Published online Feb 4, 2015. doi: 10.5492/wjccm.v4.i1.89
Peer-review started: August 31, 2014
First decision: October 14, 2014
Revised: October 21, 2014
Accepted: November 17, 2014
Article in press: November 19, 2014
Published online: February 4, 2015
Processing time: 167 Days and 20.5 Hours
AIM: To evaluate the currently available evidence on thoracic epidural anesthesia effects on splanchnic macro and microcirculation, in physiologic and pathologic conditions.
METHODS: A PubMed search was conducted using the MeSH database. Anesthesia, Epidural was always the first MeSH heading and was combined by boolean operator AND with the following headings: Circulation, Splanchnic; Intestines; Pancreas and Pancreatitis; Liver Function Tests. EMBASE, Cochrane library, ClinicalTrials.gov and clinicaltrialsregister.eu were also searched using the same terms.
RESULTS: Twenty-seven relevant studies and four ongoing trials were found. The data regarding the effects of epidural anesthesia on splanchnic perfusion are conflicting. The studies focusing on regional macro-hemodynamics in healthy animals and humans undergoing elective surgery, demonstrated no influence or worsening of regional perfusion in patients receiving thoracic epidural anesthesia (TEA). On the other hand most of the studies focusing on micro-hemodynamics, especially in pathologic low flow conditions, suggested that TEA could foster microcirculation.
CONCLUSION: The available studies in this field are heterogeneous and the results conflicting, thus it is difficult to draw decisive conclusions. However there is increasing evidence deriving from animal studies, that thoracic epidural blockade could have an important role in modifying tissue microperfusion and protecting microcirculatory weak units from ischemic damage, regardless of the effects on macro-hemodynamics.
Core tip: Effects of thoracic epidural anesthesia on splanchnic circulation are still poorly understood. The influence on macro-hemodynamics seems to vary based on the metameric extension of the blockade, the volume repletion and the hemodynamic status of the patient. Thus epidural anesthesia could reduce regional blood flow to splanchnic organs and have detrimental effects on oxygen delivery. However, there is increasing evidence, in particular deriving from animal studies, of a possible protective effect on microcirculation of the epidural blockade, especially in low flow states. In fact, despite reducing perfusion pressure, thoracic epidural anesthesia could foster perfusion of microcirculatory weak units and reduce local dysoxia.
- Citation: Siniscalchi A, Gamberini L, Laici C, Bardi T, Faenza S. Thoracic epidural anesthesia: Effects on splanchnic circulation and implications in Anesthesia and Intensive care. World J Crit Care Med 2015; 4(1): 89-104
- URL: https://www.wjgnet.com/2220-3141/full/v4/i1/89.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v4.i1.89
Thoracic epidural anesthesia is a widely used anesthetic technique providing excellent intra and postoperative analgesia. In recent years there have been efforts to further understand the effects of the sympathetic blockade this technique produces, in particular on the vascular perfusion. These effects may have a role in protecting the intestinal mucosa from injury, and promoting tissue healing, both in surgery and in pathologic scenarios such as acute pancreatitis. However, the mechanisms of organ protection and splanchnic hemodynamic effects of epidural anesthesia are not entirely clear yet, and the available evidence is conflicting.
This systematic review intends to evaluate the currently available evidence on the effects of thoracic epidural anesthesia on the splanchnic macro and microcirculation, in physiologic and pathologic conditions. Animal and human studies were taken into consideration.
A PubMed search was conducted using the MeSH database. “Anesthesia, Epidural” was always the first MeSH heading and was combined by boolean operator and with the following headings: Circulation, Splanchnic; Intestines; Liver Function Tests; Pancreas; Pancreatitis.
EMBASE and Cochrane library were also searched using the same terms.
Finally ClinicalTrials.gov and clinicaltrialsregister.eu was also searched using the term “epidural anesthesia”.
The abstracts were reviewed by three independent researchers and those not relevant to the search were excluded, only English language articles were taken into consideration. The quality of the studies was assessed using the Delphi List[1].
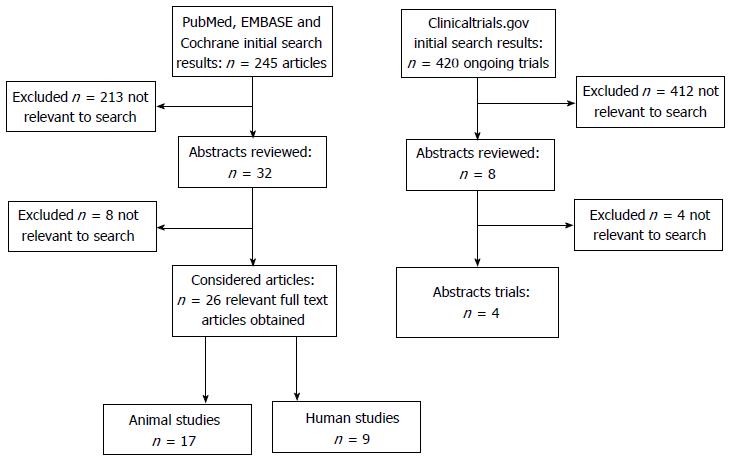
The search in Pubmed, EMBASE and Cochrane library produced a total of 245 results. Based on the review of the abstracts 219 were found not to be relevant and excluded. The full papers of the remaining 26 articles were independently reviewed by 3 researchers (Figure 1).
The Clinicaltrials.gov search produced a total of 420 studies, only 4 of these were relevant to our review. None of the trials found in clinicalregister.eu were relevant to the search terms (Figure 1).
The literature research found 26 studies related to splanchnic circulation, of these 17 were animal and 9 human studies.
Animal studies: Animal studies evaluating splanchnic regional macro-hemodynamics are synthesized in Table 1.
