Published online Dec 20, 2019. doi: 10.5492/wjccm.v8.i8.135
Peer-review started: October 15, 2019
First decision: October 25, 2019
Revised: November 28, 2019
Accepted: November 28, 2019
Article in press: November 28, 2019
Published online: December 20, 2019
Processing time: 65 Days and 10.8 Hours
Very little is known about the role of extracorporeal membrane oxygenation (ECMO) for the management of patients undergoing major aortic surgery with particular reference to aortic dissection.
To review the available literature to determine if there was any evidence.
A systematic literature search through PubMed and EMBASE was undertaken according to specific key words.
The search resulted in 29 publications relevant to the subject: 1 brief communication, 1 surgical technique report, 1 invited commentary, 1 retrospective case review, 1 observational study, 4 retrospective reviews, 13 case reports and 7 conference abstracts. A total of 194 patients were included in these publications of whom 77 survived.
Although there is no compelling evidence for or against the use of ECMO in major aortic surgery or dissection, it is enough to justify its use in this patient population despite current adverse attitude.
Core tip: The subject of our review remains controversial in the absence of clear evidence but mainly based on opinions and speculations. We believe that such a timely review may contribute to reconsider current thinking and address the subject with an open mind.
- Citation: Capoccia M, Maybauer MO. Extra-corporeal membrane oxygenation in aortic surgery and dissection: A systematic review. World J Crit Care Med 2019; 8(8): 135-147
- URL: https://www.wjgnet.com/2220-3141/full/v8/i8/135.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v8.i8.135
Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) has become an established and widely used technique to provide circulatory support for critically ill patients with refractory cardiogenic shock and cardiac arrest[1-3] although an increased left ventricular (LV) afterload may affect the intended beneficial effects[4]. The impact of VA-ECMO on LV function can be explained in terms of pressure-volume (PV) loops and Starling curves[5] following simulations based on a previously developed model[6,7]. VA-ECMO does not affect LV function directly. When LV afterload is maintained constant at a specific systemic pressure, the Starling curve generated before VA-ECMO support predicts the filling pressure related to any target stroke volume (SV) at that systemic pressure. The mechanism by which that specific pressure is achieved does not change the relationship between filling pressure and native LV SV. A maintained Starling relationship during VA-ECMO support may help predict ventricular distension and optimise the balance between LV unloading and systemic perfusion[5]. Despite the outcome of the SHOCK II trial which remains against the use of the intra-aortic balloon pump (IABP) in cardiogenic shock[8-10], a combined use of VA-ECMO and IABP has shown reduced in-hospital mortality[11,12]. In addition, the combination of VA-ECMO and the Impella device has been shown to be a useful method to offload the left ventricle[13,14]. Quantitative evaluation based on a simulation approach has confirmed the beneficial effect of adding IABP or Impella during VA-ECMO support[15].
A recent retrospective multi-centre cohort study on post-cardiotomy VA-ECMO has identified age, previous cardiac surgery, preoperative acute neurological events, aortic arch surgery and increased arterial lactate as factors associated with increased risk of early mortality following the procedure although the experience of the centre may contribute to improved results[16]. Nevertheless, there is no real focus on critical patients experiencing post-cardiotomy failure after major aortic surgery for aneurysmal disease or dissection.
Diseases of the thoracic aorta carry a high mortality with an increasing prevalence worldwide at present in the context of long-standing controversy regarding its treatment[17-19]. Current evidence suggests that acute aortic syndromes are best treated in dedicated, high-volume aortic centres[20]. Preoperative malperfusion plays a major role on early and late outcome[21-23].
Therefore, we sought to review current attitude on the use of mechanical circulatory support (MCS) following major aortic surgery with a view that it may be an option for these critical patients. The analysis has considered adult patients only.
This review has been undertaken according to a web-based literature search on PubMed and EMBASE using appropriately combined key words [extra-corporeal life support (ECLS) and aortic surgery; ECLS and aortic dissection; ECMO and aortic surgery; ECMO and aortic dissection]. The Participants, Intervention, Comparison, Outcome and Study Design (PICOS) approach for the selection of clinical studies following our systematic search has been used (Table 1). The PRISMA approach has also been considered whose main purpose is to help ensure the clarity and transparency of systematic reviews; it was developed using an evidence-based approach and is not intended as a quality assessment tool[24]. An extension of the PRISMA statement has been developed to specifically address the reporting of systematic reviews incorporating network meta-analyses[25]. PRISMA-P is intended to help the preparation and reporting of a robust protocol for a systematic review[26].
| Participants | Patients undergoing major aortic surgery for aneurysmal disease or dissection |
| Intervention | VA-ECMO in patients requiring major aortic surgery for aneurysmal disease or dissection |
| Comparison | Comparison with those who did not need ECMO support |
| Outcome | If ECMO support made a difference |
| Study design | Prospective and retrospective clinical studies; case series and case reports |
We selected all the articles including major aortic surgery involving the ascending aorta, arch, descending thoracic and abdominal aorta.
The aim of this systematic review was to determine current knowledge and experience with ECLS/ECMO support for aortic disease and whether it is appropriate for postcardiotomy failure following major aortic surgery with particular reference to aortic dissection.
