Published online Aug 7, 2020. doi: 10.5492/wjccm.v9.i3.43
Peer-review started: December 16, 2019
First decision: April 2, 2020
Revised: May 22, 2020
Accepted: June 14, 2020
Article in press: June 14, 2020
Published online: August 7, 2020
Processing time: 233 Days and 13.4 Hours
Patients with cancer have several risk factors for developing respiratory failure requiring mechanical ventilation (MV). The emergence of multidrug resistant bacteria (MDRB) has become a public health problem, creating a new burden on medical care in hospitals, particularly for patients admitted to the intensive care unit (ICU).
To describe risk factors for ventilator-acquired pneumonia (VAP) in patients with cancer and to evaluate the impact of MDRB.
A retrospective study was performed from January 2016 to December 2018 at a cancer referral center in Mexico City, which included all patients who were admitted to the ICU and required MV ≥ 48 h. They were classified as those who developed VAP versus those who did not; pathogens isolated, including MDRB. Clinical evolution at 60-d was assessed. Descriptive analysis was carried out; comparison was performed between VAP vs non-VAP and MDRB vs non-MDRB.
Two hundred sixty-three patients were included in the study; mean age was 51.9 years; 52.1% were male; 68.4% had solid tumors. There were 32 episodes of VAP with a rate of 12.2%; 11.5 episodes/1000 ventilation-days. The most frequent bacteria isolated were the following: Klebsiella spp. [n = 9, four were Extended-Spectrum Beta-Lactamase (ESBL) producers, one was Carbapenem-resistant (CR)]; Escherichia coli (n = 5, one was ESBL), and Pseudomonas aeruginosa (n = 8, two were CR). One Methicillin-susceptible Staphylococcus aureus was identified. In multivariate analysis, the sole risk factor associated for VAP was length of ICU stay (OR = 1.1; 95%CI: 1.03-1.17; P = 0.003). Sixty-day mortality was 53% in VAP and 43% without VAP (P = 0.342). There was not higher mortality in those patients with MDRB.
This study highlights the high percentage of Gram-negative bacteria, which allows the initiation of empiric antibiotic coverage for these pathogens. In this retrospective, single center, observational study, MDRB VAP was not directly linked to increased mortality at 60 days.
Core tip: This is a retrospective study to evaluate the risk factors for ventilator-associated pneumoniae (VAP) in patients with cancer who are admitted at an intensive care unit and require mechanical ventilation for > 48 h. We emphasized in microbiology etiology, particularly multidrug resistant bacteria (MDRB). We included 263 patients during 2 year-period; 32 developed VAP, with a rate of 11.5 episodes/1000 ventilation-days. Gram-negative bacteria were isolated in 95% of cases, being the rate of MDRB 24.1%. Sixty-day mortality was 53% in VAP and 43% without VAP. There was not higher mortality in patients with MDRB.
- Citation: Cornejo-Juárez P, González-Oros I, Mota-Castañeda P, Vilar-Compte D, Volkow-Fernández P. Ventilator-associated pneumonia in patients with cancer: Impact of multidrug resistant bacteria. World J Crit Care Med 2020; 9(3): 43-53
- URL: https://www.wjgnet.com/2220-3141/full/v9/i3/43.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v9.i3.43
The prognosis of malignancies has improved during recent decades, with an increase in overall survival[1,2]. However, patients with cancer have elevated risks of infections and potential complications related with treatment, particularly chemotherapy, central lines, extensive surgeries, and other factors that lead to higher morbidity and mortality[2]. Likewise, patients with cancer have several risk factors for developing respiratory failure related to infectious and non-infectious processes, such as pneumonia, lung thrombosis, sepsis, transfusion-related acute lung injury (TRALI), and lung edema[1]. Therefore, these patients sometimes require support with mechanical ventilation (MV) and admission to the intensive care unit (ICU). The development of Ventilator-Associated Pneumonia (VAP) is the most frequent ICU-acquired infection, occurring in 25%-30% of patients intubated for > 48 h, with an incremental proportional risk within the first 14 d of ventilation[3-5]. The estimated incidence of VAP range from 2-16 episodes per 1000 ventilator-days[5]. On the other hand, the emergence of multidrug resistant bacteria (MDRB) has become a public health problem, creating a new burden on medical care in hospitals, particularly for patients admitted to ICU[6].
The aim of this study was to describe the clinical characteristics, local pathogens included MDRB, risk factors, and outcomes in patients with cancer who develop VAP.
We conducted a retrospective analysis of all patients admitted to the ICU who required MV for ≥ 48 h at the Instituto Nacional de Cancerología (INCan), a cancer referral center in Mexico City, from January 1st 2016 to December 31st, 2018.
Demographic and clinical data were recorded from the clinical electronic charts of the patients and included the following age; sex; body mass index (BMI); type of neoplasm; current status of cancer (recent diagnosis; complete or partial remission, progression, or relapse); Charlson Comorbidity Index; history of chemotherapy, radiotherapy, biologic drugs, recent hospitalization, or antimicrobials used (during the last 3 mo); Sequential Organ Failure Assessment score (SOFA) and Acute Physiology Age Chronic Health Evaluation (APACHE) II at ICU admission; indication for and days of MV; tracheostomy; bronchial culture or bronchioalveolar lavage; diagnosis of VAP; bacteria isolated that were classified as susceptible, MDRB, or extreme drug-resistant (XDR) bacteria; type and number of days of antimicrobials; length of hospitalization, length of ICU stay, and 60-d outcome.
