Published online May 4, 2015. doi: 10.5492/wjccm.v4.i2.130
Peer-review started: October 10, 2014
First decision: November 27, 2014
Revised: December 20, 2014
Accepted: February 10, 2015
Article in press: February 12, 2015
Published online: May 4, 2015
Processing time: 195 Days and 0.3 Hours
Tumor lysis syndrome is an oncometabolic emergency resulting from rapid cell death. Tumor lysis syndrome can occur as a consequence of tumor targeted therapy or spontaneously. Clinicians should stratify every hospitalized cancer patient and especially those receiving chemotherapy for the risk of tumor lysis syndrome. Several aspects of prevention include adequate hydration, use of uric acid lowering therapies, use of phosphate binders and minimization of potassium intake. Patients at high risk for the development of tumor lysis syndrome should be monitored in the intensive care unit. Established tumor lysis syndrome should be treated in the intensive care unit by aggressive hydration, possible use of loop diuretics, possible use of phosphate binders, use of uric acid lowering agents and dialysis in refractory cases.
Core tip: Tumor lysis syndrome (TLS) is characterized by a massive tumor cell death leading to the development of metabolic derangements and target organ dysfunction. TLS can occur as a result of cancer treatment or spontaneously. Blood cancers constitute the vast majority of TLS cases because of the sensitivity to therapy and rapid division rates. Solid cancers comprise the minority of cases and are usually advanced if complicated by TLS. Prophylaxis is the mainstay of management and should be routinely implemented in high and intermediate risk patients. Management of established TLS includes intravenous hydration, urate lowering therapies, management of hyperkalemia and hemodialysis in refractory cases.
- Citation: Mirrakhimov AE, Voore P, Khan M, Ali AM. Tumor lysis syndrome: A clinical review. World J Crit Care Med 2015; 4(2): 130-138
- URL: https://www.wjgnet.com/2220-3141/full/v4/i2/130.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v4.i2.130
Cancer disorders constitute a diverse group of pathologies in which abnormal metabolism and life cycle lead to the profound derangement of a host’s metabolism. These cancers differ in their cellular origin, pathogenesis, clinical presentation, and management. Furthermore, cancer has been found by the Centers for Disease Control and Prevention to be the second leading cause of death among United States residents in 2011[1]. Thus, because of the high prevalence of malignant neoplasms, it is essential that clinicians are aware of the major complications of cancer itself and its management. Furthermore, it is likely that physicians will manage a greater number of cancer patients in future in the future, due to the improved survival rates of patients with cancer, ageing and growing population.
Tumor lysis syndrome (TLS) is a major oncometabolic entity requiring emergent recognition and management. TLS comprises a clinicolaboratory derangement of cellular metabolism, which can lead to severe renal impairment, cardiac arrhythmias, seizures, and death[2]. Cellular death mediated by treatment targeted at cancer (chemotherapy or another pharmacological antitumor intervention, embolization of tumor or radiation therapy) or spontaneous cellular death in rapidly dividing cancer cells (which is known as spontaneous TLS) leads to an efflux of cellular material rich in potassium, phosphorus, and uric acid into the bloodstream. However, serum calcium levels typically decrease in patients with TLS because of its binding to excess phosphorus. These key metabolic derangements mediate the acute impairment of renal function, cardiac arrhythmogenicity, central nervous system toxicity, and ultimately death.
The most widely used diagnostic criteria are those proposed by Cairo et al[2] in 2004. Based on this classification, TLS can be defined as laboratory TLS, when TLS is clinically silent and only detected through laboratory work up, and clinical TLS, when laboratory TLS is complicated by the clinical manifestations mentioned above. The diagnostic criteria proposed by Cairo et al[2] are presented in Tables 1 and 2. It is necessary to note that laboratory TLS is defined as the presence of at least two or more biochemical variables within the 3 d before chemotherapy or 7 d after chemotherapy in the face of adequate hydration and use of uric acid lowering agent. Clinical TLS is defined as the presence of at least one clinical criterion that is not believed to be attributable to the chemotherapy agent[2]. However, our group has recently mentioned that this definition is imperfect since radiation therapy may lead to TLS as well, and TLS can occur spontaneously in rapidly proliferating and bulky malignancies[3,4].
