Published online Oct 16, 2018. doi: 10.5492/wjccm.v7.i5.52
Peer-review started: June 25, 2018
First decision: July 9, 2018
Revised: August 4, 2018
Accepted: August 21, 2018
Article in press: August 21, 2018
Published online: October 16, 2018
Processing time: 113 Days and 16.3 Hours
Ascorbic acid (vitamin C) elicits pleiotropic effects in the body. Among its functions, it serves as a potent anti-oxidant, a co-factor in collagen and catecholamine synthesis, and a modulator of immune cell biology. Furthermore, an increasing body of evidence suggests that high-dose vitamin C administration improves hemodynamics, end-organ function, and may improve survival in critically ill patients. This article reviews studies that evaluate vitamin C in pre-clinical models and clinical trials with respect to its therapeutic potential.
Core tip: An increasing body of evidence suggests that high-dose vitamin C administration improves hemodynamics, end-organ function, and may improve survival in critically ill patients. This article reviews studies that evaluate vitamin C in pre-clinical models and clinical trials with respect to its therapeutic potential.
- Citation: Nabzdyk CS, Bittner EA. Vitamin C in the critically ill - indications and controversies. World J Crit Care Med 2018; 7(5): 52-61
- URL: https://www.wjgnet.com/2220-3141/full/v7/i5/52.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v7.i5.52
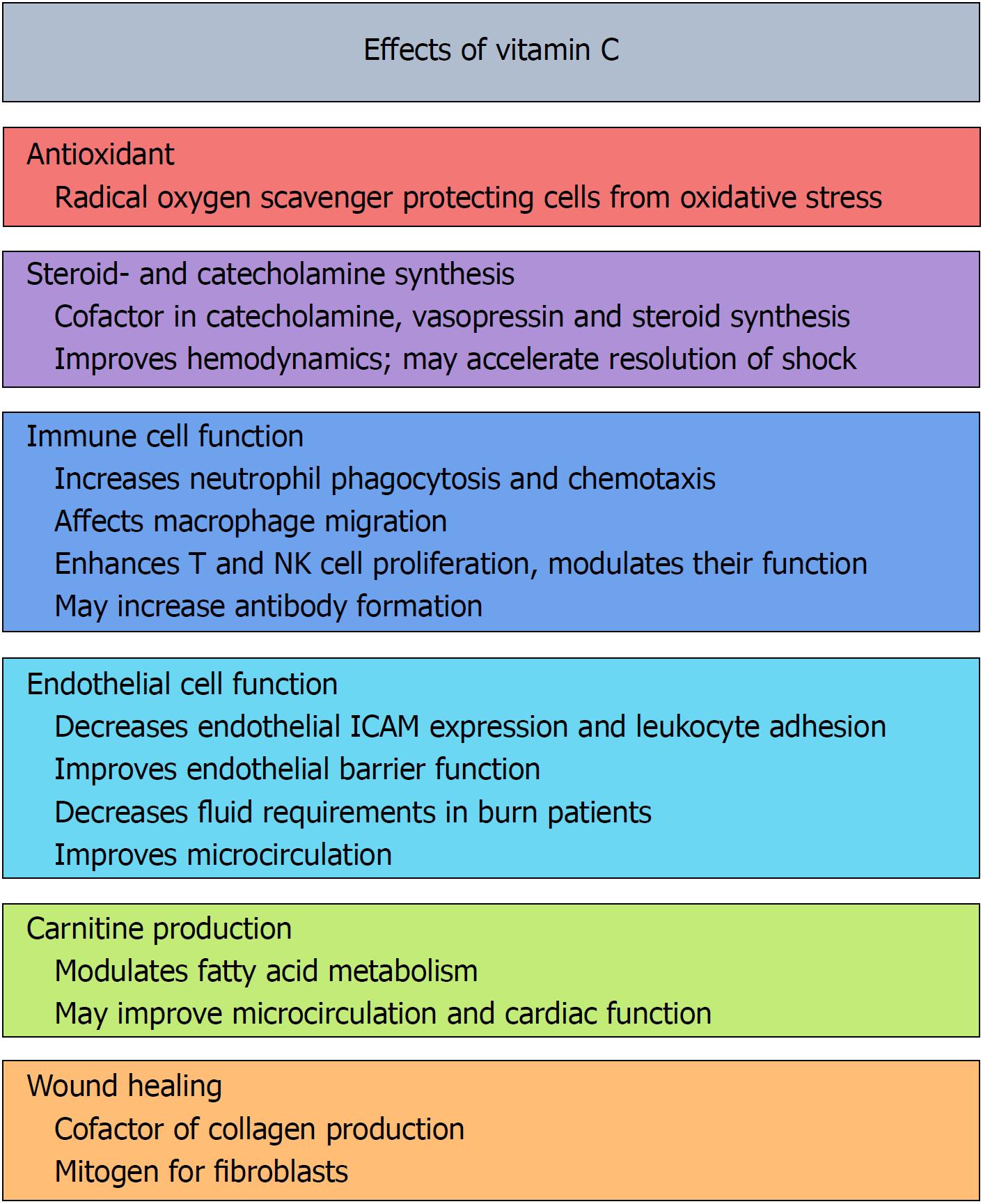
Vitamin C is one of the most well-known essential nutrients and is believed by many to confer a litany of health benefits (Figure 1). The Nobel Prize Winner Linus Pauling may have been the foremost ambassador to date who suggested that vitamin C would enhance cardiovascular health, improve the body’s immune function to overcome infections, and even help abate cancer[1-4]. These health claims created significant controversies that lasted for decades. While many of Pauling’s “more is better” claims have not been supported by rigorous scientific investigation, a growing number of benefits of vitamin C administration have been identified for medical treatment, including in the field of critical care. This mini-review will examine the evidence in support of vitamin C administration for critically ill patients and provide general recommendations for use by intensive care unit practitioners.
Vitamin C is water-soluble and circulates in the plasma. It is freely filtered by the glomerulus and reabsorbed in the proximal tubule via the first sodium-dependent vitamin C transporter (SVCT1). In the setting of hypovitaminosis C, its urinary excretion is minimal[5]. While SVCT1 regulates whole-body homeostasis of vitamin C, a high-affinity, low-capacity sodium-dependent vitamin C transporter SVCT2 protects metabolically-active cells against oxidative stress, which facilitates vitamin C accumulation where it is needed[6]. The recommended daily oral dose of vitamin C is 75 mg (adult female)/90 mg (adult male), and only ten mg of daily oral vitamin C is necessary to prevent scurvy (plasma level < 0.1 mg/dL; normal range 0.8-1.6 mg/dL). Despite meeting these recommended daily intakes, many critically ill patients exhibit decreased vitamin C plasma levels. Carr et al[7] reported hypovitaminosis C in 44 critically ill patients receiving standard intensive care unit nutrition, of which one-third had vitamin C deficiency. The degree of vitamin C deficiency was more pronounced in the septic population as compared to the non-septic critically ill. Continuous renal replacement is commonly utilized in critically ill patients and is believed to lead to a depletion of water-soluble vitamins[8-10]. A retrospective study of critically ill patients receiving continuous renal replacement revealed that 87% (13 out of 15) had vitamin C deficiencies[9].