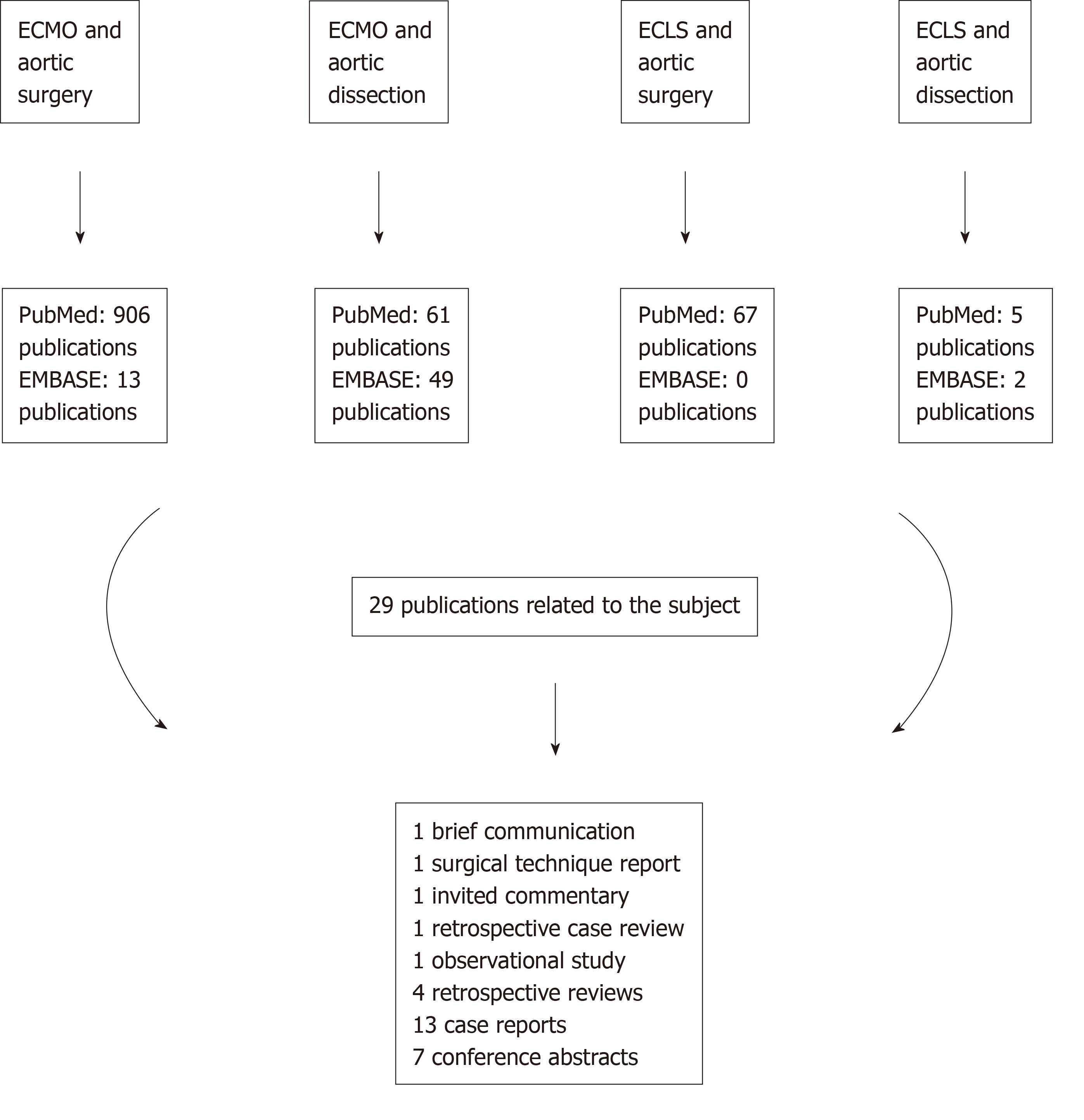
The search gave the following results (Figure 1): ECMO and aortic surgery retrieved 906 publications in PubMed and 13 publications in EMBASE; ECMO and aortic dissection retrieved 61 publications in PubMed and 49 in EMBASE; ECLS and aortic surgery retrieved 67 publications in PubMed and no publications in EMBASE; ECLS and aortic dissection retrieved 5 publications in PubMed and 2 publications in EMBASE. The overall analysis revealed 29 publications related to the subject of investigation as follows (Table 2): 1 brief communication[27], 1 surgical technique report[28], 1 invited commentary[29], 1 retrospective case review[30], 1 observational study[31], 4 retrospective studies[32-35], 13 case reports[36-48] and 7 conference abstracts[49-55]. The articles had been published between 1994 and 2019. Four publications reported key data for this review[31-34]. A total number of 194 patients had been treated with ECMO support leading to 77 surviving patients. Three publications[31,35,54] did not specify how many patients survived following ECMO support; therefore, the number of surviving patients remains incomplete. Further analysis gives a breakdown of aetiology, procedures performed and cannulation site when available (Table 3).
| Ref. | Study design/level of evidence | ECMO patients | Outcome |
| Abouliatim et al[27], 2012 | Brief Communication; Level 3 | AAA repair on ECMO support in 2 patients after failed EVAR | Both patients were discharged 12 days postoperatively |
| Lorusso et al[28], 2019 | Surgical Technique; Level 3 | 2 patients requiring elective aortic arch replacement and treated with minimally invasive central ECMO, which avoids re-sternotomy and maintains antegrade blood flow | Successful outcome for both patients. The technique is suitable only in those patients where a side-armed prosthetic graft had been used |
| Lazar et al[29], 2017 | Invited commentary; Level 3 | Comment to Sultan, 2017 with further considerations about ECMO in aortic dissection | |
| Guenther et al[30], 2014 | Retrospective Case Review; Level 3 | 6 patients with acute type A aortic dissection involving the coronary arteries treated with ECMO support | Mortality 67% (4 patients) |
| Lin et al[31], 2018 | Observational Study; Level 2- | 510 patients with TAAD between 2007 and 2018 17 required ECMO postoperatively | Comparison between low LVEF and preserved LVEF |
| Lin et al[32], 2017 | Retrospective Study; Level 2- | 162 patients underwent TAAD repair between 2008 and 2015 20 patients required ECMO support postoperatively | Mortality: ECMO group 65%; non-ECMO group 8.5% |
| Factors predicting postop ECMO: haemodynamic instability; aortic cross-clamp time; postop peak creat kinase-MB | |||
| Younger age for ECMO survivors | |||
| Zhong et al[33], 2017 | Retrospective Study; Level 2- | 5637 patients underwent major aortic surgery between 2009 and 2016 36 patients required ECMO support: 20 with TAAD; 3 Type B; 12 with thoracic aortic aneurysm; 1 with CoA (aortic coarctation) | Mortality 50% |
| Three main factors for in-hospital mortality: retrograde-flow cannulation; preop CK-MB level 100 IU/L; peak lactate level 20 mmol/L | |||
| Sultan et al[34], 2017 | Retrospective Study; Level 2- | Database review between 2004 and 2014 35 patients with Type A Aortic Dissection (TAAD) underwent ECMO support | Overall mortality 88% |
| There is no mention about indications for ECMO support; profile and co-morbidities of these patients; cannulation site (peripheral or central); cause of death | |||
| Guihaire et al[35], 2017 | Retrospective Study; Level 2- | 92 patients required ECMO support following valve surgery (66%), acute aortic dissection (10%) and CABG (9%) | Survival for patients with aortic dissection is not specified |
| Gennari et al[36], 2019 | Case Report; Level 3 | 1 patient with iatrogenic type A aortic dissection requiring ECMO support | Successful weaning off ECMO after 4 days |
| Chatterjee et al[37], 2018 | Case Report; Level 3 | 3 patients requiring ECMO support after thoraco-abdominal aneurysm repair | 1 patient discharged after 128 days but died 2 months later |
| 1 patient discharged after 35 days and alive at 3-year follow up | |||
| 1 patient discharged after 19 days and alive at 6-month follow up | |||
| Beyrouti et al[38], 2018 | Case Report; Level 3 | 1patient with aortic dissection involving the left main stem requiring ECLS and subsequently LVAD | Discharged after 27 days |
| Yukawa et al[39], 2018 | Case Report; Level 3 | Acute aortic dissection with out-of-hospital cardiac arrest requiring ECMO support | Discharged after 49 days |
| Stroehle et al[40], 2017 | Case Report; Level 3 | Traumatic aortic dissection treated with TEVAR on ECMO support | Discharged after 42 days to neuro-rehabilitation |
| Szczechowicz et al[41], 2016 | Case Report; Level 3 | 2 patients with acute type A aortic dissection complicated by right ventricular failure requiring ECMO support | First patient discharged after 27 days; second patient discharged to the ward after 8 days in ITU but no mention about how many days before discharge |
| Ishida et al[42], 2015 | Case Report; Level 3 | Two-stage procedure on ECMO support in 1 patient who sustained type A acute aortic dissection in a background of chronic thrombo-embolic pulmonary hypertension | Prolonged hospital stay; no mention how many days before discharge |
| Yavuz et al[43], 2015 | Case Report; Level 3 | ECMO following TEVAR in 1 patient | No mention about outcome |