| Variable | Value | Change from baseline value |
| Uric acid | ≥ 8 mg/dL (476 mmol/L) | 25% increase |
| Potassium | ≥ 6.0 mEq/L (or 6 mmol/L) | 25% increase |
| Phosphorus | ≥ 4.5 mg/dL (1.45 mmol/L) for adults and ≥ 6.5 mg/dL (2.1 mmol/L) for children | 25% increase |
| Calcium | ≤ 7 mg/dL (1.75 mmol/L) | 25% decrease |
| Variable | Grade 0 | Grade I | Grade II | Grade III | Grade IV | Grade V |
| Creatinine | None | 1.5 times ULN. Rise in creatinine is not attributable to chemotherapeutic agent(s) | > 1.5-3.0 times ULN. Rise in creatinine is not attributable to chemotherapeutic agent(s) | > 3.0-6.0 times ULN. Rise in creatinine is not attributable to chemotherapeutic agent(s) | > 6.0 times ULN. Rise in creatinine is not attributable to chemotherapeutic agent(s) | Death |
| Cardiac arrhythmia | None | Intervention not indicated | Nonurgent medical intervention indicated. Cardiac arrhythmias not attributable to chemotherapeutic agent(s) | Symptomatic and incompletely controlled medically or controlled with device (e.g., defibrillator). Cardiac arrhythmias not attributable to chemotherapeutic agent(s) | Life-threatening (e.g., arrhythmia associated with HF, hypotension, syncope, shock). Cardiac arrhythmias not attributable to chemotherapeutic agent(s) | Death |
| Seizures | None | - | One brief, generalized seizure; seizure(s) well controlled by anticonvulsants or infrequent focal motor seizures not interfering with ADL | Seizure in which consciousness is altered; poorly controlled seizure disorder; with breakthrough generalized seizures despite medical intervention | Seizure of any kind which are prolonged, repetitive or difficult to control (e.g., status epilepticus, intractable epilepsy) | Death |
This manuscript summarizes the current state knowledge on TLS for clinicians involved in the care of critically ill patients: first, we briefly discuss the relevant pathobiology of TLS; second, we review and discuss which patients with cancer should be deemed to be at high risk; third, we go through the clinical presentation and diagnosis of TLS, including making an appropriate differential diagnosis; fourth, the information on TLS prevention is discussed; and finally, the treatment options for full blown TLS are provided.
We searched PubMed/Medline, Scopus, Embase, and the Web of Science for articles focused on TLS from 1950 to June 2014. The search terms were: tumor lysis syndrome, tumor lysis syndrome and renal impairment, tumor lysis syndrome and cardiac arrhythmias, tumor lysis syndrome and cardiac toxicity, tumor lysis syndrome and central nervous disease, and tumor lysis syndrome and seizures, as well as combinations of these. The reference lists of the identified articles were further screened for potentially relevant articles that could have been overlooked by an electronic search. The search methodology was adapted from the scientific search guidelines published in 2011[5].
The basic understanding of the pathogenesis of TLS lies in the fact that cells and cancer cells in particular are rich in potassium, phosphorus, and uric acid. As mentioned previously, TLS can be either spontaneous when cancer cells die without the preceding chemotherapy, embolization, or radiation therapy, or secondary to cancer targeted treatment. In either case, the release of the above mentioned intracellular substances mediates the pathobiology of TLS and its complications.
Hyperkalemia is one of the key laboratory manifestations of TLS. Increased serum concentrations of potassium can adversely affect the skeletal muscle and cardiac myocardium[6,7]. Indeed, hyperkalemia can mediate severe skeletal muscle dysfunction and weakness and induce various electrocardiogram (ECG) abnormalities including peaked narrow T waves, prolongation of the PR interval, prolongation of the QRS interval, as well as sine wave morphology[8]. Ultimately, the cardiac effects of excess potassium can lead to ventricular tachyarrhythmias and death.
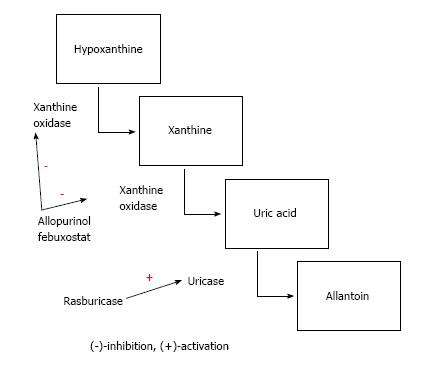
Uric acid is a byproduct of the purine nucleotides adenine and guanine, which constitute the backbone of nucleic acids[9]. Put simply, purines are metabolized initially to hypoxanthine and xanthine via enzyme xanthine oxidase to uric acid, which is a final byproduct in humans. However, some mammals have an additional enzyme called urate oxidase that converts uric acid to the much more water soluble allantoin, which is easily removed by renal system. Given a high cellular turnover in cancer for whatever reason, huge amounts of nucleic acids, purines, and eventually uric acid are released and formed. Uric acid can crystalize and obstruct the flow in the renal tubules, leading to acute kidney injury[2-4,10]. However, there are other mechanisms for uric acid mediated kidney impairment such as endothelial dysfunction and local ischemia, proinflammatory and proxidative states, and impairment of local renal repair mechanisms[10,11]. It is important to note that calcium phosphate crystals facilitate the deposition of uric acid in renal tissue[2].
