Published online Sep 20, 2025. doi: 10.5493/wjem.v15.i3.105973
Revised: April 7, 2025
Accepted: May 18, 2025
Published online: September 20, 2025
Processing time: 181 Days and 14.4 Hours
Osteoarthritis (OA) of the knee is a prevalent degenerative joint disease that sig
To compare the clinical outcomes of SVF vs nanofat therapy in patients with primary knee OA.
Conducted at Mother Cell Regenerative Centre, Trichy, over 18 months (June 2025 to December 2026), the study will enroll 30 patients, randomly assigned to two groups of 15 each. Both interventions will be administered as a single intra-articular injection under sterile conditions, with cell viability (> 85%) confirmed by a standardized assay. Group A will receive autologous SVF injections, while Group B will receive autologous nanofat injections. The primary outcome measure is the change in pain scores at 12 months using the visual analog scale (VAS). Secondary outcomes include functional improvement assessed by Osteoarthritis Outcome Score (KOOS), Western Ontario and McMaster Universities (WOMAC), and International Knee Documentation Committee (IKDC) scores, cartilage regeneration evaluated via magnetic resonance imaging with colour coded mapping of the cartilage volume (MR cartigram), and monitoring of adverse events.
This study aims to evaluate pain reduction at 12 months post-injection, using the VAS as the primary outcome. Secondary outcomes include functional improvement (KOOS, WOMAC, IKDC), cartilage regeneration (T2 cartigram), adverse event incidence, patient satisfaction (standardized questionnaires, Likert scale), and quality of life (EQ-5D). Ethical considerations follow the Declaration of Helsinki and Good Clinical Practice, with IRB approval and participant informed consent ensured. Confidentiality and data security comply with regulations, and a Data Safety Monitoring Board oversees trial safety. Results will be shared via peer-reviewed journals, presentations at international orthopedic conferences, and detailed summaries for stakeholders and participants. The trial is registered under CTRI/2024/03/064076. Findings emphasize patient-centered advancements in knee osteoarthritis management.
This trial evaluates the efficacy and safety of SVF and nanofat therapies in knee OA, addressing a significant evidence gap. It employs robust methods to enhance cartilage repair and patient quality of life. Future research should standardize dosages, protocols, and injection techniques, explore autologous/allogenic preparations, and advance radiological tools, broadening accessibility and clinical applications.
Core Tip: The double-blinded, randomized controlled trial at Mother Cell Regenerative Centre, Trichy, compares the efficacy and safety of stromal vascular fraction (SVF) vs nanofat in treating primary knee osteoarthritis. Over 18 months, 30 patients will receive either autologous SVF or nanofat injections. Primary outcome is pain reduction at 12 months, while secondary outcomes include functional improvement, cartilage regeneration, and monitoring adverse events. This study aims to provide clinical evidence for these regenerative therapies.
- Citation: Jeyaraman N, Shrivastava S, Rangarajan RV, Nallakumarasamy A, Ramasubramanian S, Muthu S, Jeyaraman M. Comparative outcome analyses of stromal vascular fraction vs nanofat in primary osteoarthritis knee: A double-blinded randomized controlled trial protocol. World J Exp Med 2025; 15(3): 105973
- URL: https://www.wjgnet.com/2220-315X/full/v15/i3/105973.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i3.105973
Osteoarthritis (OA) of the knee is a leading cause of disability worldwide, affecting millions of individuals and contributing to substantial healthcare burdens[1-4]. Characterized by the degeneration of articular cartilage, subchondral bone changes, and synovial inflammation, OA results in chronic pain, reduced joint function, and diminished quality of life[5-8]. Further, the disease by itself is not limited to cartilage or subchondral matrix but extends to involve meniscus, collateral ligaments and other joint tissue, hence called as a whole joint disease[9,10]. Traditional management strategies, including pharmacological interventions and surgical procedures, often provide symptomatic relief but fail to address the underlying cartilage damage and joint degeneration[11-14].
Recent advancements in regenerative medicine have introduced novel therapeutic approaches aimed at restoring joint integrity and function. Among these, stromal vascular fraction (SVF) and nanofat therapies have garnered significant attention due to their regenerative and anti-inflammatory properties[15-17]. SVF, derived from adipose tissue, comprises a heterogeneous population of cells, including adipose-derived mesenchymal stem cells (AD-MSCs), endothelial cells, and immune cells, which collectively contribute to tissue repair and modulation of inflammatory responses[18]. Nanofat, on the other hand, is a mechanically emulsified form of adipose tissue that retains the stromal and vascular components essential for regenerative processes[19].
Preclinical and clinical studies have demonstrated the potential of both SVF and nanofat in promoting cartilage regeneration and reducing inflammation in OA knee[17,20-27]. However, direct comparative studies evaluating the efficacy and safety of SVF vs nanofat in human patients with primary knee OA are scarce[28,29]. Establishing a comparative analysis is crucial for determining the optimal regenerative therapy, considering factors such as treatment efficacy, safety profile, preparation protocols, and cost-effectiveness. The study aims to compare the efficacy and functional outcome of stromal vascular fraction and nanofat in primary osteoarthritis knees. The objectives of the study are (1) To compare the effectiveness of stromal vascular fraction and nanofat in knee osteoarthritis; (2) To assess the functional outcome of stromal vascular fraction and nanofat in knee osteoarthritis; and (3) To study the quantitative & qualitative imaging of cartilage regeneration following the therapy.
This study is an unicentric, double-blinded, randomized controlled trial designed to compare the clinical outcomes of SVF vs nanofat therapy in patients with primary knee OA. The study will be conducted and reported in adherence to the Consolidated Standards of Reporting Trials guidelines[30]. Participants will be allocated to either the SVF group or the nanofat group in a 1:1 ratio using a computer-generated randomization sequence generated via a secure web-based system (randomization.com). Blinding will be maintained for patients, assessors, and statisticians to minimize bias.
The trial will be conducted at Mother Cell Regenerative Centre, Trichy, within the Department of Orthopaedics. The center is equipped with advanced facilities for adipose tissue-derived therapies and imaging modalities necessary for comprehensive outcome assessments.