Pneumonia was clinically suspected on the presence of new and/or progressive pulmonary infiltrates in a chest X-ray, along with two of the following criteria: Hyperthermia (≥ 38 oC) or hypothermia (≤ 36 oC); leukocytosis (≥ 12000/mL) or leucopenia (≤ 4000/mL), and purulent pulmonary secretions[7,8].
VAP was defined as pneumonia in a patient on mechanical ventilation for > 2 calendar days on the day of event, with day of ventilator placement being Day 1 and the ventilator was in place on the date of event of the day before[9]. In those patients who were admitted to the ICU with pre-existing pneumonia, the clinical worsening, and/or the appearance of new clinical data compatible with pneumonia criteria were considered to be redefined as VAP.
Endotracheal aspirate or sputum cultures together with blood cultures were performed on day one the ICU stay and later in the case of clinical deterioration or suspected pneumonia. Bronchial samples were taken by sterile aspiration through the endotracheal tube and inoculated on blood, MacConkey, Sabouraud, and chocolate agar. Bacterial identification was performed by Mass Spectrometry Especially Matrix-Assisted Laser Desorption and Ionization -Time of Flight- Mass Spectrometry (MALDI-TOF-MS; Microflex, United States). Antimicrobial susceptibility testing was performed by means of BD Automated PhoenixTM (United States) and by the Kirby-Bauer disk diffusion technique in the case of resistant strains (Clinical Laboratory Standards Institute. Microbiological data were collected from the patient’s electronic clinical chart and from Microbiology Laboratory data including cultures from the lower respiratory tract (sputum, tracheal, bronchial aspirate, or bronchioalveolar lavage). Polymicrobial pneumonia was defined when more than one pathogen was identified. The presence of MDR/XDR pathogens was recorded and defined according to Magiorakos criteria[10].
Primary outcome was VAP development. Secondary outcome was clinical evolution at 60-d.
Descriptive analysis was carried out with mean ± SD or median [Interquartile range (IQR)]. The student t-test or the Mann-Whitney U test were used to compare continuous variables as appropriate. The χ2 or Fisher exact test was utilized to compare categorical variables. Variables with P values of ≤ 0.3 in the univariate analysis were included in the multivariate analysis. A logistic regression model was performed for risk factors associated with VAP and for 60-day mortality. OR with 95%CI were calculated. P values of ≤ 0.05 were considered statistically significant. Data was analyzed using STATA (ver. 14) software. The study was approved by the INCan Institutional Review Board (REF/INCAN/CI/0922/2019).
During the study period, 736 patients were admitted to the ICU: 345 patients required MV for less than 48 h and 128 did not require intubation; 263 patients were included. Mean age was 51.9 ± 17.8 years; 188 (68.4%) were patients with solid tumors and there were 88 (31.8%) with hematologic malignancies; 123 (46.8%) were in cancer progression or relapse; eight patients had two different neoplasms. Other demographic and clinical data are shown in Table 1.
| Characteristics, n (%) | Total (n = 263) | VAP (n = 32) | Non-VAP (n = 231) | P value |
| Age (yr)1 | 51.9 ± 17.8 | 49 ± 19.7 | 52.3 ± 17.5 | 0.329 |
| Gender- Masculine | 137 (52.1) | 16 (50) | 110 (47.6) | 0.800 |
| Body mass index1 | 26.2 ± 5.6 | 24.9 ± 4.5 | 26.4 ± 5.7 | 0.188 |
| Solid tumor2 | 188 (68.1) | 25 (67.6) | 163 (68.2) | 0.938 |
| Cervical | 21 (7.6) | 2 (5.4) | 19 (7.9) | 0.749 |
| Head and neck | 21 (7.6) | 3 (8.1) | 18 (7.5) | 1 |
| Colon-rectum | 20 (7.2) | 1 (2.7) | 19 (7.9) | 0.492 |
| Breast | 18 (6.5) | 2 (5.4) | 16 (6.7) | 1 |
| Germinal | 15 (5.4) | 2 (5.4) | 13 (5.4) | 1 |
| Esophagus-stomach | 14 (5.1) | 3 (8.1) | 11 (4.6) | 0.399 |
| Sarcoma | 13 (4.7) | 2 (5.4) | 11 (4.6) | 0.688 |