It is relevant to mention that in the contemporary era most individuals at risk of TLS (at least in developed countries) or with a full-blown TLS are treated with hypouricemic agents, which minimize the impact of uric acid on the occurrence of acute kidney injury. An increase in serum phosphorus from cellular death can mediate acute kidney injury via similar mechanisms. When in excess, phosphorus tends to bind to calcium, forming the so-called calcium phosphorus product or calcium phosphate[2-4]. This product can be deposited in kidneys, mediating acute kidney injury, as well as in cardiac tissue, leading to arrhythmia. Furthermore, a secondary decrease in free calcium concentration (due to phosphorus binding) is manifested by indications of central nervous toxicity such as seizures and psychiatric complaints, prolongation of the QT interval on ECG, and muscle tetany[12]. It is interesting to observe that patients with spontaneous TLS may have lower rates of hyperphosphatemia due to phosphate uptake into rapidly dividing tumor cells[3,4]. An increase in lactate dehydrogenase (LDH) is typically seen in patients with TLS, probably because of anaerobic glucose metabolism. However, the elevation of LDH is not included in the laboratory definition of LDH and it is important to note that LDH is a very sensitive but quite nonspecific marker for TLS.
In conclusion, it is important to note that preexistent renal disease and the characteristics of certain patients increase the risk of full-blown clinical TLS. These factors will be discussed in more detail in the next section.
When assessing the risk of TLS in a particular patient, it is essential to bear in mind both the general and tumor-related predictors of risk.
An older age is associated with a reduction in the glomerular filtration rate[13]. It is likely that advanced age predisposes to TLS via a decrease in the renal reserve, and may complicate volume replacement therapy due to higher rates of cardiac dysfunction. However, it is important to keep in mind that the impact of age on the occurrence of TLS has not been specifically studied. Other general patient characteristics such as volume depletion should be assessed and corrected if present. Patients afflicted with cancer often have decreased oral intake due to the decrease in appetite and nausea. Furthermore, cancer patients often suffer from vomiting and diarrhea, which can significantly diminish their volume status. Another important aspect which we routinely assess in our patients is the use of medications capable of detrimentally affecting renal function such as non-steroidal anti-inflammatory drugs, inhibitors of the angiotensin converting enzyme, and angiotensin receptor blockers, especially in patients with decreased volume status[4]. The medication list of every patient should be reviewed and medications with a nephrotoxic renal profile should be discontinued wherever possible. It is important to consider that baseline kidney disease is a well-established risk factor for TLS[4,14]. In addition, a baseline increase in serum uric acid, phosphorus, potassium, and LDH also portends a greater risk of TLS[4]. Other general comorbid conditions such as cardiac disease, diabetes mellitus, and renal disease should be considered prior to hydration since patients with these medical problems might easily develop symptomatic volume overload.
Another aspect of the risk stratification which we use is the type and burden of malignancy. We agree with the clinical risk stratification proposed by Cairo et al[15] who stratified cancers into three risk groups: a high risk group, an intermediate risk group, and a low risk group. The high risk group of cancers include advanced Burkitt’s lymphoma/leukemia or early stage disease with elevated baseline LDH, acute lymphocytic leukemia (ALL) with white blood cell (WBC) count ≥ 100000 or less if the baseline elevation of LDH is twice the upper limit of normal (ULN), acute myeloid leukemia (AML) with WBC count ≥ 100000, diffuse large B-cell lymphoma with an elevated baseline LDH of twice ULN, and bulky disease. Intermediate risk malignancies include AML with a WBC between 25000 and 100000, ALL with WBC < 100000 and an LDH of less than twice ULN, early stage Burkitt lymphoma/leukemia with an LDH of less than twice ULN, and diffuse large B-cell lymphoma with a baseline increase in LDH of twice ULN but non-bulky disease. Low risk diseases include indolent lymphomas, chronic lymphocytic leukemia, chronic myeloid leukemia in the chronic phase, AML with WBC count < 25000 and an LDH elevated to less than twice ULN, multiple myeloma, and solid cancers. Therefore, during our risk stratification we paid extra attention to patients with Burkitt’s lymphoma/leukemia, ALL, AML, and diffuse large B cell lymphoma. Furthermore, we have recently reported that TLS in patients with solid malignancies may be higher than previously thought, and certain cancers with a sensitivity to therapy may be at higher risk for TLS, such as small cell lung cancer[4].
In summary, it is recommended that both general and cancer-related factors are included in the risk assessment of every patient. Certain patient factors such advanced age and the presence of preexistent renal and cardiac diseases warrant a closer follow up during preventive hydration.
The clinical presentation and symptomatology is directly linked to the biochemical derangements observed in this disorder. As discussed earlier, the biochemical evidence of TLS includes hypocalcemia, hyperkalemia, hyperphosphatemia, and hyperuricemia[2]. Therefore, the presentation of these biochemical disorders is typically represented by a clinical constellation of symptoms. For example, patients with TLS who have hypocalcemia may present with such symptoms as nausea, vomiting, muscular hyperactivation such as spasms and tetany, seizures, prolongation of QT interval on the ECG, cardiac dysrhythmias, and alterations of mental status[12]. Hyperphosphatemia may actually be a key mediator of acute kidney impairment as well as cardiac rhythm disturbances. Patients with hyperkalemia, if symptomatic, present with generalized fatigue, ECG abnormalities[8], and serious cardiac arrhythmias including cardiac arrest. Elevations of uric acid can lead to acute renal insult manifested as an increase in serum creatinine and decrease in urine output.