Among vitamin C’s pleiotropic functions that are of relevance to critical illness are its immune-enhancing effects, anti-oxidant properties, and potential anti-mutagenic effects[11,12]. Vitamin C has been shown to enhance neutrophil chemotaxis, phagocytosis, and thus microbial clearance[13,14]. In addition, vitamin C promotes T cell and natural killer cell proliferation and modulates their functions[13,15]. Studies on vitamin C’s effects on B cells have revealed conflicting data with regard to proliferation and differentiation[13,15]. Vitamin C appears to induce antibody production in human lymphocytes and those of guinea pigs[16,17]. In a mouse model of abdominal sepsis induced by cecal-puncture ligation, parenteral vitamin C administration improved sepsis outcomes through reversal of regulatory T cell inhibitory function[18]. Hypovitaminosis C in a sepsis model using guinea pigs was also associated with fewer macrophages in the peritoneal cavity and impaired macrophage migration[19,20]. Interestingly, the adverse effects of vitamin C deficiency were more pronounced in elderly guinea pigs[19].
In cell culture and rodent experiments, vitamin C has been shown to decrease lipid peroxidation, prevent occludin dephosphorylation, and thus diminish the loosening of tight junctions[5,21-23]. Vitamin C also improves microcirculatory flow impairment by inhibiting tumor-necrosis-factor (TNF)-induced intercellular adhesion molecule 1 expression, thereby decreasing leukocyte adhesiveness[5,24,25]. In smokers, a single bolus administration of vitamin C (3 g IV) was found to increase coronary flow reserve, which is an integrated parameter of endothelial function and vascular smooth muscle relaxation. This effect was not seen in healthy control patients[26].
Vitamin C is a cofactor in collagen synthesis, a mitogen for fibroblasts, and is believed to positively modulate proinflammatory signaling and inflammation resolution that occur in wound beds[27,28]. Vitamin C supplementation in deficient mice promotes wound healing through enhanced matrix deposition and fibroblast proliferation[27]. In addition, topical vitamin C increases dermal collagen biosynthesis in healthy volunteers[29,30]. However, vitamin C supplementation does not consistently improve pressure ulcer healing in nursing homes and hospitalized patients, and recent systematic reviews have concluded that vitamin C (often administered in conjunction with zinc and other nutrients) is ineffective in treatment for this condition[31-35].
Vitamin C is a cofactor in carnitine synthesis, a molecule that facilitates fatty acid shuttling into mitochondria, reduces oxidative stress, and promotes endothelial sprouting[36,37]. Its deficiency has been linked to cardiomyopathy and neurometabolic disease[38,39]. Despite carnitine’s essential metabolic roles, clinical data to date have not yielded convincing evidence that supplementation in critically ill patients will improve outcomes[40-42].
Vitamin C is also a cofactor in catecholamine synthesis and adrenal steroidogenesis[43,44]. Vitamin C contributes to the conversion of dopamine to norepinephrine by dopamine beta-hydroxylase[45]. Vitamin C enhances norepinephrine synthesis both by recycling tetrahydrobiopterin, a critical cofactor in catecholamine synthesis, and increasing tyrosine hydroxylase expression[46]. Furthermore, vitamin C is a cofactor for the peptidylglycine α-amidating monooxygenase that is required for the endogenous synthesis of vasopressin[47]. One study in cardiac surgical patients has suggested that pre-operative administration of vitamin C mitigates etomidate-induced adrenal suppression[48]. Thus, there has been significant interest in utilizing vitamin C for the management of hemodynamically-unstable patients[49].
While a recent review concluded that there is insufficient evidence to support the use of vitamin C to reduce cardiovascular disease risk or mortality in the general population, increasing evidence suggests that it may have a beneficial role in patients with acute coronary syndromes or undergoing cardiac surgical procedures[50]. Cardiac surgery, extracorporeal membrane oxygenation and hemodialysis produce oxidative stress, which negatively impacts morbidity and mortality[51]. Vitamin C’s ability to scavenge reactive oxygen species and increase nitric oxide production through induction of endothelial nitric oxide synthase have made it a focus of interest as a cardiovascular therapy adjunct[52]. In one study of cardiac surgical patients undergoing cardiopulmonary bypass, statistically significant reductions in plasma levels of vitamin C were found intraoperatively compared to preoperative levels, even prior to initiation of cardiopulmonary bypass (Δ16.3% compared to baseline). This decrease in vitamin C plasma levels continued after cardiopulmonary bypass and lasted for at least six days[53].
Perioperative vitamin C administration has also been shown to prevent post-operative atrial fibrillation in the majority of the studies[54-59]. Its effects appear to result in reductions in the duration of hospital and intensive care unit patient stay following cardiac surgery[54-57].
Other studies examining the effects of vitamin C administration on patients with acute myocardial infarction and undergoing coronary revascularization procedures have reported improved left ventricular ejection fraction, microcirculation, and limited infarct size in patients with acute myocardial infarction[60-62]. One recent randomized multicenter clinical trial on patients with myocardial infarction undergoing percutaneous coronary angioplasty did not show a significant improvement in infarct size or ejection fraction at the time of the intervention with vitamin C administration. However, a decline in the LVEF between 7-15 d and 2-3 mo noted in the control group was not seen in the vitamin C group[63]. The authors of this study suggested that vitamin C may have ameliorated myocardial reperfusion injury[63].
In addition to potential beneficial effects on microperfusion and myocardial protection, a growing body of evidence suggests that vitamin C administration may positively affect hemodynamic parameters and hasten freedom from vasopressors in critically ill patients[64-67]. Interestingly, some evidence suggests that vitamin C’s effects on hemodynamics may have a ceiling effect. A recently reported pharmacokinetic study by de Grooth et al[68] only found a minimal reduction in heart rate among critically ill patients randomized to receive 2 g/d vs 10 g/d of vitamin C. However, only the treatment group that received the 2 g/d of vitamin C, but not the 10 g/d treatment regimen, had a clinically-relevant decrease in norepinephrine requirements over 48 h[68].
Increased capillary leakage is a clinical hallmark of burn injury. It is associated with significant fluid and protein extravasation. The term “fluid creep” was coined to describe the phenomenon that burn patients often receive significantly more resuscitation fluid than anticipated based on Parkland formula calculations[69]. This excess fluid resuscitation can be associated with edema-related complications[70]. Endothelial damage leading to increased permeability in patients with burn injury may partly be mediated by reactive oxygen species-induced lipid peroxidation. As an antioxidant, vitamin C has been evaluated as a therapy to decrease fluid resuscitation requirements[71,72]. In a rodent model of burn injury, high-dose vitamin C appeared to improve microvascular barrier dysfunction, without affecting leukocyte activation[73]. In a study of guinea pigs with 70% third-degree burns given high dose vitamin C (170, 340 and 680 mg/kg per day), fluid requirements were significantly reduced while stable cardiac outputs were maintained[74]. In a study of dogs with burn injuries, vitamin C administration (14 mg/kg per hour) decreased lipid peroxidation and microvascular protein and fluid leakage[75]. A burn study in sheep provided additional evidence that high-dose vitamin C (250 mg/kg bolus plus 15 mg/kg per hour) could reduce fluid requirements and lipid peroxidation, as well as improve antioxidant status[76]. Preliminary studies in humans have also been promising. In a study of 37 patients with > 30% total body surface area burns, vitamin C administration (66 mg/kg per hour) reduced fluid requirements, wound edema, and increased the ratio of PaO2 to a fraction of inspired oxygen[66]. In a retrospective review of 40 patients with > 20% total body surface area, vitamin C (66 mg/kg per hour) was associated with increased urine output and decreased fluid requirements, but no change in outcomes or incidence of acute kidney injury[77]. In another small study (n = 30) of patients with second degree burns, topical vitamin C accelerated formation of granulation tissue[78].