| Ovarian | 10 (3.6) | 1 (2.7) | 9 (3.8) | 1 |
| Lung | 10 (3.6) | 1 (2.7) | 9 (3.8) | 1 |
| Prostate | 9 (3.3) | 2 (5.4) | 7 (2.9) | 0.348 |
| Liver and bile ducts | 9 (3.3) | 1 (2.7) | 8 (3.3) | 1 |
| Pancreas | 7 (2.5) | 1 (2.7) | 6 (2.5) | 1 |
| Kidney and bladder | 5 (1.8) | 2 (5.4) | 3 (1.3) | 0.136 |
| Other | 16 (5.8) | 2 (5.4) | 14 (5.9) | 1 |
| Hematological malignancies2 | 88 (31.9) | 12 (32.4) | 76 (31.8) | 0.938 |
| Lymphoblastic leukemia | 26 (9.4) | 3 (8.1) | 23 (9.6) | 1 |
| Myeloid leukemia | 12 (4.3) | 3 (8.1) | 9 (3.8) | 0.207 |
| Non-Hodgkin lymphoma | 25 (9.1) | 2 (5.4) | 23 (9.6) | 0.548 |
| Hodgkin lymphoma | 4 (1.5) | 1 (2.7) | 3 (1.2) | 0.439 |
| Multiple myeloma | 14 (5.1) | 2 (5.4) | 12 (5) | 1 |
| Other3 | 7 (2.5) | 1 (2.7) | 6 (2.5) | 1 |
| Cancer stage | ||||
| Recent diagnosis | 117 (44.5) | 11(34.4) | 105 (45.4) | 0.236 |
| Progression | 93 (35.4) | 16 (50) | 78 (33.8) | 0.07 |
| Relapse | 30 (11.4) | 2 (6.2) | 28 (12.1) | 0.551 |
| Partial remission | 21 (8) | 2 (6.2) | 19 (8.2) | 1 |
| Complete remission | 2 (0.7) | 1 (3.1) | 1 (0.4) | 0.228 |
| Chemotherapy within 3 mo | 99 (37.6) | 16 (50) | 83 (35.9) | 0.123 |
| Radiotherapy during the previous 6 mo | 23 (8.7) | 3 (94) | 20 (8.7) | 0.749 |
| Biologic antineoplastic drugs | 22 (8.4) | 6 (18.8) | 16 (6.9) | 0.155 |
| Charlson index | 3 (2, 5) | 3 (2, 5) | 3 (2, 5) | 1 |
| Hospital admission within 3-mo period | 75 (28.5) | 5 (15.6) | 70 (30.3) | 0.09 |
| Days of recent hospitalization4 | 7 (4,12) | 5 (4,9) | 7 (4,12) | 0.544 |
| Recent broad antimicrobials | 36 (13.7) | 1 (3.1) | 35 (15.1) | 0.09 |
The main cause for MV was septic shock (n = 91, 34.6%), followed by post-surgical procedure (n = 42, 16%), pneumonia (n = 38, 14.5%), and hypovolemic shock (n = 37, 14.1%). The median length of MV was 8 d (IQR 4, 12 d).
There were 32 episodes of VAP; the rate was 12.2%, with an incidence of 11.5 episodes/1000 ventilation-days. Mean days of MV until VAP diagnosis was 13.1 ± 8.8. d (Table 2).
| Characteristic – n (%) | Total (n = 263) | VAP (n = 32) | Non-VAP (n = 231) | P value |
| Length of hospitalization (d)1 | 22 (14, 34) | 32 (22, 57) | 21 (14, 32) | 0.0001 |
| Length of ICU stay (d)1 | 8 (5, 13) | 18 (9, 27) | 8 (5, 12) | < 0.0001 |
| Causes for MV | ||||
| Septic shock | 91 (34.6) | 10 (31.3) | 81 (35) | 0.843 |
| Post-surgical procedure | 42 (16) | 8 (25) | 34 (14.7) | 0.193 |
| Respiratory failure secondary to pneumonia | 37 (14) | 3 (9.4) | 34 (14.7) | 0.589 |
| Hypovolemic shock | 37 (14) | 8 (25) | 29 (12.5) | 0.09 |
| Neurologic cause | 13 (4.9) | 0 | 13 (5.6) | N/A |
| Lung tumor activity | 7 (2.7) | 1 (3.1) | 6 (2.6) | 0.601 |
| Post-CPR | 7 (2.7) | 1 (3.1) | 6 (2.6) | 0.601 |
| Acute pulmonary edema | 6 (2.3) | 0 | 6 (2.6) | N/A |
| Malignant central airway obstruction | 5 (1.9) | 0 | 5 (2.2) | N/A |
| Cardiac failure | 3 (1.1) | 1 (3.1) | 2 (0.8) | 0.323 |
| Bronchospasm | 2 (0.8) | 0 | 2 (0.8) | N/A |
| Pulmonary embolism | 2 (0.8) | 0 | 2 (0.8) | N/A |
| TRALI | 1 (0.4) | 0 | 1 (0.4) | N/A |
| Other causes | 10 (3.8) | 0 | 10 (4.3) | N/A |
| SOFA at ICU admission2 | 8.3 ± 3.4 | 8.7 ± 2.8 | 8.3 ± 3.4 | 0.477 |
| Days of mechanical ventilation1 | 8 (4, 12) | 16 (9, 27) | 7 (4, 11) | < 0.0001 |
| Tracheostomy | 68 (25.9) | 19 (59.4) | 49 (21.2) | < 0.0001 |
| Re-intubation | 27 (10.3) | 7 (21.9) | 20 (8.7) | 0.03 |
| Mortality at 60 d | 116 (44.1) | 9 (28.1) | 72 (31.7) | 0.839 |
There was a statistically significant difference between median length of ICU stay in patients with VAP (18 d; IQR 9, 27) vs those without VAP (8 d; IQR 5, 12; P < 0.001). Also, there was a difference in median length of hospitalization (32 d for VAP; IQR 22, 57 d vs 21 d for non-VAP; IQR 14, 32; P < 0.001). Mean duration of MV was significantly longer in those who developed VAP (16 d; IQR 9, 27) vs those who did not (7 d; IQR 4, 11; P < 0.001). Data is shown in Table 2.