| Amako et al[44], 2013 | Case Report; Level 3 | 1 patient with type A aortic dissection treated with ECMO support | ECMO weaned off after 65 hours uneventfully |
| Doguet et al[45], 2010 | Case Report; Level 3 | 1 patient with acute type A aortic dissection involving the coronary arteries treated with ECMO support | Discharged after 29 days postoperatively |
| Koster et al[46], 2007 | Case Report; Level 3 | 1 patient with acute type A aortic dissection requiring ECMO support who developed HIT treated successfully with bivalirudin | LV recovery during VA-ECMO support but RVAD required. Successful ECMO weaning; RVAD removed after 6 weeks |
| Fabricius et al[47], 2001 | Case Report; Level 3 | 2 patients who sustained acute type A aortic dissection during pregnancy treated with ECMO support | Successful ECMO weaning |
| Yamashita et al[48], 1994 | Case Report; Level 3 | 1 patient with acute aortic dissection treated with ECMO support | Successful ECMO weaning |
| Jorgensen et al[49], 2019 | Conference Abstract; Level 3 | Elective femoro-femoral VA-ECMO support for thoraco-abdominal aortic aneurysm repair in a 82-year-old patient | Discharged 11 days postoperatively |
| Heuts et al[50], 2017 | Conference Abstract; Level 3 | Surgical technique for ECMO insertion (the Maastricht Approach) | See Lorusso, 2019 in this table |
| Yang et al[51], 2017 | Conference Abstract; Level 3 | Retrospective analysis of 1695 patients who underwent surgery for aortic dissection between 2008 and 2015. 42 patients required VA-ECMO support | 30 patients were successfully weaned off VA-ECMO and 19 patients were discharged. |
| Higher lactate levels, pre-ECMO cardiac arrest, major haemorrhage and renal replacement therapy were related to in-hospital mortality | |||
| Goldberg et al[52], 2017 | Conference Abstract; Level 3 | 185 patients requiring repair of acute type A aortic dissection between 2005 and 2016. 4 patients required VA-ECMO support. | All 4 patients survived to hospital discharge |
| Schmidt et al[53], 2016 | Conference Abstract; Level 3 | Acute type A aortic dissection presenting as acute coronary syndrome requiring ECMO support in the cath lab as a bridge to surgical intervention | Fatal outcome |
| Nierscher et al[54], 2012 | Conference Abstract; Level 3 | Observational study of patients undergoing cardiac surgery in 2008. 35 patients required ECMO support. Only one patient with aortic dissection is reported. | Survival not specified for the patient with aortic dissection |
| Shinar et al[55], 2011 | Conference Abstract; Level 3 | Observational study over a 14-mo period of ECMO support initiated by A&E physicians. The procedure was attempted in 19 patients | Four patients were discharged without neurological injury: 2 patients after MI, one after aortic dissection with cardiac tamponade and one after profound hypothermia |
| Ref. | Study design/level of evidence | ECMO patients |
| Lin et al[31], 2018 | Observational Study; Level 2- | 510 patients with ATAAD between 2007 and 2018 |
| Entry Tear Exclusion 73.1% | ||
| Aortic Root Replacement 11.4% | ||
| Ascending Aorta Replacement 65.9% | ||
| Aortic Arch Replacement 25.3% | ||
| Hemiarch 13.3% | ||
| Total Arch 12.0% | ||
| Frozen Elephant Trunk 8.2% | ||
| Combined CABG 3.7% | ||
| 17 required ECMO support but no procedure break down is available | ||
| Lin et al[32], 2017 | Retrospective Study; Level 2- | 162 patients underwent type A aortic dissection repair between 2008 and 2015 |
| 20 patients required ECMO support as follows: | ||
| Ascending Aorta Interposition graft 6 | ||
| Aortic Root/Valve Procedure 9 | ||
| Aortic Arch Procedure 10 | ||
| Combined CABG 5 | ||
| Combined Mitral Replacement/Repair 1 | ||
| Combined Femoro-femoral crossover 1 | ||
| Zhong et al[33], 2017 | Retrospective Study; Level 2- | 5637 patients underwent major aortic surgery between 2009 and 2016 36 patients required ECMO support as follows: |
| Type A aortic dissection 20 | ||
| Type B aortic dissection 3 | ||
| Thoracic aortic aneurysm 12 | ||
| Aortic coarctation 1 | ||
| Emergency surgery 9 | ||
| Second operation 7 | ||
| Ascending aorta replacement 34 | ||
| Arch replacement 21 | ||
| Descending aorta atenting 17 | ||
| Thoraco-abdominal aorta replacement 2 | ||
| Combined valve replacement 21 | ||
| Combined CABG 16 | ||
| Central ECMO cannulation 7 | ||
| Peripheral ECMO cannulation 29 | ||
| Femoro-femoral 20 | ||
| Femoral vein to right axillary artery 7 | ||
| Femoro-femoral + right axillary artery 2 | ||
| IABP 9 | ||
| Sultan et al[34], 2017 | Retrospective Study; Level 2- | Database review between 2004 and 2014 |
| 35 patients with type A aortic dissection underwent ECMO support No procedure and cannulation break down is available | ||
| Guihaire et al[35], 2017 | Retrospective Study; Level 2- | 92 patients underwent ECMO support between January 2005 and December 2014 for post-cardiotomy cardiogenic shock as follows: |
| Valve surgery 66% | ||
| Acute Aortic Dissection 10% | ||
| CABG 9% | ||
| Break down of procedures and cannulation is not available | ||
| Nierscher et al[54], 2012 | Conference Abstract; Level 3 | 35 patients underwent ECMO support in 2008 following CABG (7), valve procedure (8), heart transplant (8), LVAD insertion (1), combined procedure (10), aortic dissection (1). |
| Cannulation was peripheral (23), central (7), subclavian artery (5). | ||
| Gennari et al[36], 2019 | Case Report; Level 3 | 1 patient with iatrogenic type A aortic dissection requiring ECMO support through peripheral cannulation. Ascending aorta replacement including right coronary sinus with interposition graft and single-vessel coronary artery bypass grafting. |