| Subjects | Ref. | Year | Title | Type of study | Scenario | No. subjects | Sensory blockade | Surrogate measure of splanchnic flow | Findings |
| Monkeys | Sivarajan et al[2] | 1976 | Systemic and regional blood flow during epidural anesthesia without epinephrine in the rhesus monkey | Prospective randomized | Anesthetized animals, epidural catheter placed L1-L2 | 9 (4 low epidural aneshtesia - level T10 vs 5 high epidural anesthesia - level T1) | higher level T10 or T1 | Radioactive microspheres and direct invasive monitoring of cardiac output | Low epidural - no difference in blood flow to major organs, while T1 epidural ↓ blood flow to liver, pancreas and gut (hepatic artery, portal vein) |
| Dogs | Meissner et al[4] | 1999 | Limited upper thoracic epidural block and splanchnic perfusion in dogs | Prospective observational | Induction of upper thoracic epidural in awake and anesthetized dogs and measurements of splanchinc perfusion | 13 (6 anesthetized, 7 no) | T1-T5 | Coloured microspheres injected in the aorta and then collected from tissue samples after autopsy | High TEA had no effect on sympathetic activity and splanchnic blood flow, nor in the awake nor anesthetized state. Propofol anaestehsia increased liver perfusion |
| Rabbits | Ai et al[6] | 2001 | Epidural anesthesia retards intestinal acidosis and reduces portal vein endotoxin concentrations during progressive hypoxia in rabbits | Prospective randomized | Progressive hypoxia in anesthetized animals | 18 (9 TEA/Lidocaine vs 9 TEA/NaCl 0.9%) | insertion point T12-L1 and 3-4 cm advancement | Portal blood flow, portal oxygen extraction ratio, portal pH, portal Lactate, intramucosal pH (pHi) of the ileum, portal endotoxin | pHi and pHart significantly higher and portal Endotoxin and Lactate significantly lower in TEA/Lido group. No diifferences in portal blood flow |
| Pigs | Vagts et al[5] | 2003 | The effects of thoracic epidural anesthesia on hepatic perfusion and oxygenation in healthy pigs during general anesthesia and surgical stress | Prospective randomized | Anesthetized and acutely instrumented pigs, assigned to 3 groups: control vs TEA plus basic fluid (BF) vs TEA plus VL | 19 (3 CTRL; 8 TEA alone; 8 TEA + VL) | T5 to T12 | Hepatic blood flow using ultrasonic transit-time perivascular flowprobes around the hepatic artery and portal vein; multiwire surface electrode placed onto the liver to measure tissue surface PO2; PDR-icg | Despite a decrease in MAP, TEA had no effect on total hepatic blood flow, liver DO2 and VO2. Liver tissue PO2 did not decrease. Lactate uptake and PDR-icg remained unchanged. Volume loading did not show any benefit with regard to hepatic perfusion, oxygenation, and function |
| Rats | Shäper et al[3] | 2010 | TEA attenuates endotoxin induced impairment of gastro intestinal organ perfusion | Prospective randomized | Sepsis model through infusion of LPS, evaluation of regional flow at 30', 60', 120' | 18 (9 TEA vs 9 sham) | T4-T11 (methilen blue spread) | Fluorecent microspheres withdrawal technique, then evaluation of microspheres in brain, heart, ileopsoas muscle, liver pancreas gut segments; determination plasma cathecolamines | TEA ↑ blood flow to GIT organs under LPS effect |
| Studies evaluating liver micro hemodynamics | |||||||||
| Rats | Freise et al[17] | 2009 | Hepatic effects of TEA in experimental severe acute pancreatitis | Prospective randomized blinded image analysis | Animal model of acute pancreatitis induced by taurocholate injection or sham lesion | 28 (7 sham + sham, 7 sham + TEA, 7 pancreat + sham, 7 pancreat + TEA) an additional 22 animals were assigned to the three group to asses hepatic apoptosis | catheter tip placed T6 | Intravital microscopy of liver left lobe, cell adehesion to sinusoid wall (rollers and stickers), apoptosis of cells by Fas-L pathway | TEA ↑ diameter of sinusoids in pancreatitis, TEA ↓ the number of parenchymal apoptotic cells in pancreatitis (Fas-L pathway), TEA does not have much influence in sham groups |
| Rats | Freise et al[18] | 2009 | TEA reduces sepsis related hepatic hyperperfusion and reduces leucocyte adehesion in septic rats | Prospective randomized blinded image analysis | Sepsis model induced with cecal ligation and perforation | 24 (8 sham + sham, 8 sepsis + sham, 8 sepsis + TEA); another 21 animals were assessed for liver failure and hemodynamics | catheter tip placed T6 | Intravital microscopy of liver left lobe, cell adehesion to sinusoid and venules, serum transaminase activity, TNFα activity | TEA ↓ sinusoid dilation in sepsis by probably restoring hepatic arterial buffer response. TEA ↓ temporary adhesion to sinusoid wall but did not affect permanent adhesion. TEA did not affect transaminase or TNF activity. No differences in hemodynamics |
Three studies[2-4] used centrally injected radioactive or colored microspheres to determine cardiac output and regional blood flow. The regional flow could be estimated by measuring organ or regional arterial blood samples radioactivity, or by microscopy after autopsy.
Sivarajan et al[2] evaluated the effects of low (T10) and high (T1) epidural anesthesia on systemic hemodynamics and regional blood flow in anesthetized monkeys. The main findings of this study were that both CO and arterial blood pressure significantly decreased in both groups and more significantly in the high epidural T1 group. Low level epidural blockade did not significantly change absolute blood flow in splanchnic organs, while high blockade produced a significant reduction in hepatic blood flow.
Schäper et al[3] studied the influence of a continuous epidural lidocaine infusion in animal models of endotoxemia, induced by continuous i.v. infusion of Escherichia Coli lipopolysaccharide (LPS). The result showed that blood flow to the gastrointestinal organs (stomach and ileum) was significantly higher in the epidural group despite a lower Mean arterial pressure (MAP). Hepatic blood flow initially decreased after the onset of the epidural blockade, but was comparable to the one in control groups in the course of LPS infusion. Finally the decrease in pH and base excess induced by endotoxemia was partially blunted by epidural blockade.
Meissner et al[4] evaluated the effects of a thoracic epidural block on splanchnic blood flow in either awake or anesthetized dogs. No difference was found between the two groups, only an increase in liver perfusion when propofol was used as anesthetic agent.
Vagts et al[5] evaluated the effects of volume loading on hepatic perfusion in animals undergoing surgery with blended anesthesia. The hepatic flow measurement was obtained using perivascular flow-probes around the hepatic artery and portal vein; liver tissue PO2, plasma disappearance rate of indocyanine green (PDRICG), total hepatic DO2 and VO2 were also recorded. The main finding of this study was that the reduction in MAP induced by thoracic epidural blockade, was not associated with a decrease in total hepatic blood flow, DO2 and parenchymal PO2. Volume loading could not modify macrohemodynamic parameters but significantly reduced portal venous oxygen content.
Ai et al[6] considered an animal model of systemic hypoxemia, finding that epidural blockade produced higher intramucosal and arterial pH, lower portal endotoxin and lower arterial lactate levels when compared to a control group. Portal blood flow remained stable during progressive hypoxia and was unaffected by epidural blockade.
Animal studies evaluating intestinal and pancreatic microhemodynamics are synthesized in Table 2.
