Published online Feb 20, 2016. doi: 10.5493/wjem.v6.i1.21
Peer-review started: July 31, 2015
First decision: September 16, 2015
Revised: December 14, 2015
Accepted: January 5, 2016
Article in press: January 7, 2016
Published online: February 20, 2016
Processing time: 200 Days and 18.8 Hours
Hepatocellular carcinoma (HCC) is the second cause of death due to malignancy in the world, following lung cancer. The geographic distribution of this disease accompanies its principal risk factors: Chronic hepatitis B virus and hepatitis C virus infection, alcoholism, aflatoxin B1 intoxication, liver cirrhosis, and some genetic attributes. Recently, type II diabetes has been shown to be a risk factor for HCC together with obesity and metabolic syndrome. Although the risk factors are quite well known and it is possible to diagnose HCC when the tumor is less than 1 cm diameter, it remains elusive at the beginning and treatment is often unsuccessful. Liver transplantation is thus far considered the best treatment for HCC as it cures HCC and the underlying liver disease. Using the Milan criteria, overall survival after liver transplantation for HCC is about 70% after 5 years. Many attempts have been made to go beyond the Milan Criteria and according to recent works reasonably good results have been achieved by using a histochemical marker such as cytokeratine 19 and the so-called “up to seven criteria” to divide patients into categories according to their risk of relapse. In addition to liver transplantation other therapies have been proposed such as resection, tumor ablation by different means, embolization and chemotherapy. An important step in the treatment of advanced HCC has been the introduction of sorafenib, the first oral, systemic drug that has provided significant improvement in survival. Treatment of HCC patients must be multidisciplinary and by using the different approaches discussed in this review it is possible to offer prolonged survival and quite good and sometimes even excellent quality of life to many patients.
Core tip: This review summarizes on the current state of the art of treatment of hepatocellular carcinoma (HCC). After a brief chapter on epidemiology, risk factors and biology of HCC, the review presents all possible therapeutic approaches for HCC, from the most effective such as liver transplantation to important but palliative treatments which can prolong patient survival such as different types of trans-artery-chemo-embolization and chemotherapy.
- Citation: Mazzanti R, Arena U, Tassi R. Hepatocellular carcinoma: Where are we? World J Exp Med 2016; 6(1): 21-36
- URL: https://www.wjgnet.com/2220-315X/full/v6/i1/21.htm
- DOI: https://dx.doi.org/10.5493/wjem.v6.i1.21
Hepatocellular carcinoma (HCC) is one of the ten most commonly occurring solid cancers worldwide and is the second cause of death from malignancy. The most recent data indicate that its incidence is still increasing in many countries whereas the most effective way of reducing mortality due to HCC is prevention[1].
These observations appear to be in contrast with what we know about this lethal cancer. Most of its risk factors are known. It is possible to diagnose HCC when the tumor is 1 cm diameter or less and it can be treated either surgically or medically. So why we are losing the battle against an enemy we know so well?
It is clear that there are some other risk factors which are not yet known. We do not understand the precise mechanisms by which liver cells become neoplastic clones without the possibility of our immune system to controlling them. Most important of all, the natural history of HCC before it has been diagnosed is unknown. Sophisticated technology for searching for cancer cells in blood has revealed that even small tumors distribute thousands of cancer cells into the circulation that then may begin the process of metastasis much earlier than was formerly thought[2]. In other words, the concept of “early cancer” that has been applied successfully to other situations, such as gastric cancer, has not yet proven for HCC. Although it is believed that not all viable cancer cells are able to metastasize, but only those with stem cells properties, we must consider these aspects before saying we can cure cancer patients[2,3]. Relapse can occur years later, well after the standard 5 years that are considered. Breast cancer is the best example of this phenomenon.
Liver cells undergo replicative activity throughout life (thus the concept of the “streaming liver”) but it has been not clarified whether this process helps to eliminate “initiated” cells or, on the contrary, helps them to arrive in the blood circulation. Moreover, since HCC occurs mostly in the cirrhotic or chronically inflamed liver, it is possible that chronic liver disease encourages the process of metastasis from tiny neoplastic nodules.
This review will focus on some of these aspects of HCC and the various current and future treatment options.
According to the most recent data, the global incidence of HCC is still increasing, although it varies throughout the world. The highest rates occur in Eastern Asia whereas the positive effect of vaccination against hepatitis B virus (HBV) infection is showing the first results in China and Taiwan now. The lowest rates are in Northern Europe and North America. These data are mainly explained on the basis that two of the risk factors for HCC, HBV and hepatitis C virus (HCV) infections, are largely diffused in Eastern Asia. In Italy, for example, HBV-related HCC is now rapidly declining, in comparison to HCV-related HCC because of the introduction of mass HBV vaccination several years ago[4,5]. Moreover, aflatoxin-B1 intoxication, an important risk factor for HCC, is quite common in those geographic areas where the incidence of HCC is high due to inadequate cereals and food storage conditions. The known risk factors for HCC are reported in Table 1.
| Age |
| Ethnicity |
| Male Gender |
| Liver cirrhosis |
| Chronic HBV infection |
| Chronic HCV infection |
| Hemochromatosis |
| Alcoholism |
| Aflatoxin-B1 intoxication |
| Tyrosinemia, galactosidemia, fructosemia |
| Alpha1 anti-trypsin deficiency |
| Genetics predisposition |
| Anabolizing hormones |
| Estrogen contraceptives |
| Obesity |
| Type II diabetes |
| Glucose overload |
| Metabolic syndrome |
| Hypothyroidism |
| Fatty liver |
| Non-alcoholic steato-hepatitis |
Some pathological aspects of the liver such as cirrhosis and fibrosis, conditions resulting from chronic inflammation due to chronic HBV and HCV infection or to alcohol intoxication, can lead to cancer through several mechanisms, some of which are linked to chronic inflammation such as hepatocyte necrosis, liver regeneration and fibrosis and others that are specific of each virus. For example, HBV causes about 70%-80% of HCC cases in East Asia, and possesses transformation activity due to some of its proteins, i.e., protein X, or by integrating part of its DNA genome into that of humans and thus altering it[6,7]. HCV, which is an RNA virus without an inverse transcriptase, cannot integrate its genome with the human, but it has several proteins that have transforming properties. In addition, HCV infects the immune system and endothelial cells, leading to immune dysfunction and chronic deregulated angiogenesis. Both conditions are known risk factors for cancer[8,9].
Alcoholism is an important risk factor for HCC because it causes fatty liver, necro-inflammation, fibrosis, liver cirrhosis and malnutrition, especially when it is associated with HCV infection. Alcohol abuse is the most important risk factor for HCC in North America and Northern Europe.
There are other risk factors for HCC, i.e., hemochromatosis, fructosemia, tyrosinemia, galactosidemia, diabetes type II, genetic propensity, or clinical conditions such as obesity or hypothyroidism. Hemochromatosis, for instance, is the most common genetic defect (1/200 born in the West) and it leads to the accumulation of iron in the body, causing heart, joint, endocrine and liver diseases. Its prevention could be a key step to preventing HCC and other fatal diseases.
In recent years, other important risk factors have been described such as type II diabetes, metabolic syndrome and non-alcoholic steato-hepatitis. Type II diabetes is so common in western countries (about 10% of the adult population) that it constitutes an enormous societal health problem. Type II diabetes also increases the risk for other types of cancer, especially the most deadly pancreatic cancer. It is also the main risk factor for heart and vessel diseases. Since its occurrence is much facilitated by obesity and the lack of physical exercise, its prevention should become a target for all healthcare systems in developed countries where large portions of the population are becoming more and more overweight and sedentary.
As mentioned above, also fatty liver significantly increases the risk of HCC[10]. This condition per se does not seem a risk factor, but when it becomes the expression of underlying metabolic disorders or other more severe diseases it may become a predisposing lesion of HCC, as in the large use of anabolic hormones to increase body mass. On the other hand, it now seems possible to exclude the use of modern contraceptive pills from this category of risk factors[11]. Estrogen actually may reduce the risk of HCC as suggested by the observation that HCC occurs more frequently in men rather women with a ratio ranging from 2:1 to 4:1.
Ionizing radiation and smoking are also risk factors for HCC although there are no specific works on these. Finally, last but not least, we cannot forget that HCC is an age-related disease. Although it can occur at every age from infants to the elderly, nevertheless its frequency increases in individuals from 30 to 70 years of age, according to the population under consideration (in much younger individuals in Africa and East Asia where HBV is prevalent, and in older subjects in Europe or America).
The process that transforms a normal liver cell into neoplastic one is not known, although changes in normal liver histology are likely to play an important role together with genetic alterations due to viruses, oxygen derived free radicals, toxic compounds and changes in the host immune system.
Cancer is a genetic- and age-related disease and HCC is not an exception. All the risk factors that we have taken into consideration may result in changes in the hepatocyte DNA and, since the process of transformation is quite long, the immune system is also involved in the development of clinically evident cancer. This means that the pathway to HCC must be long enough to cause an irreversible phenomenon. Chronic liver diseases and cirrhosis are very good examples of this old observation. There are thousands of patients who have had acute HBV or HCV infections many years earlier and then who developed HCC after a chronic disease that lasted decades in the literature. If either HBV or HCV are directly and rapidly oncogenic as experimental research shows, why does it take so long to develop HCC in humans? We know that HBV-related protein X or HCV NS3, NS5 or core proteins or others have the capacity to transform normal cells in vitro, but why does this not happen in all patients and, more importantly, why does it take 20-30 years for a neoplastic nodule to appear in man? Is it only due to our still rudimentary diagnostic tools which do not perceive tiny cancerous foci, or are some individual’s immune systems able to combat the disease, or are there more complex pathways which we do not yet understand?