Therefore, it is essential to be highly suspicious if any of the above symptoms arise in patients with cancer, especially those with tumors in a high risk group In rare instances (at least in developed countries), TLS may present prior to the diagnosis of cancer. Nevertheless, a clinician should differentiate TLS from other causes of acute kidney injury such as sepsis, obstructive renal disease, medication toxicities (including those of chemotherapeutic agents), use of contrast dye for imaging studies, and rhabdomyolysis, as well as other rarer conditions such as vasculitis and primary glomerulopathies in appropriate clinical scenarios[16]. Thus, a thorough clinical history is of paramount importance when dealing with a cancer patient who has presented with an acute decline in kidney function. The minimum amount of testing should include urinalysis and urine microscopy, comprehensive metabolic panel, uric acid, LDH, complete blood count, and renal ultrasound. The Cairo-Bishop criteria for the diagnosis of laboratory and clinical TLS are presented in Tables 1 and 2, respectively.
In conclusion, the clinical presentation of TLS is based on the constellation of individual metabolic derangements in a particular patient.
It is essential to remember that the prevention of disease is always more cost-effective than the treatment of an established disease. Therefore, it is important to address and target any underlying kidney disease and possible hypovolemia before the start of cancer targeted therapies. Patients’ management should be focused on the basis of the type of cancer and certain biochemical parameters such as LDH, phosphorus, uric acid, and potassium and serum creatinine, as discussed above. Subjects at intermediate and high risk of TLS should be monitored in a hospital setting and possibly in an intensive care unit (especially individuals at high risk of TLS). Potassium and phosphorus should be eliminated from the diet and intravenous (IV) fluids.
Several features are the mainstay of treatment for the prevention of TLS in patients undergoing active therapy. First, all patients at intermediate and high risk should be actively hydrated with IV fluids. Coiffier et al[17] recommended patients should be hydrated to maintain urine output of at least 2 mL/kg per hour to minimize the risk of acute kidney injury. The choice of the fluid varies and some recommend the use of dextrose in one quarter normal saline as the initial fluid of choice[17]. However, normal saline or lactated Ringer’s solution are alternative choices, especially if the patient has other conditions such as dehydration, hypovolemia, and hyponatremia (it is essential to remember that lactated Ringer contains potassium and normal saline is associated with hyperchloremic metabolic acidosis)[17]. Also, it is prudent to limit the calcium and potassium content of the IV fluids in such patients. Nevertheless, it is essential to note that some patients with cancer have underlying cardiorenal disease, which puts them at high risk of fluid overload and pulmonary edema. Such patients should be followed in closely monitored settings and there should be a low threshold for initiating loop diuretics if signs of fluid overload appear (shortness of breath, crackles on physical examination, desaturation, etc.). Loop diuretics are preferably used in clinical practice because of their potent diuretic properties as well as their hypokalemic effect, which can be of benefit in patients at risk of TLS. However, to the best of our knowledge there are no published scientific studies assessing the role of diuretics in the treatment of TLS.
Second, individuals at intermediate risk of TLS should be started on allopurinol at least 24 to 48 h prior to chemotherapy or radiation therapy to reduce the risk of uric acid nephropathy[17]. Patients who do not tolerate oral medication such as those with severe nausea, vomiting, or altered function of the gastrointestinal tract can be given allopurinol IV. The recommended dose of allopurinol is up to 800 mg a day orally or 100 mg per square meter, and up to 600 mg a day for IV formulation[17]. Allopurinol works by blocking the xanthine oxidase enzyme. In rare instances, allopurinol can lead to hypersensitivity reactions manifested as skin rashes, liver transaminitis, and acute kidney injury in the form of acute interstitial nephritis[18]. Another important aspect of allopurinol use is the fact that the dose should be reduced in the event of chronic kidney disease[19]. In such patients (intermediate risk, underlying renal disease, and/or history of allopurinol intolerance) the use of febuxostat may be considered, which is a relatively new xanthine oxidase inhibitor. Febuxostat does not require dose modification in patients with renal disease and does not seem to have allergy cross-reactivity with allopurinol[20]. However, febuxostat has not been specifically studied for the population at risk of TLS or in patients with established TLS. Therefore, lack of specific data on febuxostat in patients with TLS should be mentioned during the management plan discussion with the patient and significant others, whenever appropriate.