| Jorgensen et al[49], 2019 | Conference Abstract; Level 3 | 1 patient with thoraco-abdominal aortic aneurysm requiring ECMO support through peripheral cannulation. A multi-branched Gelweave Dacron graft was used. |
| Chatterjee et al[37], 2018 | Case Report; Level 3 | 3 patients requiring ECMO support after thoraco-abdominal aneurysm repair. |
| 2 patients had previous type A aortic dissection repair; 1 patient had ascending aorta and hemiarch replacement for type A aortic dissection and subsequent TEVAR procedure. ECMO cannulation between left axillary artery and femoral vein (1 patient), femoro-femoral (2 patients). | ||
| Beyrouti et al[38], 2018 | Case Report; Level 3 | 1 patient with aortic dissection involving the left main stem treated with ascending aorta interposition graft and CABG requiring ECLS through central cannulation and subsequently LVAD |
| Yukawa et al[39], 2018 | Case Report; Level 3 | Acute aortic dissection with out-of-hospital cardiac arrest requiring ECMO support through peripheral percutaneous femoral cannulation and treated with ascending aorta replacement using an interposition graft |
| Yang et al[51], 2017 | Conference Abstract; Level 3 | 1695 patients underwent repair for aortic dissection between 2008 and 2015. 42 patients required ECMO support. Procedure and cannulation break down is not available |
| Goldberg et al[52], 2017 | Conference Abstract; Level 3 | 185 patients underwent surgical intervention for acute type A aortic dissection between January 2005 and May 2016. 4 patients required VA-ECMO support. Break down of procedures, concomitant procedures and type of cannulation are not available |
| Stroehle et al[40], 2017 | Case Report; Level 3 | Traumatic aortic dissection treated with TEVAR on ECMO support |
| Schmidt et al[53], 2016 | Conference Abstract; Level 3 | Emergency ECMO insertion in the Cath Lab with findings of type A acute aortic dissection resulting in fatal outcome |
| Szczechowicz et al[41], 2016 | Case Report; Level 3 | 2 patients with acute type A aortic dissection complicated by right ventricular failure requiring ECMO support |
| Ishida et al[42], 2015 | Case Report; Level 3 | Two-stage procedure on ECMO support in 1 patient who sustained acute type A aortic dissection in a background of chronic thrombo-embolic pulmonary hypertension |
| Yavuz et al[43], 2015 | Case Report; Level 3 | ECMO following TEVAR in 1 patient |
| Guenther et al[30], 2014 | Retrospective Case Review; Level 3 | 6 patients with acute type A aortic dissection involving the coronary arteries treated with ECMO support |
| Amako et al[44], 2013 | Case Report; Level 3 | 1 patient with acute type A aortic dissection treated with ECMO support |
| Abouliatim et al[27], 2012 | Brief Communication; Level 3 | AAA repair on ECMO support in 2 patients after failed EVAR |
| Shinar et al[55], 2011 | Conference Abstract; Level 3 | 19 cases of ECMO insertion in Accident & Emergency Department through percutaneous cannulation of the femoral vessels |
| Doguet et al[45], 2010 | Case Report; Level 3 | 1 patient with acute type A aortic dissection involving the coronary arteries treated with peripheral ECMO support through femoro-femoral cannulation. CABG as concomitant procedure. |
| Koster et al[46], 2007 | Case Report; Level 3 | 1 patient with acute type A aortic dissection requiring ECMO support using bivalirudin |
| Fabricius et al[47], 2001 | Case Report; Level 3 | 2 patients who sustained acute type A aortic dissection during pregnancy treated with ECMO support |
| Yamashita et al[48], 1994 | Case Report ; Level 3 | 1 patient with acute aortic dissection treated with ECMO support |
ECMO has become increasingly available for the treatment of a diverse population of critically ill patients and recent reviews have highlighted its indications and the evidence basis to justify its use[1,56]. VA-ECMO is a suitable approach in the context of cardiac failure. Veno-venous (VV) ECMO is appropriate in the context of acute respiratory disease syndrome. More recently, ECMO has been considered in the setting of extracorporeal cardiopulmonary resuscitation. Despite increased application of the technique, overall survival rates have remained unchanged with a 50%-70% range for respiratory support and 40%-60% range for cardiac support[57,58]. Traditional configurations for ECMO support include the VV through the right internal jugular vein (Avalon cannula) and the veno-arterial (VA) either through the ascending aorta and the right atrium (central cannulation) or through the femoral vessels (peripheral cannulation)[3,59]. Hybrid ECMO configurations have been increasingly considered recently as an attempt to improve outcome. Triple cannulation such as veno-venous-arterial (VVA) or venous-arterial-venous (VAV) configurations may help with concomitant cardiac and respiratory failure. VVA ECMO consists of double venous cannulation through the right internal jugular vein and the right femoral vein for drainage with right femoral artery cannulation for perfusion. VAV ECMO consists of single venous drainage through the right femoral vein with right femoral artery and right internal jugular vein for perfusion. The VPa configuration through the insertion of a long venous cannula in the pulmonary artery, usually via the right internal jugular vein, may be a suitable option for patients with right heart failure[3].