There has recently been a surge of interest in the use of vitamin C as an adjuvant treatment for sepsis. This interest was stimulated by the findings of a cohort study by Marik et al[64] that administered a cocktail of vitamin C (1.5 g IV every 6 h), hydrocortisone (50 mg IV every 6 h) and thiamine (200 mg IV every 12 h) to 47 septic patients and found a significant reduction in SOFA scores, dependence on vasopressors, and most importantly in hospital mortality to 8.5% in the treatment arm vs 40.4% in a historic control group. These findings were consistent with small phase I double-blinded placebo-controlled trials suggesting the beneficial effects of vitamin C in patients with sepsis[67]. This trial, which randomized 24 septic patients with documented hypovitaminosis C to receive placebo, low-dose (50 mg/kg per day) or high-dose (200 mg/kg per day) parental vitamin C for four days, found significant reductions in SOFA scores and CRP plasma levels in the vitamin C-treated groups[67]. In another small trial of critically ill surgical patients, Zabet et al[65] reported a significant reduction in 28 d mortality in 14 patients with septic shock who were randomized to receive 25 mg/kg per day of ascorbic acid every 6 h for 72 h, when compared to 14 patients with septic shock who received placebo. Despite these promising findings, there are potential safety concerns worthy of consideration with vitamin C administration in the critically ill population. A recent study by De Grooth et al[68] evaluated four parenteral vitamin C repletion regimens (2 g/d vs 10 g/d; bolus vs continuous infusion) administered for 48 h to critically ill patients with multiple organ dysfunction. The patients receiving 10 g vitamin C per day had supraphysiologic vitamin C levels and hyperoxaluria, oxalate being a metabolite of vitamin C. These findings raise concern for an increased risk of oxalate nephropathy, as has been reported with high-dose vitamin C administration and more prolonged administration in the noncritically ill population[68,79,80]. This theoretical risk of oxalate nephropathy stands in contrast with the mostly reassuring data about the safety of short-term high-dose vitamin C administration[64,65,67].
At present, multiple ongoing randomized controlled trials, including the VICTAS, ACTS, and HYVCTTSSS trials, are aimed at confirming the beneficial effects of vitamin C and adjuncts in critically ill patients with sepsis[81-83].
Trauma and hemorrhagic shock can lead to significant coagulopathy and inflammation, and both are associated with increased mortality and morbidity. Given its antioxidant effects, vitamin C has long been evaluated as a protective agent to mitigate effects on proinflammatory and procoagulant pathways caused by trauma and hemorrhagic shock[84-88].
In a swine model of acute hemorrhagic shock, animals were randomized to receive either intravenous normal saline, low-dose Vitamin C (50 mg/kg), or high-dose Vitamin C (200 mg/kg). The group of animals receiving normal saline (control) showed significantly greater histological end-organ damage, including elevated acute lung injury scores and increased mRNA levels of interleukin (IL)-1β, IL-8, TNF-α, plasminogen activation inhibitor-1 and tissue factor compared with the groups receiving vitamin C. Furthermore, only a modest correction of coagulopathy was observed in the vitamin C group when compared to the normal saline group[88]. Similarly, in a rat model of hemorrhagic shock, vitamin C administration (low 100 mg/kg or high 500 mg/kg) was shown to attenuate renal injury, possibly via a SIRT1-mediated mechanism. Levels of serum creatinine, BUN, TNF-α, and IL-1β were lower in the vitamin C group when compared to a sham group. Conversely, levels of hemeoxygenase-1 (HO-1), a stress-response protein believed to play key roles in mediating protection against oxidant-mediated lung injury, were higher in kidneys treated with vitamin C. This effect appeared to occur irrespective of the vitamin C dose administered[89]. Another study of the effects of vitamin C administration (100 mg/kg) on renal function found a decrease in expression of the induced dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin protein in the tubular epithelial cells of rat kidneys. Levels of this protein are believed to correlate with the occurrence of kidney injury. Vitamin C administration prior to resuscitation was also found to decrease proinflammatory cytokine production, which mitigated renal injury[90]. Another rat model of hemorrhagic shock found that vitamin C treatment induced HO-1 expression in a variety of tissues, including kidney, lung and liver, with decreased organ injury and proinflammatory responses[91]. Likewise, vitamin C pretreatment in the setting of hemorrhagic shock appears to protect the intestinal epithelium by decreased proinflammatory cytokine expression and neutrophil infiltration. This effect was also believed to be mediated by HO-1 and was abrogated by pharmacological HO-1 inhibition[92]. Prior studies have suggested that pretreatment of rats with vitamin C (1 mg/100 g or 5 mg/100 g) decreases gastric mucosal bleeding after induction of hemorrhagic shock and retransfusion[93]. Lastly, the combination of vitamin C administration (50 mg/kg per day for 3 d) prior to inducing hemorrhage together with intravenous infusion vitamin C (50 mg/kg) following hemorrhage improved cardiovascular parameters, such as blood pressure and LV dp/dt, and decreased free radical production in a rat model of hemorrhagic hypotension[94].
These beneficial effects of vitamin C stand in contrast with those obtained in a rat model of liver injury and hemorrhagic shock, in which vitamin C preconditioning (10 mg/kg) did not improve the recovery of animals after resuscitation[95]. Likewise, a survival study in rats with hemorrhagic shock did not show a difference when lactated Ringer’s solution plus vitamin C (50 mg/kg) was administered for resuscitation, compared with lactated Ringer’s solution alone[96].
These preclinical studies point out multiple mechanisms by which vitamin C may serve as an antioxidant in hemorrhagic shock and thus could provide organ protection. However, evidence suggesting a vitamin C-mediated survival benefit is missing. To our knowledge, there is thus far no human trial data available that demonstrate a clinical benefit of vitamin C administration as an adjunct for the treatment of trauma and hemorrhagic shock.
Pain is a common problem in critically ill patients, either due to injuries secondary to infection, inflammation, trauma, surgery, cancer, or in the setting of the reactivation of herpes zoster. Evidence suggests that vitamin C acts as a cofactor for the biosynthesis of opioid peptides and as a potent anti-inflammatory agent[97,98].
Several case reports and a cohort study have reported clinical improvement in relief for patients with acute herpes zoster exacerbation who were administered vitamin C [99-101]. While a recent randomized controlled trial of high dose intravenous vitamin C (5 g iv bolus per day on day 1, 3 and 5) failed to find a reduction in acute herpes zoster pain, there was a decrease in the incidence of post-herpetic neuropathy[102]. A similarly designed study found lower plasma concentrations of vitamin C in patients with post-herpetic neuropathy than in healthy volunteers, and a reduction in spontaneous post-herpetic neuropathy pain after high-dose vitamin C treatment[103].