The sample size was calculated using the formula: n = d2Zα/22 × P × (1-P), where: Zα/2 = 1.96 (95%CI).
P = Prevalence of early OA knee grade II and III = 3.5% (0.035)[31].
d = Desired margin of error = 7% (0.07).
Calculating: n = (0.07)2(1.96)2 × 0.035 × (1 - 0.035) = 0.00493.8416 × 0.035 × 0.965 ≈ 26.47.
This estimate was based on previously reported prevalence data of early knee OA and preliminary pilot observations at our center, assuming a 95% confidence level and 80% power to detect clinically meaningful differences in pain and function. Considering a dropout rate of 15%, the total required sample size is 30 patients, divided equally into two groups of 15 each.
Participants will be randomly assigned to either the SVF or nanofat group using a web-based randomization system. Randomization will use a permuted block design (block size of four) generated via a secure web-based system (randomization.com), ensuring balance between the two groups. A study coordinator not involved in recruitment or assessment will maintain the allocation sequence and prepare identical 10 mL syringes (containing approximately 5-6 mL of injectate), making certain that labeling is neutral and does not disclose the treatment arm. The injecting physician and outcome assessors will remain blinded to allocation throughout the trial. In the event of an adverse event requiring unblinding, the study coordinator will break the code in a controlled manner, with appropriate documentation. Allocation concealment will be ensured by employing sealed opaque envelopes. Blinding will be maintained for patients, outcome assessors, and statisticians to prevent bias in outcome evaluation.
Inclusion criteria: Patients with age between 30-80 years of both sex; Patients with radiological primary osteoarthritis of knees (Kellgren Lawrence grade 2 & 3 based on X-ray findings); Patients with severe pain and under anti-inflammatory treatment without improvement > 3 months; Patients who have given consent for treatment as per our protocol; Regular visits in the out-patient department; Ability to comply with the home-based exercise regimen and scheduled follow-up visits.
Exclusion criteria: Patients with ages less than 30 and more than 80 years of both sex; Patients with advanced primary osteoarthritis of knees (Kellgren Lawrence grade 1 & 4 based on X-ray findings) and secondary osteoarthritis of knees; Patients with h/o prior corticosteroid injection at a treatment site within 3 months of duration; Patients with rheumatoid arthritis, inflammatory arthritis or polyarticular arthritis, and autoimmune diseases; Patients with hemoglobin < 10 gm/dL and platelet count < 105/μL; Patients with local infection at the site of the procedure, HIV, Hepatitis B or C, septicemia, other systemic metabolic disorders, bleeding, and other coagulation disorders.
After obtaining informed written consent from the patients enrolled in our study, they will be subjected to a thorough clinical examination to rule out the other causes of stiff and painful knee syndrome. The baseline investigations such as complete hemogram, erythrocyte sedimentation rate, C-reactive protein, renal function tests, random blood glucose, serological testing for HIV 1 & 2 and HbsAg, and radiographic analysis and MR cartigram of affected knee joint in a standing position will be done.
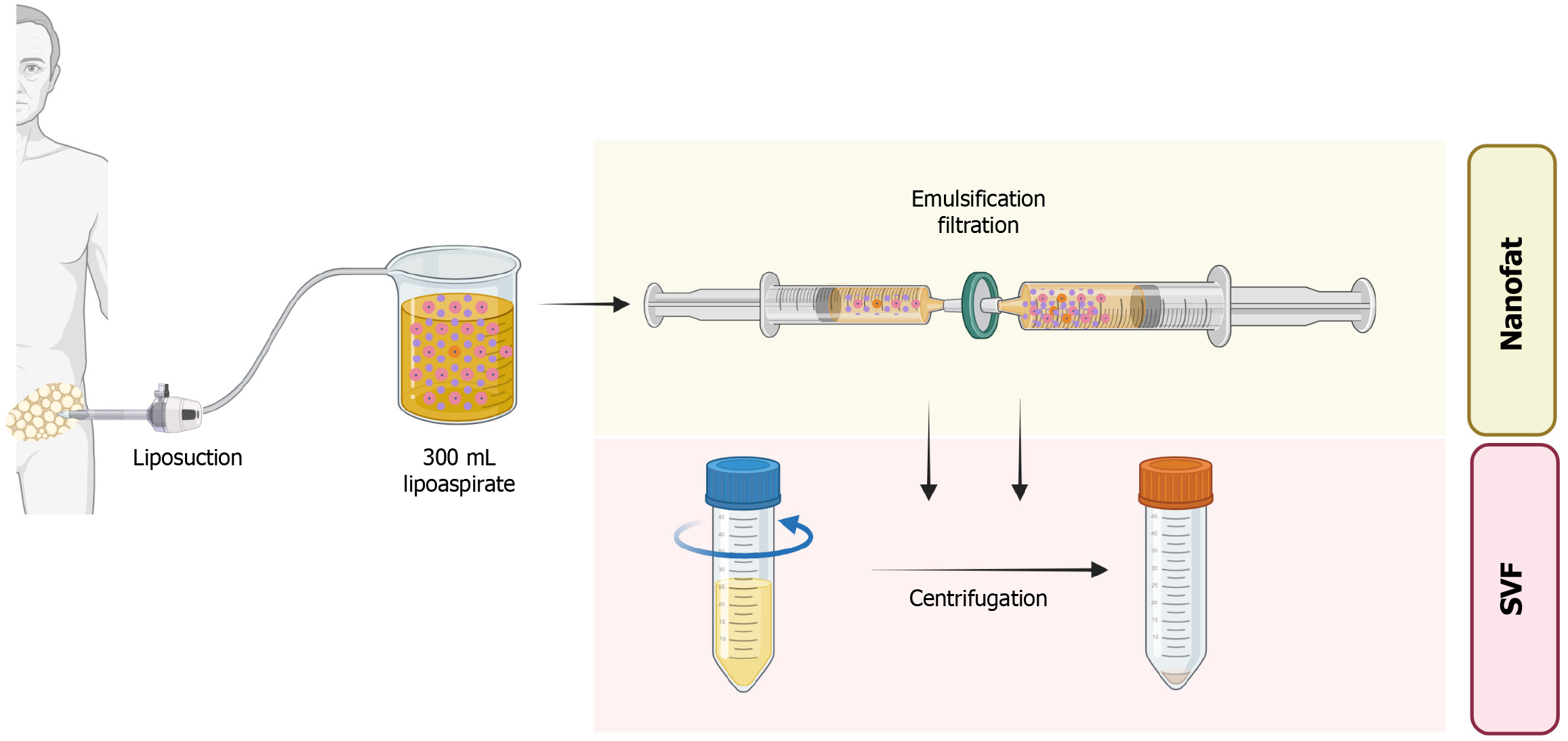
Preparation of stromal vascular fraction: After explaining the procedure to the patients in vernacular language, 300 mL of lipoaspirate will be aspirated from the lower part of the abdomen and rinsed with normal saline. The resultant adipose tissue will be converted into microfat by intrasynringing through the Leur Lok connector (30 passages) and filtration will be done to obtain nanofat. The resultant nanofat will be subjected to centrifugation at a rate of 1200 rpm for 5 minutes. The resultant column will contain an SVF pellet at the bottom of conical tubes as shown in Figure 1. A total of 5–6 mL of stromal vascular fraction (containing AD-MSCs) will be yielded with 300 mL of adipose tissue. Cell viability (> 85%) is confirmed via a trypan blue exclusion test on a small aliquot of the final pellet before intra-articular injection.
