Published online Nov 4, 2016. doi: 10.5492/wjccm.v5.i4.228
Peer-review started: April 22, 2016
First decision: June 6, 2016
Revised: July 19, 2016
Accepted: August 6, 2016
Article in press: August 8, 2016
Published online: November 4, 2016
Processing time: 196 Days and 20.7 Hours
To look into the management options of early debridement of the wound, followed by vascularized cover to bring in fresh blood supply to remaining tissue in electrical burns.
A total of 16 consecutive patients sustaining full thickness forearm burns over a period of one year were included in the study group. Debridement was undertaken within 48 h in 13 patients. Three patients were taken for debridement after 48 h. Debridement was repeated within 2-4 d after daily wound assessment and need for further debridement.
On an average two debridements (range 1-4) was required in our patients for the wound to be ready for definitive cover. Interval between each debridement ranged from 2-18 d. Fourteen patients were provided vascularized cover after final debridement (6 free flaps, 8 pedicled flaps). Functional assessment of gross hand function done at 6 wk, 2 mo, 3 mo and 6 mo follow-up.
High-tension electrical burns lead to significant morbidity. These injuries are best managed by early decompression followed by multiple serial debridements. The ideal timing of free flap coverage needs further investigation.
Core tip: High-voltage electrical injuries lead to be a significant morbidity associated with severe socioeconomic implications. There is conflicting evidence in the literature regarding progressive tissue necrosis in this devastating injury. We looked into the management options of early debridement of the wound, followed by vascularized cover to bring in fresh blood supply to remaining tissue, which can potentially prevent further progression of this pathology. We found that the phenomenon of ongoing necrosis was not halted in our study and all our early flaps failed to ingress the blood flow to the so-called ischemic zone post trauma. Electrical injuries were progressive in nature and required multiple radical debridement until the wound is ready for definitive cover.
- Citation: Mene A, Biswas G, Parashar A, Bhattacharya A. Early debridement and delayed primary vascularized cover in forearm electrical burns: A prospective study. World J Crit Care Med 2016; 5(4): 228-234
- URL: https://www.wjgnet.com/2220-3141/full/v5/i4/228.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v5.i4.228
Contact electrical injuries are a cause of significant damage to tissues, which often result in amputations to the extremities. Unlike the damage caused by thermal burns, high-voltage electrical burns tend to cause a severe and complex injury pattern. There can also be associated neurologic, cardiac, renal, gastrointestinal, ophthalmologic, and psychiatric disturbances[1]. Thus, electrical burns are associated with a significant morbidity, including high amputation rate which in turn is a cause of higher socioeconomic implications[2]. In a study by Noble et al[1], 45% of patients with electrical burns needed to change their profession and 32% were not able to return to work. Majority of the high voltage electrical injuries occur at the workplace in the adult age group. In children, injury is usually accidental occurring while playing near high voltage power supply lines[3].
The vascular endothelium provides a high resistant to the flow of electrical current; therefore, the damage was more significant along inner vascular wall. Initially, large blood vessels may appear patent, but if the endothelium was damaged mural thrombi would form, resulting in thrombosis and distal limb ischemia[4]. Thus, considering the conflicting evidence in the literature regarding progressive tissue necrosis, and high amputation rates of this devastating injury, we looked into the management options of early debridement of the wound, followed by vascularized cover to bring in fresh blood supply to remaining tissue, which was hypothesized to prevent further progression of this pathology.
This was a prospective study which was conducted over a period of 12 mo in the Department of Plastic Surgery at a tertiary level teaching hospital, to include all patients of electrical forearm burns. Those patients with associated life threatening injuries, or obviously charred limb at the time of presentation were excluded. Over a period of 1 year, 81 patients presented with history of sustaining electrical burns. Among these forty-two patients (51.85%) sustained injury to forearm, with or without involvement of other parts of body. However in 18 patients (42.8%) the limb was charred following contact and were excluded. Three (6.9%) patients with severe head injury and 5 (11.9%) patients with spinal cord injury resulting in paraplegia were excluded from the study. Thus, out of total 81 patients, 16 patients fulfilled the inclusion criteria and were included in the study. Patients presenting within 48-96 h were assessed for depth of tissue involved, both clinically and with PET findings. Those presenting later than 96 h were assessed for tissue involvement during debridement.