There were no differences between age, gender, solid or hematological neoplasm, recent chemotherapy, progression or relapse in those who developed VAP vs those who did not. The uni- and multivariate analysis is point in Table 3.
| Characteristics | Univariate | Multivariate | |||
| NAV (n = 32) | No-NAV (n = 231) | P value | OR | P value | |
| Female | 16 (50) | 121 (52.4) | 0.8 | - | |
| Male | 16 (50) | 110 (47.6) | |||
| Age < 60 yr | 21 (65.6) | 134 (58) | 0.411 | - | |
| Age ≥ 60 yr | 11 (34.4) | 97 (42) | |||
| Solid tumor | 12 (37.5) | 76 (32.9) | 0.605 | - | |
| Hematologic malignancy | 20 (62.5) | 155 (67.1) | |||
| Recent diagnosis, complete or partial remission | 14 (43.8) | 125 (54.1) | 0.271 | 1 | 0.541 |
| Progression or relapse | 18 (56.2) | 106 (45.9) | 1.3 (0.55 - 3.03) | ||
| Non-recent chemotherapy | 16 (50) | 148 (64.1) | 0.123 | 1 | 0.727 |
| Recent chemotherapy | 16 (50) | 83 (35.9) | 1.16 (0.49-2.76) | ||
| SOFA at ICU admission | 8.71 ± 2.79 | 8.26 ± 3.42 | 0.477 | - | |
| Days of hospitalization length1 | 32 (22, 57) | 21 (14, 32) | 0.0001 | 1 | 0.301 |
| 1 (0.99- 1.01) | |||||
| Days of ICU length1 | 18 (9, 27) | 8 (5, 12) | < 0.0001 | 1 | < 0.0001 |
| 1.11 (1.06-1.17) | |||||
| Alive | 10 (31.2) | 122 (52.8) | 0.02 | 1 | 0.125 |
| Death | 22 (68.8) | 109 (47.2) | 2.04 (0.82-5.12) | ||
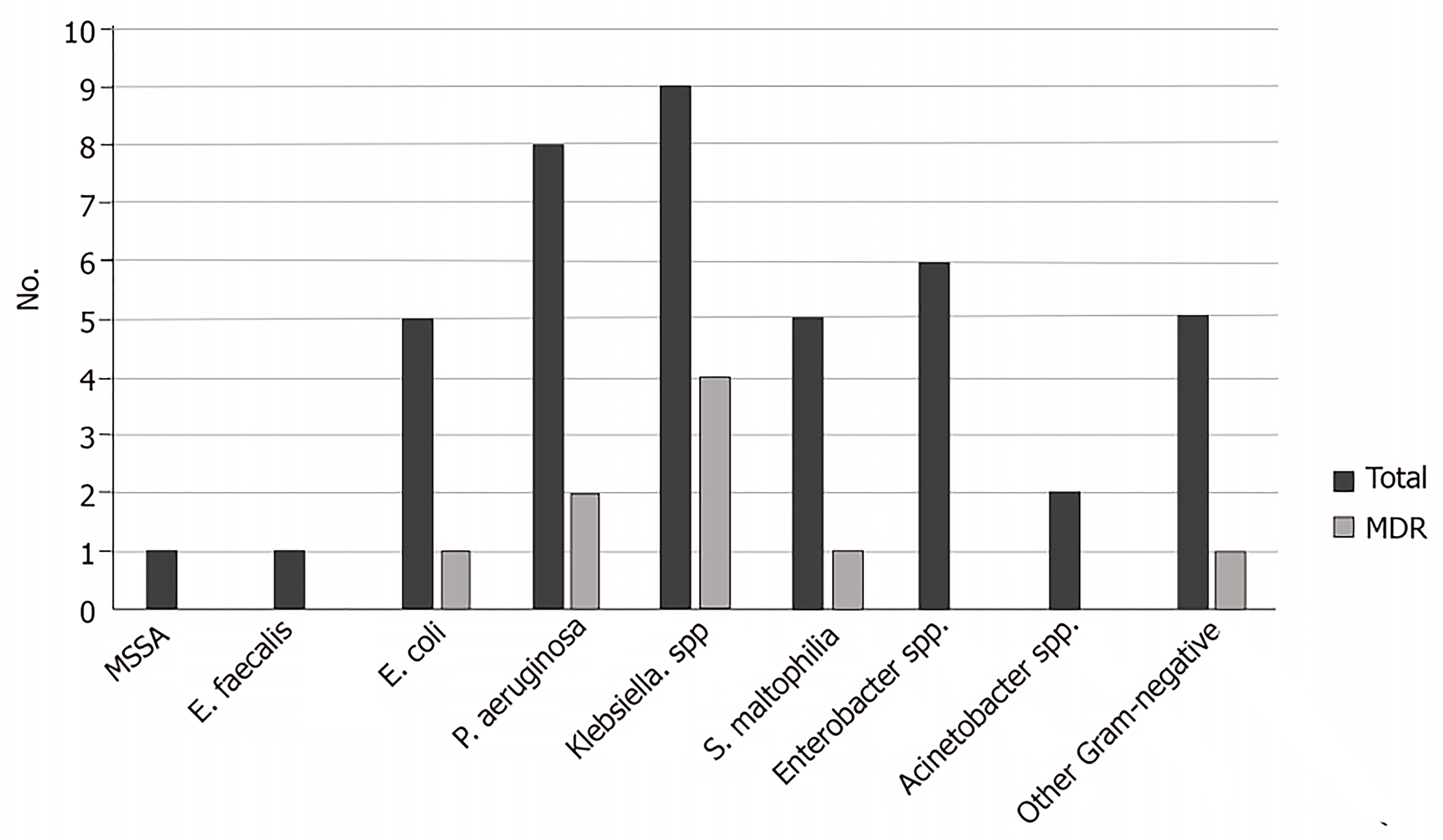
There were 42 bacteria identified in patients with VAP. In 16 (50%), only one pathogen was isolated, 11 were polymicrobial (seven cultures with two different pathogens, four with three), and five cultures were negative. The most frequent bacteria isolated were as follows: Klebsiella spp. (n = 9, 21.4%), four (44.4%) were Extended-Spectrum Beta-Lactamases (ESBL) producers, and one (11.1%) was Carbapenem-resistant (CR); Escherichia coli (n = 5, 11.9%), one (25%) was ESBL producer; Pseudomonas aeruginosa (n = 8, 19%), two (25%) were CR; and Enterobacter spp. (n = 6, 14.3%), among which none was resistant. There were two Gram-positive bacteria identified: one Enterococcus faecalis and one Methicillin-susceptible Staphylococcus aureus (MSSA) (Figure 1). The rate of MDRB was 24%. There were no differences when comparing MDRB vs susceptible, length of hospitalization, previous antibiotics, or days of MV. Patients with MDRB had a longer stay at the ICU (14.1 ± 11 d) vs patients with susceptible bacteria (10.1 ± 7.8 d; P = 0.02).