Several trials have found reductions in the development of complex regional pain syndrome after wrist and ankle surgery with vitamin C[104-107]. A study of patients with osteoarthritis-related hip or knee joint pain found that vitamin C that was administered enterally for 14 d provided modest pain relief, equivalent to approximately half the effect of nonsteroidal anti-inflammatory drugs[108]. In a randomized controlled trial of vitamin C in patients undergoing single-level posterior lumbar interbody fusion, there was no difference in postoperative pain intensity between the two groups, but vitamin C administration was associated with improved functional status[109].
A majority of the prospective and case studies of vitamin C administration for cancer-related pain have reported improvements in quality-of-life indicators such as pain, fatigue, insomnia, nausea and vomiting[110-115]. However, clinical trial data regarding vitamin C-related opioid-sparing effects in cancer patients have yielded mixed results[116-119].
Perhaps more widely investigated than any other vitamin C-related claim is the assertion of benefit for patients with cancer. In fact, a quick PubMed search of “ascorbic acid + cancer” yielded 4,376 items, 247 of which were clinical trials (as of May 2018).
Cancer patients have been recognized to have low vitamin C levels compared with healthy controls[120]. In a large randomized, placebo-controlled trial, daily intake of antioxidants, vitamins and minerals, a combination of vitamin C (120 mg/d), vitamin E, zinc, beta carotene and selenium lowered total cancer incidence and all-cause mortality in men but not women at 7.5 years[121]. A similar regimen of vitamin C and E supplementation with beta carotene did not, however, prevent the formation of colon adenomas in a randomized trial of 864 patients[122]. Another study of vitamin C and E supplementation for cancer prevention did not identify immediate or long-term effects on the risk of total cancers, prostate cancer, or other site-specific cancers[123].
A randomized clinical trial examining different doses of vitamin C (1, 2 or 4 g/d) failed to find a dose-response relationship or an association between serum ascorbic acid levels and mutagen sensitivity, which has been described as a risk factor for tobacco-related epithelial cancers[124]. Despite these clinical findings, basic science data suggest that vitamin C may have a beneficial role in cancer progression through several different mechanisms. Vitamin C was recently found to restore Tet methylcytosine dioxygenase 2 function, one of the most frequently mutated genes in hematopoietic malignancies. Through this mechanism, vitamin C may block aberrant self-renewal and leukemia progression[125]. Vitamin C also facilitates DNA oxidation in leukemia cells, rendering them more sensitive to poly ADP ribose polymerase inhibitors[125].
In cholangiocarcinoma, SVCT2 expression levels have been shown to correlate with susceptibility to vitamin C-induced cancer cell death in vitro and in vivo[126]. In separate experiments, Vitamin C has been shown to increase methotrexate-mediated hepatocellular carcinoma cell death[127]. Furthermore, vitamin C enhances the effectiveness of radiation therapy for glioblastoma and gemcitabine/epigallocatechin-3-gallate treatment for mesothelioma[128,129]. These findings are in contrast to data showing that vitamin C interferes with chemotherapy drugs such as doxorubicin, methotrexate, and cisplatin[128-131]. Moreover, vitamin C may enhance the growth of some cancers. For example, plasmocytoma cell growth is dependent on the presence of vitamin C[132]. Vitamin C exposure showed differential effects in an in vitro model of colony-forming bone marrow cell growth in patients with myelodysplastic syndrome. In this model, vitamin C responsiveness (both growth enhancement or inhibition) was associated with shorter survival when compared to patients with no response to vitamin C[133]. Adding to this complex picture is data derived from in vitro work that examined the response of HL-60 cells from an acute myeloid leukemia cell line to vitamin C. Vitamin C administration decreased oxidative stress and thus protected HL-60 cells from H2O2-induced cell death[134].
Curiously, high-dose vitamin C (0.5-5 mmol/L) has also been shown to increase the procoagulant properties of freshly isolated red blood cells via externalization of phophatidylserine, a mechanism known to lead to thrombus formation. Interestingly, this effect was more pronounced in red blood cells from cancer patients and could be confirmed in a rat model of thrombus formation[135].
In one study in terminal cancer patients, vitamin C was associated with increased quality-of-life and survival[116]. In contrast, in two double-blinded randomized controlled trials that included patients with advanced cancers (stomach, colon, pancreas, lung, breast and others), vitamin C (10 g/d) did not improve survival[136,137].
Given the complexities of cancer biology and vitamin C, the risks and benefits of initiating high-dose vitamin C therapy in critically ill oncology patients should be carefully weighed and discussed with the oncology consultant.
Vitamin C is once again a focus of intense interest with respect to its role in the treatment of critically ill patients. Evidence suggests that vitamin C administration may have a variety of beneficial effects in patients undergoing cardiac surgical procedures, during resuscitation with acute burn injury, for the treatment of sepsis, in reducing pain, and in the treatment of cancer. While many questions have yet to be answered, there is little data to suggest that short-term high-dose vitamin C would elicit major harm, except for the risk of oxalate nephropathy. In fact, evidence suggests that short-term high-dose vitamin C in selected patients may improve hemodynamic parameters, decrease fluid resuscitation requirements, reduce the incidence of perioperative atrial fibrillation, improve pain and potentially reduce sepsis-associated mortality. We eagerly await additions to the growing body of evidence that examine the role of vitamin C administration for improving outcomes for our sickest patients.
| 1. | Pauling L. Vitamin C therapy of advanced cancer. N Engl J Med. 1980;302:694-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Pauling L. Diet, nutrition, and cancer. Am J Clin Nutr. 1977;30:661-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Cameron E, Pauling L. Ascorbic acid and the glycosaminoglycans. An orthomolecular approach to cancer and other diseases. Oncology. 1973;27:181-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Pauling L. Vitamin C and common cold. JAMA. 1971;216:332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Berger MM, Oudemans-van Straaten HM. Vitamin C supplementation in the critically ill patient. Curr Opin Clin Nutr Metab Care. 2015;18:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 6. | Savini I, Rossi A, Pierro C, Avigliano L, Catani MV. SVCT1 and SVCT2: key proteins for vitamin C uptake. Amino Acids. 2008;34:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 270] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 7. | Carr AC, Rosengrave PC, Bayer S, Chambers S, Mehrtens J, Shaw GM. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit Care. 2017;21:300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 272] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 8. | Honoré PM, De Waele E, Jacobs R, Mattens S, Rose T, Joannes-Boyau O, De Regt J, Verfaillie L, Van Gorp V, Boer W. Nutritional and metabolic alterations during continuous renal replacement therapy. Blood Purif. 2013;35:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Kamel AY, Dave NJ, Zhao VM, Griffith DP, Connor MJ Jr, Ziegler TR. Micronutrient Alterations During Continuous Renal Replacement Therapy in Critically Ill Adults: A Retrospective Study. Nutr Clin Pract. 2018;33:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Story DA, Ronco C, Bellomo R. Trace element and vitamin concentrations and losses in critically ill patients treated with continuous venovenous hemofiltration. Crit Care Med. 1999;27:220-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 103] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Frei B, Stocker R, England L, Ames BN. Ascorbate: the most effective antioxidant in human blood plasma. Adv Exp Med Biol. 1990;264:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 162] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Dennis JM, Witting PK. Protective Role for Antioxidants in Acute Kidney Disease. Nutrients. 2017;9:E718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 181] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 13. | Carr AC, Maggini S. Vitamin C and Immune Function. Nutrients. 2017;9:E1211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 662] [Cited by in RCA: 1077] [Article Influence: 119.7] [Reference Citation Analysis (0)] |
