INTRODUCTION
Tuberculosis (TB) is a great threat to developing countri-es as well as developed countries, fuelled by human immunodeficiency virus infection, drug resistance and migration of population. Various T cells are integrated in the immunopathogenesis of TB. A better understanding of this issue can not only drive the progress of new immunodiagnostic tools, accelerate and facilitate the evaluation of new therapeutic methods, but also improve new treatment tools. Here, we will update the readers on the latest developments in this field and, in particular, focus on T lymphocytes, several T cell subsets, macrophages and cytokines involved in TB immunology.
INFLAMMATORY PROCESS OF TUBERCULOSIS
Histopathologically, TB displays exudative inflammation, proliferative inflammation and productive inflammation depending on the time course. Using animal experiments and an inhalation exposure system, the pathologic condition of the infected animals was followed up for 1 year[1]. Exudative inflammation was observed for the first ten days. Thereafter, granulomas, which corresponded to foci of proliferative inflammation, were formed. Cavity formation was not recognized in animal TB, except for rabbits. Using rabbit models, Dr. Arthur Dannenberg described the pathology of TB in more detail[1]. There are five stages: onset, symbiosis, early stages of caseous necrosis, interplay of cell-mediated immunity and tissue damaging delayed-type hypersensitivity, and liquefaction and cavity formation. In stage 1, tubercle bacilli are usually destroyed or inhibited by the mature resident alveolar macrophages that ingest them. If bacilli are not destroyed, they grow and eventually destroy the alveolar macrophages. In stage 2, bacilli grow logarithmically within the immature nonactivated macrophages. These macrophages enter a tubercle from the bloodstream. This stage is termed symbiosis because bacilli multiply locally without apparent damage to the host, and macrophages accumulate and divide. In stage 3, the stage at which caseous necrosis first occurs, the number of viable bacilli becomes stationary because their growth is inhibited by the immune response to tuberculin-like antigens released from bacilli. Stage 4 is the stage that usually determines whether the disease becomes clinically apparent. Cell-mediated immunity plays a major role in this situation. The cytotoxic delayed- type hypersensitivity immune response kills these macrophages, causing enlargement of the caseous center and progression of the disease. If good cell-mediated immunity develops, a mantle of highly activated macrophages surrounds the caseous necrosis. In stage 5, bacilli evade host defenses. When liquefaction of the caseous center occurs, the bacilli multiply extracellularly, frequently attaining very large numbers. The high local concentration of tuberculin-like products derived from these bacilli causes a tissue-damaging delayed-type hypersensitivity response that erodes the bronchial wall, forming a cavity.
T CELL ACTIVATION AGAINST MYCOBACTERIUM TUBERCULOSIS
In human, a TB index case may infect a contact person through cough and expectoration, so the lung is the primary route of infection and often the main tissue exhibiting TB. Infectious droplet nuclei are deposited in the alveolar spaces of the contact person where Mycobacterium tuberculosis (M. tuberculosis) can be phagocytosed by alveolar macrophages, epithelial cells, dendritic cells (DC) and neutrophils[2,3]. Alveolar macrophages and DC are then believed to transport M. tuberculosis to local lymph nodes where T cell activation occurs and expand. DC play important and indispensable roles in the initiation and maintenance of protective immune responses following mycobacterial infection. The kind of immune responses initiated by DC determines the character of immune responses mounted by the host against the pathogen. The profile of cytokines and chemokines secreted by the result of infection of DC by mycobacteria further plays an important role in defining the course of mycobacterial infection. Activation of the phagocytic host cell is much required to limit growth of M. tuberculosis; as in the absence of activation, disease outcome is extremely poor. Effective phagocyte activation requires a specific cellular response, as infected hosts lacking specific