Patients who developed VAP more frequently received cephalosporins, carbapenems, Tazobactam/Piperacillin, Vancomycin, and fluoroquinolones; furthermore, the period of administration of carbapenems was longer (Table 4).
| Antimicrobial treatment | Total (n = 263) | Non-VAP (n = 233) | VAP (n = 30) | P value |
| Antibacterial treatment | ||||
| Cephalosporins | 58 (22) | 47 (20.2) | 11 (36.7) | 0.03 |
| Days of cephalosporins12 | 6 (4, 9) | 6 (4, 9) | 4 (4, 10) | 0.856 |
| TZP | 86 (32.6) | 69 (29.6) | 17 (56.7) | 0.002 |
| Days of TZP2 | 6 (4, 9) | 7 (4, 9) | 6 (5, 7) | 0.895 |
| Aminoglycosides | 18 (6.8) | 14 (6) | 4 (13.3) | 0.134 |
| Days of aminoglycosides2 | 4 (3, 6) | 3 (3, 5) | 5 (4, 7) | 0.469 |
| Carbapenem | 228 (86.7) | 198 (85) | 30 (100) | 0.02 |
| Days of Carbapenem2 | 11 (7, 17) | 10 (6, 16) | 13 (10, 22) | 0.003 |
| Fluoroquinolones | 31 (11.8) | 23 (9.9) | 8 (26.7) | 0.006 |
| Days of fluoroquinolones2 | 10 (7, 14) | 11 (7, 14) | 9 (5, 15) | 0.586 |
| Vancomycin | 153 (58.2) | 130 (55.8) | 24 (80) | 0.01 |
| Days of vancomycin2 | 7 (4, 10) | 7 (4, 10) | 7 (4, 10) | 0.684 |
| Linezolid | 47 (17.8) | 39 (16.7) | 8 (26.7) | 0.205 |
| Days of linezolid2 | 9 (5, 12) | 8 (4, 11) | 14 (8, 21) | 0.05 |
| Clarithromycin | 68 (25.8) | 59 (25.3) | 9 (30) | 0.657 |
| Days of clarithromycin2 | 8 (7, 10) | 8 (6, 10) | 8 (8,10) | 0.505 |
| SMX/TMP | 68 (25.8) | 56 (24) | 12 (40) | 0.06 |
| Days of SMX/TMP2 | 8 (5, 13) | 12 (7, 21) | 12 (8, 14) | 0.577 |
| Colistin | 11 (4.2) | 7 (3) | 4 (13.3) | 0.02 |
| Days of colistin2 | 10 (4, 11) | 8 (3, 11) | 11 (8, 12) | 0.341 |
Univariate analysis comparing patients with VAP vs non-VAP revealed that tracheostomy and re-intubation were more frequent in VAP (27.9% vs 6.6%; P < 0.001, and 28% vs 10.6%; P = 0.03, respectively). Median length of hospitalization was longer for VAP vs non-VAP (32 d; IQR 21, 57 d vs 21 d vs IQR 14, 32; P < 0.001), in addition, the median length of ICU stay was 18 d (IQR 9, 27 vs 8 d vs IQR 5, 12; P < 0.001), and median days of MV was VAP 16 d (IQR 9, 27 vs non-VAP 7 d; IQR 4, 11; P < 0.001). In multivariate analysis, only length of ICU stay was found statistically significant (OR = 1.11; 95%CI: 1.06-1.17; P < 0.001)( Table 3).