| 14. | Ames AM, Nungester WJ. The relationship between ascorbic acid and phagocytic activity. J Bacteriol. 1947;54:53. [PubMed] |
| 15. | van Gorkom GNY, Klein Wolterink RGJ, Van Elssen CHMJ, Wieten L, Germeraad WTV, Bos GMJ. Influence of Vitamin C on Lymphocytes: An Overview. Antioxidants (Basel). 2018;7:E41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 16. | Tanaka M, Muto N, Gohda E, Yamamoto I. Enhancement by ascorbic acid 2-glucoside or repeated additions of ascorbate of mitogen-induced IgM and IgG productions by human peripheral blood lymphocytes. Jpn J Pharmacol. 1994;66:451-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Feigen GA, Smith BH, Dix CE, Flynn CJ, Peterson NS, Rosenberg LT, Pavlović S, Leibovitz B. Enhancement of antibody production and protection against systemic anaphylaxis by large doses of vitamin C. Res Commun Chem Pathol Pharmacol. 1982;38:313-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Gao YL, Lu B, Zhai JH, Liu YC, Qi HX, Yao Y, Chai YF, Shou ST. The Parenteral Vitamin C Improves Sepsis and Sepsis-Induced Multiple Organ Dysfunction Syndrome via Preventing Cellular Immunosuppression. Mediators Inflamm. 2017;2017:4024672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Ganguly R, Waldman RH. Macrophage functions in aging: effects of vitamin C deficiency. Allerg Immunol (Leipz). 1985;31:37-43. [PubMed] |
| 20. | Ganguly R, Durieux MF, Waldman RH. Macrophage function in vitamin C-deficient guinea pigs. Am J Clin Nutr. 1976;29:762-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Han M, Pendem S, Teh SL, Sukumaran DK, Wu F, Wilson JX. Ascorbate protects endothelial barrier function during septic insult: Role of protein phosphatase type 2A. Free Radic Biol Med. 2010;48:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Fisher BJ, Kraskauskas D, Martin EJ, Farkas D, Wegelin JA, Brophy D, Ward KR, Voelkel NF, Fowler AA 3rd, Natarajan R. Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. Am J Physiol Lung Cell Mol Physiol. 2012;303:L20-L32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 23. | Al-Shmgani HS, Moate RM, Macnaughton PD, Sneyd JR, Moody AJ. Effects of hyperoxia on the permeability of 16HBE14o- cell monolayers--the protective role of antioxidant vitamins E and C. FEBS J. 2013;280:4512-4521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Mo SJ, Son EW, Rhee DK, Pyo S. Modulation of TNF-alpha-induced ICAM-1 expression, NO and H2O2 production by alginate, allicin and ascorbic acid in human endothelial cells. Arch Pharm Res. 2003;26:244-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Scioli MG, Bielli A, Agostinelli S, Tarquini C, Arcuri G, Ferlosio A, Costanza G, Doldo E, Orlandi A. Antioxidant treatment prevents serum deprivation- and TNF-α-induced endothelial dysfunction through the inhibition of NADPH oxidase 4 and the restoration of β-oxidation. J Vasc Res. 2014;51:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Kaufmann PA, Gnecchi-Ruscone T, di Terlizzi M, Schäfers KP, Lüscher TF, Camici PG. Coronary heart disease in smokers: vitamin C restores coronary microcirculatory function. Circulation. 2000;102:1233-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 153] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Mohammed BM, Fisher BJ, Kraskauskas D, Ward S, Wayne JS, Brophy DF, Fowler AA 3rd, Yager DR, Natarajan R. Vitamin C promotes wound healing through novel pleiotropic mechanisms. Int Wound J. 2016;13:572-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 28. | Duarte TL, Cooke MS, Jones GD. Gene expression profiling reveals new protective roles for vitamin C in human skin cells. Free Radic Biol Med. 2009;46:78-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Nusgens BV, Humbert P, Rougier A, Richard A, Lapière CM. Stimulation of collagen biosynthesis by topically applied vitamin C. Eur J Dermatol. 2002;12:XXXII-XXXIV. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 30. | Fitzpatrick RE, Rostan EF. Double-blind, half-face study comparing topical vitamin C and vehicle for rejuvenation of photodamage. Dermatol Surg. 2002;28:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | ter Riet G, Kessels AG, Knipschild PG. Randomized clinical trial of ascorbic acid in the treatment of pressure ulcers. J Clin Epidemiol. 1995;48:1453-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 82] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Taylor TV, Rimmer S, Day B, Butcher J, Dymock IW. Ascorbic acid supplementation in the treatment of pressure-sores. Lancet. 1974;2:544-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 121] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Desneves KJ, Todorovic BE, Cassar A, Crowe TC. Treatment with supplementary arginine, vitamin C and zinc in patients with pressure ulcers: a randomised controlled trial. Clin Nutr. 2005;24:979-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 34. | Ubbink DT, Santema TB, Stoekenbroek RM. Systemic wound care: a meta-review of cochrane systematic reviews. Surg Technol Int. 2014;24:99-111. [PubMed] |
| 35. | Langer G, Fink A. Nutritional interventions for preventing and treating pressure ulcers. Cochrane Database Syst Rev. 2014;CD003216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Pekala J, Patkowska-Sokoła B, Bodkowski R, Jamroz D, Nowakowski P, Lochyński S, Librowski T. L-carnitine--metabolic functions and meaning in humans life. Curr Drug Metab. 2011;12:667-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 233] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 37. | Teuwen LA, Draoui N, Dubois C, Carmeliet P. Endothelial cell metabolism: an update anno 2017. Curr Opin Hematol. 2017;24:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Wang ZY, Liu YY, Liu GH, Lu HB, Mao CY. l-Carnitine and heart disease. Life Sci. 2018;194:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 39. | Ribas GS, Vargas CR, Wajner M. L-carnitine supplementation as a potential antioxidant therapy for inherited neurometabolic disorders. Gene. 2014;533:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 182] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 40. | Puskarich MA, Finkel MA, Karnovsky A, Jones AE, Trexel J, Harris BN, Stringer KA. Pharmacometabolomics of l-carnitine treatment response phenotypes in patients with septic shock. Ann Am Thorac Soc. 2015;12:46-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 41. | Puskarich MA, Kline JA, Krabill V, Claremont H, Jones AE. Preliminary safety and efficacy of L-carnitine infusion for the treatment of vasopressor-dependent septic shock: a randomized control trial. JPEN J Parenter Enteral Nutr. 2014;38:736-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Hatamkhani S, Karimzadeh I, Elyasi S, Farsaie S, Khalili H. Carnitine and sepsis: a review of an old clinical dilemma. J Pharm Pharm Sci. 2013;16:414-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 43. | Patak P, Willenberg HS, Bornstein SR. Vitamin C is an important cofactor for both adrenal cortex and adrenal medulla. Endocr Res. 2004;30:871-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 44. | Stone KJ, Townsley BH. The effect of L-ascorbate on catecholamine biosynthesis. Biochem J. 1973;131:611-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Bornstein SR, Yoshida-Hiroi M, Sotiriou S, Levine M, Hartwig HG, Nussbaum RL, Eisenhofer G. Impaired adrenal catecholamine system function in mice with deficiency of the ascorbic acid transporter (SVCT2). FASEB J. 2003;17:1928-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 46. | May JM, Qu ZC, Meredith ME. Mechanisms of ascorbic acid stimulation of norepinephrine synthesis in neuronal cells. Biochem Biophys Res Commun. 2012;426:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 47. | Prigge ST, Mains RE, Eipper BA, Amzel LM. New insights into copper monooxygenases and peptide amidation: structure, mechanism and function. Cell Mol Life Sci. 2000;57:1236-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 334] [Article Influence: 12.8] [Reference Citation Analysis (16)] |