| Subjects | Ref. | Year | Title | Type of study | Scenario | No. subjects | Sensory blockade | Surrogate measure of splanchnic flow | Findings |
| Rabbits | Hogan et al[7] | 1993 | Effects of epidural and systemic lidocaine on sympathetic activity and mesenteric circulation in rabbits | Prospective randomized | Anesthetized animals receiving thoraco-lumbar epidural block with different anesthetic concentrations | 32 (7 lidocaine 6 mg/kg im vs 5 lidocaine 15 mg/kg im vs 5 TEA lido 0.5% vs 8 TEA lido 1.0% vs 7 TEA lido 1.5%) | T2-L5 | Mesenteric vein diameter, sympathetic efferent nerve activity (SENA) of post ganglionic splanchnic nerve | TEA ↑ splanchnic venous capacitance and ↓ SENA |
| Rabbits | Hogan et al[8] | 1995 | Region of epidural blockade determines sympathetic and mesenteric capacitance effects in rabbits | Prospective randomized | Anesthetized and non anesthetized animals receiving either a thoracic or lumbar block with special epidural catheters limiting anesthetic spread | 26 (6 lidocaine 1% TEA vs 6 lido 1% LEA, vs 8 thoracolumbar anesthesia in spontaneous ventilation with lido 1% vs 6 thoracolumbar anesthesia with lido 1% in fully awake animals) | T11-L7 (LEA group), T4-L1 (TEA group), T1-L4 (thoracolumbar anesthesia) | Mesenteric vein diameter, sympathetic efferent nerve activity (SENA) of post ganglionic splanchnic nerve | ↑ SENA and ↓ mesenteric vein diameter after lumbar epidural anesthesia while ↓ SENA and ↑ mesenteric vein diameter after thoracic epidural anesthesia |
| Rats | Sielenkämper et al[10] | 2000 | Thoracic epidural anesthesia increases mucosal perfusion in ileum of rats | Prospective randomized | Anesthetized and mechanically ventilated rats that underwent laparotomy to obtain access to the ileum | 19: 11 bupivacaine 0.4% (TEA); 8 normal saline (CTRL) | Catheter tip placed T7-T9 | Intravital microscopy on the ileum mucosa | TEA ↑ gut mucosal blood flow and ↓ the extent of intermittent flow in the villus microcirculation |
| Rats | Adolphs et al[12] | 2003 | Thoracic epidural anesthesia attenuates hemorrhage-induced impairment of intestinal perfusion in rats | Prospective randomized | Hemorragic shock model (PAM 30 mmHg for 60 min) induced by withdrawal of blood and subsequent retransfusion for resuscitation | 32 (4 groups of 8); epidural lidocaine 2% (TEA) or normal saline (CTRL), muscolaris or mucosa evaluated | catheter tip placed T11-T12 | Intravital microscopy with fluorescein (FCD = functional capillary density and erythrocyte velocity in the mucosa and muscularis of distal ileum) | TEA ↑ intestinal microvascular perfusion and ↓ hypotension-induced impairment of capillary perfusion in the muscularis, ↓ systemic acidemia during hypotension and ↓ leukocyte rolling after resuscitation |
| Rats | Adolphs et al[11] | 2004 | Effects of thoracic epidural anaesthesia on intestinal microvascular perfusion in a rodent model of normotensive endotoxaemia | Prospective randomized | Normotensive endotoxaemia model through LPS infusion in anesthetized animals | 32 (8 no TEA vs 24 TEA) +/- E.coli LPS infusion +/- epidural lidocaine 2% or saline infusion, muscolaris or mucosa evaluated | catheter tip placed T11-T12 | Intravital microscopy with fluorescein (densities of perfused and non-perfused capillaries and erythrocyte velocity in both the mucosa and the muscularis of the terminal ileum) | TEA ↓ MAP and HR, ↑ muscularis and ↓ mucosal microvascular perfusion |
| Dogs | Schwarte et al[15] | 2004 | Effects of thoracic epidural anaesthesia on microvascular gastric mucosal oxygenation in physiological and compromised circulatory conditions in dogs | Prospective randomized | Chronically instrumented and anaesthetized dogs. Animals were studied under physiological and compromised circulatory conditions (PEEP 10 cm H(2)O), both with and without fluid resuscitation | 12 (6 lidocaine vs 6 saline) | catheter tip placed T10, thoracolumbar - paresis of the ocular nictitating membrane, sensory block up to the neck region, and motor block of the limbs | Gastric mucosal oxygenation by measuring microvascular haemoglobin oxygen saturation (µHbO2) using reflectance spectrophotometry | Under physiological conditions, TEA preserved gastricmucosal oxygenation but aggravated its reduction during impaired circulatory conditions, thereby preserving the correlation between gastric mucosal and systemic oxygenation. Fluid resuscitation completely restored these variables |
| Rabbits | Kosugi et al[9] | 2005 | Epidural analgesia prevents endotoxin-induced gut mucosal injury in rabbits | Prospective randomized | Normotensive endotoxaemia model through LPS infusion in anesthetized animals | PROTOCOL 1: 28 = 14 saline (C = CONTROL) vs 14 lidocaine (E = EPIDURAL); PROTOCOL 2: 20, into groups C or E (10 each group) | catheter placed via T11-T12 interspace | PROTOCOL 1: Measurements of systemic and splanchnic variables using catheter inserted through the mesenteric vein and perivascular probe attached around the portal vein. Intramucosal pH using tonometer catheter surgically inserted into the terminal ileum. Mucosal edema and microstructure of the terminal ileum using tissue sampling to determine wet-to-dry weight ratio and histological analysis (histopathological injury scores of gut mucosa). PROTOCOL 2: gut permeability using fluorescence spectrometry | The application of epidural analgesia in endotoxemic hosts attenuates the progression of intramucosal acidosis, the increase of intestinal permeability, and the structural alterations of intestinal villi, possibly throught the restoration of microcirculation, despite a significant decrease of perfusion pressure and arterial oxygen content |
| Rats | Freise et al[13] | 2006 | Thoracic epidural analgesia augments ileal mucosal capillary perfusion and improves survival in severe acute pancreatitis in rats | Prospective randomized | Animal model of acute pancreatitis (AP) induced by taurocholate injection or sham lesion | 28 (4 groups of 7): sham + saline TEA (Sham) vs AP + saline TEA (PANC) vs AP + TEA (EPI) vs AP + delayed TEA (delayed EPI). Outcome protocol: (n = 30): 15 AP vs 15 TEA | catheter tip placed T6 | Intravital microscopy of the ileal mucosa | TEA ↓ intercapillary area (↑ local perfusion) ↓ IL-6 and serum lactate and ↓ 66% mortality |
| Rats | Daudel et al[14] | 2007 | Continuous thoracic epidural anesthesia improves gut mucosal microcirculation in rats with sepsis | Prospective randomized, blinded image analysis | Sepsis model induced with cecal ligation and perforation (CLP) | 27 (10 CLP/TEA vs 9 CLP/Control vs 8 sham laparotomy) | catheter tip placed T6 | Intravital videomicroscopy performed on villi of ileum mucosa | Smaller intercapillary area hence ↑ villus perfusion in CLP/TEA vs CLP/Control. Diameter of terminal arterioles and red blood cell velocity didn't differ |
| Pigs | Bachmann et al[16] | 2013 | Effects of thoracic epidural anesthesia on survival and microcirculation in severe acute pancreatitis: a randomized experimental trial | Prospective randomized | Animal model of SAP induced by intraductal injection of glycodesoxycholic acid in the main pancreatic duct followed by closure | 34: 17 bupivacaine via TEA after induction of SAP (TEA) vs 17 no TEA (control) | catheter introduced T7-T8 and advanced 2 cm (documented by epidurogram) | Continuous measurement of the tissue oxygen tension (tpO2) using a flexible polarographic measuring probe placed in the pancreatic head and pancreatic microcirculation using Laser-Doppler imager during a period of 6 h after induction SAP. Histopathologic tissue damage (histopathologic severity score of acute pancreatis) by postmortem examination of the animals sacrificed after 7 d of observation | TEA improved survival as well as pancreatic microcirculation and tissue oxygenation resulting in reduced histopathologic tissue-damage |
Hogan et al[7,8], in two studies on rabbits, measured sympathetic efferent nerve activity, through surgically implanted electrodes in a postganglionic splanchnic nerve, and in vivo mesenteric vein diameter, to estimate venous capacitance.