Preparation of nanofat: After explaining the procedure to the patients in vernacular language, 300 mL of lipoaspirate will be aspirated from the lower part of the abdomen and rinsed with normal saline. The resultant adipose tissue will be converted into microfat by intrasynringing through the Leur Lok connector (30 passages) and filtration will be done to obtain nanofat as shown in Figure 1. A total of 5-6 mL of nanofat (containing AD-MSCs) will be yielded with 300 mL of adipose tissue. We will likewise evaluate cell viability (> 85%) using the same trypan blue assay, confirming an adequate regenerative cell population in the final nanofat product. Further, flow cytometry of both nanofat and SVF will be done to quantify specific cellular populations present in SVF and confirm the absence of significant cellular content in nanofat.
Pre-procedural protocol: After obtaining a radiograph and magnetic resonance (MR) cartigram of the affected knee joint, the patient will be informed regarding the procedure, informed and written consent will be obtained and the patient will be taught active quadriceps exercises.
Intra-procedural protocol: Group A (n = 15) – One dose of 5-6 mL (cellular dosage of 5.0 × 107 cells with the viability of > 85%) of SVF injectate will be injected on each knee joint on day 0 under sterile conditions. The injection will be performed via a superolateral or anterolateral approach under ultrasound guidance with a 20-gauge needle, ensuring precise intra-articular placement.
Group B (n = 15) – One dose of 5-6 mL of nano fat injectate will be injected on each knee joint on day 0 under sterile conditions. The same ultrasound-guided superolateral or anterolateral approach with a 20-gauge needle will be used for nanofat injection.
All syringes will be covered by a non-transparent sleeve or label to obscure any differences in color or consistency. The same caliber needle will be used for both interventions. Neither the patient nor the injector will see the final contents prior to injection. After the procedure, containing dressing and crepe bandage will be applied. After 10 minutes of post-procedure, gentle knee mobilization exercises will be done for equal distribution of injected solution into the joint space. The patients will be trained for home-based active quadriceps and knee strengthening programs. All the patients will be advised to bear weight on a sequential weight bearing protocol after SVF or nano fat injection and the pain will be combated with ice pack application. NSAIDs and painkillers will be avoided in all patients.
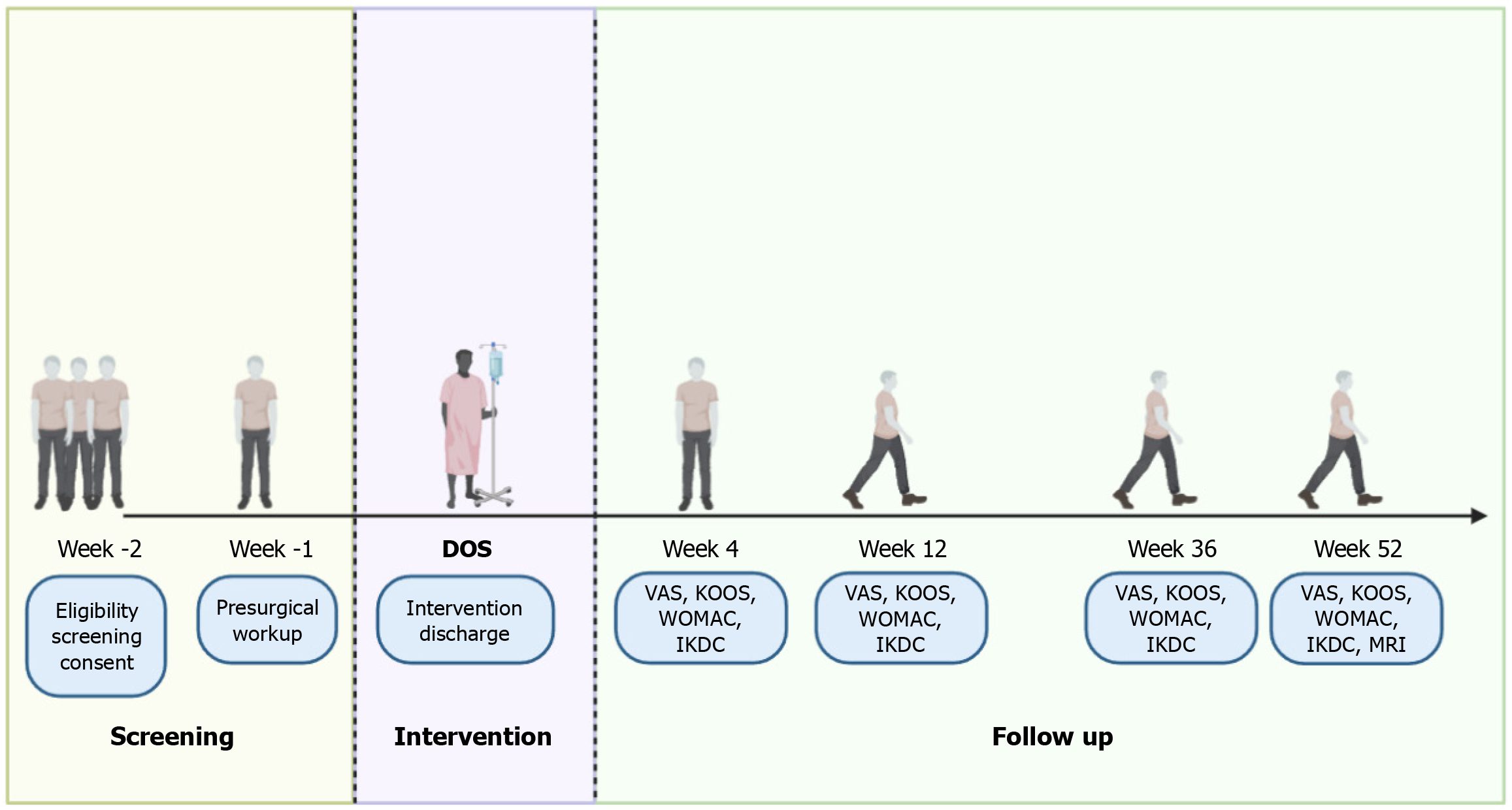
Post-procedural protocol: Once SVF/Nanofat is injected into knee joints for osteoarthritis, the patients are advised to take adequate rest and ice fomentation therapy.Patients are advised to avoid full weight bearing for 48 to 72 hours and to perform active range of motion of knees along with active quadriceps exercises. Patients are advised to perform partial weight bearing from 3rd day to 7th post-op day and full weight bearing at the end of 1st week. Regular follow-up radiographs are performed at the end of 1, 3, 6, and 12 months. Functional scoring will be performed pre-procedurally and post-procedurally at the end of 1, 3, 6, and 12 months with visual analog scale (VAS), Knee injury and Osteoarthritis Outcome Score (KOOS), Western Ontario and McMaster Universities (WOMAC), and International Knee Documentation Committee (IKDC) scores[32-35]. Patients will be subjected for MR cartigram of the knee to check the cartilage thickness after 1 year as shown in Figure 2.
The standardized MR cartigram protocol will include T2 mapping and proton density sequences to assess cartilage thickness and integrity. Cartilage repair will be analyzed by two blinded musculoskeletal radiologists using a semi-quantitative grading system.