| 48. | Das D, Sen C, Goswami A. Effect of Vitamin C on adrenal suppression by etomidate induction in patients undergoing cardiac surgery: A randomized controlled trial. Ann Card Anaesth. 2016;19:410-417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 49. | Carr AC, Shaw GM, Fowler AA, Natarajan R. Ascorbate-dependent vasopressor synthesis: a rationale for vitamin C administration in severe sepsis and septic shock? Crit Care. 2015;19:418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 180] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 50. | Moser MA, Chun OK. Vitamin C and Heart Health: A Review Based on Findings from Epidemiologic Studies. Int J Mol Sci. 2016;17:E1328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 51. | McDonald CI, Fraser JF, Coombes JS, Fung YL. Oxidative stress during extracorporeal circulation. Eur J Cardiothorac Surg. 2014;46:937-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 52. | Wilson JX. Mechanism of action of vitamin C in sepsis: ascorbate modulates redox signaling in endothelium. Biofactors. 2009;35:5-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 53. | Rodemeister S, Duquesne M, Adolph M, Nohr D, Biesalski HK, Unertl K. Massive and long-lasting decrease in vitamin C plasma levels as a consequence of extracorporeal circulation. Nutrition. 2014;30:673-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 54. | Hu X, Yuan L, Wang H, Li C, Cai J, Hu Y, Ma C. Efficacy and safety of vitamin C for atrial fibrillation after cardiac surgery: A meta-analysis with trial sequential analysis of randomized controlled trials. Int J Surg. 2017;37:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 55. | Hemilä H, Suonsyrjä T. Vitamin C for preventing atrial fibrillation in high risk patients: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2017;17:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 56. | Antonic M, Lipovec R, Gregorcic F, Juric P, Kosir G. Perioperative ascorbic acid supplementation does not reduce the incidence of postoperative atrial fibrillation in on-pump coronary artery bypass graft patients. J Cardiol. 2017;69:98-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 57. | Baker WL, Coleman CI. Meta-analysis of ascorbic acid for prevention of postoperative atrial fibrillation after cardiac surgery. Am J Health Syst Pharm. 2016;73:2056-2066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 58. | Dehghani MR, Majidi N, Rahmani A, Asgari B, Rezaei Y. Effect of oral vitamin C on atrial fibrillation development after isolated coronary artery bypass grafting surgery: A prospective randomized clinical trial. Cardiol J. 2014;21:492-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 59. |
Carnes CA, Chung MK, Nakayama T, Nakayama H, Baliga RS, Piao S, Kanderian A, Pa |
| 60. | Valls N, Gormaz JG, Aguayo R, González J, Brito R, Hasson D, Libuy M, Ramos C, Carrasco R, Prieto JC. Amelioration of persistent left ventricular function impairment through increased plasma ascorbate levels following myocardial infarction. Redox Rep. 2016;21:75-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 61. | Basili S, Tanzilli G, Mangieri E, Raparelli V, Di Santo S, Pignatelli P, Violi F. Intravenous ascorbic acid infusion improves myocardial perfusion grade during elective percutaneous coronary intervention: relationship with oxidative stress markers. JACC Cardiovasc Interv. 2010;3:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 62. | Wang ZJ, Hu WK, Liu YY, Shi DM, Cheng WJ, Guo YH, Yang Q, Zhao YX, Zhou YJ. The effect of intravenous vitamin C infusion on periprocedural myocardial injury for patients undergoing elective percutaneous coronary intervention. Can J Cardiol. 2014;30:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 63. | Ramos C, Brito R, González-Montero J, Valls N, Gormaz JG, Prieto JC, Aguayo R, Puentes Á, Noriega V, Pereira G, Palavecino T, Rodrigo R. Effects of a novel ascorbate-based protocol on infarct size and ventricle function in acute myocardial infarction patients undergoing percutaneous coronary angioplasty. Arch Med Sci. 2017;13:558-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 64. | Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest. 2017;151:1229-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 726] [Cited by in RCA: 657] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 65. | Zabet MH, Mohammadi M, Ramezani M, Khalili H. Effect of high-dose Ascorbic acid on vasopressor’s requirement in septic shock. J Res Pharm Pract. 2016;5:94-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 198] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 66. | Tanaka H, Matsuda T, Miyagantani Y, Yukioka T, Matsuda H, Shimazaki S. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: a randomized, prospective study. Arch Surg. 2000;135:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 297] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 67. | Fowler AA, Syed AA, Knowlson S, Sculthorpe R, Farthing D, DeWilde C, Farthing CA, Larus TL, Martin E, Brophy DF. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. 2014;12:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 410] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 68. | de Grooth HJ, Manubulu-Choo WP, Zandvliet AS, Spoelstra-de Man AME, Girbes AR, Swart EL, Oudemans-van Straaten HM. Vitamin C Pharmacokinetics in Critically Ill Patients: A Randomized Trial of Four IV Regimens. Chest. 2018;153:1368-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 69. | Saffle JI. The phenomenon of “fluid creep” in acute burn resuscitation. J Burn Care Res. 2007;28:382-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 210] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 70. | Saffle JR. Fluid Creep and Over-resuscitation. Crit Care Clin. 2016;32:587-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 71. | Rizzo JA, Rowan MP, Driscoll IR, Chung KK, Friedman BC. Vitamin C in Burn Resuscitation. Crit Care Clin. 2016;32:539-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 72. | Cartotto R, Greenhalgh DG, Cancio C. Burn State of the Science: Fluid Resuscitation. J Burn Care Res. 2017;38:e596-e604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 73. | Kremer T, Harenberg P, Hernekamp F, Riedel K, Gebhardt MM, Germann G, Heitmann C, Walther A. High-dose vitamin C treatment reduces capillary leakage after burn plasma transfer in rats. J Burn Care Res. 2010;31:470-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 74. | Matsuda T, Tanaka H, Williams S, Hanumadass M, Abcarian H, Reyes H. Reduced fluid volume requirement for resuscitation of third-degree burns with high-dose vitamin C. J Burn Care Rehabil. 