The first protocol[7] compared the effects of a thoraco-lumbar epidural block using different lidocaine concentrations (T2-L5), with injection of i.m. lidocaine. The results showed a reduction in sympathetic efferent nerve activity, and an increase in mesenteric vein diameter in animals treated with epidural lidocaine.
The second study[8] investigated the different effects of a Thoracic (T4-L1) Thoracolumbar (T1-L4) and Lumbar (T11-L7) epidural block. The results showed that Thoracic and Thoracolumbar blocks reduced sympathetic tone and increased mesenteric vein diameter, whilst a lumbar block produced opposite effects.
Kosugi et al[9] investigated the effect of epidural analgesia on intestinal macro and micro-hemodynamics and the alterations in gut barrier function elicited by continuous endotoxin infusion in rabbits. The histopathological evaluation of the intestinal mucosa samples, showed that epidural anesthesia reduced injury. Moreover higher intramucosal pH, and reduced mucosal permeability, were recorded in animals treated with thoracic epidural blockade, despite a decrease in perfusion pressure and arterial oxygen content.
Intravital microscopy of the ileus was used as an indirect measure of splanchnic flow in 5 studies[10-14].
Sielenkämper et al[10] found, in anesthetized rats treated with epidural bupivacaine, an increase in arteriolar red blood cell velocity, expressing an increase in gut mucosal blood flow despite a lower MAP. Also, intercapillary area calculated for continuously perfused capillaries, was reduced in the TEA group, indicating a decrease in intermittent blood flow in the villus microcirculation.
Adolphs et al[11,12] investigated the effect of thoracic epidural anesthesia in hemorrhagic hypotension and normotensive endotoxemia in rats.
In the hemorrhagic hypotension model[12], the pH and base excess, and muscularis layer capillary perfusion, were significantly improved in the group receiving epidural anesthesia. Moreover, leukocyte rolling after resuscitation was attenuated in thoracic epidural anesthesia (TEA) group, indicating a reduction in postischemic tissue injury.
In the normotensive endotoxemia model[11], despite a lower MAP and an overall decrease in mucosal functional capillary density, epidural infusion of lidocaine reduced the non-perfused capillaries in the muscularis layer after 120 min of continuous LPS infusion. Moreover, erythrocyte velocity decreased in the mucosa and muscularis layer during endotoxemia, but was not influenced by epidural blockade.
Freise et al[13] in a rodent model of acute pancreatitis, found a decrease in ileal mucosa intercapillary area, IL-6 and lactate levels, in animals treated with both immediate or delayed epidural injection of local anesthetic. These results indicate that a thoracic epidural block improved local perfusion. This group had also lower scores of pancreatic injury and a 66% decrease in mortality.
Daudel et al[14] induced sepsis by cecal ligation and perforation in rats, finding a significant decrease in intercapillary area in the group treated with TEA, without differences in terminal arterioles diameter and red blood cells velocity.
Schwarte et al[15] evaluated the effects on gastric mucosal oxygenation of epidural anesthesia and volume loading, in either normal or compromised circulation, in dogs. Hemodynamic failure was induced with high PEEP levels.
In healthy animals, TEA induced a reduction in MAP and DO2, preserving gastric mucosal oxygenation. In compromised circulatory conditions, TEA aggravated the reduction of gastric mucosal oxygenation; volume loading restored both DO2 and mucosal oxygenation. TEA maintained a constant relationship between gastric mucosal and systemic oxygenation.
Bachmann et al[16], evaluated a porcine model of acute pancreatitis finding that TEA enhanced pancreatic microcirculation and oxygenation, and reduced histopathologic scores of tissue damage. Also, 7 d mortality was lower in animals treated with TEA.
Animal studies evaluating hepatic microhemodynamics are synthesized in Table 1.
Two studies by Freise et al[17,18] used intravital microscopy to assess hepatic microcirculation in different pathologic animal models.
In acute pancreatitis in rats[17], TEA prevented the vasoconstriction of sinusoids, but could not reduce the number of non perfused sinusoids. The number of parenchymal apoptotic cells was reduced by TEA, probably by inhibition of the Fas ligand pathway, without effects on leukocyte adhesion. In healthy animals, TEA did not exert any effect on the evaluated microcirculation parameters.
In a sepsis model induced by cecal ligation and perforation[18], TEA was able to normalize the increase in blood flow to the liver and to decrease temporary leucocyte adhesion to the venular endothelium, but not the vasoconstriction of hepatic vasculature and temporary sinusoidal leukocyte adhesion, both induced by sepsis.
Human studies: Human studies are synthesized in Table 3.
| Ref. | Year | Title | Type of study | Scenario | No. subjects | Sensory blockade | Surrogate measure of splanchnic flow | Findings |
| Lundberg et al[19] | 1990 | Intestinal hemodynamics during laparotomy: effects of thoracic epidural anesthesia and dopamine in humans | Prospective observational | Patients undergoing abdominal aorto-bifemoral reconstruction | 9 | Catheter inserted T7-T8 or T8-T9 and advanced 2-3 cm | Superior mesenteric artery blood flow (SMABF) via electromagnetic flow probe, mesenteric arteriovenous oxygen difference mesenteric venous lactate | ↓ SMABF and ↓ MAP only restored by dopamine infusion |
| Tanaka et al[23] | 1997 | The effect of dopamine on hepatic blood flow in patients undergoing epidural anesthesia | Prospective controlled | Patients ASA 1-2 undergoing elective gynecological surgery. Normotension maintained either with HES infusion or HES + dopamine | 28 (7 no TEA vs 14 TEA + HES vs 7 TEA + HES + dopamine) | Upper T5 | Hepatic blood flow using Plasma Disappearance Rate of indocyanine green (PDR-icg) | ↓ PDR-icg in TEA + HES group, = PDR-icg in TEA + HES + dopamine group |
| Väisänen et al[25] | 1998 | Epidural analgesia with bupivacaine does not improve splanchnic tissue perfusion after aortic reconstruction surgery | Prospective randomized controlled | Patients undergoing elective aortic reconstruction surgery | 20 (10 TEA vs 10 controls) | Catheter inserted T12-L1 and advanced 5 cm | Gastric and sigmoid mucosal PCO2, pHi. Splanchnic blood flow direct invasive measure by cannulation of hepatic vein and dye diluition method (indocyanine green) | No differences |