The statistical analysis will be done with Statistical Package for Social Sciences software Version 26, IBM Corp, Chicago, Illinois, United States. Both an intention-to-treat analysis and a per-protocol analysis will be performed to account for any missing data or dropouts, preserving the benefits of randomization while also examining treatment efficacy in those who fully adhere. To determine the effect of the intervention on the outcome variables, a paired t-test will be done to compare pre-procedure and post-procedure scores at the end of 1st, 2nd, 6th, and 12th-month follow-up scores for the VAS, KOOS, WOMAC, and IKDC scores for each group. Numerical variables will be compared by using the Kruskal-Wallis test. On the other hand, intra-group comparisons will be performed by using the Friedman test. If data are normally distributed, repeated-measures ANOVA with post-hoc Bonferroni corrections will be applied. If data deviate from normality, the Friedman test with pairwise Wilcoxon signed-rank tests will be employed. Categorical variables were compared by using Fisher's exact test. An alpha level of P < 0.05 will be used to determine statistical significance.
Primary outcome: Change in pain scores at 12 months post-injection, measured using the VAS.
Secondary outcomes: (1) Functional improvement assessment by KOOS, WOMAC, and IKDC scores at various intervals; (2) Cartilage regeneration evaluation via MR carigram at 12 months; (3) Incidence of adverse events related to the treatments; (4) Patient satisfaction measurement through standardized questionnaires; (5) Quality of life improvements assessment using the EQ-5D scores; and (6) Patient satisfaction will be quantified using a 5-point Likert scale (ranging from ‘very unsatisfied’ to ‘very satisfied’), documented at each follow-up interval.
Cartilage integrity will be assessed using T2 cartigram, a well-established MRI technique that provides a quantitative evaluation of cartilage health[36]. This method measures the T2 relaxation time of cartilage tissue, offering insights into the biochemical composition, particularly the hydration status and collagen fiber organization. The choice of a T2 cartigram is based on its ability to detect early degenerative changes in cartilage, which are critical in the context of knee osteoarthritis.
This study will adhere to the Declaration of Helsinki and Good Clinical Practice guidelines. Ethical approval has been obtained from the institutional review board (IRB No. Plasmaart Resto Ethics Committee PR23NJS016 dated 28.10.2023). The clinical trial is registered under Clinical Trial Registry of India (CTRI) number CTRI/2024/03/064076 dated 13.03.2024. All participants will provide informed consent before enrollment. Confidentiality of participant data will be maintained, and data will be stored securely in compliance with relevant data protection regulations. Any serious adverse events will be reported to the institutional review board within 24 hours, and a Data Safety Monitoring Board will oversee ongoing trial safety.
The results of this trial will be disseminated through publication in peer-reviewed medical journals and presentations at international orthopedic and regenerative medicine conferences. Additionally, findings will be shared with healthcare stakeholders and participants through detailed reports and summaries.
OA of the knee remains a significant public health concern, contributing to substantial morbidity and decreased quality of life among the aging population[2,37-42]. Current therapeutic approaches, including pharmacological interventions and surgical procedures, primarily offer symptomatic relief without addressing the underlying degenerative changes in the joint[11,43-46]. This study aims to compare the efficacy and functional outcomes of two promising regenerative therapies—SVF and nanofat—in the treatment of primary knee OA. The discussion herein contextualizes our study within the existing body of literature, explores the potential implications of our findings, and delineates the strengths and limitations of our research design.
Our literature review underscores the therapeutic potential of both SVF and nanofat in cartilage regeneration and symptom alleviation in OA. Various studies have consistently demonstrated that SVF, rich in AD-MSCs, possesses regenerative and anti-inflammatory properties that facilitate cartilage repair and modulate the inflammatory milieu within the joint[47-49]. Similarly, nanofat therapy, characterized by its mechanically emulsified adipose tissue, has shown promise in enhancing chondrocyte viability and promoting anabolic processes while reducing catabolic markers in OA models[25,50-52].