All patients sustaining these injuries were males and the mean age was 26 years. All injuries were due to high voltage electric contact over hand and forearm. Those presenting within 48 h (n = 6) were infused with Lactated Ringer’s infusion with monitoring of urine output, ECG monitoring, and circulatory assessment at 1 hourly intervals as per standard burn resuscitation protocols. Baseline investigations were done on admission and were monitored daily for 7 d. Assessment of urine for myoglobin and renal parameters were done on admission and repeated everyday if deranged. ECG was performed at admission to rule out cardiac irregularities and to assess for hyperkalemia. Those with deranged renal function (n = 2, 12.5%) were assessed and managed in collaboration with nephrologist.
The area of burn was examined and dimensions measured from bony landmarks. The graphical tracing of the injured area was used to measure the length and breadth of the wound while depth of involvement was assessed mainly by identifying structures involved during debridement. Distal limb viability was assessed by examining presence or absence of distal pulsations, capillary refill time and pulse oximetry readings compared with opposite healthy limb if uninjured. In the presence of compromise in the circulation, subjective neurosensory disturbance, pain with passive stretch of intrinsic musculature, decrease in oximetry readings to affected digits or extremity, liberal fasciotomy was done with standard defined incisions to release the compartment pressure. Circulatory status was examined and noted at 4 hourly intervals for 24 h and 8 hourly thereafter for 4 d.
Of 16 patients in the study group only 4 patients presented within 96 h and were assessed with PET FDG scan for the depth of tissue involvement. The scan was performed using a Discovery STE-16 PET-CT scanner (GE Healthcare, Milwaukee, United States) after intravenous injection of 370-444 MBq (10-12 mCi) of F-18 FDG. Debridement was undertaken within 48 h in 13 patients. The presence of obvious necrosis was indication for necrectomy. Three patients were taken for debridement after 48 h. One out of 3 had only fasciotomy wound with viable muscles clinically. On primary wound assessment in 2 patients, muscles were not obviously necrotic and were reassessed before debridement. Procedure was conducted under regional or general anesthesia, using tourniquet in all cases. Extent of involvement of muscles, tendons, nerves and vessels were noted. Bleeding, colour and contractility of muscles, along with status of tendons nerves and vessels were noted after tourniquet release.
Debridement was repeated within 2-4 d after daily wound assessment and need for further debridement. On an average two debridements (range 1-4) was required in our patients for the wound to be ready for definitive cover. Interval between each debridements ranged from 2-18 d.
Flap cover was planned depending upon status of the wound. Distant pedicled flaps and free flaps were used to resurface these defects. Locoregional flaps were not available for use due to the nature of the injuries. A total of 8 distant pedicled and 6 free flaps were undertaken. The functional and aesthetic outcome was assessed at 6 wk, 2 mo, 3 mo and 6 mo follow-up. Clinical assessment was done for median, ulnar, and radial nerves, and touch sensations at respective nerve territory were noted. ROM both active and passive of all joints of hand was documented. Patients were provided with activities of daily living (ADL) charts at 3 mo follow-up and asked to score depending on the scale of easy to difficulty in performing outlined tasks. These were scored as 1-no difficulty in performing the activity, 2-mild difficulty, 3-moderate difficulty, 4-severe difficulty, 5-cannot do at all. These activities were reassessed at 6 mo follow-up and any improvement or change of score was noted.
The statistical methods used in this study were approved in ethics committee/institute review board which has biostatistician as a co-opted member.
A total of 16 patients satisfied the inclusion criteria and constituted the study group. Patient’s age ranged from 10-45 years, average age being 26 years. All of these 16 patients were males. Incidence of electrical injuries during work was 81.25%. Ten patients were farmers working at the field near transformers, three were electricians injured while repairing high voltage lines. The three pediatric age group patients had contact with high-tension cables running nearby, while playing or flying kite.