1991;12:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 48] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 75. | Matsuda T, Tanaka H, Yuasa H, Forrest R, Matsuda H, Hanumadass M, Reyes H. The effects of high-dose vitamin C therapy on postburn lipid peroxidation. J Burn Care Rehabil. 1993;14:624-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 76. | Dubick MA, Williams C, Elgjo GI, Kramer GC. High-dose vitamin C infusion reduces fluid requirements in the resuscitation of burn-injured sheep. Shock. 2005;24:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 77. | Kahn SA, Beers RJ, Lentz CW. Resuscitation after severe burn injury using high-dose ascorbic acid: a retrospective review. J Burn Care Res. 2011;32:110-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 78. | Hrsfmmvy T. The Effects of Topical Vitamin C Solution on Burn Wounds Granulation: A Randomized Clinical Trial. J Biomed Health. 2016;1:1-5. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 79. | Lamarche J, Nair R, Peguero A, Courville C. Vitamin C-induced oxalate nephropathy. Int J Nephrol. 2011;2011:146927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 80. | Rathi S, Kern W, Lau K. Vitamin C-induced hyperoxaluria causing reversible tubulointerstitial nephritis and chronic renal failure: a case report. J Med Case Rep. 2007;1:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 81. | Donnino M. Ascorbic Acid, Corticosteroids, and Thiamine in Sepsis (ACTS) Trial. [accessed 2018 Aug 8]. In ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03389555 ClinicalTrials.gov Identifier: NCT01750697. |
| 82. | Stefanovic S. The Effect of Vitamin C, Thiamine and Hydrocortisone on Clinical Course and Outcome in Patients With Severe Sepsis and Septic Shock. [accessed 2018 Aug 8]. In ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03335124 ClinicalTrials.gov Identifier: NCT03335124. |
| 83. | Zhujiang Hospital. Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Sepsis and Septic Shock (HYVCTTSSS). [accessed 2018 Aug 8]. In ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03258684 ClinicalTrials.gov Identifier: NCT03258684. |
| 84. | De Pasqualini CD. The effect of ascorbic acid on hemorrhagic shock in the guinea pig. Am J Physiol. 1946;147:598-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 85. | Strawitz JG, Temple RL, Hift H. The effect of methylene blue and ascorbic acid in hemorrhagic shock. Surg Forum. 1958;9:54-58. [PubMed] |
| 86. | Gomez OA, Santome JA. ASCORBIC Acid And Hemorrhagic Shock. Ii. Changes In The Whole Adrenal Gland And In The Adrenal Cortex. Acta Physiol Lat Am. 1963;13:155-158. [PubMed] |
| 87. | Santome JA, Gomez OA. Ascorbic Acid And Hemorrhagic Shock. I. Changes In Plasma And In Whole Blood. Acta Physiol Lat Am. 1963;13:150-154. [PubMed] |
| 88. | Reynolds PS, Fisher BJ, McCarter J, Sweeney C, Martin EJ, Middleton P, Ellenberg M, Fowler E, Brophy DF, Fowler AA 3rd, Spiess BD, Natarajan R. Interventional vitamin C: A strategy for attenuation of coagulopathy and inflammation in a swine multiple injuries model. J Trauma Acute Care Surg. 2018;85:S57-S67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 89. | Qi MZ, Yao Y, Xie RL, Sun SL, Sun WW, Wang JL, Chen Y, Zhao B, Chen EZ, Mao EQ. Intravenous Vitamin C attenuates hemorrhagic shock-related renal injury through the induction of SIRT1 in rats. Biochem Biophys Res Commun. 2018;501:358-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 90. | Ma L, Fei J, Chen Y, Zhao B, Yang ZT, Wang L, Sheng HQ, Chen EZ, Mao EQ. Vitamin C Attenuates Hemorrhagic Shock-induced Dendritic Cell-specific Intercellular Adhesion Molecule 3-grabbing Nonintegrin Expression in Tubular Epithelial Cells and Renal Injury in Rats. Chin Med J (Engl). 2016;129:1731-1736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 91. | Zhao B, Fei J, Chen Y, Ying YL, Ma L, Song XQ, Huang J, Chen EZ, Mao EQ. Vitamin C treatment attenuates hemorrhagic shock related multi-organ injuries through the induction of heme oxygenase-1. BMC Complement Altern Med. 2014;14:442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 92. | Zhao B, Fei J, Chen Y, Ying YL, Ma L, Song XQ, Wang L, Chen EZ, Mao EQ. Pharmacological preconditioning with vitamin C attenuates intestinal injury via the induction of heme oxygenase-1 after hemorrhagic shock in rats. PLoS One. 2014;9:e99134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 93. | Ekman T, Risberg B, Bagge U. Ascorbate reduces gastric bleeding after hemorrhagic shock and retransfusion in rats. Eur Surg Res. 1994;26:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 94. | Bhandari B, Kohli SK, Lal V. Protective role of ascorbic acid in hemorrhage-induced cardiovascular depression. Indian J Physiol Pharmacol. 2014;58:371-375. [PubMed] |
| 95. | Minor T, Niessen F, Klauke H, Isselhard W. No evidence for a protective effect of ascorbic acid on free radical generation and liver injury after hemorrhagic shock in rats. Shock. 1996;5:280-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 96. | Daughters K, Waxman K, Gassel A, Zommer S. Anti-oxidant treatment for shock: vitamin E but not vitamin C improves survival. Am Surg. 1996;62:789-792. [PubMed] |
| 97. | Carr AC, McCall C. The role of vitamin C in the treatment of pain: new insights. J Transl Med. 2017;15:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 98. | Mikirova N, Casciari J, Rogers A, Taylor P. Effect of high-dose intravenous vitamin C on inflammation in cancer patients. J Transl Med. 2012;10:189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 99. | Schencking M, Sandholzer H, Frese T. Intravenous administration of vitamin C in the treatment of herpetic neuralgia: two case reports. Med Sci Monit. 2010;16:CS58-CS61. [PubMed] |
| 100. | Byun SH, Jeon Y. Administration of Vitamin C in a Patient with Herpes Zoster - A case report -. Korean J Pain. 2011;24:108-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 101. | Schencking M, Vollbracht C, Weiss G, Lebert J, Biller A, Goyvaerts B, Kraft K. Intravenous vitamin C in the treatment of shingles: results of a multicenter prospective cohort study. Med Sci Monit. 2012;18:CR215-CR224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 102. | Kim MS, Kim DJ, Na CH, Shin BS. A Study of Intravenous Administration of Vitamin C in the Treatment of Acute Herpetic Pain and Postherpetic Neuralgia. Ann Dermatol. 2016;28:677-683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 103. | Chen JY, Chang CY, Feng PH, Chu CC, So EC, Hu ML. Plasma vitamin C is lower in postherpetic neuralgia patients and administration of vitamin C reduces spontaneous pain but not brush-evoked pain. Clin J Pain. 2009;25:562-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 104. | Besse JL, Gadeyne S, Galand-Desmé S, Lerat JL, Moyen B. Effect of vitamin C on prevention of complex regional pain syndrome type I in foot and ankle surgery. Foot Ankle Surg. 2009;15:179-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 105. | Zollinger PE, Tuinebreijer WE, Kreis RW, Breederveld RS. Effect of vitamin C on frequency of reflex sympathetic dystrophy in wrist fractures: a randomised trial. Lancet. 1999;354:2025-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 199] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 106. | Cazeneuve JF, Leborgne JM, Kermad K, Hassan Y. [Vitamin C and prevention of reflex sympathetic dystrophy following surgical management of distal radius fractures]. Acta Orthop Belg. 2002;68:481-484. [PubMed] |
| 107. | Zollinger PE, Tuinebreijer WE, Breederveld RS, Kreis RW. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? A randomized, controlled, multicenter dose-response study. J Bone Joint Surg Am. 2007;89:1424-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 108. | Jensen NH. [Reduced pain from osteoarthritis in hip joint or knee joint during treatment with calcium ascorbate. A randomized, placebo-controlled cross-over trial in general practice]. Ugeskr Laeger. 2003;165:2563-2566. [PubMed] |