One hundred sixteen patients (44.1%) died during the first 60 d: 17 (53%) with VAP vs 99 (43%) without VAP (P = 0.342). No differences were found between hematologic patients (n = 42, 47.7%), vs those with solid tumors (n = 74, 42.3%; P = 0.401). There was no difference in outcome in patients with MDRB (P = 1). Univariate and multivariate analysis demonstrated that a recent history of chemotherapy (OR = 2.16; 95%CI: 1.24-3.76) and tracheostomy (OR = 2.52; 95%CI: 1.24-5.13) were predictive risk factors for 60-d mortality (Table 5).
| Characteristics | Univariate | Multivariate | |||
| Alive (n = 147) | Death (n = 116) | P value | OR | P value | |
| Female | 79 (53.7) | 58 (50) | 0.546 | - | |
| Male | 68 (46.3) | 58 (50) | |||
| Age < 60 yr | 83 (56.5) | 72 (62.1) | 0.358 | - | |
| Age ≥ 60 yr | 64 (43.5) | 44 (37.9) | |||
| Solid tumor | 101 (68.7) | 74 (63.8) | 0.401 | - | |
| Hematologic malignancy | 46 (31.3) | 42 (36.2) | |||
| Recent diagnosis, complete or partial remission | 85 (57.8) | 54 (46.6) | 0.069 | 1 | 0.237 |
| Progression or relapse | 62 (42.2) | 62 (53.4) | 1.38 (0.81-2.37) | ||
| Non-recent chemotherapy | 103 (70.1) | 61 (52.6) | 0.003 | 1 | 0.006 |
| Recent chemotherapy | 44 (29.9) | 55 (47.4) | 2.16 (1.24-3.76) | ||
| SOFA at ICU admission | 8.45 ± 3.45 | 8.15 ± 3.2 | 0.471 | - | |
| Non-tracheostomy | 115 (78.2) | 80 (69) | 0.088 | 1 | 0.01 |
| Required tracheostomy | 32 (21.8) | 36 (31) | 2.52 (1.24-5.13) | ||
| Days of ICU length | 8 (6, 13) | 8 (5, 15) | 0.457 | - | |
| Days of mechanical ventilation | 7 (4, 11) | 9 (5, 14) | 0.029 | 1 | 0.15 |
| 1.04 (1.008-1.07) | |||||
| Non-VAP | 132 (89.8) | 99 (85.3) | 0.342 | - | |
| VAP | 15 (10.2) | 17 (14.7) | |||
This study sought to describe the characteristics of patients with cancer admitted to the ICU who required MV and developed VAP, analyzing risk factors for 60-d mortality.
It is important to note that almost two thirds of the patients had a solid tumor and one third had received chemotherapy within the last 3 mo. It is relevant to highlight that 46.8% of patients were on cancer relapse or progression, because policies in our hospital include the admission at the ICU of patients who have an expectation of survival more than 3 mo, an adequate functional state, and if they are receiving the first or second line of neoplastic treatment even if they are not in remission. Regarding the risk factors analyzed in relation to cancer such as solid tumor vs hematological, clinical stage of cancer, or recent chemotherapy, there was no relationship with the development of VAP. The median of Charlson Comorbidity Index was 3 for the whole group, that corresponds to one-year mortality rate of 52%. SOFA index was less than 10 in all patients, without differences between VAP vs non-VAP, that indicates between one or two organ failures, and a mortality percentage between 10% and 25%.
The incidence of VAP varies among different series, the latter related to the characteristics of ICU and type of hospitals, and ranges between 2.1 and 24.5 cases/1000 ventilator-days[4,11]. Specifically, a study performed in patients with cancer, VAP was reported in 42/1000 ventilator-days[11]. The incidence we found in this study was 12.2% and 11.5 cases/1000 ventilator-days, lower than those reported in these previous studies[4,11].
VAP is associated with longer hospital and ICU stays, higher hospital-related costs, and greater in-hospital mortality[4]. We also described longer ICU and hospital stays and more days of MV in patients with VAP, more often requiring tracheostomy and re-intubation. These findings would be explained by effect-cause bias, because patients with VAP are patients who are more difficult to extubate, they require a tracheostomy more frequently, more days of antibiotics, and this leads to more days of hospitalization. An important finding in this study was that patients with VAP more frequently received broad-spectrum antibiotics (particularly cephalosporins, Tazobactam/Piperacillin, carbapenems, and Vancomycin). It is noteworthy that frequent causes for ICU admission were septic shock and respiratory failure secondary to pneumonia; thus, broad-spectrum antibiotics are usually initiated empirically in these patients.