components of the acquired response have a poor outcome[4]. While acquired cellular protection is expressed rapidly following systemic challenge with M. tuberculosis, it is less rapid in the lung. Slow expression of protection in the lung allows mycobacteria to grow and modulate the infection site. Until recently it has not been clear whether the slow response to aerosol delivery of bacteria resulted from limited availability of antigen or inhibition of antigen-presentation by M. tuberculosis. Several studies show that the first T cell activation occurs in the draining lymph node (DLN) of the lung 8-10 d following initial challenge. The activation of T cells correlated temporally with the arrival of bacteria and availability of antigen in the DLN, however conditions for T cell activation were unique to the DLN as the presence of antigen-producing bacteria in the lung and spleen did not result in initial activation of T cells[5,6]. While delivery of lipopolysaccharide (LPS) to the M. tuberculosis-infected lung failed to accelerate T cell priming[5], increasing the bacterial dose did accelerate the response modestly suggesting that both antigen burden and refractory cells serve to slow the response. Thus, protective memory cells will not become activated until they recognize antigen, i.e., more than 8 d post infection. Once T cells become activated they differentiate into effector T cells that migrate to the lung. By day 14 of infection, when activated T cells first arrive in the lung, bacteria are within alveolar macrophages, myeloid DC and neutrophils[4]. T cells can recognize antigen within the mycobacterially-infected lung but the antigen presentation is not optimal. It takes time for the protective T cells to reach sufficient numbers to stop bacterial growth. T cells can be divided into two subsets, Th1 and Th2, on the basis of the cytokines they produce. In tuberculosis, Th1 plays a major role in defense against tuberculosis. Th1 cells suppress Th2 cells. CD4 + T cells have unambiguously been identified as the most important lymphocyte subset for mediating protection. CD4 T lymphocytes differentiate in the peripheral tissues to adopt a variety of fates such as the Th-1 cells, which produce interferon (IFN)-γ to down-regulate Th2 responses and Th-2 cells, which produce interleukin (IL)-4. CD8 T lymphocytes produce predominantly IFN-γ. Though CD4 response is greater than the CD8 response, the latter can provide protection in the absence of CD4 help[7]. During active TB there is a local pulmonary immune response characterized by α/β T cells and strongly enhanced M. tuberculosis antigen-specific Th1 responses, with large amounts of locally secreted IFN-γ[8].
ALVEOLAR MACROPHAGES IN TUBERCULOSIS DEVELOPMENT
When tubercle bacilli reach alveoli, they are phagocytosed by resident alveolar macrophages. Though tubercle bacilli are killed by alveolar macrophages, tubercle bacilli can also kill macrophages through apoptosis. What is the fate of tubercle bacilli once they enter the phagosomes of macrophages Alveolar macrophages of aerially infected guinea pigs were collected by bronchoalveolar lavage. At 12 d after infection, one out of about 10 000 alveolar macrophages of various sizes contained many tubercle bacilli[9]. This indicates that certain alveolar macrophages permit M. tuberculosis to replicate in the phagosomes, although most of tubercle bacilli are killed by activated alveolar macrophages. It will be of great interest to examine the survival mechanism of M. tuberculosis at the single-cell level, but we still do not know why macrophages targeted by tubercle bacilli cannot kill the bacilli.
IFN-γ knockout mice were infected with avirulent H37Ra or BCG Pasteur, multinucleated giant cells were recognized in the granulomatous lesions. The lesions also contained tubercle bacilli and consisted of multinucleated cell clusters, being immunopositive with anti-Mac-3 antibody. The multinucleated giant cells were transformed alveolar macrophages. We subsequently infected various cytokine-knockout mice with M. TB, but no Langhans’ multinucleated giant cells were recognized the granulomas. Therefore, it seems that formation of multinucleated giant cells requires optimal combinations and concentrations of various cytokines, and the level of IFN-γ, at least, has to be significantly low.
