz RA, Xavier-Elsas P, de Luca B, Masid-de-Brito D, Cauduro PS, Arcanjo LC, dos Santos AC, de Oliveira IC, Gaspar-Elsas MI. 5-lipoxygenase-dependent recruitment of neutrophils and macrophages by eotaxin-stimulated murine eosinophils. Mediators Inflamm. 2014;2014:102160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol. 2007;119:1303-1310; quiz 1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 310] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 34. | Horton MA, Larson KA, Lee JJ, Lee NA. Cloning of the murine eosinophil peroxidase gene (mEPO): characterization of a conserved subgroup of mammalian hematopoietic peroxidases. J Leukoc Biol. 1996;60:285-294. [PubMed] |
| 35. | Ten RM, Pease LR, McKean DJ, Bell MP, Gleich GJ. Molecular cloning of the human eosinophil peroxidase.Evidence for the existence of a peroxidase multigene family. J Exp Med. 1989;169:1757-1769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 147] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Gaspar-Elsas MI, Queto T, Vasconcelos Z, Jones CP, Lannes-Vieira J, Xavier-Elsas P. Evidence for a regulatory role of alpha 4-integrins in the maturation of eosinophils generated from the bone marrow in the presence of dexamethasone. Clin Exp Allergy. 2009;39:1187-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Sitkauskiene B, Rådinger M, Bossios A, Johansson AK, Sakalauskas R, Lötvall J. Airway allergen exposure stimulates bone marrow eosinophilia partly via IL-9. Respir Res. 2005;6:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Dyer KD, Percopo CM, Rosenberg HF. IL-33 promotes eosinophilia in vivo and antagonizes IL-5-dependent eosinophil hematopoiesis ex vivo. Immunol Lett. 2013;150:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Inman MD. Bone marrow events in animal models of allergic inflammation and hyperresponsiveness. J Allergy Clin Immunol. 2000;106:S235-S241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Chung KF. Targeting the interleukin pathway in the treatment of asthma. Lancet. 2015;386:1086-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 200] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 41. | Yu C, Cantor AB, Yang H, Browne C, Wells RA, Fujiwara Y, Orkin SH. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med. 2002;195:1387-1395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 472] [Cited by in RCA: 497] [Article Influence: 20.7] [Reference Citation Analysis (1)] |
| 42. | Matsuoka K, Shitara H, Taya C, Kohno K, Kikkawa Y, Yonekawa H. Novel basophil- or eosinophil-depleted mouse models for functional analyses of allergic inflammation. PLoS One. 2013;8:e60958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Giembycz MA, Lindsay MA. Pharmacology of the eosinophil. Pharmacol Rev. 1999;51:213-340. [PubMed] |
| 44. | Martinez-Moczygemba M, Huston DP. Biology of common beta receptor-signaling cytokines: IL-3, IL-5, and GM-CSF. J Allergy Clin Immunol. 2003;112:653-655; quiz 666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 169] [Reference Citation Analysis (0)] |
| 45. | Masid-de-Brito D, Xavier-Elsas P, Luz RA, Queto T, Almeida da Silva CL, Lopes RS, Vieira BM, Gaspar-Elsas MI. Essential roles of endogenous glucocorticoids and TNF/TNFR1 in promoting bone-marrow eosinopoiesis in ovalbumin-sensitized, airway-challenged mice. Life Sci. 2014;94:74-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 46. | Gaspar Elsas MI, Maximiano ES, Joseph D, Bonomo A, Vargaftig BB, Xavier Elsas P. Isolation and characterization of hemopoietic cells from lungs of allergic mice. Chest. 2003;123:345S-348S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Masid-de-Brito D, Queto T, Gaspar-Elsas MI, Xavier-Elsas P. Roles of 5-lipoxygenase and cysteinyl-leukotriene type 1 receptors in the hematological response to allergen challenge and its prevention by diethylcarbamazine in a murine model of asthma. Mediators Inflamm. 