Some studies have described Gram-negative bacilli as the most common group of VAP-associated pathogens, accounting for over 50% of cases; Acinetobacter baumannii, Pseudomonas aeruginosa, in addition to S. aureus[4,12]. We found that 95% of Gram-negative bacteria in this series were Klebsiella spp., P. aeruginosa, Enterobacter spp., and E. coli the most common pathogens. It is important to emphasize that there were only two Gram-positive bacteria identified. Additionally, we found that 34.3% of the infections were polymicrobial, similar to 40% reported in other studies[3].
Likewise, an increase has been described in the isolation of Gram-negative MDRB strains in patients with VAP[13]. Nevertheless, we identified only 21.4% of MDRB strains as follows: ESBL-Klebsiella spp. in 44.4%; ESBL-E. coli in 25%; P. aeruginosa CR in 25%, and Klebsiella spp. in 11.1%. The rate of MDRB described in this study was similar to that which we have previously reported in health care-associated infections in the same ICU during 2013 and 2014 (24%)[14]. The National Healthcare Surveillance Network in the United States in 2014 found the following higher rates of MDR in patients with VAP: 37% of Methicillin-resistant S. aureus (MRSA); 31.1% CR-P. aeruginosa, and 14% CR-Klebsiella pneumoniae. A study performed to assess the microbiological profile and MDR Gram-negative bacteria in the ICU during 2010-2011, showed Citrobacter and K. pneumoniae as the most common isolated pathogens, with a high prevalence of carbapenemase- producing bacteria (48%)[15], considerably higher than the results found in our study.
MDRB strains have been related with widespread use of antimicrobials, prolonged use of MV, longer length of hospitalization, and prior antibiotic therapy[12]. In this study, only longer ICU stay was more frequent in patients with these bacteria (P = 0.02).
Sixty-day mortality was reported in 44.1% (48.8% in hematological and 43.4% in patients with solid tumors; P = 0.457). In a previous study performed in the same ICU, the mortality rate for patients with MV was 34.4% (73% for hematological patients and 34.3% for patients with solid tumors)[16], this lower mortality can be related because, in the last study, we included all patients with MV, regardless of ventilation time.
Bundle implementation reduces the rate of VAP; this is the most efficacious measure when compliance rates are high, and includes education and training, hand hygiene, head positioning (> 30o), cuff- pressure maintenance, avoidance of elective changes of circuits, humidifiers, and endotracheal tubes, oral chlorhexidine gluconate, aspiration of subglottic secretions, selective decontamination of the oropharynx tract, and a short course of systemic antibiotics during the intubation of patients with previous decreased consciousness[17,18]. In our hospital, the previous measures, except for the last two, are performed routinely; adherence to prevention bundles is monitored by a nurse from the Infection Control Department who is assigned to the ICU. In addition to the latter prevention measures, enhancing antimicrobial stewardship programs is a simple and cost-effective way to improve clinical outcomes, maintaining quality of care and contributing to the decrease of VAP episodes[19].
There are some imitations of this study. First, it was retrospective, and second was conducted at only one center, it could have the bias inherent to this type of design. However, the hospital is one of the biggest in the region, and the number of patients treated each year is also large. Third, the number of episodes of VAP were not many, which could have influenced not to find significant differences in some of the risk factors studied. On the other hand, the study’s main strength is the example of how a study such as the one we present, contributes to reinforcing policies of antimicrobial stewardship within a hospital tailored by the results.
In conclusion, the rate of VAP was similar to that reported in other studies conducted in immunosuppressed patients. However, it is important to highlight the elevated percentage of Gram-negative bacteria as a cause of pneumonia, which permits beginning empiric antibiotic coverage for these pathogens, without the need to cover Gram-positive bacteria, particularly Vancomycin for Methicillin-resistant S. aureus. In this retrospective, single center, observational study, MDRB VAP was not directly linked to increased mortality at 60 d.
Patients with cancer have several risk factors for developing respiratory failure requiring mechanical ventilation (MV). The emergence of multidrug resistant bacteria (MDRB) has become a public health problem, creating a new burden on medical care in hospitals, particularly for patients admitted to the intensive care unit (ICU).
To establish and/or modify guidelines for the initiation of empirical antimicrobial treatment in cancer patients who develop VAP.
To describe in the patient with cancer which are the risk factors for developing ventilator-acquired pneumonia, and if there is a higher incidence of episodes secondary to multidrug-resistant bacteria.
A retrospective study carried out over a two-year period, that included all patients with mechanical ventilation who were admitted to the ICU, and we analyzed those who developed an episode of VAP and the bacteria involved.
Two hundred sixty-three patients were included; two thirds with a solid tumor. There were 32 episodes of VAP; 11.5 episodes/1000 ventilation-days. Gram-negative bacteria were involved in 95%of cases, 24% were MDRB. There were no differences in mortality between those patients with VAP vs non-VAP, neither when MDRB vs non-MDRB were compared. Length of ICU was documented as risk factor for VAP. Recent chemotherapy and tracheostomy were predictive risk factors for 60-d mortality.