Nevertheless, despite the availability of allopurinol, there is a significant number of patients who still develop significant kidney damage due to uric acid toxicity. As discussed above, some mammals (but not humans) possess urate oxidase or uricase enzyme, which is capable of converting xanthine into allantoin. This is an important step since allantoin is easily excreted substance. A medication mimicking urate oxidase named rasburicase was approved by Food and Drug Association in 2012 for use in subjects at risk of TLS[21]. Coiffier et al[22] enrolled 100 patients with aggressive non-Hodgkin lymphoma to investigate whether the use of rasburicase is a safe and effective method of preventing TLS in a high risk group. Indeed, this investigation showed that rasburicase led to the normalization of uric acid within four hours of its administration, and it was well tolerated. Rasburicase provides much better control of uric acid than allopurinol (87% compared to 66%, respectively) as demonstrated in a study by Cortes et al[23]. However, on the development of clinical TLS, no change between rasburicase and allopurinol was demonstrated[23]. Similarly, in a recent meta-analysis published by Lopez-Olivo et al[24], rasburicase was found to be effective in reducing uric acid levels, but it is unclear whether it led to better outcomes for clinical TLS.
Rasburicase should be used in individuals who are at high risk of developing TLS and in patients whose baseline uric acid is higher than 7.5 mg/dL (446 mmol/L)[17]. The dosage of rasburicase is based on the underlying risk of TLS. Thus, in patients at high risk the recommended dose is 0.2 mg/kg daily and in patients with intermediate risk and whose baseline uric acid level is ≤ 7.5 mg/dL the suggested dose of rasburicase is 0.15 mg/kg daily[17]. However, it is essential to mention that several small studies, most of which are retrospective in nature, have demonstrated that a single dose of rasburicase was effective[25-27]. The dose of the rasburicase administered varies, but 6 mg of rasburicase has been shown to be effective[27] and has provided uric acid control for 48 h after administration[26]. Given the high cost of rasburicase, this may decrease the cost of treatment. This approach should be reserved for subjects at intermediate risk of TLS and allopurinol should usually be started simultaneously with rasburicase, unless contraindicated. Purine metabolism and the sites of action of allopurinol, febuxostat, and rasburicase are presented in Figure 1.
Despite being a safe agent, rasburicase should not be used in pregnant or lactating patients due to limited data on safety (pregnancy category C drug) and excretion into breast milk. Furthermore, it should not be used on patients with glucose 6 phosphate dehydrogenase deficiency due to the high risk of hemolysis and methemoglobinemia[24,28].
Urine alkalization is another way of managing patients at risk of TLS. The rationale for this approach lies in the fact that an alkaline urine pH promotes uric acid solubility and its removal[29]. Typically, a carbonic anhydrase inhibitor acetazolamide or sodium bicarbonate are used to reach a urine pH of at least 6.5. However, this approach has not been shown to be superior to the administration of normal saline alone[29]. Furthermore, as mentioned above, in the current era of the widespread use of uric acid lowering agents for TLS prevention, the role of calcium phosphate toxicity increases as a mediator of kidney damage in patients with TLS. In contrast to uric acid crystal deposition in acidic pH, the crystals of calcium phosphate more readily precipitate in alkaline pH, making this approach to alkalization potentially dangerous[30]. Also, an alkaline pH promotes calcium binding to albumin, which can be very dangerous in patients with TLS who tend to have lower calcium levels at baseline, leading to neuromuscular and cardiac toxicity. Therefore, the current role of urine alkalization is of limited value and not recommended for routine use in patients at risk of TLS. It is also important to note that the use of phosphate binders in the prevention of TLS was not specifically studied in the literature. The decision to start phosphate binders should be decided on a case by case basis and always discussed with the patient prior to its initiation. The interested reader is referred to a recently published review on phosphate binders[31].
Certain parameters should be monitored in individuals at high risk for TLS such as uric acid, phosphorus, potassium, and LDH 4 h after the initiation of chemotherapy or radiation therapy. The discontinuation of prophylaxis should be considered after the completion of cancer-related treatment when serum markers (uric acid, potassium, phosphorus, calcium, LDH, and creatinine) are within normal limits for at least two consecutive measurements several hours apart. It is reasonable to monitor patients for at least 24 h after discontinuation of TLS prophylaxis to ensure no development of TLS.
The treatment of fully blown TLS is based on the same principles as its prevention. Patients with laboratory TLS and cardiorenal comorbid conditions, as well as patients with clinical TLS, should be admitted to an intensive care unit (ICU). Patients with TLS, unless anuric, should receive aggressive IV fluids with the goal of a urine output of at least 2 mL/kg per hour, as described above. Individuals deemed to be at increased risk of fluid overload, such as patients with cardiac and baseline renal disease, we consider the administration of IV loop diuretics such as furosemide to decrease the risk of pulmonary edema and augment urine output. Administration of loop diuretics may also improve control of hyperkalemia in patients with TLS. However, the role of loop diuretics is not based on solid data; thus, it should be approached on an individual basis.