Consider the recent data published by two of the top scientists worldwide on colon cancer: The main risk factor according to their data is bad luck. After thousands of papers published on this issue, billions of dollars spent on research on it, the bitter conclusion is that the most important factor, but not the only one, in getting colon or lung cancer is bad luck[12]. What a terrible conclusion!
The liver is an example that fits quite well what Tomasetti and Vogelstein have shown[12]. Since liver stem cells have quite intense replicative activity and since the greater the replication, the higher the risk that one or more daughter cells have mutation in their DNA, it makes sense that the risk of acquiring HCC is higher in the presence of risk factors, such as HBV or HCV infection, alcoholism, aging, excessive iron, ionizing radiation, and smoking, however HCC also occurs in subjects who do not have any of the above risk factors.
Vaccination against HBV infection, prevention of HCV-infected transfusions, prevention of alcoholism or aflatoxin intoxication are crucial factors in the prevention of HCC. And we should also mention that besides the risk factors, there are also protective conditions that could help us to understand better how cancer develops. The most important protective factor for HCC is vaccination against HBV infection. Prevention of chronic HBV carrier status and chronic liver disease is crucial as shown recently by the decrease in HCC in Taiwan and China after the adoption of mass vaccination[4]. Reducing the HBV load in patients with liver cirrhosis and chronic hepatitis reduces also reduces the risk of HCC[13]. There are other factors that can help protect from HCC, such as regular consumption of vegetables and coffee drinking (3-4 cups/d). Vitamin D and calcium intake may also exert a protective role although results are controversial[14-17] (Table 2). It is also possible that other dietary factors act as protective factors, including consumption of phenols contained in red wine, green tea, olive oil, but these data require confirmation[18].
| HBV vaccination |
| Physical exercise |
| Balanced diet |
| High vegetable consumption |
| Calcium |
| Vitamin D |
| Coffee |
Despite recent advances in surveillance and management of HCC, its molecular biology is quite elusive. A complete definition of molecular events that lead to hepato-carcinogenesis is a major challenge in the clinical management of this disease. There is no doubt, however, that liver cirrhosis, chronic hepatitis, chronic HBV and HCV infections, and intoxication by alcohol, iron and aflatoxin-B1 all play a major role in causing HCC.
Hepato-carcinogenesis is a multi-step process that begins from an altered hepatic microenvironment, typically related to chronic liver disease, characterized by massive inflammation and fibrosis that are responsible for the deregulation of several signaling pathways and accumulation of genetic alterations in normal hepatocytes[19-21]. The latter are finally transformed into dysplastic lesions causing early carcinoma which finally progresses to HCC[22]. What seems particularly important in liver oncogenesis in humans is the lag time from the beginning of the inflammatory process or the exposure to risk factors and the occurrence of HCC. This review does not address specific aspects that could be involved in liver oncogenesis, but we do present some updated references that can provide more thorough information to the interested reader.
HCC is an extremely heterogeneous solid cancer[23] and factors such as platelet derived growth factor, transforming growth factors (TGFs), vascular endothelial growth factor (VEGF), hepatocyte growth factor, reactive oxygen species (ROS) and cellular factors such as hepatocytes, stem like, stellate and inflammatory cells are involved[24,25]. We believe that ROS and the oxidative stress that are associated with inflammation are of particular importance[26]. HCV infection, alcohol abuse or iron intoxication, for instance, all cause ROS and reactive nitrogen species (RNS) that can eventually overcome the liver’s defense against oxidative stress, thus damaging DNA and cell proteins[27]. HBV-related inflammation also causes oxidative stress due to both the inflammation itself and protein X that stresses the endoplasmic reticulum via cAMP-responsive element-binding protein (CREBH)[26]. The presence of transforming proteins such as HBVx protein or HCV-related NS3, NS5, or core proteins may all lead to hepatocyte transformation. Intense angiogenesis in the liver during chronic HCV infection is also important and thus these patients are at greater risk of developing HCC[28,29].
Understanding the precise molecular processes occurring during these steps may offer a better therapeutic approach to HCC. Unfortunately, our understanding of how things occur in vivo is not sufficient to drive safe and effective therapy, which is the main reason why traditional chemo- and hormone therapies have produced discouraging results[30]. From a molecular point of view HCC is a heterogeneous disease that shows heterogeneity between different tumor nodules in the same patient and differences in the same nodule itself[31,32]. This may reflect the existence of distinct pools of cancer stem-like cells with different oncogenicity and independent genomic evolution. Thus, not only does each patient have his/her own HCC, but each nodule may be genetically unique[32-35]. With regard to this point, unfortunately the theory about the importance of cancer stem-like cells in HCC has produced little. There is a controversy regarding what they are and where they come from in HCC as well as in other cancers[36-39]. Like many, we think that they come from oval cells that, after prolonged proliferative activity, evolve towards a sort of erroneous differentiation. It is possible that oxidative stress causes DNA damage that results in initiation of the process. However, this is pure speculation, as we simply do not know! What we know very well is that without liver cirrhosis, chronic HBV and HCV infections or iron, ethanol or aflatoxin B1 intoxication, HCC is a rare disease.
Genome-wide surveys have been used to try to better understand HCC molecular mechanisms. Pathways commonly altered by genetic alterations (somatic mutations or homozygous deletions) include the Wnt/beta-catenin pathway, the p53 pathway, phosphatidylinositol 3-kinase (PI3K)/Ras signaling pathways, oxidative and endoplasmic reticulum stress modulators and processes responsible for chromatin remodeling[40]. Interestingly, different mutations segregated by the etiology of the underlying liver disease suggest that the carcinogenesis and development of mutations causing cancer may vary, depending on pre-existing liver disease. For example, inactivation of genes involved in chromatin remodeling is more common in patients with alcoholic cirrhosis, while p53 pathway deregulation is predominant in HBV-positive patients and Ras in those affected by HCV[40-43].
Similar results have recently been obtained through transcriptome analysis of liver cancer that has shown a deregulation in some oncogenic signaling pathways such as those of TGF-β1, MYC, PI3K/Akt, Wnt/β-catenin, NOTCH and MET[41-43].
Some of these HCC hallmarks have been analyzed in depth and their role as a potential target for HCC treatment is under investigation. VEGF and its receptors are known to be key mediators in HCC initiation, growth and diffusion[44,45]. Increased VEGF expression from low to high grade dysplastic nodules or advanced HCC and the correlation of VEGF levels with tumor stage and risk of recurrence, support this hypothesis[44-46]. We have confirmed the importance of angiogenic phenotype in HCV-related HCC[47]. Fibroblast growth factor (FGF) and its family of receptors are recognized as a central player in augmenting HCC growth, invasion, and angiogenesis, making it an intriguing candidate for HCC therapy. FGF activation is involved in neovascularization[48]. Increased serum levels of FGF or activation of its receptors are associated with recurrence, treatment resistance, and poor prognosis[49] while synergistic interactions between FGF and VEGF have been shown to be a resistance mechanism to the antiangiogenic effects of VEGF-targeted agents[50].
Several other pathways have been shown to be involved in HCC pathogenesis. In particular, promising results come from the PI3K/Akt/mTOR, EGFR, Ras/Raf/mitogen-activated protein kinase (MEK)/extracellular-signal-regulated kinase (ERK), and IGFR pathways[51,52].
The PI3K/Akt/mTOR pathway is a major intracellular signaling cascade that is involved in the regulation of cell proliferation, growth and survival, through activation of tyrosine kinase receptors, such as VEGFR, EGFR, PDGFR, and IGFR. Nearly 50% of patients with HCC exhibit activation of the mTOR pathway, which may be partially attributable to activation signals from receptor tyrosine kinases such as IGFR and/or EGFR pathways. mTOR pathway activation is associated with aggressive HCC and decreased survival in these patients[53]. Despite promising results targeting mTOR, a substantial benefit in HCC patients has not been shown in clinical practice.
The Ras/Raf/MEK/ERK signaling cascade is another important intracellular pathway altered in HCC such as the Wnt pathway, however, while Raf inhibitors such as sorafenib have been developed in HCC therapy, Wnt inhibitors have not yet been developed.
cMET, a tyrosine kinase receptor with its ligand hepatocyte growth factor, has been implicated in HCC and probably in multidrug resistance[54] and represents a target that is undergoing intense investigation. Randomized phase II data of the cMET inhibitor, tivantinib, has demonstrated activity in patients with advanced HCC who progress on sorafenib with elevated expression of cMET by immunohistochemistry. Tivantinib is undergoing phase III investigation in this subgroup of patients[55,56].
Given the molecular heterogeneity of HCC, the challenge is to identify in which patients any given alteration is critical. It is unlikely that any of these targeted agents will yield clinical success without selecting the patients whose tumors are most dependent on these pathways and are therefore most likely to benefit, and the identification of predictive markers of response is essential for the successful development of new targeted agents.
If we exclude the so-called “incidental” occurrence of HCC that is found by chance in subjects undergoing ultrasound scan and/or computerized tomography (CT) for other reasons, most HCC cases are suspected and diagnosed in a program of clinical controls or planned controls in patients known to be at risk for the disease. It is possible to diagnose HCC using different imaging techniques such as ultrasound, CT, nuclear magnetic resonance (NMR), but confirmation comes only with histology. There is a general agreement now that ultrasound should be the first tool for detecting HCC nodules in patients admitted to follow-up because of chronic liver disease or for the existence of an at-risk condition. Blood tests are much less useful, and they may help more in patient follow-up rather than in diagnosis. It is possible to find diagnostic algorithms made by experts after extensive discussion in the international literature[57,58]. However, we believe that confirmation of the presence of HCC by histology should be searched in all cases where image means (CT and/or NMR) are not conclusive or legal controversy may arise when such confirmation is lacking.