2014;2014:403970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Xavier-Elsas P, Silva CL, Pinto L, Queto T, Vieira BM, Aranha MG, De Luca B, Masid-de-Brito D, Luz RA, Lopes RS. Modulation of the effects of lung immune response on bone marrow by oral antigen exposure. Biomed Res Int. 2013;2013:474132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Han ST, Mosher DF. IL-5 induces suspended eosinophils to undergo unique global reorganization associated with priming. Am J Respir Cell Mol Biol. 2014;50:654-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Lintomen L, Elsas MI, Maximiano ES, de Paula Neto HA, Joseph D, Vargaftig BB, Elsas PX. Allergenic sensitization prevents upregulation of haemopoiesis by cyclo-oxygenase inhibitors in mice. Br J Pharmacol. 2002;135:1315-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 51. | Gaspar Elsas MI, Maximiano ES, Joseph D, Alves L, Topilko A, Vargaftig BB, Xavier Elsas P. Upregulation by glucocorticoids of responses to eosinopoietic cytokines in bone-marrow from normal and allergic mice. Br J Pharmacol. 2000;129:1543-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 52. | Elsas PX, Neto HA, Cheraim AB, Magalhães ES, Accioly MT, Carvalho VF, e Silva PM, Vargaftig BB, Cunha FQ, Gaspar Elsas MI. Induction of bone-marrow eosinophilia in mice submitted to surgery is dependent on stress-induced secretion of glucocorticoids. Br J Pharmacol. 2004;143:541-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 53. | Xavier-Elsas P, da Silva CL, Vieira BM, Masid-de-Brito D, Queto T, de Luca B, Vieira TS, Gaspar-Elsas MI. The In Vivo Granulopoietic Response to Dexamethasone Injection Is Abolished in Perforin-Deficient Mutant Mice and Corrected by Lymphocyte Transfer from Nonsensitized Wild-Type Donors. Mediators Inflamm. 2015;2015:495430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 54. | Queto T, Xavier-Elsas P, Gardel MA, de Luca B, Barradas M, Masid D, E Silva PM, Peixoto CA, Vasconcelos ZM, Dias EP. Inducible nitric oxide synthase/CD95L-dependent suppression of pulmonary and bone marrow eosinophilia by diethylcarbamazine. Am J Respir Crit Care Med. 2010;181:429-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Gregory B, Kirchem A, Phipps S, Gevaert P, Pridgeon C, Rankin SM, Robinson DS. Differential regulation of human eosinophil IL-3, IL-5, and GM-CSF receptor alpha-chain expression by cytokines: IL-3, IL-5, and GM-CSF down-regulate IL-5 receptor alpha expression with loss of IL-5 responsiveness, but up-regulate IL-3 receptor alpha expression. J Immunol. 2003;170:5359-5366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 56. | Upham JW, Sehmi R, Hayes LM, Howie K, Lundahl J, Denburg JA. Retinoic acid modulates IL-5 receptor expression and selectively inhibits eosinophil-basophil differentiation of hemopoietic progenitor cells. J Allergy Clin Immunol. 2002;109:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Guyre PM, Yeager MP, Munck A. Glucocorticoid Effects on Immune Responses. The Hypothalamus-Pituitary-Adrenal Axis. del Rey A, Chrousos GP, Besedovsky HO, editors. USA: Elsevier BV 2008; 147-166. |
| 58. | Liu LY, Coe CL, Swenson CA, Kelly EA, Kita H, Busse WW. School examinations enhance airway inflammation to antigen challenge. Am J Respir Crit Care Med. 2002;165:1062-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 204] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 59. | Okuyama K, Dobashi K, Miyasaka T, Yamazaki N, Kikuchi T, Sora I, Takayanagi M, Kita H, Ohno I. The involvement of glucocorticoids in psychological stress-induced exacerbations of experimental allergic asthma. Int Arch Allergy Immunol. 2014;163:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Rosenberg SL, Miller GE, Brehm JM, Celedón JC. Stress and asthma: novel insights on genetic, epigenetic, and immunologic mechanisms. J Allergy Clin Immunol. 2014;134:1009-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 61. | Wright RJ, Cohen RT, Cohen S. The impact of stress on the development and expression of atopy. Curr Opin Allergy Clin Immunol. 2005;5:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 238] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 62. | Busillo JM, Cidlowski JA. The five Rs of glucocorticoid action during inflammation: ready, reinforce, repress, resolve, and restore. Trends Endocrinol Metab. 2013;24:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 252] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 63. | Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335:2-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1343] [Cited by in RCA: 1303] [Article Influence: 86.9] [Reference Citation Analysis (0)] |