In addition to their regenerative potential, both SVF and nanofat harness significant immunomodulatory capabilities[53,54]. Adipose tissue serves as a reservoir of diverse cell types—including mesenchymal stromal cells, endothelial progenitor cells, and various immune cells—that collectively secrete a broad array of cytokines and growth factors[18]. These secretions, notably interleukin-10 and transforming growth factor-β, help modulate the local inflammatory environment. By tempering inflammatory cascades and encouraging anabolic activity, these factors not only alleviate pain but also create conditions conducive to cartilage repair. Moreover, the heterogeneous cell composition inherent to adipose-derived preparations facilitates a dynamic immunoregulatory response[55,56]. The interplay between pro-inflammatory and anti-inflammatory mediators, along with a shift from M1 to M2 macrophage phenotypes and enhanced regulatory T-cell activity, contributes to a reduction in synovial inflammation[57-62]. This coordinated immunomodulation may play a pivotal role in mitigating joint degradation and slowing osteoarthritic progression, thereby augmenting the overall efficacy of both SVF and nanofat therapies.
Notably, comparative studies are limited, making our randomized controlled trial a crucial addition to the literature. Previous research indicates that both SVF and nanofat can significantly improve pain and functional outcomes in OA patients. For instance, SVF therapy has been associated with sustained pain relief and functional improvement over extended follow-up periods, with minimal adverse events reported[20-24]. On the other hand, nanofat injections have demonstrated significant improvements in pain scores and delayed the need for total knee arthroplasty in OA patients[17,25-27,52]. However, direct comparisons between these two therapies are sparse, necessitating studies like ours to establish their relative efficacy and safety profiles.
The outcomes of this study hold significant implications for clinical practice. Should SVF demonstrate superior efficacy in pain reduction and cartilage regeneration compared to nanofat, it could position SVF as the preferred regenerative therapy for primary knee OA. Conversely, if nanofat shows comparable or enhanced outcomes, it may offer a more cost-effective and easily prepared alternative, particularly beneficial in resource-constrained settings. Furthermore, understanding the differential effects on cartilage morphology via MRI could inform personalized treatment strategies, optimizing therapeutic outcomes based on individual patient profiles.
Several strengths underpin the robustness of our study design:
Randomized controlled design: The double-blind, randomized allocation minimizes selection bias and confounding, enhancing the internal validity of the findings.
Comprehensive outcome measures: By evaluating primary outcomes (pain scores via VAS) and multiple secondary outcomes (functional scores, cartilage regeneration on MRI, adverse events), the study provides a holistic assessment of therapeutic efficacy and safety.
Standardized protocols: Detailed intervention protocols for both SVF and nanofat ensure consistency in treatment administration, facilitating reproducibility and reliability of results. We have followed the standardized methods of preparation of SVF and nanofat as detailed earlier[19,21,63,64].
Ethical considerations: Adherence to ethical guidelines, including informed consent and data confidentiality, ensures the protection of participant rights and the integrity of the research process.
An essential strength lies in the double-blind structure, which reduces both observer and participant bias in subjective outcome measures such as VAS pain scores. By incorporating a robust randomization scheme—using a permuted block algorithm and neutral syringe labeling—we minimize the potential for allocation imbalances and ensure that neither patients nor clinical staff can predict group assignments. This meticulous approach is especially critical when evaluating treatments like SVF and nanofat, whose differences might be visually evident. Through careful masking of the injectate and strict allocation concealment, the current design offers a high level of internal validity.
Despite its strengths, the study is subject to certain limitations:
Sample size: With a total of 30 patients, the study may lack sufficient power to detect smaller effect sizes and generalize findings across diverse populations. Future studies with larger cohorts are warranted to validate our results.
Short follow-up duration: An 18-month study period may not capture long-term outcomes and potential delayed adverse events. Extending follow-up durations in subsequent research could provide more comprehensive insights into the durability of treatment effects.
Single-center design: Conducting the trial at a single center may limit the generalizability of the findings to other clinical settings with different patient demographics and treatment practices.
Variability in SVF and Nanofat preparations: Although standardized protocols are employed, inherent variability in adipose tissue-derived cell preparations may influence therapeutic outcomes. Inherent differences in the liposuction technique or adipose tissue microenvironment among participants may influence cell yields. Although standardized protocols are employed, such variability could affect the regenerative potential in each group, which may in turn limit the generalizability of our findings.
Blinding challenges: While efforts are made to maintain blinding, the distinct nature of SVF and nanofat preparations may inadvertently unblind participants or clinicians, potentially introducing bias.
Another notable limitation concerns the inherent challenge of masking treatments that may differ in appearance, consistency, or color. Despite using opaque syringe covers and a standardized injection protocol, subtle visual or tactile differences might inadvertently unblind the clinician or participant. Although the randomization technique reduces selection bias, larger multi-center trials would offer stronger external validity and better capture the nuances of real-world patient populations.
Building on the findings of this study, future research should consider the following avenues:
Larger, multi-center trials: Expanding the sample size and involving multiple centers can enhance the generalizability and robustness of the findings, providing a more comprehensive understanding of SVF and nanofat efficacy across varied populations.
Long-term follow-up: Extending the follow-up period beyond 18 months will allow for the assessment of long-term benefits and potential late-onset adverse events, ensuring the sustained efficacy and safety of the therapies.
Mechanistic studies: Investigating the molecular and cellular mechanisms underlying the regenerative effects of SVF and nanofat can elucidate their pathways of action, optimizing therapeutic strategies and potentially enhancing efficacy.
Comparative effectiveness research: Direct comparisons with other regenerative therapies, such as platelet-rich plasma or cultured MSCs, can position SVF and nanofat within the broader landscape of regenerative medicine, guiding clinical decision-making. Further research should also explore the use of objective biomarkers (e.g., serum or synovial fluid inflammatory markers) to correlate clinical improvement with local tissue changes, refining our understanding of the mechanisms behind SVF and nanofat efficacy.