As mentioned above, HCC can first be observed by ultrasound scan, which is an inexpensive, easy, fast, and readily available technology in every part of the globe. Most scientific societies for the study of liver diseases agree that a 6-mo interval is the most convenient for checking up the development of HCC in liver patients[57,58] (Table 3).
| Society | Target population | Target population |
| APASL, 2010 | Chronic hepatitis | Cirrhosis |
| No recommendation | HBV and HCV cirrhosis | |
| AASLD, 2011 | HBV-positive patients | HBV and/or HCV cirrhotics |
| Male Asian HBV+ > 40 yr | Fourth stage PBC | |
| African > 20 yr; Familial predisposition + for HCC | a1-antitrypsin deficit | |
| Autoimmune hepatitis | ||
| NASH | ||
| JSH, 2011 | HBV and HCV chronic hepatitis | Non viral cirrhosis |
| HBV and HCV cirrhosis | ||
| EASL, 2012 | HBV + active hepatitis | Child A and B cirrhosis |
| HCV + hepatitis with advanced fibrosis F3 (Metavir) | Child C cirrhosis with indication to liver transplantation |
Some authors suggest that surveillance becomes convenient when the ratio between cost and benefit achieves 3 extra mo of life at a cost of less than 50000 dollars[59]. The 6-mo interval comes from a large number of studies that have shown that the doubling time of HCC is about 6-mo, although very few of them have shown a clear gain in survival as compared to groups of patients who did not undergo programmed check-up[60].
The effectiveness of ultrasound in detecting any form of HCC is quite good (94%), but its sensitivity drops to 63% when the target to be detected is small[61,62]. Because no ethical committee would permit a comparative study between controlled and uncontrolled cirrhotic patients, we think that the 6-mo interval is reasonable to follow-up patients. This interval becomes useless for assessing the response to treatment or to check for relapse in patients who have been treated for HCC. In addition, in patients where ultrasound has shown lesions of less than 1 cm diameter it should be repeated with a much shorter interval than 6-mo (we use 3-mo, normally). It is clear that once liver patients have been treated for HCC, they should be carefully followed-up with intervals inferior to 6-mo, either to follow how the treated nodule(s) is doing or to assess new lesions.
There is another exception that must be considered and it concerns those who are on the waiting list for liver transplantation. The occurrence of a small nodule of suspected HCC puts these patients into the priority line for transplantation according to the Milan criteria[61,62].
Contrast-enhanced ultrasound does not increase the sensitivity for small HCC, but it helps in differentiating some lesions[63]. It is common that most of those with liver nodules undergo ultrasound-guided needle biopsy to assess the histology of the lesion. Expert pathologists are needed to solve sometimes difficult diagnoses and the use of ultrasound scan, CT, or even NMR are very helpful in some cases. Before biopsy there are other techniques that help clinicians to reach the correct diagnosis.
Radiologists have studied the behavior of blood distribution inside the tumor, differentiating the artery phase from the portal phase (early and tardive). By using liver specific contrast it is possible to observe how the liver handles the contrast, suggesting the presence of HCC tissue or benign lesions. HCC is commonly a hyper-vascular lesion and contrasted CT can distinguish a rapid wash–in phase of the tumor (artery supply) from liver tissue that surrounds the HCC nodule. In patients with chronic liver disease the occurrence of a lesion having such characteristics strongly suggests the presence of HCC independently from other signs. Sometimes lesions are hypo-vascularized, especially those with a diameter of less than 2 cm. In these cases the most sensitive and specific technique is NMR with hepato-specific contrast that distinguishes the hypo-vascular lesion from other aspects of the liver, taking advantage of the absence of any metabolism of the contrast by neoplastic cells[64-68].
In patients with chronic liver disease, especially with liver cirrhosis, it is relatively common to observe lesions of less than 1 cm diameter by ultrasound and CT scan. These lesions are difficult to diagnose, even when using ultrasound-guided biopsy because of the occurrence of false negatives. It is more prudent to wait and watch before doing anything, unless the patient is on the waiting list for liver transplantation. As mentioned before, this occurrence can move the patient into priority line for liver transplant. In patients with Child-Pugh stage A liver cirrhosis, repeated ultrasound check-ups must be done and if the lesion tends to grow, it may be considered for needle biopsy by expert hands. Once liver histology has been obtained, the pathologist should perform many specific tests such as CD 34, CK7, glypican 3, HSP-70 and others to assess the malignancy of the tissue according to guidelines of the European Association for the Study of the Liver, or the European Organization for Research and Treatment of Cancer. There is controversy among different scientific societies regarding use of contrasted ultrasound in differentiating HCC, though CEUS can better identify a small lesion in a cirrhotic liver.
An issue that has been discussed intensely concerns whether liver biopsy of suspected nodules should be performed. From what was said above, we are in favor of liver biopsy in cases where not invasive techniques are not conclusive[69]. In addition, now that target therapy is becoming a reality, we think that good quality molecular study of each biopsy could add the knowledge that is required for administering the right target therapy. Circulating cancer cells could be the alternative in the future, but at present they do not give certainty about the presence and the site of HCC[70,71].
As regards the use of biochemical blood parameters such as alpha-fetoprotein, or fucosylate alpha-phetoprotein or des-gamma-prothrombin, we agree with most western researchers that these parameters have limited utility in diagnosing HCC. They are especially misleading in screening programs because of poor sensitivity and specificity. On the contrary, they can be useful once HCC has been diagnosed to follow up the results of treatment. While this manuscript was in preparation a study concerning a possible new blood indicator for the presence of HCC in cirrhotic patients was published[72]. Only time will tell whether this new indicator for the presence of HCC will become of general use or will be disappointing, like many others.
As most solid cancers, HCC is asymptomatic for several months and sometimes for years. This means that HCC should be searched for in all known chronic liver disease patients with adequate diagnostic tools at regular intervals.
When HCC occurs in patients with an apparently healthy liver, the diagnosis is usually late and the patient has advanced disease.
Symptoms, when present, are those of chronic hepatitis with the addition of pain in the right superior quarter of the abdomen, increased volume, and more fatigue. If a liver disease patient presents with a change in mood, edema in the legs, swollen abdomen due to ascites, rapid occurrence or deterioration of portal hypertension with esophageal or rectal varices, hemorrhoids or bleeding, the physician should consider that HCC may be developing. Decompensation of diabetes can also be a sign of the presence of HCC. Ultrasound scan without and with liver-specific contrast should be done rapidly and followed by a CT scan with contrast medium.
Early detection of liver nodules and diagnosis of HCC is crucial to ensuring that the patient have the greatest possibility of being cured or, at least, of prolonging survival and improving quality of life. It must be remembered that only 15%-35% of those who develop HCC can undergo surgery.
A patient’s prognosis strongly depends upon the condition of his/her liver at diagnosis. Because of this, several authors have tried to make a “road map” to assist in selecting the proper therapy for the right patient.
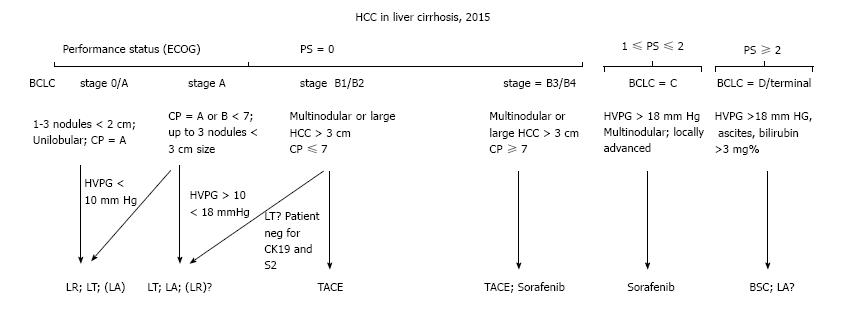
As in all patients with cancer, staging is crucial before deciding any treatment. In addition, since more than 90% of HCC patients have an important underlying liver disease, careful staging of it must also be performed to avoid cancer treatment that damages the liver reserve. In the last 50 years several systems have been proposed to stage liver disease with or without HCC. With others we have developed in Italy a system to estimate the prognosis of patients affected by HCC and liver cirrhosis, the Cancer of the Liver Italian Program that, according to our own results and in the opinion of many other researchers has been very precise in defining the prognosis of patients[73]. However, the Barcelona Clinic Liver Classification (BCLC) system with few modifications is more frequently used now because it also takes into consideration the choice of treatment. The BCLC staging system divides HCC associated with liver cirrhosis into: Very early stage (0 stage), early stage (stage A), intermediate stage (stage B), advanced stage (stage C) and terminal stage (stage D) and, compared to others, has the advantage of taking into account not only tumor burden, stage of liver cirrhosis, liver function, physical condition but also the efficacy of available treatment[74]. Patients have the best treatment opportunities at Centers with vast experience who utilize the BCLC system, which has recently been updated because of stage B heterogeneity, so B stage can now be divided into four subgroups, B1, B2, B3, B4 according to Child Pugh, Milan criteria, number and size of lesions, and ECOG performance status (Figure 1)[75,76].
The treatment of HCC is difficult and requires a multidisciplinary approach, whereby specialists in gastroenterology, hepatology, radiology, oncology, surgery and others need to bring their expertise to provide patients with the best and most updated therapies. It is possible to differentiate treatments into two main categories: Those that are potentially curative and those that are palliative.
In this category we consider three types of approach: Liver transplantation, liver resection, and liver ablation. Each of these approaches has indications and contraindications, and need to be discussed carefully and tailored to each individual patient.