| 64. | Zen M, Canova M, Campana C, Bettio S, Nalotto L, Rampudda M, Ramonda R, Iaccarino L, Doria A. The kaleidoscope of glucorticoid effects on immune system. Autoimmun Rev. 2011;10:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 208] [Article Influence: 13.9] [Reference Citation Analysis (1)] |
| 65. | Cyr MM, Baatjes AJ, Dorman SC, Crawford L, Sehmi R, Foley R, Alam R, Byrne PO, Denburg JA. In vitro effects of budesonide on eosinophil-basophil lineage commitment. Open Respir Med J. 2008;2:60-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 66. | Balzano G, Fuschillo S, Gaudiosi C. Leukotriene receptor antagonists in the treatment of asthma: an update. Allergy. 2002;57 Suppl 72:16-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | Peters-Golden M, Sampson AP. Cysteinyl leukotriene interactions with other mediators and with glucocorticosteroids during airway inflammation. J Allergy Clin Immunol. 2003;111:S37-42; discussion S43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 68. | Bautz F, Denzlinger C, Kanz L, Möhle R. Chemotaxis and transendothelial migration of CD34(+) hematopoietic progenitor cells induced by the inflammatory mediator leukotriene D4 are mediated by the 7-transmembrane receptor CysLT1. Blood. 2001;97:3433-3440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 69. | Braccioni F, Dorman SC, O’byrne PM, Inman MD, Denburg JA, Parameswaran K, Baatjes AJ, Foley R, Gauvreau GM. The effect of cysteinyl leukotrienes on growth of eosinophil progenitors from peripheral blood and bone marrow of atopic subjects. J Allergy Clin Immunol. 2002;110:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 70. | Elsas PX, Queto T, Mendonça-Sales SC, Elsas MI, Kanaoka Y, Lam BK. Cysteinyl leukotrienes mediate the enhancing effects of indomethacin and aspirin on eosinophil production in murine bone marrow cultures. Br J Pharmacol. 2008;153:528-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 71. | Parameswaran K, Watson R, Gauvreau GM, Sehmi R, O’Byrne PM. The effect of pranlukast on allergen-induced bone marrow eosinophilopoiesis in subjects with asthma. Am J Respir Crit Care Med. 2004;169:915-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 72. | Jones CP, Paula Neto HA, Assreuy J, Vargaftig BB, Gaspar Elsas MI, Elsas PX. Prostaglandin E2 and dexamethasone regulate eosinophil differentiation and survival through a nitric oxide- and CD95-dependent pathway. Nitric Oxide. 2004;11:184-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 73. | de Luca B, Xavier-Elsas P, Barradas M, Luz RA, Queto T, Jones C, Arruda MA, Cunha TM, Cunha FQ, Gaspar-Elsas MI. Essential roles of PKA, iNOS, CD95/CD95L, and terminal caspases in suppression of eosinopoiesis by PGE2 and other cAMP-elevating agents. ScientificWorldJournal. 2013;2013:208705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 74. | Yudina Y, Parhamifar L, Bengtsson AM, Juhas M, Sjölander A. Regulation of the eicosanoid pathway by tumour necrosis factor alpha and leukotriene D4 in intestinal epithelial cells. Prostaglandins Leukot Essent Fatty Acids. 2008;79:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 75. | Ketchell RI, D’Amato M, Jensen MW, O’Connor BJ. Contrasting effects of allergen challenge on airway responsiveness to cysteinyl leukotriene D(4) and methacholine in mild asthma. Thorax. 2002;57:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Louboutin JP, Song GB S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