| Spackman et al[26] | 2000 | Effect of epidural blockade on indicators of splanchnic perfusion and gut function in critically ill patients with peritonitis: a randomised comparison of epidural bupivacaine with systemic morphine | Double-blinded, prospective, randomised, controlled | Critically ill patients admitted in ICU with peritonitis (and systemic sepsis) and adynamic small bowel following abdominal surgery | 21 (10 intravenous morphine vs 11 epidural bupivacaine) | Low thoracic or high lumbar epidural catheter insertion | Gastric tonometry: gastric intramucosal pH (pHig) and the intramucosal-arterial PCO2 gradient (Pg-PaCO2) | Significant improvements in gastric mucosal perfusion (a rise in Pg-PaCO2 and a fall in pHig in the morphine group and a significant difference between groups in the Pg-PaCO2 trends) and in the ultrasound appearance of the small bowel in the epidural group |
| Gould et al[20] | 2002 | Effect of thoracic epidural anaesthesia on colonic blood flow | Prospective observational | Patients undergoing elective anterior resection for rectal cancer | 15 | Cahteter inserted T9-T10 | Doppler flowmetry for inferior mesenteric artery flow and Laser Doppler flowmetry for serosal red cell flux | ↓ inferior mesenteric artery flow and ↓ serosal red cell flux significantly correlated to ↓ MAP reverted only by vasoconstrictors usage |
| Michelet et al[22] | 2007 | Effect of thoracic epidural analgesia on gastric blood flow after oesophagectomy | Prospective controlled | Patients undergoing elective radical oesophagectomy, postoperative evaluation | 27 (18 TEA vs 9 controls) | C8-T11 | Gastric mucosal blood flow (GMBF) measured using laser Doppler flowmetry at 1 and 18 h post surgery | ↑GMBF in TEA group without correlation with MAP or CI |
| Kortgen et al[27] | 2009 | Thoracic but not lumbar epidural anaesthesia increases liver blood flow after major abdominal surgery | Prospective | Patients undergoing major abdominal surgery | 34 (17 TEA vs 17 LEA) | Thoracic catheters between T5-T6 and T9-T10, lumbar catheters between L1-L2 and L4-L5 | Blood lactate levels, central venous oxygen saturation (ScvO2), PDR-icg | TEA but not LEA ↑ PDR-icg |
| Meierhenrich et al[21] | 2009 | The effects of thoracic epidural anesthesia on hepatic blood flow in patients under general anesthesia | Prospective controlled | Patients undergoing major pancreatic surgery | 30 (15 TEA vs 5 TEA + Norepinephrine vs 10 no TEA) | T4-T11 | Hepatic blood flow index and hepatic stroke volume index in the right and middle hepatic vein by use of multiplane TEE | ↓ Hepatic venous blood flow. The combination of thoracic TEA with continuous infusion of NE seems to induce a further decrease in hepatic blood flow. CO was not affected by TEA |
| Trepenaitis et al[24] | 2010 | The influence of thoracic epidural anesthesia on liver hemodynamics in patients under general anesthesia | Prospective randomized | Patients undergoing upper abdominal surgery for carcinoma of the stomach, papilla of Vater, and pancreas | 50 (40 TEA vs 10 controls) | T5-T12 | Hepatic blood flow using Plasma Disappearance Rate of indocyanine green (PDR-icg) | ↓ PDR-icg in TEA group, even if ephedrine was administered to correct hypotension. ↑ PDR-icg in patients receiving general anetshesia. CO was unaffected |
Of the 9 human studies taken into consideration, 4 evaluated splanchnic hemodynamics by direct measures of blood flow, 5 measured derived parameters such as gastric tonometry, intramucosal pH or PDRICG.
Three studies which used direct hemodynamic measures found a reduction in blood flow caused by TEA, even though different measuring techniques and different vessels were considered. One study showed that TEA improved microcirculatory parameters whilst worsening macro-hemodynamics.
Lundberg et al[19] measured superior mesenteric artery blood flow via an electromagnetic flow probe. In this study TEA reduced vascular resistance and blood flow in the superior mesenteric artery, with no change in measured CO. These hemodynamic changes were successfully corrected by dopamine infusion.
Gould et al[20] used doppler flowmetry to measure inferior mesenteric artery blood flow and laser doppler flowmetry to evaluate red cells flux. Results showed that TEA produced a reduction of blood flow and red cells flux in the inferior mesenteric artery, which was strictly correlated with the fall in MAP. The hemodynamic changes registered could be corrected by vasopressors infusion but not by goal directed fluid therapy.
Meierhenrich et al[21] used transesophageal echography to estimate blood flow in the hepatic veins, finding a reduction in estimated liver blood flow after the induction of a thoracic epidural blockade. The reduction in blood flow was resistant to the correction of hypotension using vasopressors.
Michelet et al[22] used doppler flowmetry to evaluate gastric mucosal blood flow in patients undergoing oesophagectomy. In this scenario TEA improved the microcirculatory perfusion of the gastric tube at 1 and 18 h after surgery, a result which did not correlate with the measured macro-hemodynamic parameters.
The six studies using surrogate hemodynamic parameters had conflicting results. Tanaka et al[23] used PDR-icg as indirect measure of hepatic blood flow, finding that TEA reduced blood flow to the liver, fluid resuscitation alone could not reverse this effect but had to be associated with dopamine infusion. Trepenaitis et al[24] found a reduction of PDRICG in patients undergoing upper abdominal surgery with TEA. This data was not associated with a fall in CO and could not be reversed by administration of vasopressors.
Väisänen et al[25] found no influence of TEA on gastric and sigmoidal PCO2 gap in patients undergoing elective abdominal aortic surgery.
Spackman et al[26] evaluated the effects of TEA on a group of critically ill patients with surgically treated peritonitis, they found better pHi and PCO2 gap in patients treated with epidural infusion of bupivacaine.
Kortgen et al[27] found an increase in liver blood flow measured with PDRICG in patients undergoing major abdominal surgery and treated with thoracic epidural anesthesia in addition to general anesthesia. This finding couldn’t be replicated by using lumbar epidural anesthesia and general anesthesia as anesthetic technique.
The quality of animal and human studies assessed through Delphi List is synthesized in Table 4, Table 5 and Table 6.