Liver transplantation is widely considered the only real potentially curative approach that provides treatment of both HCC and the underlying liver cirrhosis. Its gold standard indication is early unresectable HCC in patients with compensated liver cirrhosis or HCC with no more than 3 nodules, Stage 0 or A according to the BCLC system. Recent reports show that 70% of transplanted patients for HCC are alive after 5 years, especially when transplantation was performed using the Milan criteria[77-79]. This percentage of success is extremely good when considering that most of these patients had liver cirrhosis and HCC. Unfortunately, only a minority of patients can undergo liver transplantation even when it is indicated. The number of donors is largely insufficient to cope with the worldwide need and the shortage of organs makes waiting lists longer each day in every country, so that many patients become unsuitable for transplantation or die while awaiting transplant. Many attempts have been made to go beyond the limits of the Milan criteria and results are under scrutiny now. One large review involving 770 consecutive transplanted patients for HCC shows that performing transplantation beyond the Milan criteria attains a 57% general rate of survival, after 5 years, with a rate of recurrence of 35%[80]. However, by using tissue cytokeratin 19 (CK 19) and the sum of the size of the largest tumor size plus the number of nodules up to 7, as for S2 patients, as prognostic markers, it was possible to divide the entire group of patients in two: (1) one group of individuals who were negative for CK 19 and were not included in the S2 group; and (2) another group resulting positive or to be in the S2 group. The results were quite different and impressive: The first group had a 64% survival rate and 19% relapse compared to the other group where survival dropped to 45% with a rate of recurrence of 53%[80].
There are several reviews that address the issue of selection of patients to insert on the waiting list for transplantation[81,82]. In this general review it is sufficient to say that patients with early or very early unresectable HCC and liver cirrhosis with good liver reserve function (Child Pugh A) (stage 0 or A according to the BCLC system) are excellent candidates for liver transplant. Those who have poor reserve of liver function or too advanced HCC, stage C of the BCLC system, are not good candidates because of the surgery risk and/or frequent and/or rapid recurrence or appearance of metastases. Finally, there is a group of heterogeneous patients, included in the intermediate stage according to the BCLC system (stage B), where the decision on whether to submit patients to transplantation must be discussed among experts with different expertise. One possible pathway is indicated in Figure 1. Patients with Stage D disease are not candidates for transplantation nor for active treatment of HCC.
There are other problems with liver transplant: One is that many candidates for transplantation have chronic HBV or HCV infections that recur after transplantation and may quite rapidly reproduce liver cirrhosis. Effective treatments have been available for HBV infection for several years, including use of interferon, lamivudine and other antiviral drugs[83-85]. Treatment is usually effective and eradicates the infection in most. Therapy should start before surgery and be continued for several months. It is more difficult to deal with HCV infection, especially that due to HCV genotype 1 that is particularly resistant to antiviral treatment with peg-interferon and ribavirin. Relapse of chronic hepatitis and liver cirrhosis occurs rapidly and, in addition, these patients show significantly worse prognosis than others[86]. Accordingly, active HCV infection has been a reason to exclude patients from transplantation in many centers. However, major progress has been made in the last 2 years in the treatment of chronic HCV infection including that due to genotype 1, through introduction of drugs such as sofosbuvir, ledipasvir, elbasvir and others that specifically target HCV proteins[87,88]. These drugs are able to clear the virus in most HCV patients though bitter discussion is in progress in several countries over the cost of these drugs and on their generalized use. It is too early to ascertain whether these new antiviral drugs have solved the problem. It must be kept in mind that HCV infects several sanctuaries of the body and since transplanted patients are chronically treated with immunosuppressive drugs, it is not possible to exclude later relapse of infection. Moreover, reactivation of HBV infection has been reported in patients on treatment with these new anti-HCV drugs and the issue is still open to possible surprises[89].
To overcome the shortage of donors and to cut - waiting list time for recipients of a new liver it has been proposed to split the available deceased-donor livers (DDLT) or to take part of the liver from live donors (LDLT) (usually a relative of the patient). The results appear good according to what has been published especially in Asia, without any statistically significant increase in recurrence of HCC as compared to traditional cadaveric donors[90-93]. Complications of liver surgery of live donors make this option still debatable especially considering a mortality rate that ranges from 0.1% to 0.3% of the donor[93,94].
What should be done to stop HCC progression while patients are on the transplant waiting list? The ideal approach would destroy the neoplastic tissue as much as possible without damaging the rest of the liver, without reducing functional reserve and, most importantly, without increasing the number of circulating liver cancer cells that cause metastases. Radiofrequency ablation (RFA) that causes almost full coagulative necrosis of the nodule in a single shot might be the best procedure, at least for small lesions. However, the use of trans-hepatic artery embolization (TAE) or trans-hepatic artery chemo-embolization (TACE) (see later) that theoretically deprive HCC of artery blood flow, necrotizing most of the tumor while allowing the presence of other neoplastic nodules to be assessed during the same procedure, are generally used. Many papers published on this issue show that TACE provides good results and increases overall survival compared to transplantation alone or TACE alone[95]. However, there are no controlled randomized studies comparing TACE and RFA as neoadjuvant treatment prior to liver transplant. In addition, many centers are now using a different technique of TACE that uses beads eluting anticancer drugs or ionizing microspheres charged with Yttrium-99 to embolize the artery that feeds the tumor. Results are similar in terms of overall survival, although bead-TACE seems to produce fewer side effects than traditional TACE[95-98]. Bead-TACE also appears useful in downstaging HCC or in maintaining it at a steady stage while patients are awaiting transplant[99,100].
Liver resection is the other surgical option for treatment of HCC[101,102]. Liver surgery in patients with cirrhosis or chronic liver disease requires expert surgeons and teams trained to work together in these patients, who may develop liver failure after large resection. In addition, recurrence of HCC within 3 years is quite common (> 50%) even after the introduction of ultrasound during surgery. Other procedures have been proposed to improve the outcome of patients with HCC and liver cirrhosis who undergo liver resection. The adoption of the Milan criteria used for liver transplantation and NMR with gadoxetic acid-enhanced MRI during surgery can improve detection of small lesions by 16% and makes surgery more precise and radical[103,104].
Liver resection has some advantages over transplantation (organ saving, costs, expertise, etc.) and in addition leaves open the possibility of later transplantation, thus allowing many patients to gain time. Unfortunately, liver resection does not treat the underlying cirrhosis that is a pre-neoplastic lesion and it can be too risky for patients with advanced HCC and/or liver disease (stage C according to the BCLC). Even very selective resection can cause liver decompensation and/or gastrointestinal bleeding, especially in patients with high values (>10 mmHg) of portal hypertension and gastro-esophageal varices. This limitation has been disputed recently as it excludes too many patients (about one-fourth) who could benefit from surgery from effective treatment[105].
Liver resection in patients with BCLC Stage B can be done quite safely by avoiding to operate those with serum bilirubin over 2 mg/dL and/or ascites. In selected patients, resection leads to an overall survival > 85% at 5 years, making this type of surgery the best option for treating early or very early HCC in well compensated liver cirrhosis (Child Pugh A).
Liver ablation performed using different techniques can be used in patients where surgery is not possible or too risky. Ablative techniques performed by experts in selected patients can allow survival comparable to transplantation even if more recent data have questioned this conclusion[106-112]. Of the ablative techniques, Percutaneous ethanol injection (PEI) was the first to be used in patients with small lesion(s) (< 3 cm diameter) HCC. Excellent results have been observed with recurrence rates at 5 years similar to resection. PEI consists in injecting ethanol inside the tumor mass to cause coagulative necrosis of all neoplastic nodules. PEI has been the most successful technique in treating small (less than 3 cm diameter) HCC nodules. It is easy, inexpensive, requires minimal equipment and expertise, and has been shown to prolong survival of these patients so much as to be comparable to surgery. PEI is so convenient and easy that it could be done at the patient’s home even in poor countries where health systems are weak. Ethanol destroys cancer tissue and diffuses well in the cirrhotic liver, and the procedure can be repeated several times on the same nodule or on new ones. The side effects are minimal, no general anesthesia is required and the procedure can be performed in an ambulatory situation. The use of this simple and inexpensive procedure has shown that it is possible to prolong survival of many patients and allow them to maintain an excellent quality of life[106-112]. PEI can be used in patients with more than one nodule, usually no more than 3, and because it can easily be repeated on the already treated nodule, offers patients a simple and inexpensive treatment of their disease.
The ablative technique obtained by PEI has been improved by using RFA that can destroy cancer tissue in only one step. This procedure, more complex and expensive than PEI, has the advantage that it can treat cancer lesions with a larger diameter than PEI can (usually < 5 cm diameter) and many of them with one single application. Because of the pain involved in the procedure, RFA is more commonly performed with the patient under light anesthesia. Side effects occur more commonly than with PEI and but are rarely severe.
A comparison between PEI and RFA shows that when patients are carefully selected, the results in terms of overall survival are similar, though disease recurrence can be observed more frequently in patients treated by PEI[113,114].
Ablation of the tumor can be obtained by other means, such as acetic acid or laser, but what is crucial is the expertise of the operator and good patient selection.
As mentioned earlier, most HCC patients cannot undergo surgery and so, for them, especially those with locally advanced disease, the most effective and safe treatment is that which aims to destroy the most cancer tissue without affecting liver reserve function.
The procedures that can be used are PEI, RFA, TAE, TACE, in association or not with different forms of chemotherapy.
PEI and RFA are so easy and safe that they can be performed even in patients with advanced stage of liver cirrhosis (stage C according to the BCLC system). The main risks are infection and bleeding in patients with severe defects in blood coagulation (pro-thrombin time < 60% and platelet counts < 60000/mL3). The risk of diffusing cancer cells along the needle passage is real, but in our experience is a rare event.