Our literature search revealed a limited number of relevant articles as expected. ECMO support following major aortic surgery has not been usually recommended because of its potential to further exacerbate lesions of the aortic wall and increased bleeding with delayed thrombosis of the false lumen due to the use of anticoagulation[60-62]. Nevertheless, 3 retrospective studies[32-34] and 1 observational study[31] (Table 2) have shown the feasibility of ECMO support in patients undergoing major aortic surgery for aneurysmal disease and dissection in contrast to current scepticism[29]. In many countries the argument is to make for a balance between the costs involved in running ECMO support and select those patients who would benefit the most from a period of circulatory support following repair for acute aortic dissection. Monitoring the outcome of those patients who required ECMO support postoperatively and develop a specific database may be the way forward to shed further lights on the role of ECMO support in patients undergoing major aortic surgery. Although 1 retrospective study[34] has reported 88% mortality rate in 35 patients who underwent ECMO support following surgical treatment for type A aortic dissection, there is no mention about indications for ECMO support; profile and co-morbidities of these patients; cannulation site (peripheral or central); cause of death. Twenty-seven patients received ECMO support on the day of surgery and 8 patients required ECMO support on postoperative day 1 or later. Most unusual, 4 additional patients with type A aortic dissection underwent ECMO support without surgical intervention but none of them survived. The other two retrospective studies[32,33] are more detailed with more favourable outcome in line with the extra corporeal life support organization registry[57,58]. One study[33] included 36 patients who required VA-ECMO for post-cardiotomy failure following major aortic surgery. In-hospital mortality was 50% with multi-organ failure being the main cause of death. Preoperative levels of CK-MB > 100 IU/L and peak lactate levels > 20 mmol/L were considered relevant factors for in-hospital mortality. Retrograde flow cannulation was identified as another key factor for reduced survival compared to antegrade cannulation although the risk for early mortality is related to the preoperative clinical and haemodynamic status rather than the cannulation technique[62]. The other study[32] compared short- and long-term outcomes between patients who required ECMO support and those who did not. In-hospital mortality was higher in the ECMO group (65%) compared to the non-ECMO group (8.5%). Preoperative haemodynamic instability, aortic cross-clamp time and postoperative peak CK-MB were identified as predicting factors for postoperative ECMO support. ECMO survivors had younger age and less postoperative blood transfusion. Interestingly, those patients who survived after ECMO support following repair for acute type A aortic dissection showed a long-term survival rate comparable to patients who did not require ECMO support postoperatively. These findings were confirmed by a very detailed observational study[31] comparing patients with and without LV systolic dysfunction who underwent surgical intervention for acute type A aortic dissection. A total of 510 patients were considered: 86 with LV systolic dysfunction (group I) and 424 patients with preserved LV systolic function (group II). ECMO support was required in 7 patients from group I and in 10 patients from group II. The overall mortality was 79 patients out of 510: 20 from group I and 59 from group II. Multivariate analysis confirmed that a preoperative serum creatinine greater than 1.5 mg/dL and the requirement for ECMO support intra-operatively were significant independent predictors of in-hospital mortality but survival following ECMO support was not specified. Although patients with preoperative LV systolic dysfunction showed higher surgical risk for in-hospital mortality, their 3-year cumulative survival rate (77.8%) was comparable with those with preserved LV systolic function (82.1%). Serial echocardiographic assessment did not show further deterioration of LV systolic function during the 3-year follow-up.
To summarise the key factors related to the need for postoperative ECMO support and outcome, the following have been identified.
Factors predicting the need for postoperative ECMO support[31,32,34]: Preoperative haemodynamic instability; Myocardial infarction; Aortic cross-clamp time; Cardiopulmonary bypass time; Biventricular systolic dysfunction; Inadequate myocardial protection; Postoperative peak CK-MB; Propagation of the dissection into the coronary arteries.
Factors related with survival following ECMO support[32,33]: Younger age; Reduced postoperative blood transfusion; Lower level of preoperative CK-MB; Higher rate of antegrade cannulation; Lower lactate levels at 12 h; Lower rate of continuous renal replacement therapy; Longer intensive care stay.
Factors related with adverse outcome[31,33]: Retrograde flow cannulation; Peak lactate levels > 20 mmol/L; Preoperative CK-MB > 100 IU/L; Combined aortic arch replacement; Postoperative need of continuous renal replacement therapy; Prolonged inotropic support; Visceral ischaemia; Limb ischaemia.
In conclusion, although there is no compelling evidence in favour or against the use of ECMO support following major aortic surgery for aneurysmal disease or acute aortic dissection, it is enough to justify its use in those patients who develop haemodynamic instability refractory to inotropic support.
Extra-corporeal membrane oxygenation (ECMO) support following major aortic surgery with particular reference to aortic dissection remains controversial without clear direction. We aim to shed some lights on the subject in order to make an impact and give a clear view that may well lead to further studies.