| 109. | Lee GW, Yang HS, Yeom JS, Ahn MW. The Efficacy of Vitamin C on Postoperative Outcomes after Posterior Lumbar Interbody Fusion: A Randomized, Placebo-Controlled Trial. Clin Orthop Surg. 2017;9:317-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 110. | Carr AC, Vissers MC, Cook J. Relief from cancer chemotherapy side effects with pharmacologic vitamin C. N Z Med J. 2014;127:66-70. [PubMed] |
| 111. | Carr AC, Vissers MC, Cook J. Parenteral vitamin C for palliative care of terminal cancer patients. N Z Med J. 2014;127:84-86. [PubMed] |
| 112. | Ma Y, Chapman J, Levine M, Polireddy K, Drisko J, Chen Q. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci Transl Med. 2014;6:222ra18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 357] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 113. | Hoffer LJ, Robitaille L, Zakarian R, Melnychuk D, Kavan P, Agulnik J, Cohen V, Small D, Miller WH Jr. High-dose intravenous vitamin C combined with cytotoxic chemotherapy in patients with advanced cancer: a phase I-II clinical trial. PLoS One. 2015;10:e0120228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 114. | Stephenson CM, Levin RD, Spector T, Lis CG. Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother Pharmacol. 2013;72:139-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 115. | Ragnhammar P, Hafström L, Nygren P, Glimelius B; SBU-group. Swedish Council of Technology Assessment in Health Care. A systematic overview of chemotherapy effects in colorectal cancer. Acta Oncol. 2001;40:282-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 162] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 116. | Murata A, Morishige F, Yamaguchi H. Prolongation of survival times of terminal cancer patients by administration of large doses of ascorbate. Int J Vitam Nutr Res Suppl. 1982;23:103-113. [PubMed] |
| 117. | Pinkerton E, Good P, Gibbons K, Hardy J. An open-label pilot study of oral vitamin C as an opioid-sparing agent in patients with chronic pain secondary to cancer. Support Care Cancer. 2017;25:341-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 118. | Cameron E, Campbell A. The orthomolecular treatment of cancer. II. Clinical trial of high-dose ascorbic acid supplements in advanced human cancer. Chem Biol Interact. 1974;9:285-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 206] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 119. | Günes-Bayir A, Kiziltan HS. Palliative Vitamin C Application in Patients with Radiotherapy-Resistant Bone Metastases: A Retrospective Study. Nutr Cancer. 2015;67:921-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 120. | Mayland CR, Bennett MI, Allan K. Vitamin C deficiency in cancer patients. Palliat Med. 2005;19:17-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 131] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 121. | Hercberg S, Galan P, Preziosi P, Bertrais S, Mennen L, Malvy D, Roussel AM, Favier A, Briançon S. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 663] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 122. | Greenberg ER, Baron JA, Tosteson TD, Freeman DH Jr, Beck GJ, Bond JH, Colacchio TA, Coller JA, Frankl HD, Haile RW. A clinical trial of antioxidant vitamins to prevent colorectal adenoma. Polyp Prevention Study Group. N Engl J Med. 1994;331:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 447] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 123. | Wang L, Sesso HD, Glynn RJ, Christen WG, Bubes V, Manson JE, Buring JE, Gaziano JM. Vitamin E and C supplementation and risk of cancer in men: posttrial follow-up in the Physicians’ Health Study II randomized trial. Am J Clin Nutr. 2014;100:915-923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 124. | King TM, Trizna Z, Wu X, Amos CI, Fueger RH, Fueger JJ, Fritsche HA, Hsu TC, Winn R, Spitz MR. A clinical trial to evaluate the effect of vitamin C supplementation on in vitro mutagen sensitivity. The University of Texas M. D. Anderson Clinical Community Oncology Program Network. Cancer Epidemiol Biomarkers Prev. 1997;6:537-542. [PubMed] |
| 125. | Cimmino L, Dolgalev I, Wang Y, Yoshimi A, Martin GH, Wang J, Ng V, Xia B, Witkowski MT, Mitchell-Flack M. Restoration of TET2 Function Blocks Aberrant Self-Renewal and Leukemia Progression. Cell. 2017;170:1079-1095.e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 564] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 126. | Wang C, Lv H, Yang W, Li T, Fang T, Lv G, Han Q, Dong L, Jiang T, Jiang B. SVCT-2 determines the sensitivity to ascorbate-induced cell death in cholangiocarcinoma cell lines and patient derived xenografts. Cancer Lett. 2017;398:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 127. | Yiang GT, Chou PL, Hung YT, Chen JN, Chang WJ, Yu YL, Wei CW. Vitamin C enhances anticancer activity in methotrexatetreated Hep3B hepatocellular carcinoma cells. Oncol Rep. 2014;32:1057-1063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 128. | Martinotti S, Ranzato E, Burlando B. In vitro screening of synergistic ascorbate-drug combinations for the treatment of malignant mesothelioma. Toxicol In Vitro. 2011;25:1568-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 129. | Herst PM, Broadley KW, Harper JL, McConnell MJ. Pharmacological concentrations of ascorbate radiosensitize glioblastoma multiforme primary cells by increasing oxidative DNA damage and inhibiting G2/M arrest. Free Radic Biol Med. 2012;52:1486-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 130. | Ong PS, Chan SY, Ho PC. Differential augmentative effects of buthionine sulfoximine and ascorbic acid in As2O3-induced ovarian cancer cell death: oxidative stress-independent and -dependent cytotoxic potentiation. Int J Oncol. 2011;38:1731-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 131. | Heaney ML, Gardner JR, Karasavvas N, Golde DW, Scheinberg DA, Smith EA, O’Connor OA. Vitamin C antagonizes the cytotoxic effects of antineoplastic drugs. Cancer Res. 2008;68:8031-8038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 145] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 132. | Park CH, Bergsagel DE, McCulloch EA. Ascorbic acid: a culture requirement for colony formation by mouse plasmacytoma cells. Science. 1971;174:720-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 133. | Park CH, Kimler BF, Bodensteiner D, Lynch SR, Hassanein RS. In vitro growth modulation by L-ascorbic acid of colony-forming cells from bone marrow of patients with myelodysplastic syndromes. Cancer Res. 1992;52:4458-4466. [PubMed] |
| 134. | Guaiquil VH, Vera JC, Golde DW. Mechanism of vitamin C inhibition of cell death induced by oxidative stress in glutathione-depleted HL-60 cells. J Biol Chem. 2001;276:40955-40961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 107] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 135. | Kim K, Bae ON, Koh SH, Kang S, Lim KM, Noh JY, Shin S, Kim I, Chung JH. High-Dose Vitamin C Injection to Cancer Patients May Promote Thrombosis Through Procoagulant Activation of Erythrocytes. Toxicol Sci. 2015;147:350-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 136. | Creagan ET, Moertel CG, O’Fallon JR, Schutt AJ, O’Connell MJ, Rubin J, Frytak S. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N Engl J Med. 1979;301:687-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 378] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 137. | Moertel CG, Fleming TR, Creagan ET, Rubin J, O’Connell MJ, Ames MM. High-dose vitamin C vs placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double-blind comparison. N Engl J Med. 1985;312:137-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 382] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Critical care medicine
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Lin JA, Llompart-Pou JA, Kumar N, Willms DC S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Yin SY