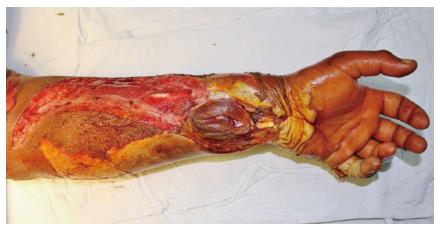
All patients were right handed and all injuries were due to high voltage. The dominant hand was affected in 12 of the 16 patients. Bilateral limbs were involved in seven patients Seven (7/16 - 44%) patients required fasciotomy. Fasciotomy was done under local anesthesia in emergency room with standard defined incisions to release the compartment pressure. Of 16 patients, derangement of renal function was observed in two patients (12.5%). One patient required multiple sittings of haemodialysis and other patient was managed conservatively with forced diuresis and urine alkalinization. On assessment of wound prior to debridement the average surface area involved was found to be 40.37 cm2 (range 5-82.5 cm2). The volar forearm was involved in 14 patients; while 2 patients had involvement of hand, and thumb (Figure 1). The hand as a whole was viable in all patients, except two patients who had non-viable little finger which required debridement. Patients had involvement of either median, ulnar nerve. Radial nerve was not involved as injury was predominantly on volar aspect of forearm. Three patients showed involvement of combined median and ulnar nerve. Involvement of only median nerve was seen in two patients, while only ulnar nerve was involved in six patients.
PET scan was done in four patients for assessment of involvement of deeper tissues focusing on accuracy and predictability of PET in assessing this. FDG uptake was adequately seen in upper third proximal muscle bellies of volar forearm. Pronater quadratus was involved in three cases, i.e., showed no glucose uptake. Flexor digitorum profundus was involved in all four patients. Flexor digitorum superficialis was injured in three-patients. The debridement findings in these patients correlated well with PET findings.
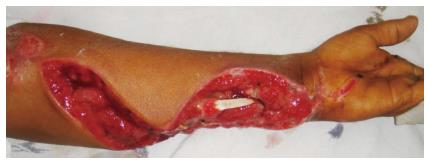
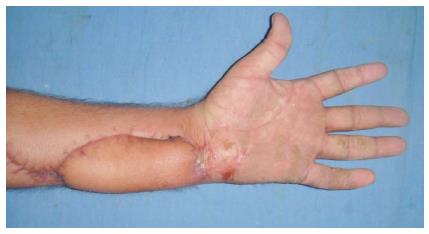
Debridement was undertaken under regional or general anesthesia, tourniquet was used in all cases. Debridement was considered to be complete when clinically there was no evidence of obvious necrotic tissues or slough in the wound (Figure 2). Patients underwent regular dressings and removal of obvious necrotic tissues during dressings. Debridement was repeated under anesthesia and tourniquet control in 10 patients and in six patients single debridement was done. Seven patients with bilateral limb involvement required more than one debridement, range 2-4 (mean 3 debridements). Three patients with unilateral limb involvement required more than one debridement (mean 2). The mean surface area of the wound after final debridement was measured 47 cm2 (range 5-96 cm2) from initial measurement of 40.37 cm2 showing 15.9% increase. Fourteen patients were provided vascularized cover after final debridement (6 free flaps, 8 pedicled flaps) (Figure 3); two patients had viable muscles exposed post fasciotomy, which were covered with skin graft.
Free flaps were selected on the basis of size and shape of wound, tissue requirement, availability of the recipient vessels and general condition of patient. Distant pedicled flap was selected in cases with recipient vessels involvement, associated proximal injury leading to impaired venous return of distal part, and associated co morbidities. Of the 6 free flaps, anterolateral thigh flap (n-2), latissimus dorsi flap (n-1), thoracodorsal artery perforator flap (n-2), and lateral arm flap (n-1) were done. In four patients, ulnar artery was used as a recipient vessel, while in two patients radial artery was used as a recipient vessel. Three out of six free flaps necrosed. All patients in whom flaps failed had undergone single debridement before flap transfer. The status of wound prior to flap transfer in these three patients showed apparently healthy looking and viable muscles. The vessels including radial and ulnar artery were patent proximally. All three flaps were transferred between 2-8 d post injury. Earliest free flap was done 2 d after debridement in which flap necrosed. Three flaps, which survived, were transferred after two weeks of injury. In case of distant pedicled flaps, 2 groin flaps, 1 bipedicled abdominal flap, 5 thoraco-umbilical flaps were used.
Functional assessment of gross hand function done at 6 wk, 2 mo, 3 mo and 6 mo follow-up. Patients were provided with ADL charts and asked to score depending on the scale of easy to difficulty in performing outlined tasks. The ADL score in all 16 patients were between 3-5. There was no progression and/or improvements in scores at three and six months follow-up.