Standardization of preparation protocols: Developing universally accepted protocols for SVF and nanofat preparation can reduce variability, ensuring consistent therapeutic outcomes and facilitating widespread clinical adoption.
This randomized controlled trial addresses a critical gap in the comparative clinical evidence for the efficacy and safety of SVF and nanofat therapies in the management of primary knee OA. As a condition associated with significant morbidity and diminished functional quality of life in the adult population, knee OA demands innovative treatment approaches. Regenerative therapies, particularly SVF and nanofat, hold significant promise in promoting cartilage repair and alleviating symptoms. By employing a robust study design, comprehensive outcome measures, and stringent adherence to ethical standards, this trial seeks to inform optimal regenerative treatment strategies and improve patient outcomes and quality of life.
The findings of this study will contribute meaningfully to the field of regenerative medicine in orthopedics. However, there remains considerable scope for further research to advance the field. Future studies should focus on the standardization of dosage and injection frequency for both SVF and nanofat therapies, alongside the development of universal protocols for preparation methods and injection techniques. Comparative investigations into autologous vs allogenic preparations are needed to optimize therapeutic efficacy. Additionally, the integration of advanced radiological tools to document cartilage regeneration comprehensively will enhance evidence-based practices. Exploration of allogenic formulations may further improve the accessibility and scalability of these promising therapies, ultimately broadening their clinical application.
| 1. | Scheuing WJ, Reginato AM, Deeb M, Acer Kasman S. The burden of osteoarthritis: Is it a rising problem? Best Pract Res Clin Rheumatol. 2023;37:101836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 2. | Leifer VP, Katz JN, Losina E. The burden of OA-health services and economics. Osteoarthritis Cartilage. 2022;30:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 289] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 3. | Cui A, Li H, Wang D, Zhong J, Chen Y, Lu H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine. 2020;29-30:100587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 351] [Cited by in RCA: 858] [Article Influence: 143.0] [Reference Citation Analysis (0)] |
| 4. | Langworthy M, Dasa V, Spitzer AI. Knee osteoarthritis: disease burden, available treatments, and emerging options. Ther Adv Musculoskelet Dis. 2024;16:1759720X241273009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 5. | Primorac D, Molnar V, Rod E, Jeleč Ž, Čukelj F, Matišić V, Vrdoljak T, Hudetz D, Hajsok H, Borić I. Knee Osteoarthritis: A Review of Pathogenesis and State-Of-The-Art Non-Operative Therapeutic Considerations. Genes (Basel). 2020;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 307] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 6. | Tong L, Yu H, Huang X, Shen J, Xiao G, Chen L, Wang H, Xing L, Chen D. Current understanding of osteoarthritis pathogenesis and relevant new approaches. Bone Res. 2022;10:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 276] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 7. | Du X, Liu ZY, Tao XX, Mei YL, Zhou DQ, Cheng K, Gao SL, Shi HY, Song C, Zhang XM. Research Progress on the Pathogenesis of Knee Osteoarthritis. Orthop Surg. 2023;15:2213-2224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 75] [Reference Citation Analysis (0)] |
| 8. | Giorgino R, Albano D, Fusco S, Peretti GM, Mangiavini L, Messina C. Knee Osteoarthritis: Epidemiology, Pathogenesis, and Mesenchymal Stem Cells: What Else Is New? An Update. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 216] [Reference Citation Analysis (0)] |
| 9. | Fontanella CG, Belluzzi E, Pozzuoli A, Scioni M, Olivotto E, Reale D, Ruggieri P, De Caro R, Ramonda R, Carniel EL, Favero M, Macchi V. Exploring Anatomo-Morphometric Characteristics of Infrapatellar, Suprapatellar Fat Pad, and Knee Ligaments in Osteoarthritis Compared to Post-Traumatic Lesions. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 10. | Muthu S. Osteoarthritis, an old wine in a new bottle! World J Orthop. 2023;14:1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Block JA, Cherny D. Management of Knee Osteoarthritis: What Internists Need to Know. Rheum Dis Clin North Am. 2022;48:549-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 12. | McGrory BJ, Weber KL, Jevsevar DS, Sevarino K. Surgical Management of Osteoarthritis of the Knee: Evidence-based Guideline. J Am Acad Orthop Surg. 2016;24:e87-e93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Srivastava AK; Surgical Management of Osteoarthritis of the Knee Work Group, Staff of the American Academy of Orthopaedic Surgeons. American Academy of Orthopaedic Surgeons Clinical Practice Guideline Summary of Surgical Management of Osteoarthritis of the Knee. J Am Acad Orthop Surg. 2023;31:1211-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 14. | Mintarjo JA, Poerwanto E, Tedyanto EH. Current Non-surgical Management of Knee Osteoarthritis. Cureus. 2023;15:e40966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 15. | Jeyaraman M, Muthu S, Ganie PA. Does the Source of Mesenchymal Stem Cell Have an Effect in the Management of Osteoarthritis of the Knee? Meta-Analysis of Randomized Controlled Trials. Cartilage. 2021;13:1532S-1547S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 16. | Zampogna B, Parisi FR, Ferrini A, Zampoli A, Papalia GF, Shanmugasundaram S, Papalia R. Safety and efficacy of autologous adipose-derived stem cells for knee osteoarthritis in the elderly population: A systematic review. J Clin Orthop Trauma. 2024;59:102804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Jeyaraman N, Shrivastava S, Ravi VR, Nallakumarasamy A, Jeyaraman M. Current status of nanofat in the management of knee osteoarthritis: A systematic review. World J Orthop. 2025;16:99690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (7)] |
| 18. | Sharma S, Muthu S, Jeyaraman M, Ranjan R, Jha SK. Translational products of adipose tissue-derived mesenchymal stem cells: Bench to bedside applications. World J Stem Cells. 2021;13:1360-1381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (2)] |