As described above, the clinical spectrum of TLS includes laboratory abnormalities such as hyperkalemia, hyperphosphatemia, hyperuricemia, and hypocalcemia, which present clinically as cardiac arrhythmias, tetany, seizures, and acute kidney injury. We will briefly discuss the management of each laboratory abnormality one at a time. Hyperkalemia is a dangerous abnormality which may lead to muscle fatigue and cardiac toxicity and arrest[8,32]. The reader is referred to a detailed review on the management of hyperkalemia[32]. However, the management of hyperkalemia should always start from a 12-lead ECG. As discussed above, hyperkalemia may present with peaked narrow-based T waves, prolongation of the PR interval, loss of P waves, prolongation of the QRS interval, and the appearance of so called sine waves[8]. Certain therapies are available for the management of hyperkalemia such as IV calcium products, IV insulin +/- dextrose, inhalational beta 2 agonists, IV sodium bicarbonate, cation exchange resins, and hemodialysis[32]. Calcium in the form of gluconate or chloride should be administered IV (a typical dose is either 1 g of calcium gluconate and 500 mg to 1 g of calcium chloride) with certain ECG changes such loss of P waves and prolongation of the QRS interval[32]. It is important to note that calcium chloride contains more calcium than calcium gluconate and should preferably be administered via a central line. IV calcium works by blocking the potassium effect on the cardiac cell membrane. However, it is essential to mention that in subjects with TLS calcium should be administered cautiously and ideally only in patients with severe ECG changes, malignant cardiac dysrhythmias, and cardiac arrest, and also in patients with severe neurological dysfunction such as seizures due to the possibility of forming a calcium phosphate product, which may lead to acute kidney injury[33]. Furthermore, calcium products do not lower potassium levels and they must be used in conjunction with modalities which lower the serum concentration of potassium.
Albuterol, the most commonly used beta 2 agonist, which works by driving potassium into the cells, should be administered by a dose of 10 mg to 20 mg diluted in 4 mL of normal saline and nebulized during 10 min with a peak effect 90 min after administration[32]. Generally, albuterol should be combined with IV insulin, with or without dextrose. Usually, 10 units of regular insulin should be administered, and if the serum glucose is < 250 mg/dL 50 mL of 50% dextrose should be administered [32]. If the serum glucose is > 250, the administration of 50% dextrose is not necessary. IV insulin drives potassium into the cells in a similar way to albuterol and starts working 10 min after administration, peaks in 1 h, and lasts up to 6 h[32]. A drop of potassium should be expected of up to 1.5 mmol/L after administration of both insulin and albuterol combined[32]. The third option for reducing potassium is the administration of IV sodium bicarbonate in a dose of 50 mEq, which works by pushing potassium into the cells in exchange for hydrogen ions[32]. However, it is necessary to remember that IV sodium bicarbonate is a weak agent with the best possible effect observed in patients with hyperkalemia and metabolic acidosis[32]. Sodium bicarbonate should not be used as a sole agent in reducing elevated potassium. Furthermore, there are at least two factors that should be considered when using sodium bicarbonate in patients with TLS: first, alkalization may further decrease the free calcium concentration due to the greater binding of calcium to albumin, which might further decrease the physiologically active calcium; and second, urine alkalization might facilitate the deposition of calcium phosphate crystals in the kidney. Therefore, the general use of sodium bicarbonate in patients with hyperkalemia in the TLS setting is not recommended.
Another option for reducing potassium is the use of cation exchange resins such as sodium polystyrene sulfonate[32]. Sodium polystyrene sulfonate works in the intestinal tract by binding potassium and exchanging it with sodium[32]. The clinical effect of cation exchange resins typically starts within 2 h of administration and lasts up to 6 to 8 h. Sodium polystyrene sulfonate can be administered orally or as enema. The oral dose ranges from 15 to 45 g and can be repeated every 6 h as needed, while the enema is administered as 50 g of sodium polystyrene sulfonate mixed with water as a tap water enema. It is essential to note that sodium polystyrene sulfonate should not be used in patients with intestinal ileus or obstruction, and in post-operative patients due to higher risk of intestinal ischemia and necrosis[32]. Also, whenever possible, patients with TLS should receive aggressive IV hydration (as with patients without end-stage renal disease who produce urine), and if needed with loop diuretics to minimize the chances of fluid overload as this will also promote the normalization of serum potassium. However, patients with refractory hyperkalemia should be strongly considered for renal replacement therapy, typically hemodialysis. In emergent cases where there is no permanent dialysis access, a short term dialysis catheter should be inserted.