In several patients where surgery is not indicated, TAE and TACE can be employed to embolize the artery feeding the HCC. Patients for this procedure must be selected very carefully and the individual performing the procedure needs to be expert (usually a radiologist) in superselective embolization[114,115]. Necrosis of the tumor is rarely complete because of tumor angiogenesis that forms bunches of new small vessels that feed the cancer nodule. In addition, in many cases significant blood supply comes from portal blood and the liver cancer cells are ischemia resistant[116,117]. Nevertheless, the growth of the tumor may be impaired for weeks, making TACE or TAE the most commonly performed pre-transplantation procedure and the most used technique in HCC of large dimension and/or with too many nodules to be ablated.
Several authors have reported TACE prolonging survival also in patients with intermediate or advanced HCC. Considering that there are no other possible procedures in many patients, chemoembolization must be regarded as palliative but also active treatment[118,119].
Recently, two retrospective works have compared liver resection and TACE in patients with BCLC stage B. In a Chinese study, resection appears as safe as TACE and provides better overall survival at 3 years (59% vs 29%) and 5 years (37% vs 14%) with a highly statistically significant difference (P < 0.001)[119]. In the other study, from Spain, the authors analyzed their results according to the revised BCLC classification system that divides stage B into 4 substages (B1, B2, B3 and B4) on the basis of Child Pugh score, inclusion in the Milan criteria and furthermore, size and number of lesions under or over 7, and Performance Status (P.S.) according to ECOG. They found that liver resection provides particularly good results in the B1 group (Child Pugh score 5-7, within Milan Criteria and < 7, and P.S. = 0) with an overall survival of 62.9% after 5 years and a recurrence rate of 25% as opposed to a recurrence rate of 60% in the TACE group (P < 0.018). B3 (Child Pugh score = 7, beyond Milan criteria or > 7 and P.S. = 0) or B4 (Child Pugh score 8 or 9, any Milan criteria or number, P.S. >0) patients had the worst prognosis after 5 years (15.4% as overall survival). These authors concluded that modern surgical techniques and good selection of patients with liver cirrhosis and HCC in BCLC stages B1 or B2 (Child Pugh 5 or 6, beyond Milan Criteria but number < 7, P.S. = 0) according to the new BCLC system can provide particularly good results with liver resection in terms of survival and recurrence rate compared to similar patients treated with TACE[120].
In the last decade, the use of radio-labeled microspheres or embolizing beads that carry anticancer agents such as doxorubicin or cisplatin has been proposed to treat advanced HCC. The results have been encouraging and similar to those obtained by traditional TACE or TAE in terms of anticancer activity. Side effects appear less frequent and of minor gravity, making this new BEAD-TACE procedure one of the most favored in many centers[121-124].
Since all these treatments must be considered palliative and locally active, to improve their efficacy, it has been proposed to associate them with systemic chemotherapy.
In intermediate or in selected advanced HCC patients who cannot be treated by ablative or embolizing techniques or where HCC is already extrahepatic, the only systemic chemotherapy that has been proved to be effective is sorafenib[125-127]. This drug (800 mg/d, orally) is a tyrosine kinase activity inhibitor that acts mainly as antiangiogenic to slow down the growth of the tumor. The response rate is not particularly high (about 4%) and toxicity may be relevant. Sorafenib has been described as decompensating liver cirrhosis, and then its dosage must be reduced to 600 mg/d or 400 mg/d. In a few cases therapy must be withdrawn and it is possible to reintroduce it at a lower dosage once liver function has recovered. Sorafenib can be too toxic in patients with advanced liver cirrhosis, provoking side effects that include mucositis, skin toxicity, fatigue, bleeding and liver failure[128,129]. It should be considered that drug resistance to sorafenib and other inhibitors of tyrosine-kinase activity has been reported[130]. It is also possible to try to reverse drug resistance by using other drugs that interfere with mechanisms of drug resistance[131]. Agents such as sunitinib, erlotinib, and many others have been proposed but toxicity or drug resistance have limited their use and they have been abandoned so far for the treatment of HCC.
Recently, as already mentioned when talking about the biology of HCC, and following experimental data showing the importance of the cMET pathway in HCC, a phase II randomized study was carried out using tivantinib, a specific inhibitor of the cMET, as second-line treatment after sorafenib. The results of this study show that especially in HCC showing MET expression tivantinib is active and significantly slows tumor growth. The authors conclude that a phase III study is warranted to see the real impact of this new drug in advanced HCC[55,56].
Several authors have tried to improve the efficacy of these palliative treatments by associating sorafenib with TACE, TAE, ablation, or surgery[132-135]. Sorafenib has also been used as adjuvant chemotherapy in patients after complete resection of HCC to prevent relapse or as neoadjuvant chemotherapy before liver transplantation or resection. Though some encouraging preliminary results have emerged, none of these studies have shown clear advantages and must be regarded as experimental protocols which need to be repeated as controlled and much larger studies[136].
Many years ago we performed a pilot study (phase II) by using traditional intrahepatic artery chemotherapy using 5-fluorouracil in patients who were not suitable for surgery or ablative techniques or after relapse of HCC. This drug was well tolerated and our results, in terms of tumor response and survival, were positive compared with historic controls and in line with what others have recently shown, but the lack of randomized controls and small number of patients enrolled strongly limited the overall significance of this study[137].
In conclusion, HCC is at present one of the most challenging cancers in clinical practice[138]. Though our understanding of its risk factors and how it develops have improved in the last decade, so much remains to be clarified. Currently the best way to significantly reduce the death rate remains prevention of HBV and HCV infection and alcoholism.
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20723] [Article Influence: 1883.9] [Reference Citation Analysis (23)] |
| 2. | Ji J, Zheng X, Forgues M, Yamashita T, Wauthier EL, Reid LM, Wen X, Song Y, Wei JS, Khan J. Identification of microRNAs specific for epithelial cell adhesion molecule-positive tumor cells in hepatocellular carcinoma. Hepatology. 2015;62:829-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Islam F, Gopalan V, Smith RA, Lam AK. Translational potential of cancer stem cells: A review of the detection of cancer stem cells and their roles in cancer recurrence and cancer treatment. Exp Cell Res. 2015;335:135-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 2015;19:223-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 679] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 5. | Mele A, Stroffolini T, Zanetti AR. Hepatitis B in Italy: where we are ten years after the introduction of mass vaccination. J Med Virol. 2002;67:440-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Mazzanti R, Gramantieri L, Bolondi L. Hepatocellular carcinoma: epidemiology and clinical aspects. Mol Aspects Med. 2008;29:130-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Kew MC. Hepatitis B virus x protein in the pathogenesis of hepatitis B virus-induced hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26 Suppl 1:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 217] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 8. | Ali N, Allam H, May R, Sureban SM, Bronze MS, Bader T, Umar S, Anant S, Houchen CW. Hepatitis C virus-induced cancer stem cell-like signatures in cell culture and murine tumor xenografts. J Virol. 2011;85:12292-12303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Machida K, Tsukamoto H, Mkrtchyan H, Duan L, Dynnyk A, Liu HM, Asahina K, Govindarajan S, Ray R, Ou JH. Toll-like receptor 4 mediates synergism between alcohol and HCV in hepatic oncogenesis involving stem cell marker Nanog. Proc Natl Acad Sci USA. 2009;106:1548-1553. [PubMed] |
| 10. | Shetty K, Chen J, Shin JH, Jogunoori W, Mishra L. Pathogenesis of Hepatocellular carcinoma. Development in non alcoholic fatty liver disease. Curr Hepatol Rep. 2015;14:119-127. [PubMed] |
| 11. | McGlynn KA, Sahasrabuddhe VV, Campbell PT, Graubard BI, Chen J, Schwartz LM, Petrick JL, Alavanja MC, Andreotti G, Boggs DA. Reproductive factors, exogenous hormone use and risk of hepatocellular carcinoma among US women: results from the Liver Cancer Pooling Project. Br J Cancer. 2015;112:1266-1272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Tomasetti C, Vogelstein B. Cancer risk: role of environment—response. Science. 2015;347:729-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Sinn DH, Lee J, Goo J, Kim K, Gwak GY, Paik YH, Choi MS, Lee JH, Koh KC, Yoo BC. Hepatocellular carcinoma risk in chronic hepatitis B virus-infected compensated cirrhosis patients with low viral load. Hepatology. 2015;62:694-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 14. | Yang Y, Zhang D, Feng N, Chen G, Liu J, Chen G, Zhu Y. Increased intake of vegetables, but not fruit, reduces risk for hepatocellular carcinoma: a meta-analysis. Gastroenterology. 2014;147:1031-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Bamia C, Lagiou P, Jenab M, Trichopoulou A, Fedirko V, Aleksandrova K, Pischon T, Overvad K, Olsen A, Tjønneland A. Coffee, tea and decaffeinated coffee in relation to hepatocellular carcinoma in a European population: multicentre, prospective cohort study. Int J Cancer. 2015;136:1899-1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Bamia C, Lagiou P, Jenab M, Aleksandrova K, Fedirko V, Trichopoulos D, Overvad K, Tjønneland A, Olsen A, Clavel-Chapelon F. Fruit and vegetable consumption in relation to hepatocellular carcinoma in a multi-centre, European cohort study. Br J Cancer. 2015;112:1273-1282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Krstic G, Wetterslev J, Gluud C. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev. 2014;6:CD007469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 18. | Zamora-Ros R, Fedirko V, Trichopoulou A, González CA, Bamia C, Trepo E, Nöthlings U, Duarte-Salles T, Serafini M, Bredsdorff L. Dietary flavonoid, lignan and antioxidant capacity and risk of hepatocellular carcinoma in the European prospective investigation into cancer and nutrition study. Int J Cancer. 2013;133:2429-2443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1097] [Cited by in RCA: 1104] [Article Influence: 46.0] [Reference Citation Analysis (1)] |