We believe that a clear direction based on evidence may change current attitude.
Although ECMO support is not perfect, it does work when appropriately considered and performed. We believe it may become an additional option in aortic surgery.
The methods have been already described in the article.
The results are promising and may lead to further studies to improve outcomes.
There is enough evidence to support our statement although we would like to think that further studies can be pursued to confirm our initial findings.
There is potential to support further studies in the future.
| 1. | Ng GW, Yuen HJ, Sin KC, Leung AK, Au Yeung KW, Lai KY. Clinical use of venoarterial extracorporeal membrane oxygenation. Hong Kong Med J. 2017;23:282-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Khorsandi M, Dougherty S, Bouamra O, Pai V, Curry P, Tsui S, Clark S, Westaby S, Al-Attar N, Zamvar V. Extra-corporeal membrane oxygenation for refractory cardiogenic shock after adult cardiac surgery: a systematic review and meta-analysis. J Cardiothorac Surg. 2017;12:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 3. | Brasseur A, Scolletta S, Lorusso R, Taccone FS. Hybrid extracorporeal membrane oxygenation. J Thorac Dis. 2018;10:S707-S715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 4. | Meani P, Gelsomino S, Natour E, Johnson DM, Rocca HB, Pappalardo F, Bidar E, Makhoul M, Raffa G, Heuts S, Lozekoot P, Kats S, Sluijpers N, Schreurs R, Delnoij T, Montalti A, Sels JW, van de Poll M, Roekaerts P, Poels T, Korver E, Babar Z, Maessen J, Lorusso R. Modalities and Effects of Left Ventricle Unloading on Extracorporeal Life support: a Review of the Current Literature. Eur J Heart Fail. 2017;19 Suppl 2:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 185] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 5. | Dickstein ML. The Starling Relationship and Veno-Arterial ECMO: Ventricular Distension Explained. ASAIO J. 2018;64:497-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 6. | Santamore WP, Burkhoff D. Hemodynamic consequences of ventricular interaction as assessed by model analysis. Am J Physiol. 1991;260:H146-H157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 45] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Burkhoff D, Tyberg JV. Why does pulmonary venous pressure rise after onset of LV dysfunction: a theoretical analysis. Am J Physiol. 1993;265:H1819-H1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 74] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Thiele H, Schuler G, Neumann FJ, Hausleiter J, Olbrich HG, Schwarz B, Hennersdorf M, Empen K, Fuernau G, Desch S, de Waha S, Eitel I, Hambrecht R, Böhm M, Kurowski V, Lauer B, Minden HH, Figulla HR, Braun-Dullaeus RC, Strasser RH, Rochor K, Maier SK, Möllmann H, Schneider S, Ebelt H, Werdan K, Zeymer U. Intraaortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock: design and rationale of the Intraaortic Balloon Pump in Cardiogenic Shock II (IABP-SHOCK II) trial. Am Heart J. 2012;163:938-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, de Waha A, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Lauer B, Böhm M, Ebelt H, Schneider S, Werdan K, Schuler G; Intraaortic Balloon Pump in cardiogenic shock II (IABP-SHOCK II) trial investigators. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet. 2013;382:1638-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 699] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 10. | Thiele H, Zeymer U, Thelemann N, Neumann FJ, Hausleiter J, Abdel-Wahab M, Meyer-Saraei R, Fuernau G, Eitel I, Hambrecht R, Böhm M, Werdan K, Felix SB, Hennersdorf M, Schneider S, Ouarrak T, Desch S, de Waha-Thiele S; IABPSHOCK II Trial (Intraaortic Balloon Pump in Cardiogenic Shock II) Investigators. Intraaortic Balloon Pump in Cardiogenic Shock Complicating Acute Myocardial Infarction: Long-Term 6-Year Outcome of the Randomized IABP-SHOCK II Trial. Circulation. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 293] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 11. | Li Y, Yan S, Gao S, Liu M, Lou S, Liu G, Ji B, Gao B. Effect of an intra-aortic balloon pump with venoarterial extracorporeal membrane oxygenation on mortality of patients with cardiogenic shock: a systematic review and meta-analysis†. Eur J Cardiothorac Surg. 2019;55:395-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 12. | Chen K, Hou J, Tang H, Hu S. Concurrent initiation of intra-aortic balloon pumping with extracorporeal membrane oxygenation reduced in-hospital mortality in postcardiotomy cardiogenic shock. Ann Intensive Care. 2019;9:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Cheng A, Swartz MF, Massey HT. Impella to unload the left ventricle during peripheral extracorporeal membrane oxygenation. ASAIO J. 2013;59:533-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 14. | Patel SM, Lipinski J, Al-Kindi SG, Patel T, Saric P, Li J, Nadeem F, Ladas T, Alaiti A, Phillips A, Medalion B, Deo S, Elgudin Y, Costa MA, Osman MN, Attizzani GF, Oliveira GH, Sareyyupoglu B, Bezerra HG. Simultaneous Venoarterial Extracorporeal Membrane Oxygenation and Percutaneous Left Ventricular Decompression Therapy with Impella Is Associated with Improved Outcomes in Refractory Cardiogenic Shock. ASAIO J. 2019;65:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 183] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 15. | Donker DW, Brodie D, Henriques JPS, Broomé M. Left Ventricular Unloading During Veno-Arterial ECMO: A Simulation Study. ASAIO J. 2019;65:11-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 16. | Biancari F, Dalén M, Fiore A, Ruggieri VG, Saeed D, Jónsson K, Gatti G, Zipfel S, Perrotti A, Bounader K, Loforte A, Lechiancole A, Pol M, Spadaccio C, Pettinari M, Ragnarsson S, Alkhamees K, Mariscalco G, Welp H; PC-ECMO Study Group. Multicenter study on postcardiotomy venoarterial extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 17. | Bonser RS, Ranasinghe AM, Loubani M, Evans JD, Thalji NM, Bachet JE, Carrel TP, Czerny M, Di Bartolomeo R, Grabenwöger M, Lonn L, Mestres CA, Schepens MA, Weigang E. Evidence, lack of evidence, controversy, and debate in the provision and performance of the surgery of acute type A aortic dissection. J Am Coll Cardiol. 