CYTOKINES IN PROTECTION AGAINST TB
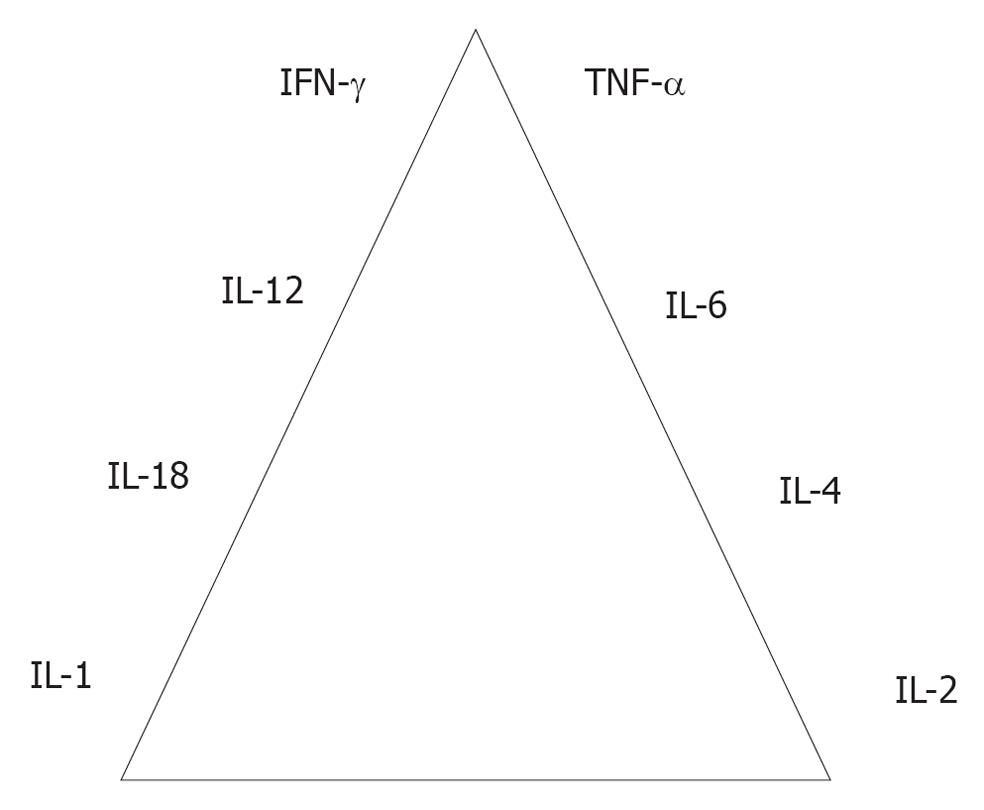
IFN-γ and tumor necrosis factor (TNF) have long been implicated as regulators of T cell responses in mycobacterial disease[10]. The technique of gene targeting (knockout) has swept through biomedical research. IFN-γ, TNF-α, interferon response factor (IRF)-1, nuclear factor (NF)-IL6, NF-κB p50, signal transducer and activator of transcription (STAT)1 and STAT4 knockout mice succumbed to M. tuberculosis infection over time. There appears to be a cytokine and transcription factor hierarchy in experimental tuberculosis. The results indicate that these molecules play major roles in defense against the disease, IFN-γ and TNF-α being the leading players in this respect[11].
Figure 1 shows the cytokine hierarchy associated with important molecules in experimental tuberculosis. The transcription factors such as NF-κB, STAT1, STAT4, IRF-1 and NF-IL6 are important molecules in defense against tuberculosis. The molecules [toll-like receptor (TLR)2, TLR6 and MyD-88] are less important in defense against tuberculosis in our hands[11].
Figure 1 Cytokine hierarchy in immunology of tuberculosis.
IFN: Interferon; IL: Interleukin; TNF: Tumor necrosis factor.