The aims of treatment in electrical burns are to achieve good final function with good cosmetic appearance along with early return to a normal productive life. The aim of present study was to assess whether the extent of forearm injury and tissue damage was progressive and to determine whether early debridement and vascularized cover prevented progressive tissue necrosis and halted further progression.
Electrical injury usually affects young age group individuals; the incidence is high amongst males as compared to females because of work related nature of injury. The age group involved in various studies ranged from 10-50 years[5-7]. In our study, we observed 81.25% of the injuries to occur during work. Majority of our patients were farmers injured while working in the fields near transformers. We also observed a clear relationship between hand dominance and limb involved as 75% of our patients had dominant limb affected by injury supporting the observation that most of injuries occurred at workplace.
There is a significant variation in the rate of escharotomy/fasciotomy in electrical burns, which ranges from 9.2% to 54%[1,4,5]. Although, some studies advocate an immediate decompression[8], this concept has been questioned by others[9]. Mann et al[9] advocated selective decompression only in the presence of signs of compartment syndrome. They have based their algorithm on a continuous clinical evaluation and they opine that selective, non-immediate decompression may preserve tissue thus contributing to lower amputation rates. They did fasciotomy in 22% of their patients within 24 h. We opted for decompressive fasciotomy within 48 h of injury in 56% of patients.
d’Amato et al[3] and Ferreiro et al[5] in their individual studies supported the fact of early and radical debridement of all the necrotic tissues in electrical burns. d’Amato et al[3] also suggested the value of extended fasciotomy to access the viability of muscles. They said debride when necessary, and relieve the pathologic elevation of compartment pressures in an attempt to prevent ongoing neuromuscular damage. These observations support the need of early exploration with wide exposure of deep muscle compartments to assess the extent of injury in high-voltage burns. In present study, we did fasciotomy in nine patients with signs of increased compartment pressure and found that neuromuscular damage could be prevented with extended fasciotomies.
Scheker et al[8] were also of opinion that a wound should be radically debrided and cover should be provided immediately, they have proven this method of treatment to be superior to conservative debridement and delayed vascularized cover. They concluded that immediate reconstruction of severe upper extremity injuries is associated with increased function, lesser complications and a shorter hospital stay. According to authors, immediate reconstruction also permits earlier mobilization thereby preventing the tendon adhesions. We opted for radical debridement in our patients; however, the ongoing progression of tissue necrosis prevented us from immediate reconstruction.
García-Sánchez et al[2] observed myoglobinuria in 70% of their patients, while Mann et al[9] had reported 53.2% patients had myoglobinuria. In neither of these studies, authors observed their patients requiring haemodialysis for renal failure. Ferreiro et al[5] required hemodialysis in 3% of their patients. We observed two patients with myoglobinuria, one of which required haemodialysis. Both patients showed improvement in their renal functions after debridement. Thus, we observed that low urine output, and persistent acidosis is indication for exploration and debridement rather than conservative management of myoglobinuria.
Ferreiro et al[5] reported that the most frequently affected nerves were the ulnar and the median nerves. In their series, the incidence of peripheral neurological injury was 30%. The permanent peripheral neurological injury was located at the point of entry of the current in 88% of the cases and there was no recovery of sensations or movement during a period of six months after the trauma. Mazzetto-Betti et al[7] had ulnar clawing in 22% and median claw in 5% of cases. Analyzing hand sensation in their study, the radial nerve was the least affected among the patients. Sensation improvement was worst for the median nerve. In present study, we observed 38% patients with injury to ulnar nerve, while median nerve was affected in 12.5% of patients. Combined median and ulnar nerve was seen in 19% patients while none of our patients had involvement of radial nerve because of predominant involvement of volar forearm in our patients.
Burn wound depth is a significant determinant of patient treatment and morbidity. Devgan et al[10] reviewed modalities for the assessment of burn wound depth and concluded that Indo cyanine green video angiography or Laser Doppler Imaging is appropriate to best assess the depth of acute burn wounds. Nettelblad et al[11] used MRI as diagnostic modality in two patients with electrical burns. They suggested that the alteration of tissue signal exhibited by necrosed muscle is not specific to the injury mechanism. Smith et al[12] investigated the use of 18FDG PET scanning for assessment of skeletal muscle viability. They concluded that FDG-PET scanning could determine skeletal muscle viability in patients with peripheral vascular disease and in patients following free-flap transfer. In our study, we used FDG PET for assessment of muscle viability in four patients who presented within 96 h of injury. The numbers were less because of varied timing of presentation of patients. The findings at debridement correlated well with PET findings and the investigation aided the surgeon during debridement. The tool can be used in conjunction with clinical evaluation for deep muscle necrosis in electrical burns but further studies are needed in this context to reach any definitive conclusion.