One aspect of the management of elevated phosphorus in patients with TLS includes the restriction in phosphorus intake, both in diet and IV fluids. It is necessary to mention that phosphate binders may be used in patients with hyperphosphatemia in the TLS setting. Phosphate binders include calcium containing medications such as calcium acetate and calcium carbonate, as well as non-calcium phosphate binders such as sevelamer and lanthanum[31]. Phosphate binders should be taken with each meal and work by reducing the intestinal absorption of phosphorus[31]. Calcium containing phosphate binders should theoretically be the first choice given the frequent presence of hypocalcemia in patients with TLS. However, there are no published scientific studies investigating the role of phosphate binders in the TLS setting. Hemodialysis or renal replacement therapy should be considered in patients with refractory hyperphosphatemia, in patients with symptomatic hypocalcemia, and with an elevated calcium phosphorus product of at least 70 mg2/dL2. The calcium phosphorus produced is calculated simply by multiplying the serum calcium and the phosphorus concentration. As discussed above, patients with TLS who have hypocalcemia should not be generally treated with calcium supplementation, given the higher risk of calcium phosphate crystallization and organ injury. However, calcium should be administered in the case of malignant cardiac arrhythmia (such as ventricular tachycardia or fibrillation), cardiac arrest, and seizure disorder. In the cardiac arrest setting, it is important to follow the advanced cardiac life support (ACLS) guidelines for its management and to exclude other possible causes of cardiac arrest such as hyperkalemia (common in TLS), hypokalemia, hypovolemia, acidosis (common in TLS, and which may be an indication for renal replacement therapy), hypothermia, tension pneumothorax, cardiac tamponade, thrombosis of the coronary and/or pulmonary circulation, as well as toxin exposure[34]. In the same way, the approach to seizure in the TLS setting should include exclusion of hypoglycemia (and corrected if present), other metabolic abnormalities (hypo- or hypernatremia, hypomagnesemia), brain vascular abnormalities (hemorrhagic and ischemic strokes, subarachnoid hemorrhage, etc.), brain tumors or metastatic disease, toxin exposure (such as amphetamines, cocaine, tricyclic antidepressants, etc.), alcohol withdrawal, benzodiazepine withdrawal, brain infection, and others[35].
Briefly, elevated levels of uric acid should be treated with rasburicase, unless contraindicated, in doses of at least 0.2 mg/kg once or twice a day. Allopurinol should only be considered if rasburicase is contraindicated or unavailable. Furthermore, it is essential to remember that allopurinol may actually increase the risk of acute kidney injury, given the increased production of xanthine, which is a poorly soluble bypass uric acid metabolite, as discussed above. In such patients early consideration of renal replacement therapy is advisable.
In conclusion, hemodialysis or other forms of renal replacement therapy should be considered in patients who are anuric, who have refractory hyperkalemia, symptomatic hypocalcemia, and with a calcium phosphorus product of at least 70.
TLS is an oncometabolic emergency resulting from rapid cell death. TLS can occur as a consequence of tumor targeted therapy (chemotherapy, embolization therapy, and radiation therapy) or spontaneously. Clinicians should stratify every hospitalized cancer patient, especially those receiving chemotherapy, for the risk of TLS. Some aspects of prevention include adequate hydration, use of uric acid lowering therapies, use of phosphate binders, and the minimization of potassium intake. Patients at high risk for the development of TLS should be monitored in the ICU.
Treatment of established TLS should be taken in the ICU and includes aggressive hydration, the possible use of loop diuretics (especially for the patients prone to fluid overload), use of phosphate binders, use of uric acid lowering agents (preferably rasburicase), and dialysis in refractory cases.
| 1. | Child Trends DATA BANK. Life expectancy. Available from: http: //www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_06.pdf. |
| 2. | Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 605] [Cited by in RCA: 686] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 3. | Ali AM, Barbaryan A, Zdunek T, Khan M, Voore P, Mirrakhimov AE. Spontaneous tumor lysis syndrome in a patient with cholangiocarcinoma. J Gastrointest Oncol. 2014;5:E46-E49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 4. | Mirrakhimov AE, Ali AM, Khan M, Barbaryan A. Tumor Lysis Syndrome in Solid Tumors: An up to Date Review of the Literature. Rare Tumors. 2014;6:5389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD. Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int. 2011;31:1409-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 519] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 6. | Espay AJ. Neurologic complications of electrolyte disturbances and acid-base balance. Handb Clin Neurol. 2014;119:365-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | McCullough PA, Beaver TM, Bennett-Guerrero E, Emmett M, Fonarow GC, Goyal A, Herzog CA, Kosiborod M, Palmer BF. Acute and chronic cardiovascular effects of hyperkalemia: new insights into prevention and clinical management. Rev Cardiovasc Med. 2014;15:11-23. [PubMed] |
| 8. | Wagner GS, Strauss DG. Marriott’s Practical Electrocardiography. 12th ed. New York, NY: LWW 2013; . |