| 20. | Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1461] [Cited by in RCA: 1576] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 21. | Villanueva A, Newell P, Chiang DY, Friedman SL, Llovet JM. Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis. 2007;27:55-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 418] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 22. | Kojiro M, Roskams T. Early hepatocellular carcinoma and dysplastic nodules. Semin Liver Dis. 2005;25:133-142. [PubMed] |
| 23. | Li L, Wang H. Heterogeneity of liver cancer and personalized therapy. Cancer Lett. 2015;July 23; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 239] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 24. | Chen C, Wang G. Mechanisms of hepatocellular carcinoma and challenges and opportunities for molecular targeted therapy. World J Hepatol. 2015;7:1964-1970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (4)] |
| 25. | Chiba T, Suzuki E, Saito T, Ogasawara S, Ooka Y, Tawada A, Iwama A, Yokosuka O. Biological features and biomarkers in hepatocellular carcinoma. World J Hepatol. 2015;7:2020-2028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Cho HK, Kim SY, Kyaw YY, Win AA, Koo SH, Kim HH, Cheong J. HBx induces the proliferation of hepatocellular carcinoma cells via AP1 over-expressed as a result of ER stress. Biochem J. 2015;466:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Ohnishi S, Ma N, Thanan R, Pinlaor S, Hammam O, Murata M, Kawanishi S. DNA damage in inflammation-related carcinogenesis and cancer stem cells. Oxid Med Cell Longev. 2013;2013:387014. [PubMed] |
| 28. | Mazzanti R, Messerini L, Monsacchi L, Buzzelli G, Zignego AL, Foschi M, Monti M, Laffi G, Morbidelli L, Fantappié O. Chronic viral hepatitis induced by hepatitis C but not hepatitis B virus infection correlates with increased liver angiogenesis. Hepatology. 1997;25:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Mazzanti R, Messerini L, Comin CE, Fedeli L, Ganne-Carrie N, Beaugrand M. Liver angiogenesis as a risk factor for hepatocellular carcinoma development in hepatitis C virus cirrhotic patients. World J Gastroenterol. 2007;13:5009-5014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Chen J, Gao J. Advances in the study of molecularly targeted agents to treat hepatocellular carcinoma. Drug Discov Ther. 2014;8:154-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 1127] [Article Influence: 93.9] [Reference Citation Analysis (1)] |
| 32. | Tao Y, Ruan J, Yeh SH, Lu X, Wang Y, Zhai W, Cai J, Ling S, Gong Q, Chong Z. Rapid growth of a hepatocellular carcinoma and the driving mutations revealed by cell-population genetic analysis of whole-genome data. Proc Natl Acad Sci USA. 2011;108:12042-12047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 33. | Guichard C, Amaddeo G, Imbeaud S, Ladeiro Y, Pelletier L, Maad IB, Calderaro J, Bioulac-Sage P, Letexier M, Degos F. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet. 2012;44:694-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 1176] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 34. | Ferber MJ, Montoya DP, Yu C, Aderca I, McGee A, Thorland EC, Nagorney DM, Gostout BS, Burgart LJ, Boix L. Integrations of the hepatitis B virus (HBV) and human papillomavirus (HPV) into the human telomerase reverse transcriptase (hTERT) gene in liver and cervical cancers. Oncogene. 2003;22:3813-3820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 165] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 35. | Fujimoto A, Totoki Y, Abe T, Boroevich KA, Hosoda F, Nguyen HH, Aoki M, Hosono N, Kubo M, Miya F. Whole-genome sequencing of liver cancers identifies etiological influences on mutation patterns and recurrent mutations in chromatin regulators. Nat Genet. 2012;44:760-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 726] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 36. | Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, Zheng BJ, Guan XY. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542-2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 899] [Cited by in RCA: 934] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 37. | Oishi N, Yamashita T, Kaneko S. Molecular biology of liver cancer stem cells. Liver Cancer. 2014;3:71-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 38. | Blaylock RL. Cancer microenvironment, inflammation and cancer stem cells: A hypothesis for a paradigm change and new targets in cancer control. Surg Neurol Int. 2015;6:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Anfuso B, El-Khobar KE, Sukowati CH, Tiribelli C. The multiple origin of cancer stem cells in hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2015;39 Suppl 1:S92-S97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 40. | Sung WK, Zheng H, Li S, Chen R, Liu X, Li Y, Lee NP, Lee WH, Ariyaratne PN, Tennakoon C. Genome-wide survey of recurrent HBV integration in hepatocellular carcinoma. Nat Genet. 2012;44:765-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 747] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 41. | Totoki Y, Tatsuno K, Yamamoto S, Arai Y, Hosoda F, Ishikawa S, Tsutsumi S, Sonoda K, Totsuka H, Shirakihara T. High-resolution characterization of a hepatocellular carcinoma genome. Nat Genet. 2011;43:464-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 228] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 42. | Li M, Zhao H, Zhang X, Wood LD, Anders RA, Choti MA, Pawlik TM, Daniel HD, Kannangai R, Offerhaus GJ. Inactivating mutations of the chromatin remodeling gene ARID2 in hepatocellular carcinoma. Nat Genet. 2011;43:828-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 345] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 43. | Marquardt JU, Seo D, Andersen JB, Gillen MC, Kim MS, Conner EA, Galle PR, Factor VM, Park YN, Thorgeirsson SS. Sequential transcriptome analysis of human liver cancer indicates late stage acquisition of malignant traits. J Hepatol. 2014;60:346-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 44. | Sanz-Cameno P, Trapero-Marugán M, Chaparro M, Jones EA, Moreno-Otero R. Angiogenesis: from chronic liver inflammation to hepatocellular carcinoma. J Oncol. 2010;2010:272170. [PubMed] |
| 45. | Park YN, Kim YB, Yang KM, Park C. Increased expression of vascular endothelial growth factor and angiogenesis in the early stage of multistep hepatocarcinogenesis. Arch Pathol Lab Med. 2000;124:1061-1065. [PubMed] |
| 46. | Poon RT, Ng IO, Lau C, Zhu LX, Yu WC, Lo CM, Fan ST, Wong J. Serum vascular endothelial growth factor predicts venous invasion in hepatocellular carcinoma: a prospective study. Ann Surg. 2001;233:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 186] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 47. | Cipriani G, Mazzanti R. Treatment with inhibitors of angiogenesis in advanced hepatocellular carcinoma: a new tool in our hands or simply a hope? Dig Liver Dis. 2005;37:230-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 48. | Presta M, Dell’Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16:159-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 910] [Cited by in RCA: 972] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 49. | Poon RT, Ng IO, Lau C, Yu WC, Fan ST, Wong J. Correlation of serum basic fibroblast growth factor levels with clinicopathologic features and postoperative recurrence in hepatocellular carcinoma. Am J Surg. 2001;182:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 109] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 50. | Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2319] [Cited by in RCA: 2299] [Article Influence: 127.7] [Reference Citation Analysis (0)] |
| 51. | Finn RS. Emerging targeted strategies in advanced hepatocellular carcinoma. Semin Liver Dis. 2013;33 Suppl 1:S11-S19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 52. | Villanueva A, Chiang DY, Newell P, Peix J, Thung S, Alsinet C, Tovar V, Roayaie S, Minguez B, Sole M. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135:1972-1983, 1983.e1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 564] [Cited by in RCA: 601] [Article Influence: 33.4] [Reference Citation Analysis (1)] |
| 53. | Schmitz KJ, Wohlschlaeger J, Lang H, Sotiropoulos GC, Malago M, Steveling K, Reis H, Cicinnati VR, Schmid KW, Baba HA. Activation of the ERK and AKT signalling pathway predicts poor prognosis in hepatocellular carcinoma and ERK activation in cancer tissue is associated with hepatitis C virus infection. J Hepatol. 2008;48:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 246] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 54. | Lasagna N, Fantappiè O, Solazzo M, Morbidelli L, Marchetti S, Cipriani G, Ziche M, Mazzanti R. Hepatocyte growth factor and inducible nitric oxide synthase are involved in multidrug resistance-induced angiogenesis in hepatocellular carcinoma cell lines. Cancer Res. 2006;66:2673-2682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 55. | Santoro A, Porta C, Rimassa L, Borbath I, Daniele B, Finn RS, Raoul JL, Schwartz LH, He AR, Trojan J. Metiv-HCC: A phase III clinical trial evaluating tivantinib (ARQ 197), a MET inhibitor, versus placebo as second-line patients (pts) with MET-high inoperable hepatocellular carcinoma (HCC). J Clin Oncol. 2013;31 suppl:abstr TPS4159 Available from: http://meetinglibrary.asco.org/content/111110-132. |
| 56. | Santoro A, Rimassa L, Borbath I, Daniele B, Salvagni S, Van Laethem JL, Van Vlierberghe H, Trojan J, Kolligs FT, Weiss A. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 study. Lancet Oncol. 2013;14:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 466] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 57. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6630] [Article Influence: 442.0] [Reference Citation Analysis (1)] |