| Hogan et al[7] | Hogan et al [8] | Sielenkämper et al [10] | Adolphs et al [12] | Adolphs et al [11] | Schwarte et al [15] | Kosugi et al [9] | Freiseet al[13] | Daudelet al[14] | Bachmann et al [16] | |
| Treatment allocation | ||||||||||
| (1) Was a method of randomization performed? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the treatment allocation concealed? | No | No | Yes | Yes | Yes | Yes | No | Yes | No | No |
| Were the groups similar at baseline regarding the most important progNostic indicators? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the eligibility criteria specified? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Was the outcome assessor blinded? | No | No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Was the care providor blinded? | No | No | No | No | No | No | No | No | No | No |
| Was the patient blinded? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Were point estimates and measures of variability presented for the primary outcome measures? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Did the analysis include an intention-to- treat analysis? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Lundberg et al[19] | Tanaka et al [23] | Väisänen et al [25] | Spackman et al [26] | Gouldet al[20] | Micheletet al[22] | Kortgen et al [27] | Meierhenrich et al [21] | Trepenaitis et al [24] | |
| Treatment allocation | |||||||||
| Was a method of randomization performed? | No | No | No | Yes | N/A | No | No | No | No |
| Was the treatment allocation concealed? | No | No | No | Yes | N/A | No | No | No | No |
| Were the groups similar at baseline regarding the most important progNostic indicators? | N/A | Yes | Don't know | Yes | N/A | Yes | No | Yes | Yes |
| Were the eligibility criteria specified? | No | Yes | No | Yes | Yes | Yes | No | Yes | Yes |
| Was the outcome assessor blinded? | No | No | No | Yes | N/A | Don't know | Don't know | Yes | No |
| Was the care providor blinded? | No | No | No | No | N/A | No | No | No | No |
| Was the patient blinded? | No | Don't know | No | Yes | N/A | No | No | No | No |
| Were point estimates and measures of vari ability presented for the primary outcome measures? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Did the analysis include an intention-to-treat analysis? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Sivarajanet al[2] | Meissner et al [4] | Ai et al [6] | Vagtset al[5] | Shäperet al[3] | Freiseet al[17] | Freiseet al[18] | |
| Treatment allocation | |||||||
| Was a method of randomization performed? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the treatment allocation concealed? | No | No | No | Yes | No | Yes | Yes |
| Were the groups similar at baseline regarding the most important progNostic indicators? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the eligibility criteria specified? | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Was the outcome assessor blinded? | No | No | don't kNow | No | Yes | Yes | Yes |
| Was the care providor blinded? | No | No | No | No | No | Yes | Yes |
| Was the patient blinded? | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Were point estimates and measures of variability presented for the primary outcome measures? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Did the analysis include an intention-to-treat analysis? | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
The Animal studies considered in this review, in particular the most recent ones, are well designed; groups were homogeneous, methods were minutely described, and outcome assessors were frequently blinded. However, the high variability of surrogate measures used to estimate splanchnic blood flow in each study, is the biggest limit to the common interpretation of their results.
The quality of human studies was in general low. Most of the studies considered did not use randomization criteria, and the control groups were often composed by patients not eligible for epidural anesthesia, or who refused it. Patients were undergoing different surgical procedures, so that selection bias could not be excluded. Moreover, the outcome assessor was frequently not blinded. The outlined considerations limit the reliability of these studies, and underline the urgent need for well designed RCTs.
Clinical trials search found 4 works of interest, which are synthesized in Table 7.
| Title | Start year | Scenario | No. subjects | Current primary outcome measures | Current secondary outcome measures | Findings |
| Effect of Epidural Anesthesia on Pancreatic Perfusion and Clinical Outcome in Patients With Severe Acute Pancreatitis | July 2005 | Acute pancreatitis with Ranson Criteria over 2, and/or CRP over 100, and or pancreatic necrosis on CT scan | 35 (epidural anesthesia with carbostesin and fentanyl vs PCA with fentanyl) | Number of patients with adverse events related to epidural anesthesia, pancreatic perfusion measured by computerized tomography | Clinical outcome, Lenght of stay, admission to intensive care unit, need for surgery | n/d |
| Epidural Analgesia for Pancreatitis (Epipan Study) | April 2014 | Patients admitted to the ICU for acute pancreatitis | 148 (PCEA with Ropivacaine and sufentanyl vs conventional analgesia - acetaminophen, nefopam, tramadol, opidoids) | Ventilator-free days | Duration of invasive and/or non invasive mechanical ventilation, incidence of various complications, biological inflammatory response, cost analysis, incidence of intolerance to enteral feeding, effectiveness of pain management, duration of EA | n/d |
| Study of Effectiveness of Thoracic Epidural Analgesia for the Prevention of Acute Pancreatitis After ERCP Procedures | January 2008 | Patients undergoing therapeutic ERCP for the first time without clinical signs of acute pancreatitis | 491 (standard premedication + TEA vs standard premedication) | prevention of post-ERCP pancreatitis | Not provided | n/d |
| The Effects of Local Infiltration Versus Epidural Following Liver Resection 2 (LIVER 2) | December 2012 | Patients undergoing open hepatic resection for benign or malignant conditions | 100 (EA vs wound catheter) | Length of stay | Pain Scores, Molecular response to surgery, Central Venous Pressure, estimated Blood Loss, Operative field asessment, Pringle time, Quality of Life (EQ-5D), Morphine consumption, IV Fluid volume, Complications, Post-operative blood tests | n/d |
Two clinical trials are evaluating the effects of thoracic epidural blockade on the clinical outcome of patients with acute pancreatitis.
One examines the hypothesis that thoracic epidural anesthesia for therapeutic ERCP could have a role in preventing post-ERCP pancreatitis.
Another study is comparing epidural anesthesia and the use of a wound catheter for post liver resection pain management. Amongst the secondary endpoints of this study are the molecular response to surgery, surgical and medical complications, and postoperative liver blood test results, which could all be modified by the microvascular effects of epidural blockade.
Splanchnic blood flow is regulated by intrinsic and extrinsic mechanisms. Intrinsic factors include local myogenic and metabolic control, locally produced vasoactive substances and local reflexes. The main extrinsic factors are the sympathetic innervation and the circulating vasoactive substances.
Epidural blockade can interfere with all these factors, either by direct block of sympathetic efferents or by the systemic effects of circulating local anesthetics.
A thoracic epidural block influences the systemic hemodynamics by reducing the intestinal vascular resistance and the stimulation of the adrenal gland and the renin-angiotensin axis[28].
The extension of the blockade seems to be a key factor in determining the splanchnic circulation response to epidural anesthesia. In fact the sympathetic innervation to the celiac, superior and inferior mesenteric ganglia originates in the T5-T11 region of the spinal cord, hence a low epidural block could not ensure a sufficient spread of local anesthetic to include all the efferent sympathetic innervation to the gut. Moreover the sympathetic activity in the regions not involved by the epidural blockade could be increased[29]. For these reasons an epidural block limited to the upper thoracic region, could potentially result in splanchnic sympathetic hyperactivity and foster splanchnic ischemia. Meissner et al[4] tested this hypothesis and found no modifications in intestinal blood flow during epidural anesthesia extending to the T1-T5 metameres in dogs, indicating that other local or systemic mechanisms could counteract the sympathetic hyperactivity and maintain a normal blood flow.
The data available in current literature regarding the effects of thoracic epidural anesthesia on splanchnic perfusion are conflicting. Studies focusing on regional macro-hemodynamics in healthy animals[2,5] and humans undergoing elective surgery[19-21,23-25] demonstrated no influence or worsening of regional perfusion in subject receiving thoracic epidural anesthesia. On the other hand most of the studies focusing on micro-hemodynamics[7-12,17,18], especially those focusing on pathologic low flow conditions, suggested that TEA could foster microcirculation despite a reduction in mean perfusion pressure.
The Gut receives its blood supply from three great vessels: the celiac artery, and the superior and inferior mesenteric arteries. The branchings of these three vessels result in a common set of mesenteric arteries evolving in two orders of arterioles, located in the superficial submucosa, forming a highly interconnected system to perfuse the small, third order arterioles.
Third order arterioles perfuse one or several villi, submucosal glands, crypt regions and the corresponding muscle layer, forming a mesh-like subepithelial capillary plexus. First and second order arterioles account for about 65% of the intestinal vascular resistance in rats, an additional 20% resistance resides in the capillaries and venules, so that terminal arterioles can govern only 15% of blood flow modifications[30]. One or two veins drain each villus. The parallel arrangement of these vessels produces a countercurrent mechanism that is the basis for the oxygen shunting phenomena that account for the extreme sensitivity of the apical region of the villi to hypoxia[31].