2011;58:2455-2474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 184] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 18. | Bottle A, Mariscalco G, Shaw MA, Benedetto U, Saratzis A, Mariani S, Bashir M, Aylin P, Jenkins D, Oo AY, Murphy GJ; UK Aortic Forum. Unwarranted Variation in the Quality of Care for Patients With Diseases of the Thoracic Aorta. J Am Heart Assoc. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Mariscalco G, Bilal H, Catarino P, Hadjinikolaou L, Kuduvalli M, Field M, Mascaro J, Oo AY, Quarto C, Kuo J, Tsang G; UK Aortic Group. Reflection From UK Aortic Group: Frozen Elephant Trunk Technique as Optimal Solution in Type A Acute Aortic Dissection. Semin Thorac Cardiovasc Surg. 2019;31:686-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Mariscalco G, Maselli D, Zanobini M, Ahmed A, Bruno VD, Benedetto U, Gherli R, Gherli T, Nicolini F. Aortic centres should represent the standard of care for acute aortic syndrome. Eur J Prev Cardiol. 2018;25:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Girdauskas E, Kuntze T, Borger MA, Falk V, Mohr FW. Surgical risk of preoperative malperfusion in acute type A aortic dissection. J Thorac Cardiovasc Surg. 2009;138:1363-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 22. | Czerny M, Schoenhoff F, Etz C, Englberger L, Khaladj N, Zierer A, Weigang E, Hoffmann I, Blettner M, Carrel TP. The Impact of Pre-Operative Malperfusion on Outcome in Acute Type A Aortic Dissection: Results From the GERAADA Registry. J Am Coll Cardiol. 2015;65:2628-2635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 296] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 23. | Narayan P, Rogers CA, Benedetto U, Caputo M, Angelini GD, Bryan AJ. Malperfusion rather than merely timing of operative repair determines early and late outcome in type A aortic dissection. J Thorac Cardiovasc Surg. 2017;154:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6253] [Cited by in RCA: 7865] [Article Influence: 462.6] [Reference Citation Analysis (3)] |
| 25. | Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catalá-López F, Gøtzsche PC, Dickersin K, Boutron I, Altman DG, Moher D. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4165] [Cited by in RCA: 5849] [Article Influence: 531.7] [Reference Citation Analysis (1)] |
| 26. | Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15040] [Cited by in RCA: 16658] [Article Influence: 1514.4] [Reference Citation Analysis (1)] |
| 27. | Abouliatim I, Paramythiotis A, Harmouche M, Ternisien E, Verhoye JP. Extracorporeal membrane oxygenation support for abdominal aortic aneurysms surgery in high-risk patients. Interact Cardiovasc Thorac Surg. 2012;14:215-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Lorusso R, Bidar E, Natour E, Heuts S. Minimally invasive management of central ECMO after ascending aortic surgery. J Card Surg. 2019;34:131-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Lazar HL. The use of extracorporeal membrane oxygenation in type A aortic dissections-Long run for a short slide? J Card Surg. 2017;32:826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Guenther SP, Peterss S, Reichelt A, Born F, Fischer M, Pichlmaier M, Hagl C, Khaladj N. Diagnosis of coronary affection in patients with AADA and treatment of postcardiotomy myocardial failure using extracorporeal life support (ECLS). Heart Surg Forum. 2014;17:E253-E257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Lin CY, Lee KT, Ni MY, Tseng CN, Lee HA, Su IL, Ho HP, Tsai FC. Impact of reduced left ventricular function on repairing acute type A aortic dissection: Outcome and risk factors analysis from a single institutional experience. Medicine (Baltimore). 2018;97:e12165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Lin TW, Tsai MT, Hu YN, Lin WH, Wang WM, Luo CY, Roan JN. Postoperative Extracorporeal Membrane Oxygenation Support for Acute Type A Aortic Dissection. Ann Thorac Surg. 2017;104:827-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Zhong Z, Jiang C, Yang F, Hao X, Xing J, Wang H, Hou X. Veno-Arterial Extracorporeal Membrane Oxygenation Support in Patients Undergoing Aortic Surgery. Artif Organs. 2017;41:1113-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Sultan I, Habertheuer A, Wallen T, Siki M, Szeto W, Bavaria JE, Williams M, Vallabhajosyula P. The role of extracorporeal membrane oxygenator therapy in the setting of Type A aortic dissection. J Card Surg. 2017;32:822-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Guihaire J, Dang Van S, Rouze S, Rosier S, Roisne A, Langanay T, Corbineau H, Verhoye JP, Flécher E. Clinical outcomes in patients after extracorporeal membrane oxygenation support for post-cardiotomy cardiogenic shock: a single-centre experience of 92 cases. Interact Cardiovasc Thorac Surg. 2017;25:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 36. | Gennari M, Polvani G, Agrifoglio M. Favorable outcome of mechanical support for iatrogenic aortic dissection. Asian Cardiovasc Thorac Ann. 2019;27:55-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 37. | Chatterjee S, Mulvoy W, Preventza O, de la Cruz KI, LeMaire SA, Coselli JS. ECMO for Acute Respiratory Distress Syndrome After Thoracoabdominal Aortic Aneurysm Repair. Ann Thorac Surg. 2018;106:e171-e172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Beyrouti HE, Kornberger A, Halloum N, Beiras-Fernandez A, Vahl CF. Early LVAD Implantation in a Patient with Left Ventricular Failure after Aortic Dissection with Left Main Stem Involvement. Ann Thorac Cardiovasc Surg. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 39. | Yukawa T, Sugiyama K, Miyazaki K, Tanabe T, Ishikawa S, Hamabe Y. Treatment of a patient with acute aortic dissection using extracorporeal cardiopulmonary resuscitation after an out-of-hospital cardiac arrest: a case report. Acute Med Surg. 2018;5:189-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Stroehle M, Lederer W, Schmid S, Glodny B, Chemelli AP, Wiedermann FJ. Aortic stent graft placement under extracorporeal membrane oxygenation in severe multiple trauma. Clin Case Rep. 2017;5:1604-1607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | Szczechowicz M, Weymann A, Karck M, Szabo G. Right Ventricular Failure Following Acute Type A Aortic Dissection Successfully Treated with ECMO: Report of Two Cases. J Clin Case Rep. 2016;6:12. |