| 58. | Roeb E. Hepatocellular carcinoma - current aspects of screening, surveillance and therapeutic strategies (revised EASL-EORTC recommendations). Zentralbl Chir. 2014;139:175-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 59. | Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosis. Am J Med. 1996;101:422-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 267] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 60. | Santi V, Trevisani F, Gramenzi A, Grignaschi A, Mirici-Cappa F, Del Poggio P, Di Nolfo MA, Benvegnù L, Farinati F, Zoli M. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol. 2010;53:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 243] [Article Influence: 15.2] [Reference Citation Analysis (1)] |
| 61. | Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, Marrero JA. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30:37-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 589] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 62. | Trevisani F, Santi V, Gramenzi A, Di Nolfo MA, Del Poggio P, Benvegnù L, Rapaccini G, Farinati F, Zoli M, Borzio F. Surveillance for early diagnosis of hepatocellular carcinoma: is it effective in intermediate/advanced cirrhosis? Am J Gastroenterol. 2007;102:2448-2457; quiz 2458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 63. | Terzi E, Salvatore V, Negrini G, Piscaglia R. Ongoing challenges in the diagnosis of hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2015;Nov 24; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 64. | Forner A, Vilana R, Ayuso C, Bianchi L, Solé M, Ayuso JR, Boix L, Sala M, Varela M, Llovet JM. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 736] [Article Influence: 40.9] [Reference Citation Analysis (1)] |
| 65. | Fleisher JM. Occupational and non-occupational risk factors in relation to an excess of primary liver cancer observed among residents of Brooklyn, New York. Cancer. 1990;65:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 66. | Chou R, Cuevas C, Fu R, Devine B, Wasson N, Ginsburg A, Zakher B, Pappas M, Graham E, Sullivan SD. Imaging Techniques for the Diagnosis of Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Ann Intern Med. 2015;162:697-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 67. | Bruix J, Han KH, Gores G, Llovet JM, Mazzaferro V. Liver cancer: Approaching a personalized care. J Hepatol. 2015;62:S144-S156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 233] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 68. | Colagrande S, Regini F, Taliani GG, Nardi C, Inghilesi AL. Advanced hepatocellular carcinoma and sorafenib: Diagnosis, indications, clinical and radiological follow-up. World J Hepatol. 2015;7:1041-1053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (2)] |
| 69. | Tejeda-Maldonado J, García-Juárez I, Aguirre-Valadez J, González-Aguirre A, Vilatobá-Chapa M, Armengol-Alonso A, Escobar-Penagos F, Torre A, Sánchez-Ávila JF, Carrillo-Pérez DL. Diagnosis and treatment of hepatocellular carcinoma: An update. World J Hepatol. 2015;7:362-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 70. | Attwa MH, El-Etreby SA. Guide for diagnosis and treatment of hepatocellular carcinoma. World J Hepatol. 2015;7:1632-1651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (9)] |
| 71. | Waghray A, Murali AR, Menon KN. Hepatocellular carcinoma: From diagnosis to treatment. World J Hepatol. 2015;7:1020-1029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 72. | Sadeghi M, Lahdou I, Oweira H, Daniel V, Terness P, Schmidt J, Weiss KH, Longerich T, Schemmer P, Opelz G. Serum levels of chemokines CCL4 and CCL5 in cirrhotic patients indicate the presence of hepatocellular carcinoma. Br J Cancer. 2015;113:756-762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 73. | Daniele B, De Sio I, Izzo F, Capuano G, Andreana A, Mazzanti R, Aiello A, Vallone P, Fiore F, Gaeta GB. Hepatic resection and percutaneous ethanol injection as treatments of small hepatocellular carcinoma: a Cancer of the Liver Italian Program (CLIP 08) retrospective case-control study. J Clin Gastroenterol. 2003;36:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 74. | Llovet JM, Fuster J, Bruix J. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004;10:S115-S120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 516] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 75. | Scaffaro LA, Stella SF, Alvares-Da-Silva MR, Kruel CD. Survival rates according to barcelona clinic liver cancer sub-staging system after transarterial embolization for intermediate hepatocellular carcinoma. World J Hepatol. 2015;7:628-632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 76. | Wang JH, Kee KM, Lin CY, Hung CH, Chen CH, Lee CM, Lu SN. Validation and modification of a proposed substaging system for patients with intermediate hepatocellular carcinoma. J Gastroenterol Hepatol. 2015;30:358-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 77. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [PubMed] |
| 78. | Rahimi RS, Trotter JF. Liver transplantation for hepatocellular carcinoma: outcomes and treatment options for recurrence. Ann Gastroenterol. 2015;28:323-330. [PubMed] |
| 79. | Kumaran V. Role of liver transplantation for hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4:S97-S103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 80. | Miltiadous O, Sia D, Hoshida Y, Fiel MI, Harrington AN, Thung SN, Tan PS, Dong H, Revill K, Chang CY. Progenitor cell markers predict outcome of patients with hepatocellular carcinoma beyond Milan criteria undergoing liver transplantation. J Hepatol. 2015;63:1368-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 81. | Kanda T, Ogasawara S, Chiba T, Haga Y, Omata M, Yokosuka O. Current management of patients with hepatocellular carcinoma. World J Hepatol. 2015;7:1913-1920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 82. | Hsu C, Chen BB, Chen CH, Ho MC, Cheng JC, Kokudo N, Murakami T, Yeo W, Seong J, Jia JD. Consensus Development from the 5th Asia-Pacific Primary Liver Cancer Expert Meeting (APPLE 2014). Liver Cancer. 2015;4:96-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 83. | Cohen GS, Black M. Multidisciplinary management of hepatocellular carcinoma: a model for therapy. J Multidiscip Healthc. 2013;6:189-195. [PubMed] |
| 84. | Suk-Fong Lok A. Hepatitis B: 50 years after the discovery of Australia antigen. J Viral Hepat. 2015;Aug 17; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 85. | Younossi ZM, Stepanova M, Saab S, Ahmed A, Lam B, Srishord M, Venkatesan C, Wai H, Henry L. The impact of viral hepatitis-related hepatocellular carcinoma to post-transplant outcomes. J Viral Hepat. 2015;Aug 20; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 86. | Dugum M, Hanouneh I, Lopez R, Aucejo F, Eghtesad B, Zein N. Hepatocellular Carcinoma in the Setting of Chronic Hepatitis B Virus Infection: Tumor Recurrence and Survival Rates After Liver Transplantation. Transplant Proc. 2015;47:1939-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 87. | Gane EJ, Hyland RH, An D, Svarovskaia E, Pang PS, Brainard D, Stedman CA. Efficacy of Ledipasvir and Sofosbuvir, With or Without Ribavirin, for 12 Weeks in Patients With HCV Genotype 3 or 6 Infection. Gastroenterology. 2015;149:1454-1461.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 88. | Younossi ZM, Stepanova M, Marcellin P, Afdhal N, Kowdley KV, Zeuzem S, Hunt SL. Treatment with ledipasvir and sofosbuvir improves patient-reported outcomes: Results from the ION-1, -2, and -3 clinical trials. Hepatology. 2015;61:1798-1808. [PubMed] |
| 89. | Takayama H, Sato T, Ikeda F, Fujiki S. Reactivation of hepatitis B virus during interferon-free therapy with daclatasvir and asunaprevir in patient with hepatitis B virus/hepatitis C virus co-infection. Hepatol Res. 2015;Aug 22; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 90. | Xiao GQ, Song JL, Shen S, Yang JY, Yan LN. Living donor liver transplantation does not increase tumor recurrence of hepatocellular carcinoma compared to deceased donor transplantation. World J Gastroenterol. 2014;20:10953-10959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (3)] |
| 91. | Shukla A, Vadeyar H, Rela M, Shah S. Liver Transplantation: East versus West. J Clin Exp Hepatol. 2013;3:243-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 92. | Bhangui P, Vibert E, Majno P, Salloum C, Andreani P, Zocrato J, Ichai P, Saliba F, Adam R, Castaing D. Intention-to-treat analysis of liver transplantation for hepatocellular carcinoma: living versus deceased donor transplantation. Hepatology. 2011;53:1570-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 93. | Sandhu L, Sandroussi C, Guba M, Selzner M, Ghanekar A, Cattral MS, McGilvray ID, Levy G, Greig PD, Renner EL. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma: comparable survival and recurrence. Liver Transpl. 2012;18:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 94. | Kaido T, Uemoto S. Does living donation have advantages over deceased donation in liver transplantation? J Gastroenterol Hepatol. 2010;25:1598-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 95. | Facciorusso A, Licinio R, Muscatiello N, Di Leo A, Barone M. Transarterial chemoembolization: Evidences from the literature and applications in hepatocellular carcinoma patients. World J Hepatol. 2015;7:2009-2019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 96. | Nicolini D, Svegliati-Baroni G, Candelari R, Mincarelli C, Mandolesi A, Bearzi I, Mocchegiani F, Vecchi A, Montalti R, Benedetti A. Doxorubicin-eluting bead vs conventional transcatheter arterial chemoembolization for hepatocellular carcinoma before liver transplantation. World J Gastroenterol. 2013;19:5622-5632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 97. | Megìas Vericat JE, Garcia Marcos R, Lopez Briz E, Gòmez Munoz F, Ramos Ruiz J, Martinez Rodrigo JJ, Poveda Andrés JL. Trans-arterial Chemoembolization with Doxorubicin-eluting Particles versus Conventional Trans-arterial Chemoembolization in Unresectable Hepatocellular Carcinoma: a Study of Effectiveness, Safety and Costs. Radiologia. 2015;57:496-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 98. | Kloeckner R, Weinmann A, Prinz F, Pinto dos Santos D, Ruckes C, Dueber C, Pitton MB. Conventional transarterial chemoembolization versus drug-eluting bead transarterial chemoembolization for the treatment of hepatocellular carcinoma. BMC Cancer. 2015;15:465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 99. | Orlacchio A, Chegai F, Merolla S, Francioso S, Giudice CD, Angelico M, Tisone G, Simonetti G. Downstaging disease in patients with hepatocellular carcinoma outside up-to-seven criteria: Strategies using degradable starch microspheres transcatheter arterial chemo-embolization. World J Hepatol. 