| 19. | Jeyaraman M, Muthu S, Sharma S, Ganta C, Ranjan R, Jha SK. Nanofat: A therapeutic paradigm in regenerative medicine. World J Stem Cells. 2021;13:1733-1746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (2)] |
| 20. | Tantuway V, Thomas W, Parikh MB, Sharma R, Jeyaraman N, Jeyaraman M. Clinical Outcome of Minimally Manipulated, Mechanically Isolated Autologous Adipose Tissue-Derived Stromal Vascular Fraction (Sahaj Therapy®) in Knee Osteoarthritis-Randomized Controlled Trial. Indian J Orthop. 2023;57:1646-1658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Jeyaraman M, Jeyaraman N, Jayakumar T, Ramasubramanian S, Ranjan R, Jha SK, Gupta A. Efficacy of stromal vascular fraction for knee osteoarthritis: A prospective, single-centre, non-randomized study with 2 years follow-up. World J Orthop. 2024;15:457-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Zhang S, Xu H, He B, Fan M, Xiao M, Zhang J, Chen D, Tong P, Mao Q. Mid-term prognosis of the stromal vascular fraction for knee osteoarthritis: a minimum 5-year follow-up study. Stem Cell Res Ther. 2022;13:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 23. | Ren B, Chang Y, Liu R, Xiao F, Xu J, Li L, Li T, Ruan Z, Bao Y, Lin J, Zhou J, Liao W, Pan Z, Xu H, Tian J, Cai L, Zheng XX. Clinical phase I/II trial of SVF therapy for cartilage regeneration: A cellular therapy with novel 3D MRI imaging for evaluating chondral defect of knee osteoarthritis. Front Cell Dev Biol. 2023;11:1106279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 24. | Kim YS, Oh SM, Suh DS, Tak DH, Kwon YB, Koh YG. Cartilage lesion size and number of stromal vascular fraction (SVF) cells strongly influenced the SVF implantation outcomes in patients with knee osteoarthritis. J Exp Orthop. 2023;10:28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 25. | Chen Z, Ge Y, Zhou L, Li T, Yan B, Chen J, Huang J, Du W, Lv S, Tong P, Shan L. Pain relief and cartilage repair by Nanofat against osteoarthritis: preclinical and clinical evidence. Stem Cell Res Ther. 2021;12:477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 26. | Han Z, Bai L, Zhou J, Qian Y, Tang Y, Han Q, Zhang X, Zhang M, Yang X, Cui W, Hao Y. Nanofat functionalized injectable super-lubricating microfluidic microspheres for treatment of osteoarthritis. Biomaterials. 2022;285:121545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 27. | Smyshlyaev IA, Gilfanov SI, Batuchtina EV, Popov I, Vasilyev VS, Pulin AA, Eremin II. Osteoarthritis of the Knee: Comparison Between Intra-articular Injection of Adipose-Derived Stromal Vascular Fraction and Nanofat. In: Kalaaji A, editors. Plastic and Aesthetic Regenerative Surgery and Fat Grafting. Cham: Springer, 2022: 1683-1700. [DOI] [Full Text] |
| 28. | Maeda T, Sobajima S, Matsumoto T, Tsubosaka M, Matsushita T, Iwaguro H, Kuroda R. Comparison of short-term clinical outcomes of intra-articular injection of micro-fragmented adipose tissue and stromal vascular fraction cells for knee osteoarthritis treatment: A retrospective single-center cohort study. Regen Ther. 2025;29:91-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Han JH, Jung M, Chung K, Moon HS, Jung SH, Byun J, Kim SH. Intra-articular Stromal Vascular Fraction and Mesenchymal Stem Cell Injections Show Variable Efficacy and Higher Potential Complications Compared to Corticosteroid and Hyaluronic Acid in Treatment of Knee Osteoarthritis: A Meta-analysis of Randomized Controlled Trials. Arthroscopy. 2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Cuschieri S. The CONSORT statement. Saudi J Anaesth. 2019;13:S27-S30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 295] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 31. | Driban JB, Bannuru RR, Eaton CB, Spector TD, Hart DJ, McAlindon TE, Lu B, Lo GH, Arden NK. The incidence and characteristics of accelerated knee osteoarthritis among women: the Chingford cohort. BMC Musculoskelet Disord. 2020;21:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Muthu S, Vadranapu S. Variations in quantifying patient reported outcome measures to estimate treatment effect. World J Methodol. 2025;15:97078. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (11)] |
| 33. | Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1182] [Cited by in RCA: 1783] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 34. | Riddle DL, Perera RA. The WOMAC Pain Scale and Crosstalk From Co-occurring Pain Sites in People With Knee Pain: A Causal Modeling Study. Phys Ther. 2020;100:1872-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Collins NJ, Misra D, Felson DT, Crossley KM, Roos EM. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS). Arthritis Care Res (Hoboken). 2011;63 Suppl 11:S208-S228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 909] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 36. | Zhao H, Li H, Liang S, Wang X, Yang F. T2 mapping for knee cartilage degeneration in young patients with mild symptoms. BMC Med Imaging. 2022;22:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 37. | Yahaya I, Wright T, Babatunde OO, Corp N, Helliwell T, Dikomitis L, Mallen CD. Prevalence of osteoarthritis in lower middle- and low-income countries: a systematic review and meta-analysis. Rheumatol Int. 2021;41:1221-1231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 38. | Barbour KE, Sagawa N, Boudreau RM, Winger ME, Cauley JA, Nevitt MC, Fujii T, Patel KV, Strotmeyer ES. Knee Osteoarthritis and the Risk of Medically Treated Injurious Falls Among Older Adults: A Community-Based US Cohort Study. Arthritis Care Res (Hoboken). 2019;71:865-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Palazzo C, Nguyen C, Lefevre-Colau MM, Rannou F, Poiraudeau S. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 585] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 40. | GBD 2021 Osteoarthritis Collaborators. Global, regional, and national burden of osteoarthritis, 1990-2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023;5:e508-e522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1052] [Cited by in RCA: 995] [Article Influence: 331.7] [Reference Citation Analysis (1)] |