In low flow states, ischemia/reperfusion injury to the intestinal mucosa could damage the intestinal barrier and promote bacterial migration.
In sepsis and septic shock, both micro and macro-hemodynamics undergo profound alterations. However, the restoration of normal global hemodynamic and oxygen derived variables is not necessarily correlated to a correction of tissue dysoxia. This condition of oxygen extraction deficit could be correlated to metabolic disturbances or to regional hypoxia.
Microvascular flow distribution becomes highly heterogeneous during sepsis and septic shock, microcirculatory weak units can become hypoxic while other units can be overperfused.
These alterations are probably related to the presence of inflammatory mediators and microcirculatory emboli that impair microvascular autoregulation and increase oxygen shunting. This explains the finding of a venous PO2 higher than regional capillary PO2 (PO2 gap) in sepsis models[32].
These observations are progressively changing the primary endpoint for resuscitation procedures: from global hemodynamic and oxygen derived variables, to microcirculatory oxygenation. In fact, administration of vasopressors, despite correcting macrohemodynamic and oxygen delivery, could have counterproductive effects on microcirculation. This is the rationale for ongoing experimental therapies using vasodilators or oxygen carrying solutions to support dysoxic weak units[33].
Thoracic epidural blockade applied to animal[3,9,14] and human[26] models of sepsis appeared to be effective in fostering microvascular circulation or at least modify its distribution[11].
Regional sympathetic blockade could counteract the above mentioned mechanisms of heterogeneous flow distribution, restoring oxygenation to weak units, and thus contributing to the survival of the intestinal barrier[9,34].
In non septic low flow states, the correction of the hemodynamic status appears to restore oxygenation if a damage to the microcirculation has not developed yet.
Epidural anesthesia in this context could help maintaining microvascular perfusion, reducing ischemia/reperfusion injury and inflammation, as demonstrated by Adolphs et al[12].
However, Schwarte et al[15] found that TEA produced a reduction in gastric mucosal oxygenation in dog models of hemodynamic dysfunction induced by high PEEP levels. In this study the extension of the epidural block was thoraco-lumbar, which produced a significant fall in blood pressure compared to the control group. This was not the case in the study by Adolphs et al[11], where hemodynamic disturbances were more limited. Overall TEA appears to have a protective role for intestinal microcirculation in hemorrhagic low flow states, until macro-hemodynamics are maintained.
Pancreatic circulation is organized in a continuous network called insulo-acinar portal system. The pancreatic lobule is served by a single end artery, that first supplies blood to islets, and then continues as vasa efferentia to supply acini.
The autoregulation of this system is both hormonal and neural. The blood flow is strictly correlated to exocrine secretion, and modulated by various gastro-entero-pancreatic hormones.
This particular anatomy is very susceptible to ischemia, and it appears to have an important role in the development of acute pancreatitis[35].
During acute pancreatitis, microvascular perfusion is altered in accordance to the severity of the disease[36], and regional macro-hemodynamic blood flow appears not to correlate with microcirculation[37]. Pancreatic blood flow, red cell velocity, and functional capillary density all decrease; end artery vasoconstriction and increased shunts lead to ischemia, necrosis and circulatory stasis[38]. Moreover, local immune reaction and oxygen free radicals contribute to lobular and endothelial necrosis.
Animal models of acute pancreatitis treated with thoracic epidural blockade[13,16], showed reduced histologic signs of pancreatic necrosis and a restoration of continuous capillary perfusion and arteriolar blood flow, moreover, survival rate was significantly higher in both of the studies.
The microcirculatory effects induced by TEA could contribute in interrupting the ischemic injury involved in the beginning and progression of pancreatic lesions.
Nowadays TEA is increasingly used in humans as an effective analgesic technique for acute pancreatitis and it appears to be safe[39]. The currently ongoing clinical trials are expected to shed light on whether TEA can influence the prognosis of acute pancreatitis.
Liver circulation is characterized by a double afferent flow from the portal vein and the hepatic artery. In physiologic conditions, the portal vein drains the digestive tract below the diaphragm, spleen, and pancreas and supplies approximately two thirds of the hepatic blood flow, whilst the hepatic artery supplies one third.
Regulation of this dual blood supply, is strictly regulated by the hepatic arterial buffer response (HABR). This mechanism is responsible for maintaining a constant total hepatic blood flow by adjusting the hepatic arterial flow in relation to the modifications of portal flow.
Mechanisms regulating HABR are not fully understood, adenosine seems to be an important mediator of hepatic artery resistance, and reduction in adenosine wash out consensual to a drop in portal blood flow could enhance arterial flow. However the mechanism appears to be more complex and other mediators could be involved[40].
The studies evaluating the effects of TEA on liver macro hemodynamics in normal conditions, found no difference[5], or a reduction[2], in total hepatic blood flow. It must be noted that, when comparing the results of these two studies, the extension of epidural blockade could have influenced the outcome. In fact Vagts et al[5] (cit) considered the effects of a T5-T12 block, while Sivarajan et al[2] (cit) compared two levels of sensory blockade, finding a reduction of total hepatic blood flow only in the high level sensory blockade group (T1).
Total liver blood flow in sepsis and septic shock is usually increased, proportionally to the cardiac output[41]. This is associated with a decreased oxygen hemoglobin saturation in the hepatic veins. The reasons behind these phenomena are probably an impairment of the HABR mechanism, and a mismatch between oxygen distribution and metabolic demand.
Microvascular circulation appears to be uncoupled from systemic circulation in this context. In fact, despite the hyperdynamic circulatory state, microvascular flow appears to be unchanged or even decreased[42,43].
Intrahepatic blood flow is redistributed, blood is channeled away from contracted to dilated vessels reducing the perfused sinusoidal area. Imbalances in nitric oxide production may be the origin of these modifications. Moreover, sinusoidal and Kupffer cells are activated by contact with leukocytes and toxins, and react by producing cytokines which further impair microcirculation[44,45].
The review of the literature found only one study[18] considering the effect of epidural blockade on liver circulation in septic animals. In this scenario of late sepsis, TEA appeared to ameliorate sinusoidal hyperflow and reduce temporary venular leukocyte adhesion.
The authors suggested that TEA could have a role in restoring the impaired HABR, reducing the immune activation, through a direct reduction in hepatic sympathetic activity, which seems to have a role in the regulation of liver immunity. Also TEA, by fostering intestinal barrier function, would have an indirect role in preserving liver microcirculation and function.
The data obtained in the animal studies on acute pancreatitis also suggest a protective effect of TEA on liver circulation[17]. In fact TEA prevented sinusoids constriction and reduced liver cells apoptosis.
In conclusion, epidural anesthesia has been increasingly used in the last decades as an effective analgesic technique. This method produces a central sympathetic blockade which has strong effects on macro and microcirculation, by reducing autonomic efference and modifying the endocrine profile.