ROLE OF NEUTROPHILS
The role of neutrophils in the development of tuberculosis remained unknown for a long time. We utilized LPS-induced transient neutrophilia in the lungs[9]. LPS (50 μg/mL) was administered intratracheally to male Fischer rats, which were then infected with M. tuberculosis via an airborne route. Intratracheal injection of LPS significantly blocked the development of pulmonary granulomas and significantly reduced the number of pulmonary colony-forming units (CFU). Treatment with amphotericin B (an LPS inhibitor) or neutralizing anti-rat neutrophil antibody reversed the development of pulmonary lesions. LPS-induced transient neutrophilia prevented early mycobacterial infection. The timing of LPS administration was important. When given intratracheally at least 10 d after aerial infection, LPS did not prevent the development of tuberculosis. Neutrophils obtained by bronchoalveolar lavage killed M. tuberculosis bacilli. These results indicate clearly that neutrophils participate actively in defense against early-phase tuberculosis.
ROLE OF NATURAL KILLER CELLS
Natural killer (NK) cells are innate lymphocytes which are a first line of defense against infection. NK cells can kill autologous infected cells without prior sensitization, and are believed to play a pivotal role in innate immunity to microbial pathogens. In mouse model, NK cells are activated and produce IFN-γ during the early response to pulmonary tuberculosis[9] and NK cell-produced IFN-γ regulates the anti-mycobacterial resistance mediated by neutrophils[12]. However animal models do not give a clear answer to whether NK cells is important in M. tuberculosis infection in vivo. Depletion of NK cells had no effect on bacterial replication in the lung of immunocompetent mice[13], suggesting that NK cells may be redundant in the presence of intact adaptive immunity. Surprisingly, IFN-γ knockout mice, which are impaired in their ability to clear mycobacteria, cleared them as effectively as wild-type mice when NK cells were depleted, suggesting that NK cells can inhibit protective immunity[14].
Human NK cells use the NKp46, the natural cytotoxicity receptors (NCRs) and NKG2D receptors to lyse M. tuberculosis-infected monocytes and alveolar macrophages[15], through damage of infected cells and secretion of cytokines, such as IFN-γ[16]. Inhibitory receptors of NK cells include killer immunoglobulin-like receptors (KIRs) and the NKG2A: CD94 dimer and NK cell activation can also be triggered by loss of inhibitory ligands from the cell surface. In addition, NK cells can also be activated by cytokines, including type I interferons, IL-12 and IL-18. NK cells are a potent and early source of cytokines, particularly IFN-γ, but they can also produce Th2-associated cytokines, such as IL-5 and IL-13, and the regulatory cytokine IL-10[17]. NK cell NKp46 expression and cytotoxicity are reduced in freshly isolated peripheral blood mononuclear cells (PBMCs) from tuberculosis patients, which may be attributable to suppression by monocytes and IL-10. Recent studies have found that NK cells produce IL-22[18], which was induced by IL-15 and DAP-10, an adaptor protein that is known to be involved in NK cell activation, in response to M. tuberculosis. Rohan Dhiman et al[19] also found that IL-22 can restrict growth of M. tuberculosis in macrophages by enhancing phagolysosomal fusion[19]. Nonetheless to fully understand the importance of NK cells in M. TB infection it may be necessary to differentiate their contributions at different stages of disease.