2015;7:1694-1700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 100. | Manini MA, Sangiovanni A, Martinetti L, Viganò D, La Mura V, Aghemo A, Iavarone M, Crespi S, Nicolini A, Colombo M. Transarterial chemoembolization with drug-eluting beads is effective for the maintenance of the Milan-in status in patients with a small hepatocellular carcinoma. Liver Transpl. 2015;21:1259-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 101. | Ramesh H. Resection for hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4:S90-S96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 102. | Pang TC, Lam VW. Surgical management of hepatocellular carcinoma. World J Hepatol. 2015;7:245-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 103. | Tanaka S, Noguchi N, Ochiai T, Kudo A, Nakamura N, Ito K, Kawamura T, Teramoto K, Arii S. Outcomes and recurrence of initially resectable hepatocellular carcinoma meeting milan criteria: Rationale for partial hepatectomy as first strategy. J Am Coll Surg. 2007;204:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 104. | Kim HD, Lim YS, Han S, An J, Kim GA, Kim SY, Lee SJ, Won HJ, Byun JH. Evaluation of early-stage hepatocellular carcinoma by magnetic resonance imaging with gadoxetic acid detects additional lesions and increases overall survival. Gastroenterology. 2015;148:1371-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 105. | Cucchetti A, Cescon M, Golfieri R, Piscaglia F, Renzulli M, Neri F, Cappelli A, Mazotti F, Mosconi C, Colecchia A. Hepatic venous pressure gradient in the preoperative assessment of patients with resectable hepatocellular carcinoma. J Hepatol. 2015;Aug 29; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 106. | Mazzanti R, Arena U, Pantaleo P, Antonuzzo L, Cipriani G, Neri B, Giordano C, Lanini F, Marchetti S, Gentilini P. Survival and prognostic factors in patients with hepatocellular carcinoma treated by percutaneous ethanol injection: a 10-year experience. Can J Gastroenterol. 2004;18:611-618. [PubMed] |
| 107. | Livraghi T. Single HCC smaller than 2 cm: surgery or ablation: interventional oncologist’s perspective. J Hepatobiliary Pancreat Sci. 2010;17:425-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 108. | Ruzzenente A, Guglielmi A, Sandri M, Campagnaro T, Valdegamberi A, Conci S, Bagante F, Turcato G, D’Onofrio M, Iacono C. Surgical resection versus local ablation for HCC on cirrhosis: results from a propensity case-matched study. J Gastrointest Surg. 2012;16:301-311; discussion 311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 109. | Kang TW, Kim JM, Rhim H, Lee MW, Kim YS, Lim HK, Choi D, Song KD, Kwon CH, Joh JW. Small Hepatocellular Carcinoma: Radiofrequency Ablation versus Nonanatomic Resection--Propensity Score Analyses of Long-term Outcomes. Radiology. 2015;275:908-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 110. | Ni JY, Xu LF, Sun HL, Zhou JX, Chen YT, Luo JH. Percutaneous ablation therapy versus surgical resection in the treatment for early-stage hepatocellular carcinoma: a meta-analysis of 21,494 patients. J Cancer Res Clin Oncol. 2013;139:2021-2033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 111. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4519] [Article Influence: 215.2] [Reference Citation Analysis (4)] |
| 112. | Pompili M, De Matthaeis N, Saviano A, De Sio I, Francica G, Brunello F, Cantamessa A, Giorgio A, Scognamiglio U, Fornari F. Single hepatocellular carcinoma smaller than 2 cm: are ethanol injection and radiofrequency ablation equally effective? Anticancer Res. 2015;35:325-332. [PubMed] |
| 113. | Yang B, Zan RY, Wang SY, Li XL, Wei ML, Guo WH, You X, Li J, Liao ZY. Radiofrequency ablation versus percutaneous ethanol injection for hepatocellular carcinoma: a meta-analysis of randomized controlled trials. World J Surg Oncol. 2015;13:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 114. | Xu C, Lv PH, Huang XE, Wang SX, Sun L, Wang FA. Efficacy of Transarterial Chemoembolization Combined with Radiofrequency Ablation in Treatment of Hepatocellular Carcinoma. Asian Pac J Cancer Prev. 2015;16:6159-6162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 115. | Sieghart W, Hucke F, Peck-Radosavljevic M. Transarterial chemoembolization: modalities, indication, and patient selection. J Hepatol. 2015;62:1187-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 257] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 116. | Peng XH, Karna P, Cao Z, Jiang BH, Zhou M, Yang L. Cross-talk between epidermal growth factor receptor and hypoxia-inducible factor-1alpha signal pathways increases resistance to apoptosis by up-regulating survivin gene expression. J Biol Chem. 2006;281:25903-25914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 245] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 117. | Lv Y, Zhao S, Han J, Zheng L, Yang Z, Zhao L. Hypoxia-inducible factor-1α induces multidrug resistance protein in colon cancer. Onco Targets Ther. 2015;8:1941-1948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 118. | Hiraoka A, Kumada T, Michitaka K, Toyoda H, Tada T, Ishikawa T, Itobayashi E, Shimada N, Takaguchi K, Takizawa D. Is there a survival benefit in interventional radiology for hepatocellular carcinoma in patients with Child-Pugh C liver cirrhosis?: A multicenter study. Hepatol Res. 2015;Aug 29; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 119. | Zhong JH, Xiang BD, Gong WF, Ke Y, Mo QG, Ma L, Liu X, Li LQ. Comparison of long-term survival of patients with BCLC stage B hepatocellular carcinoma after liver resection or transarterial chemoembolization. PLoS One. 2013;8:e68193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 120. | Ciria R, López-Cillero P, Gallardo AB, Cabrera J, Pleguezuelo M, Ayllón MD, Luque A, Zurera L, Espejo JJ, Rodríguez-Perálvarez M. Optimizing the management of patients with BCLC stage-B hepatocellular carcinoma: Modern surgical resection as a feasible alternative to transarterial chemoemolization. Eur J Surg Oncol. 2015;41:1153-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 121. | Facciorusso A, Mariani L, Sposito C, Spreafico C, Bongini M, Morosi C, Cascella T, Marchianò A, Camerini T, Bhoori S. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 2015;Sep 2; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 122. | Mosconi C, Cappelli A, Pettinato C, Golfieri R. Radioembolization with Yttrium-90 microspheres in hepatocellular carcinoma: Role and perspectives. World J Hepatol. 2015;7:738-752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 123. | Martin R, Geller D, Espat J, Kooby D, Sellars M, Goldstein R, Imagawa D, Scoggins C. Safety and efficacy of trans arterial chemoembolization with drug-eluting beads in hepatocellular cancer: a systematic review. Hepatogastroenterology. 2012;59:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 124. | Nishikawa H, Kita R, Kimura T, Osaki Y. Transcatheter arterial embolic therapies for hepatocellular carcinoma: a literature review. Anticancer Res. 2014;34:6877-6886. [PubMed] |
| 125. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10533] [Article Influence: 585.2] [Reference Citation Analysis (9)] |
| 126. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4740] [Article Influence: 263.3] [Reference Citation Analysis (0)] |
| 127. | Kane RC, Farrell AT, Madabushi R, Booth B, Chattopadhyay S, Sridhara R, Justice R, Pazdur R. Sorafenib for the treatment of unresectable hepatocellular carcinoma. Oncologist. 2009;14:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 162] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 128. | Brandi G, de Rosa F, Calzà L, Girolamo SD, Tufoni M, Ricci CS, Cirignotta F, Caraceni P, Biasco G. Can the tyrosine kinase inhibitors trigger metabolic encephalopathy in cirrhotic patients? Liver Int. 2013;33:488-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 129. | Terada T, Noda S, Inui K. Management of dose variability and side effects for individualized cancer pharmacotherapy with tyrosine kinase inhibitors. Pharmacol Ther. 2015;152:125-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 130. | Zhai B, Sun XY. Mechanisms of resistance to sorafenib and the corresponding strategies in hepatocellular carcinoma. World J Hepatol. 2013;5:345-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (5)] |
| 131. | Zhai B, Hu F, Jiang X, Xu J, Zhao D, Liu B, Pan S, Dong X, Tan G, Wei Z. Inhibition of Akt reverses the acquired resistance to sorafenib by switching protective autophagy to autophagic cell death in hepatocellular carcinoma. Mol Cancer Ther. 2014;13:1589-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 237] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 132. | Kawaoka T, Aikata H, Hyogo H, Morio R, Morio K, Hatooka M, Fukuhara T, Kobayashi T, Naeshiro N, Miyaki D. Comparison of hepatic arterial infusion chemotherapy versus sorafenib monotherapy in patients with advanced hepatocellular carcinoma. J Dig Dis. 2015;16:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 133. | Wang Z, Zhang G, Wu J, Jia M. Adjuvant therapy for hepatocellular carcinoma: current situation and prospect. Drug Discov Ther. 2013;7:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 134. | Miyahara K, Nouso K, Yamamoto K. Chemotherapy for advanced hepatocellular carcinoma in the sorafenib age. World J Gastroenterol. 2014;20:4151-4159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 135. | Chao Y, Chung YH, Han G, Yoon JH, Yang J, Wang J, Shao GL, Kim BI, Lee TY. The combination of transcatheter arterial chemoembolization and sorafenib is well tolerated and effective in Asian patients with hepatocellular carcinoma: final results of the START trial. Int J Cancer. 2015;136:1458-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 136. | Villanueva A, Portela A, Sayols S, Battiston C, Hoshida Y, Méndez-González J, Imbeaud S, Letouzé E, Hernandez-Gea V, Cornella H. DNA methylation-based prognosis and epidrivers in hepatocellular carcinoma. Hepatology. 2015;61:1945-1956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 355] [Cited by in RCA: 334] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 137. | Mazzanti R, Giallombardo AL, Mini E, Nobili S, Neri B, Arena U, Pantaleo P, Fabbroni V, Ghilardi M, Gattai R. Treatment of locally advanced hepatocellular carcinoma by hepatic intra-artery chemotherapy: a pilot study. Dig Liver Dis. 2004;36:278-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 138. | Llovet JM, Villanueva A, Lachenmayer A, Finn RS. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol. 2015;12:436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Markopoulos AK, Tiribelli C, Varona MA, Yankol Y S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