| 42. | Ishida K, Masuda M, Ishizaka T, Matsumiya G. Successful staged operation for acute aortic dissection and chronic thromboembolic pulmonary hypertension. Eur J Cardiothorac Surg. 2015;47:575-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 43. | Yavuz S, Arikan AA, Ozbudak E, İrkil S, Hosten T, Gumustas S, Berki KT. Concomitant Persistent Atelectasis following TEVAR Due to a Descending Aortic Aneurysm: Hybrid Endovascular Repair and ECMO Therapy. Heart Surg Forum. 2015;18:E188-E191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 44. | Amako M, Akasu K, Oda T, Zaima Y, Yasunaga H. A Case of Acute Aortic Dissection with Severe Aortic Regurgitation Successfully Treated by Postoperative Extracorporeal Membrane Oxygenation. Jpn J Vasc Surg. 2013;22:984-988. |
| 45. | Doguet F, Vierne C, Leguillou V, Bessou JP. Place of extracorporeal membrane oxygenation in acute aortic dissection. Interact Cardiovasc Thorac Surg. 2010;11:708-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Koster A, Weng Y, Böttcher W, Gromann T, Kuppe H, Hetzer R. Successful use of bivalirudin as anticoagulant for ECMO in a patient with acute HIT. Ann Thorac Surg. 2007;83:1865-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 47. | Fabricius AM, Autschbach R, Doll N, Mohr W. Acute aortic dissection during pregnancy. Thorac Cardiovasc Surg. 2001;49:56-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Yamashita T, Kozawa S, Okada M, Ohta T, Ataka K, Kitade T. [A case of acute aortic dissection with aortic regurgitation successfully treated by postoperative ECMO]. Kyobu Geka. 1994;47:283-287. [PubMed] |
| 49. | Jorgensen MS, Farres H, Sorrells WS, Erben Y, Martin AK, Pham SM, Hakaim A. Utilization of Intraoperative Extracorporeal Membrane Oxygenation Bypass to Reduce Visceral Vessel Ischemia During Open Thoracoabdominal Aortic Aneurysm Repair. J Vasc Surg. 2019;69:e118. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 50. | Heuts S, Gelsomino S, Natour E, Lozekoot P, Johnson D, Bidar E, Kats S, Sluijpers N, Makhoul M, Schreurs R, Gilbers M, Poels T, Weerwind P, Ganushchak Y, Korver E, Babar Z, Maessen J, Lorusso R, Meani P, Delnoij T, Sels JW, Van De Poll M, Montalti A, Roekaerts P. Minimally invasive central arterial cannulation management in ECMO patients after complex aortic surgery: The Maastricht approach. Eur J Heart Fail. 2017;19 Suppl 2:11-12. |
| 51. | Yang F, Hou D, Hou X. Venoarterial extracorporeal membrane oxygenation support for early refractory cardiogenic shock and cardiac arrest after aortic surgery. Eur J Heart Fail. 2017;19 Suppl 2:7-8. |
| 52. | Goldberg JB, Kai M, Malekan R, Tang G, Lansman SL, Spielvogel D. Extracorporeal membrane oxygenation after acute type a aortic dissection repair decreases the mortality rate and enhances survival. Innovations: Technology and Techniques in Cardiothoracic and Vascular Surgery. 2017;12:S38. |
| 53. | Schmidt TR, Baquero G, Hansen J, Mahidhar R. Ascending aortic dissection: A rare but fatal mechanism for anterior ST-elevation myocardial infarction. J Am Coll Cardiol. 2016;67:1157. |
| 54. | Nierscher FJ, Zaludik J, Hiesmayr M, Lasnigg A, Ehrlich M. ECMO in cardiac surgery: Outcome, mortality and costs. Appl Cardiopulm Pathophysiol. 2012;16 Suppl I:261-262. |
| 55. | Shinar Z, Bellezzo J. Emergency physician initiated ECMO: Our experience. Circulation. 2011;124:2374. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 56. | Squiers JJ, Lima B, DiMaio JM. Contemporary extracorporeal membrane oxygenation therapy in adults: Fundamental principles and systematic review of the evidence. J Thorac Cardiovasc Surg. 2016;152:20-32. [RCA] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 57. | Clark JB, Wang S, Palanzo DA, Wise R, Baer LD, Brehm C, Ündar A. Current techniques and outcomes in extracorporeal life support. Artificial Organs. 2015;39:926–930. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 58. | Life support organization registry report, International summary, January 2015. Ann Arbor, MI: Extracorporeal Life Support Organization 2015: 1-26. |
| 59. | Pavlushkov E, Berman M, Valchanov K. Cannulation techniques for extracorporeal life support. Ann Transl Med. 2017;5:70. [RCA] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 60. | Easo J, Weigang E, Hölzl PPF, Horst M, Hoffmann I, Blettner M, Dapunt OE. Influence of operative strategy for the aortic arch in DeBakey type I aortic dissection — analysis of the German Registry for Acute Aortic Dissection type A (GERAADA). Ann Cardiothorac Surg. 2013;2:175–180. [RCA] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 61. | Rylski B, Czerny M, Beyersdorf F, Kari FA, Siepe M, Adachi H, Yamaguchi A, Itagaki R, Kimura N. Is right axillary artery cannulation safe in type A aortic dissection with involvement of the innominate artery? J Thorac Cardiovasc Surg. 2016;152:801–807. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 62. | Klotz S, Bucsky BS, Richardt D, Petersen M, Sievers HH. Is the outcome in acute aortic dissection type A influenced by of femoral versus central cannulation? Ann Cardiothorac Surg. 2016;5:310–316. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited Manuscript
Specialty type: Critical care medicine
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jha NK S-Editor: Ma RY L-Editor: A E-Editor: Liu MY