©The Author(s) 2015.
World J Exp Med. Aug 20, 2015; 5(3): 164-181
Published online Aug 20, 2015. doi: 10.5493/wjem.v5.i3.164
Published online Aug 20, 2015. doi: 10.5493/wjem.v5.i3.164
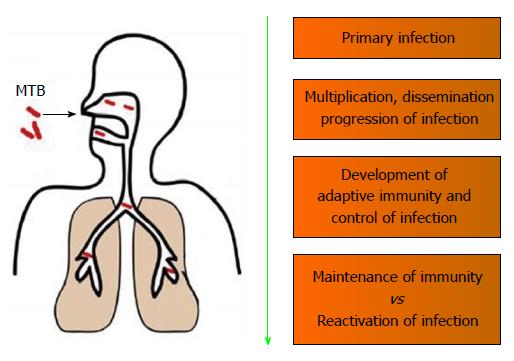
Figure 1 Spectrum of host immune responses against Mycobacterium tuberculosis.
MTB: Mycobacterium tuberculosis.
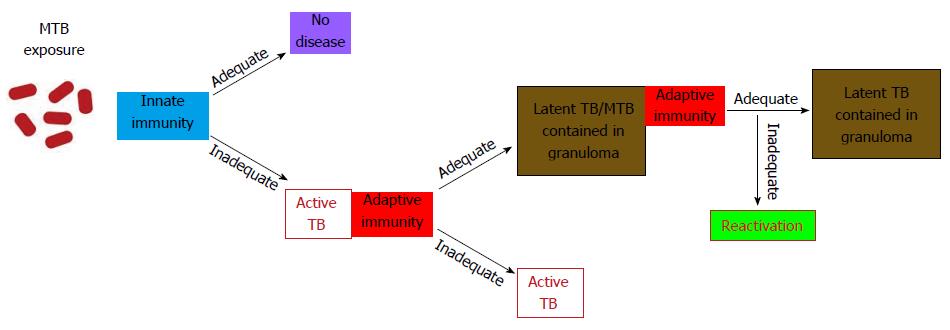
Figure 2 Latent tuberculosis pathogenesis and transmission profile.
Most people are adequately protected by innate immunity against MTB infection, however, the infection progresses when the innate immune response levels are inadequate. The development of specific immunity leads to the containment of MTB in granulomas as asymptomatic latent infection. However, inadequate adaptive response at any time progresses to the reactivation and development of full-blown active tuberculosis. MTB: Mycobacterium tuberculosis.
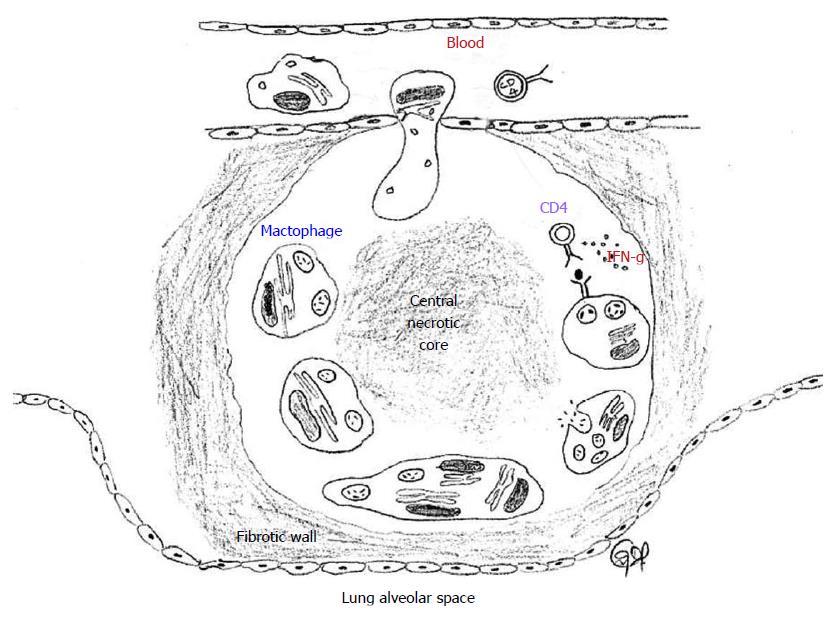
Figure 3 Tuberculosis granuloma.