| 9. | Álvarez-Lario B, Macarrón-Vicente J. Uric acid and evolution. Rheumatology (Oxford). 2010;49:2010-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 263] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 10. | Chaudhary K, Malhotra K, Sowers J, Aroor A. Uric Acid - key ingredient in the recipe for cardiorenal metabolic syndrome. Cardiorenal Med. 2013;3:208-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 11. | Han HJ, Lim MJ, Lee YJ, Lee JH, Yang IS, Taub M. Uric acid inhibits renal proximal tubule cell proliferation via at least two signaling pathways involving PKC, MAPK, cPLA2, and NF-kappaB. Am J Physiol Renal Physiol. 2007;292:F373-F381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Tohme JF, Bilezikian JP. Hypocalcemic emergencies. Endocrinol Metab Clin North Am. 1993;22:363-375. [PubMed] |
| 13. | Esposito C, Plati A, Mazzullo T, Fasoli G, De Mauri A, Grosjean F, Mangione F, Castoldi F, Serpieri N, Cornacchia F. Renal function and functional reserve in healthy elderly individuals. J Nephrol. 2007;20:617-625. [PubMed] |
| 14. | Montesinos P, Lorenzo I, Martín G, Sanz J, Pérez-Sirvent ML, Martínez D, Ortí G, Algarra L, Martínez J, Moscardó F. Tumor lysis syndrome in patients with acute myeloid leukemia: identification of risk factors and development of a predictive model. Haematologica. 2008;93:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Cairo MS, Coiffier B, Reiter A, Younes A. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149:578-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 356] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 16. | Lam AQ, Humphreys BD. Onco-nephrology: AKI in the cancer patient. Clin J Am Soc Nephrol. 2012;7:1692-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008;26:2767-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 459] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 18. | Keenan RT. Safety of urate-lowering therapies: managing the risks to gain the benefits. Rheum Dis Clin North Am. 2012;38:663-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Thurston MM, Phillips BB, Bourg CA. Safety and efficacy of allopurinol in chronic kidney disease. Ann Pharmacother. 2013;47:1507-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Uloric® Product Monograph. Febuxostat Tablets, 80 mg. Xanthine Oxidase Inhibitor. Oakville, Ontario, Canada: Takeda Canada Inc 2013; . |
| 21. | Department of health and human services, Food and Drug Administration. Rasburicase Product Approval Information - Licensing Action 7/12/02. Malvern, PA: Sanofi-Synthelabo, Inc 2012; . |
| 22. | Coiffier B, Mounier N, Bologna S, Fermé C, Tilly H, Sonet A, Christian B, Casasnovas O, Jourdan E, Belhadj K. Efficacy and safety of rasburicase (recombinant urate oxidase) for the prevention and treatment of hyperuricemia during induction chemotherapy of aggressive non-Hodgkin’s lymphoma: results of the GRAAL1 (Groupe d’Etude des Lymphomes de l’Adulte Trial on Rasburicase Activity in Adult Lymphoma) study. J Clin Oncol. 2003;21:4402-4406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 157] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Cortes J, Moore JO, Maziarz RT, Wetzler M, Craig M, Matous J, Luger S, Dey BR, Schiller GJ, Pham D. Control of plasma uric acid in adults at risk for tumor Lysis syndrome: efficacy and safety of rasburicase alone and rasburicase followed by allopurinol compared with allopurinol alone--results of a multicenter phase III study. J Clin Oncol. 2010;28:4207-4213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 24. | Lopez-Olivo MA, Pratt G, Palla SL, Salahudeen A. Rasburicase in tumor lysis syndrome of the adult: a systematic review and meta-analysis. Am J Kidney Dis. 2013;62:481-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Reeves DJ, Bestul DJ. Evaluation of a single fixed dose of rasburicase 7.5 mg for the treatment of hyperuricemia in adults with cancer. Pharmacotherapy. 2008;28:685-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Campara M, Shord SS, Haaf CM. Single-dose rasburicase for tumour lysis syndrome in adults: weight-based approach. J Clin Pharm Ther. 2009;34:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | McBride A, Lathon SC, Boehmer L, Augustin KM, Butler SK, Westervelt P. Comparative evaluation of single fixed dosing and weight-based dosing of rasburicase for tumor lysis syndrome. Pharmacotherapy. 2013;33:295-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Sonbol MB, Yadav H, Vaidya R, Rana V, Witzig TE. Methemoglobinemia and hemolysis in a patient with G6PD deficiency treated with rasburicase. Am J Hematol. 2013;88:152-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Conger JD, Falk SA. Intrarenal dynamics in the pathogenesis and prevention of acute urate nephropathy. J Clin Invest. 1977;59:786-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 88] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Worcester EM, Coe FL. Clinical practice. Calcium kidney stones. N Engl J Med. 2010;363:954-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 247] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 31. | Malberti F. Hyperphosphataemia: treatment options. Drugs. 2013;73:673-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Maxwell AP, Linden K, O’Donnell S, Hamilton PK, McVeigh GE. Management of hyperkalaemia. J R Coll Physicians Edinb. 2013;43:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364:1844-1854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 705] [Cited by in RCA: 625] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 34. | Neumar RW, Otto CW, Link MS, Kronick SL, Shuster M, Callaway CW, Kudenchuk PJ, Ornato JP, McNally B, Silvers SM. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S729-S767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 907] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 35. | Angus-Leppan H. First seizures in adults. BMJ. 2014;348:g2470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
P- Reviewer: Lin J, Muensterer OHJ S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/