ROLE OF NKT CELLS
Certain T subsets, such as NKT cells and γδ T cells, have features of innate immune cells including a partially activated phenotype, a rapid response following detection of infected cells, and the modulation of other cell types. Together with NK cells, these cell subsets are functionally defined as innate lymphocytes. CD1d-restricted invariant NKT (iNKT) cells are a conserved subset of T cells that express an invariant T cell receptor (TCR) α chain (Vα24-Jα18 in humans, and Vα14-Jα18 in mice) paired with TCR β chains encoded by one or a few Vβ gene segments (Vβ11 in humans, and predominantly Vβ2, 7 and 8 in mice). These cells show different phenotypes and functions[20]. Many iNKT cells are CD4+, and they have been mainly associated with the induction of Th2 cytokines such as IL-4, IL-5, IL-13. This subset is believed to play a prominent role in suppression of autoimmune or chronic inflammatory diseases, and in promoting allergic conditions such as asthma. Few iNKT cells are CD8+, and most of those express only the CD8α subunit, which means that they likely express only CD8αα homodimers. An additional fraction of iNKT cells are negative for both CD4 and CD8 (DN T cells). They have been found to produce predominantly IFN-γ and other Th1-associated cytokines. Studies of human iNKT cells have shown that they have the ability to kill M. tuberculosis organisms within infected macrophages, possibly through their production of the peptide granulysin[21]. Im et al[22] found that the percentages of iNKT cells among total circulating T cells in TB patients were not significantly different compared to those in healthy controls. However, TB patients showed a selective reduction of the proinflammatory CD4-CD8- (DN) iNKT cells with a proportionate increase in the CD4+ iNKT cells. The mouse model of tuberculosis has been used by Sada-Ovalle et al[23] to find that iNKT cells have a direct bactericidal effect on M. tuberculosis, and protect mice against aerosol M. tuberculosis infection[23]. Their activation requires CD1d expression by infected macrophages as well as IL-12 and IL-18. In addition, pharmacological activation of iNKT cells with the synthetic ligand aGalCer often enhances host resistance to infection. iNKT cell use several mechanisms to modify host immunity. These include induction of DC maturation, secondary activation of effector cells (NK cells) or recruitment of inflammatory cells to the site of infection[24,25]. Thus, by being an early producer of IFN-γ and suppressing intracellular bacterial growth, iNKT cells function as an important part of the early immune response against M. tuberculosis that affect both the innate and the adaptive arms of the immune response.
ROLE OF γΔ T CELLS
Antigen-specific γδ T cells represent an early innate defense that may play a role in antimycobacterial immunity. Studies done in humans and animal models have demonstrated complex patterns of γδ T cell immune responses during early mycobacterial infections and chronic TB. Like αβ T lymphocytes, γδ T cells carry antigen TCR that vary in the physical properties of their ligand-binding sites. γδ T cells are frequently activated by a variety of pathogens including M. tuberculosis[26]. Mice lacking γδ T cells succumb more rapidly than control mice following intravenous challenge with virulent M. tuberculosis; however, such a difference has not been observed following infection by the aerosol route. γδ T cells constitute a whole system of functionally specialized subsets that have been implicated in the innate responses against tumors and pathogens, the regulation of immune responses, cell recruitment and activation, and tissue repair[27]. Human alveolar macrophages and monocytes can serve as antigen presentation cells (APCs) for γδ T cells. Furthermore, the predominance of Vγ9Vδ2 T cells in TB disease has been confirmed[28]. When MTB-activated CD4+ and γδ T cells from healthy tuberculin-positive donors were analyzed for cytokine production in response to M. tuberculosis -infected monocytes, both groups secreted large amounts of IFN-γ[29]. Previous studies have also demonstrated an increased proliferative activity of Vγ9Vδ2 T cells from patients with TB[30], but reduced production of IFN-γ, compared with that of healthy tuberculin-positive donors[31]. Additionally, Dieli et al[32] reported that decrease of Vγ9Vδ2 T cell effector functions involves not only IFN-γ production but also expression of granulysin[32].
CONCLUSION
γδ T cells appear to combine properties of both adaptive and innate immunities. The identification of unusual compounds that are recognized by human γδ T cells but not by αβ T cells has recently stimulated great interest in the development of γδ T cell-based therapies.
Peer reviewer: Fabian Benencia, PhD, Assistant Professor of Immunology, Department of Biomedical Sciences, College of Osteopathic Medicine and BME, Russ College of Engineering and Technology, Academic Research Center, Ohio University Athens, OH 45701, United States
S- Editor Li JY L- Editor A E- Editor Zheng XM