A granuloma sequesters MTB infected macrophages and is surrounded by immune cells, predominantly CD4+ helper T lymphocytes. Some infected macrophages fuse to form foamy giant cells. The infected macrophages and giant cells present antigens to T cells and activate them to produce a variety of cytokines and chemokynes, and also kills the infected macrophage and the MTB. The chemokines also serve to recruit additional T cells to the granuloma from the circulating blood. IFN-γ activates the macrophages to kill the intracellular MTB by generating reactive oxygen species and reactive nitrogen species intracellularly. The center of the granuloma is filled with cell debris and both live and dead MTB spilled from dead macrophages (caseation), all of which form a central hypoxic necrotic core. A sheath of collagen fibers produced from lung fibroblasts surround the granuloma. MTB: Mycobacterium tuberculosis.
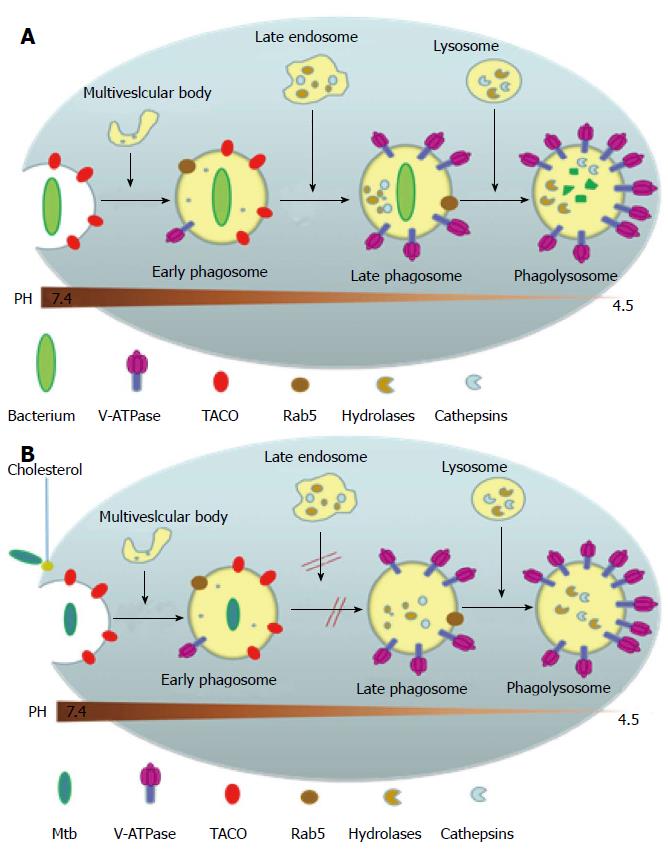
Figure 4 Phagosomal maturation or arrest following pathogen uptake (A) or Mycobacterium tuberculosis uptake (B), respectively.
(A) shows that upon entering into the body, most non-pathogenic microbes are phagocytized by the macrophage into a phagosome which then goes on to mature by fusing with the vesicles of the endocytic pathway and to finally fuse with lysosomes. These phagosomes undergo acidification due to the presence of proton-ATPase molecules from vacuolar membranes and the lysosomes, and this increased level of acidification activates the lysosomally derived acid hydrolases, cathepsins and other enzymes, along with reactive oxygen and nitrogen intermediates, to destroy the pathogen. Phagocytosis also initially triggers the recruitment of TACO around the particle to be ingested, as a result of the latter’s initial association with cell cortex microtubules, but is released prior to the lysosomal delivery of the bacteria; (B) Shows the effect of M. tuberculosis on phagosome maturation. Cholesterol serves as a docking site for the mycobacteria and its cell surface receptor there by facilitating its phagocytosis at cholesterol-rich regions. Cholesterol plays a crucial role in not only the entry of mycobacteria into macrophages but also mediates the phagososomal association of TACO (Coronin 1), a coat protein associated with cholesterol-rich regions which is actively retained on the phagosomal membrane housing the mycobacteria through a yet unknown mechanism, which prevents the degradation of the mycobacteria in the lysosomes. TACO: Tryptophan-aspartate containing coat protein.
Figure 5 The organization of the mce operons.
The operon structures of the four mce operons with their constituent genes are shown. The grey arrows represent yrbE genes and the blue arrows represent the mce genes.