| 41. | He Y, Jiang W, Wang W. Global burden of osteoarthritis in adults aged 30 to 44 years, 1990 to 2019: results from the Global Burden of Disease Study 2019. BMC Musculoskelet Disord. 2024;25:303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 42. | Long H, Liu Q, Yin H, Wang K, Diao N, Zhang Y, Lin J, Guo A. Prevalence Trends of Site-Specific Osteoarthritis From 1990 to 2019: Findings From the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022;74:1172-1183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 732] [Article Influence: 183.0] [Reference Citation Analysis (0)] |
| 43. | Martel-Pelletier J, Maheu E, Pelletier JP, Alekseeva L, Mkinsi O, Branco J, Monod P, Planta F, Reginster JY, Rannou F. A new decision tree for diagnosis of osteoarthritis in primary care: international consensus of experts. Aging Clin Exp Res. 2019;31:19-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 44. | Karasavvidis T, Hirschmann MT, Kort NP, Terzidis I, Totlis T. Home-based management of knee osteoarthritis during COVID-19 pandemic: literature review and evidence-based recommendations. J Exp Orthop. 2020;7:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Buelt A, Narducci DM. Osteoarthritis Management: Updated Guidelines from the American College of Rheumatology and Arthritis Foundation. Am Fam Physician. 2021;103:120-121. [PubMed] |
| 46. | Madry H. Surgical therapy in osteoarthritis. Osteoarthritis Cartilage. 2022;30:1019-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 47. | Ude CC, Shah S, Ogueri KS, Nair LS, Laurencin CT. Stromal Vascular Fraction for Osteoarthritis of the Knee Regenerative Engineering. Regen Eng Transl Med. 2022;8:210-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 48. | Yokota N, Yamakawa M, Shirata T, Kimura T, Kaneshima H. Clinical results following intra-articular injection of adipose-derived stromal vascular fraction cells in patients with osteoarthritis of the knee. Regen Ther. 2017;6:108-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 49. | Yang WT, Ke CY, Yeh KT, Huang SG, Lin ZY, Wu WT, Lee RP. Stromal-vascular fraction and adipose-derived stem cell therapies improve cartilage regeneration in osteoarthritis-induced rats. Sci Rep. 2022;12:2828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 50. | Cohen SR, Tiryaki T, Womack HA, Canikyan S, Schlaudraff KU, Scheflan M. Cellular Optimization of Nanofat: Comparison of Two Nanofat Processing Devices in Terms of Cell Count and Viability. Aesthet Surg J Open Forum. 2019;1:ojz028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 51. | Quintero Sierra LA, Biswas R, Conti A, Busato A, Ossanna R, Zingaretti N, Parodi PC, Conti G, Riccio M, Sbarbati A, De Francesco F. Highly Pluripotent Adipose-Derived Stem Cell-Enriched Nanofat: A Novel Translational System in Stem Cell Therapy. Cell Transplant. 2023;32:9636897231175968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 52. | Cicione C, Vadalà G, Di Giacomo G, Tilotta V, Ambrosio L, Russo F, Zampogna B, Cannata F, Papalia R, Denaro V. Micro-fragmented and nanofat adipose tissue derivatives: In vitro qualitative and quantitative analysis. Front Bioeng Biotechnol. 2023;11:911600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 53. | Girard P, Dulong J, Duisit J, Mocquard C, Le Gallou S, Chaput B, Lupon E, Watier E, Varin A, Tarte K, Bertheuil N. Modified nanofat grafting: Stromal vascular fraction simple and efficient mechanical isolation technique and perspectives in clinical recellularization applications. Front Bioeng Biotechnol. 2022;10:895735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 54. | Goncharov EN, Koval OA, Igorevich EI, Encarnacion Ramirez MJ, Nurmukhametov R, Valentinovich KK, Montemurro N. Analyzing the Clinical Potential of Stromal Vascular Fraction: A Comprehensive Literature Review. Medicina (Kaunas). 2024;60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 55. | Walker JT, Cooper TT, Dunmore-Buyze J, Serack FE, Brooks C, Grant A, Drangova M, Lajoie G, Dekaban GA, Flynn LE. Syngeneic adipose-derived stromal cells modulate the immune response but have limited persistence within decellularized adipose tissue implants in C57BL/6 mice. Acta Biomater. 2025;195:169-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 56. | Laloze J, Fiévet L, Desmoulière A. Adipose-Derived Mesenchymal Stromal Cells in Regenerative Medicine: State of Play, Current Clinical Trials, and Future Prospects. Adv Wound Care (New Rochelle). 2021;10:24-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 57. | Corvera S. Cellular Heterogeneity in Adipose Tissues. Annu Rev Physiol. 2021;83:257-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 58. | Al-Ghadban S, Artiles M, Bunnell BA. Adipose Stem Cells in Regenerative Medicine: Looking Forward. Front Bioeng Biotechnol. 2021;9:837464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 59. | Qin Y, Ge G, Yang P, Wang L, Qiao Y, Pan G, Yang H, Bai J, Cui W, Geng D. An Update on Adipose-Derived Stem Cells for Regenerative Medicine: Where Challenge Meets Opportunity. Adv Sci (Weinh). 2023;10:e2207334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 144] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 60. | Ceccarelli S, Pontecorvi P, Anastasiadou E, Napoli C, Marchese C. Immunomodulatory Effect of Adipose-Derived Stem Cells: The Cutting Edge of Clinical Application. Front Cell Dev Biol. 2020;8:236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 61. | Seo Y, Shin TH, Kim HS. Current Strategies to Enhance Adipose Stem Cell Function: An Update. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 62. | Wang Y, Fang J, Liu B, Shao C, Shi Y. Reciprocal regulation of mesenchymal stem cells and immune responses. Cell Stem Cell. 2022;29:1515-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 211] [Reference Citation Analysis (0)] |
| 63. | Jeyaraman N, Shrivastava S, Ravi VR, Nallakumarasamy A, Pundkar A, Jeyaraman M. Understanding and controlling the variables for stromal vascular fraction therapy. World J Stem Cells. 2024;16:784-798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (2)] |
| 64. | Mundluru VK, Naidu MJ, Mundluru RT, Jeyaraman N, Muthu S, Ramasubramanian S, Jeyaraman M. Non-enzymatic methods for isolation of stromal vascular fraction and adipose-derived stem cells: A systematic review. World J Methodol. 2024;14:94562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (6)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/