The rate of VAP was similar to that reported in other studies. We described an elevated percentage of Gram-negative bacteria as a cause of pneumonia, which permits beginning empiric antibiotic coverage for these pathogens. MDRB were found in a quarter of the episodes, and were not linked to increased mortality at 60 d.
To perform a monitoring for a longer period of time will allow evaluating the evolution of bacterial resistance, and establishing whether, with a greater number of cases, it can impact the mortality of these patients.
Infection Control and Hospital Epidemiology Team.
| 1. | Belenguer-Muncharaz A, Albert-Rodrigo L, Ferrandiz-Sellés A, Cebrián-Graullera G. [Ten-year evolution of mechanical ventilation in acute respiratory failure in the hematogical patient admitted to the intensive care unit]. Med Intensiva. 2013;37:452-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 2. | Park SA, Cho SS, Kwak GJ. Factors influencing ventilator-associated pneumonia in cancer patients. Asian Pac J Cancer Prev. 2014;15:5787-5791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Patil HV, Patil VC. Incidence, bacteriology, and clinical outcome of ventilator-associated pneumonia at tertiary care hospital. J Nat Sci Biol Med. 2017;8:46-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Liu Y, Di Y, Fu S. Risk factors for ventilator-associated pneumonia among patients undergoing major oncological surgery for head and neck cancer. Front Med. 2017;11:239-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Sarda C, Fazal F, Rello J. Management of ventilator-associated pneumonia (VAP) caused by resistant gram-negative bacteria: which is the best strategy to treat? Expert Rev Respir Med. 2019;13:787-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Aly NY, Al-Mousa HH, Al Asar el SM. Nosocomial infections in a medical-surgical intensive care unit. Med Princ Pract. 2008;17:373-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O'Grady NP, Bartlett JG, Carratalà J, El Solh AA, Ewig S, Fey PD, File TM, Restrepo MI, Roberts JA, Waterer GW, Cruse P, Knight SL, Brozek JL. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2156] [Cited by in RCA: 2391] [Article Influence: 239.1] [Reference Citation Analysis (0)] |
| 8. | Nair GB, Niederman MS. Ventilator-associated pneumonia: present understanding and ongoing debates. Intensive Care Med. 2015;41:34-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Centers for Disease Control and Prevention. Pneumonia (Ventilator-associated [VAP] and non-ventilator-associated Pneumonia [PNEU]) Event. [published January 2020]. Available from: https://www.cdc.gov/nhsn/pdfs/pscmanual/6pscvapcurrent.pdf. |
| 10. | Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Stoclin A, Rotolo F, Hicheri Y, Mons M, Chachaty E, Gachot B, Pignon JP, Wartelle M, Blot F. Ventilator-associated pneumonia and bloodstream infections in intensive care unit cancer patients: a retrospective 12-year study on 3388 prospectively monitored patients. Support Care Cancer. 2020;28:193-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Yalçınsoy M, Salturk C, Takır HB, Kutlu SB, Oguz A, Aksoy E, Balcı M, Kargın F, Mocin OY, Adıguzel N, Gungor G, Karakurt Z. Case fatality rate related to nosocomial and ventilator-associated pneumonia in an ICU: a single-centre retrospective cohort study. Wien Klin Wochenschr. 2016;128:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Roberts KL, Micek ST, Juang P, Kollef MH. Controversies and advances in the management of ventilator associated pneumonia. Expert Rev Respir Med. 2017;11:875-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Cornejo-Juárez P, Vilar-Compte D, García-Horton A, López-Velázquez M, Ñamendys-Silva S, Volkow-Fernández P. Hospital-acquired infections at an oncological intensive care cancer unit: differences between solid and hematological cancer patients. BMC Infect Dis. 2016;16:274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Thakuria B, Singh P, Agrawal S, Asthana V. Profile of infective microorganisms causing ventilator-associated pneumonia: A clinical study from resource limited intensive care unit. J Anaesthesiol Clin Pharmacol. 2013;29:361-366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Namendys-Silva SA, Jarquin-Badiola YD, García-Guillén FJ, Texcocano-Becerra J, Cázares-Mejía R, Herrera-Gómez A. Mechanical ventilation in critically ill cancer patients. Heart Lung. 2015;44:85-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Ochoa-Hein E, Choi SJ, Gómez-Santillán JA, Oyervides-Alvarado JA, Galindo-Fraga A, Rivero-Sigarroa E, Hernández-Gilsoul T, Domínguez-Cherit JG. Near-zero ventilator-associated pneumonia rates after implementation of a multimodal preventive strategy in a Mexican hospital. Am J Infect Control. 2020;48:446-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | O'Grady NP, Murray PR, Ames N. Preventing ventilator-associated pneumonia: does the evidence support the practice? JAMA. 2012;307:2534-2539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Borgatta B, Rello J. How to approach and treat VAP in ICU patients. BMC Infect Dis. 2014;14:211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Critical care medicine
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gajic O, Santomauro M, Turner AM S-Editor: Gong ZM L-Editor: A E-Editor:Li JH