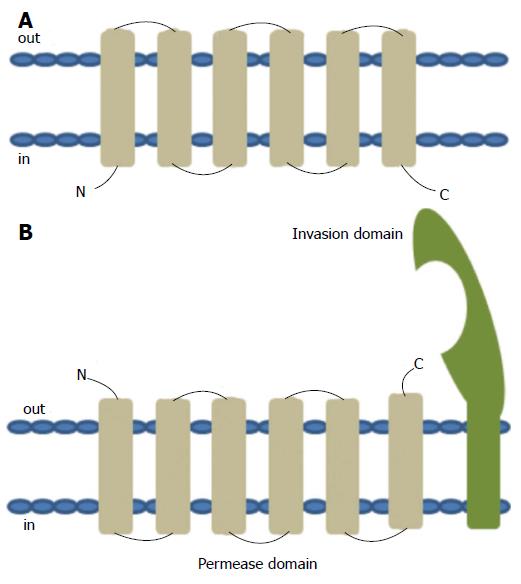
Figure 6 Topology of typical ATP binding cassette transmembrane permease (A) and the predicted topology of YRBE permease and MCE invasion domain (B).
ATP binding cassette (ABC) transport systems in both prokaryotes and eukaryotes consists of four domains, two cytoplasmic ABC domains and two hydrophobic membrane spanning permease domains (A). Each of the membrane spanning domains typically contains 6 hydrophobic transmembrane segments anchored in the cell membrane with the C- and N-termini located towards the cytoplasmic side. ABC transporters in prokaryotes that function as importers also typically require additional extracytoplasmic helper proteins called ‘substrate binding proteins’ for function. Each of the mce operons contains two YRBE domains that have structural homology to the permease domain of the ABC transporters (B). Each YRBE permease contains five or six transmembrane segments with the C-terminal end located on the extracytoplasmic side and the N-terminal end being either cytoplasmic or extracellular. The MCE protein, MCE4A, consists of a patch of 400 amino acid residues, with a hydrophobic stretch anchored in the cell membrane and a 22-amino acid invasion domain exposed outside to the membrane, which is predicted to be structurally similar to the substrate binding domain of ABC importers.
Figure 7 Molecular beacon technology.
Molecular beacons are hairpin shaped antisense RNAs that does not fluoresce in the absence of mRNA binding (left) while the fluorescence increases when the beacon hybridizes with its target mRNA (right).
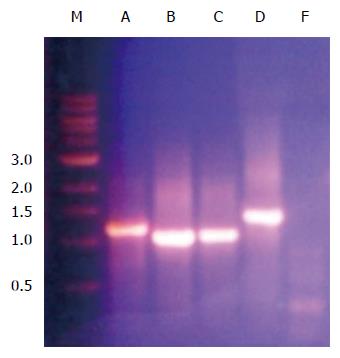
Figure 8 Polymerase chain reaction amplification of mce4A, mce4B, mce4C, mce4D and mce4F of Mycobacterium tuberculosis.
Mce4 operon genes were polymerase chain reaction amplified from M. tuberculosis H37Rv using gene specific primers and resolved on 1% agarose gel. The forward primer spanned the first 21 nucleotides from the beginning of the open reading frame and the reverse primer covered 21 nucleotides spanning the complementary strand to the 3’ end of the gene. The termination codon was omitted so that the product, MCE4A-F, will be expressed with a 6XHis tag and a myc tag.
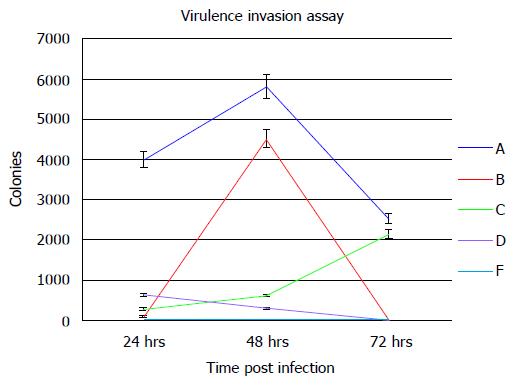
Figure 9 Mtb-MCE4 proteins confer virulence to Escherichia coli.
Escherichia coli-Mtbmce4 clones were used to infect 2 × 106 MCF7 epithelial cells at an MOI of 10:1 for 2 h. The level of infection was assessed by counting bacterial colony numbers at 24 , 48 and 72 h post-infection (n = 3).
- Citation: George R, Cavalcante R, Jr CC, Marques E, Waugh JB, Unlap MT. Use of siRNA molecular beacons to detect and attenuate mycobacterial infection in macrophages. World J Exp Med 2015; 5(3): 164-181
- URL: https://www.wjgnet.com/2220-315X/full/v5/i3/164.htm
- DOI: https://dx.doi.org/10.5493/wjem.v5.i3.164