The distant microvascular tissue transfer primarily aims at improving limb salvage rates in electrical burns. The transfer of well vascularized distant tissue in an ischemic bed can potentially lead to preservation of tissues. However, the best timing for free microvascular tissue transfer in electrical burn injuries remains debatable. Sauerbier et al[13] reported a higher free flap failure rate of 24% in electrical burns during primary reconstruction (within 5-21 d after trauma). In their later publication, they described a “vulnerable phase” of 3 wk after trauma in which vascular instability may compromise free flap success[14]. In contrast to this finding, Koul et al[15] found no variation in flap survival when performing microvascular tissue transfer in this vulnerable phase. Similar findings were reported earlier by Ninkovic et al[16]. Our own results favor the hypothesis of a vulnerable phase. In our series, flap failure due to microvascular thrombosis was seen exclusively in flaps transferred during the first 2 weeks. In our study, we found that the injury was progressive in nature and eventually required multiple radical debridements until the wound was ready for definitive cover. The phenomenon of ongoing necrosis was not found to be halted in our study and all our early flaps failed to ingress the blood flow to the so-called ischemic zone post trauma. Shen et al[17] also observed the phenomenon of distal flap necrosis and wound bed necrosis in early coverage of forearm electrical burns. Dega et al[18] were also able to achieve stable wound bed in patients of electrical burns after 12 d.
In present study, we observed average 61 d of hospital stay, which was directly related to number of procedures patient required for definitive wound cover. Handschin et al[19] reported average 44 d of hospital stay and Noble et al[1] in their study reported hospital stay 24.5 ± 21 d in electrical injuries.
The return to work data in the existing literature is rare and is difficult to interpret. A retrospective review by Mazzetto-Betti et al[7] reports that 72% patients retired or changed their job. Kidd et al[6] however reported that average time to return to work was 101 d post injury. Six patients in our study, returned to work after average 98 d post injury. Amongst these, three were students, two were farmers, and they adapted to use their contra-lateral uninjured limb. One patient was electrician who changed his job and presently doing farming with uninjured limb. None of our patients with bilateral involvement returned to work.
Following analysis of 16 patients of forearm electrical burns, it was concluded from the study that: (1) most common victims of electrical injury were young adult males. It affected the dominant limb more commonly and injury predominantly occurred at the workplace; (2) fasciotomy performed within 48 h of injury with slightest evidence of compartment syndrome was found to be limb saving; (3) there was a direct correlation between the point of entry of the current and the resulting neurological injury; (4) the clinical and PET assessment of deeper soft tissue involvement showed that injury affected periosseus tissues more than superficial muscles and skin; (5) electrical injuries were progressive in nature and required multiple radical debridement until the wound is ready for definitive cover; (6) the phenomenon of ongoing necrosis was not halted in our study and all our early flaps failed to ingress the blood flow to the so-called ischemic zone post trauma; and (7) distant pedicled flaps offered a viable and safe option for early coverage of hand and forearm defect in the presence of recipient vessel injury.
To conclude, high-tension electrical burns represent a severe injury with significant morbidity and the time tested concepts of early fasciotomy followed by repeated debridements remain the procedure of choice. The ideal timing of free flap coverage for these wounds needs further investigation.
High-voltage electrical burns are found to be associated with a significant morbidity leading to severe socioeconomic implications. Considering the conflicting evidence in the literature regarding progressive tissue necrosis in this devastating injury, the authors looked into the management options of early debridement of the wound, followed by vascularized cover to bring in fresh blood supply to remaining tissue, which can potentially prevent further progression of this pathology.
The timing of flap coverage in electrical burns is debatable because of presence of progressive tissue necrosis following electrical insult. Flaps done at first debridement can fail because of progression of tissue necrosis.
The authors found that the phenomenon of ongoing necrosis was not halted and all their early flaps failed to ingress the blood flow to the so-called ischemic zone post trauma. Serial debridements were required to achieve viable tissue bed. In addition distant pedicled flaps also allowed for safe and early coverage of hand and forearm defect and this method of reconstruction represented alternative to free tissue transfer in the presence of recipient vessel injury.
The study results suggest that electrical burns do lead to progressive tissue necrosis and any flap coverage should be contemplated after this process has stabilized.
Progressive tissue necrosis is seen in electrical burns because of progressive microvascular thrombosis. This requires multiple stages of debridements.
The study on electrical burns is relatively well-presented. It is an interesting article in this field.
| 1. | Noble J, Gomez M, Fish JS. Quality of life and return to work following electrical burns. Burns. 2006;32:159-164. [PubMed] |
| 2. | García-Sánchez V, Gomez Morell P. Electric burns: high- and low-tension injuries. Burns. 1999;25:357-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | d’Amato TA, Kaplan IB, Britt LD. High-voltage electrical injury: a role for mandatory exploration of deep muscle compartments. J Natl Med Assoc. 1994;86:535-537. [PubMed] |
| 4. | Herrera FA, Hassanein AH, Potenza B, Dobke M, Angle N. Bilateral upper extremity vascular injury as a result of a high-voltage electrical burn. Ann Vasc Surg. 2010;24:825.e1-825.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Ferreiro I, Meléndez J, Regalado J, Béjar FJ, Gabilondo FJ. Factors influencing the sequelae of high tension electrical injuries. Burns. 1998;24:649-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Kidd M, Hultman CS, Van Aalst J, Calvert C, Peck MD, Cairns BA. The contemporary management of electrical injuries: resuscitation, reconstruction, rehabilitation. Ann Plast Surg. 2007;58:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Mazzetto-Betti KC, Amâncio AC, Farina JA, Barros ME, Fonseca MC. High-voltage electrical burn injuries: functional upper extremity assessment. Burns. 2009;35:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Scheker LR, Ahmed O. Radical debridement, free flap coverage, and immediate reconstruction of the upper extremity. Hand Clin. 2007;23:23-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Mann R, Gibran N, Engrav L, Heimbach D. Is immediate decompression of high voltage electrical injuries to the upper extremity always necessary? J Trauma. 1996;40:584-587; discussion 584-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Devgan L, Bhat S, Aylward S, Spence RJ. Modalities for the assessment of burn wound depth. J Burns Wounds. 2006;5:e2. [PubMed] |
| 11. | Nettelblad H, Thuomas KA, Sjöberg F. Magnetic resonance imaging: a new diagnostic aid in the care of high-voltage electrical burns. Burns. 1996;22:117-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Smith GT, Wilson TS, Hunter K, Besozzi MC, Hubner KF, Reath DB, Goldman MH, Buonocore E. Assessment of skeletal muscle viability by PET. J Nucl Med. 1995;36:1408-1414. [PubMed] |
| 13. | Sauerbier M, Ofer N, Germann G, Baumeister S. Microvascular reconstruction in burn and electrical burn injuries of the severely traumatized upper extremity. Plast Reconstr Surg. 2007;119:605-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Ofer N, Baumeister S, Megerle K, Germann G, Sauerbier M. Current concepts of microvascular reconstruction for limb salvage in electrical burn injuries. J Plast Reconstr Aesthet Surg. 2007;60:724-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Koul AR, Patil RK, Philip VK. Early use of microvascular free tissue transfer in the management of electrical injuries. Burns. 2008;34:681-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Ninkovic M, Deetjen H, Ohler K, Anderl H. Emergency free tissue transfer for severe upper extremity injuries. J Hand Surg Br. 1995;20:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Shen YM, Hu XH, Mi HR, Yu DN, Qin FJ, Chen H, Wang H, Zhang GA. Early treatment of high-voltage electric burn wound in the limbs. Zhonghua Shaoshang Zazhi. 2011;27:173-177. [PubMed] |
| 18. | Dega S, Gnaneswar SG, Rao PR, Ramani P, Krishna DM. Electrical burn injuries. Some unusual clinical situations and management. Burns. 2007;33:653-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Handschin AE, Vetter S, Jung FJ, Guggenheim M, Künzi W, Giovanoli P. A case-matched controlled study on high-voltage electrical injuries vs thermal burns. J Burn Care Res. 2009;30:400-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Critical care medicine
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Inchauspe AA, Losanoff JE, Mocellin S S- Editor: Gong XM L- Editor: A E- Editor: Wu HL