In recent years there have been efforts to further understand the underlying mechanisms of this technique. The available studies to date are heterogeneous and show conflicting results, making it difficult to gather decisive conclusions. A recent review by Richards et al[46] investigated the effects of TEA on spanchnic blood flow, focusing in particular on its potential implications in abdominal surgery. Their analysis, which comprised some of the studies that have been taken into consideration in the present review, also found the results to be inconsistent when suggesting a protective or detrimental effect of TEA on splanchnic circulation.
Overall the studies we considered also suggest possible new therapeutic applications of TEA, especially if micro-hemodynamics are taken into consideration.
Thoracic epidural anesthesia appears to reduce regional blood flow in relation to its effects on the vascular resistance, but at the same time it seems to foster microcirculation, especially in pathologic low flow states, or in conditions involving a degree of microvascular dysfunction. In these scenarios epidural anesthesia seems to restore blood flow to microcirculatory weak units, ameliorating tissue dysoxia and resistance to hypoperfusion.
However, given the variable extension that the epidural blockade can have, a wide extension of the blockade could impair macro-circulation enough to reduce regional DO2 under tissue requirements thus worsening hypoxia.
Human studies mostly evaluated macro-hemodynamics in patients undergoing surgery. Currently ongoing clinical trials could identify interesting applications in the prevention and treatment of acute pancreatitis, which have been strongly suggested by animal studies.
Further studies should investigate: (1) what is the extension of epidural blockade and local anesthetic concentration which can grant better micro-perfusion without significant hemodynamic impairment; (2) the effects on mortality and risk of catheter infection in septic animals treated with epidural anesthesia; (3) the effect of epidural blockade on the perioperative splanchnic organs function tests to assess whether epidural anesthesia can reduce perioperative organ injury or whether its macro-hemodynamic effects are relevant in inducing organ injury; and (4) the effect of epidural anesthesia on hepatic arterial buffer response, given the fact that this mechanism appears to be implicated in the constriction of the hepatic artery and the hemodynamic alterations developing after a liver resection, which could have a role in promoting Small for Size Syndrome[47].
Thoracic epidural anesthesia is a broadly used analgesic technique, however its eventual therapeutic effects in different fields are still matter of debate.
Macro and microcirculatory effects that result from the interaction between the analgesic technique and the neuro-endocrine system are suggested to have some therapeutic effect in animal models, studies on humans are scanty and use different methods for measuring hemodynamic variables, hence a thorough comparison is difficult. The hotspot of this Systematic Review is to evaluate up to date literature considering macro and microcirculatory effects of thoracic epidural anesthesia on splanchnic circulation and their possible therapeutic implications.
This Review considered the peculiarities of each particular regional circulation of the abdominal organs in order to evaluate possible effects of thoracic epidural anesthesia in both physiologic and pathologic conditions, this could change the way we use this technique, making it glide into a more comprehensive therapeutic view of its usage in medical and surgical conditions.
This Review points out some research fields that would be of interest in everyday clinical practice. In fact, on the basis of some rodent models, it appears that epidural anesthesia could reduce the mortality of acute pancreatitis, so that it could be used as a simultaneously analgesic and therapeutic procedure for this pathology. Moreover it has profound effects on hepatic circulation that could influence the function of this organ when it is object of surgical interventions. At last, some interesting implications in abdominal sepsis conditions are discussed.
The study was well designed and carried out. The data and conclusions are convincing.
| 1. | Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM, Knipschild PG. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51:1235-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1565] [Cited by in RCA: 1701] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 2. | Sivarajan M, Amory DW, Lindbloom LE. Systemic and regional blood flow during epidural anesthesia without epinephrine in the rhesus monkey. Anesthesiology. 1976;45:300-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Schäper J, Ahmed R, Perschel FH, Schäfer M, Habazettl H, Welte M. Thoracic epidural anesthesia attenuates endotoxin-induced impairment of gastrointestinal organ perfusion. Anesthesiology. 2010;113:126-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Meissner A, Weber TP, Van Aken H, Rolf N. Limited upper thoracic epidural block and splanchnic perfusion in dogs. Anesth Analg. 1999;89:1378-1381. [PubMed] |
| 5. | Vagts DA, Iber T, Puccini M, Szabo B, Haberstroh J, Villinger F, Geiger K, Nöldge-Schomburg GF. The effects of thoracic epidural anesthesia on hepatic perfusion and oxygenation in healthy pigs during general anesthesia and surgical stress. Anesth Analg. 2003;97:1824-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Ai K, Kotake Y, Satoh T, Serita R, Takeda J, Morisaki H. Epidural anesthesia retards intestinal acidosis and reduces portal vein endotoxin concentrations during progressive hypoxia in rabbits. Anesthesiology. 2001;94:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Hogan QH, Stadnicka A, Stekiel TA, Bosnjak ZJ, Kampine JP. Effects of epidural and systemic lidocaine on sympathetic activity and mesenteric circulation in rabbits. Anesthesiology. 1993;79:1250-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Hogan QH, Stekiel TA, Stadnicka A, Bosnjak ZJ, Kampine JP. Region of epidural blockade determines sympathetic and mesenteric capacitance effects in rabbits. Anesthesiology. 1995;83:604-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Kosugi S, Morisaki H, Satoh T, Ai K, Yamamoto M, Soejima J, Serita R, Kotake Y, Ishizaka A, Takeda J. Epidural analgesia prevents endotoxin-induced gut mucosal injury in rabbits. Anesth Analg. 2005;101:265-272, table of contents. [PubMed] |
| 10. | Sielenkämper A, Brodner G, Van Aken H. Epidural anesthesia and splanchnic perfusion. Can J Anaesth. 2001;48:611-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Adolphs J, Schmidt DK, Korsukewitz I, Kamin B, Habazettl H, Schäfer M, Welte M. Effects of thoracic epidural anaesthesia on intestinal microvascular perfusion in a rodent model of normotensive endotoxaemia. Intensive Care Med. 2004;30:2094-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Adolphs J, Schmidt DK, Mousa SA, Kamin B, Korsukewitz I, Habazettl H, Schäfer M, Welte M. Thoracic epidural anesthesia attenuates hemorrhage-induced impairment of intestinal perfusion in rats. Anesthesiology. 2003;99:685-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Freise H, Lauer S, Anthonsen S, Hlouschek V, Minin E, Fischer LG, Lerch MM, Van Aken HK, Sielenkämper AW. Thoracic epidural analgesia augments ileal mucosal capillary perfusion and improves survival in severe acute pancreatitis in rats. Anesthesiology. 2006;105:354-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Daudel F, Freise H, Westphal M, Stubbe HD, Lauer S, Bone HG, Van Aken H, Sielenkämper AW. Continuous thoracic epidural anesthesia improves gut mucosal microcirculation in rats with sepsis. Shock. 2007;28:610-614. [PubMed] |
| 15. | Schwarte LA, Picker O, Höhne C, Fournell A, Scheeren TW. Effects of thoracic epidural anaesthesia on microvascular gastric mucosal oxygenation in physiological and compromised circulatory conditions in dogs. Br J Anaesth. 2004;93:552-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Bachmann KA, Trepte CJ, Tomkötter L, Hinsch A, Stork J, Bergmann W, Heidelmann L, Strate T, Goetz AE, Reuter DA. Effects of thoracic epidural anesthesia on survival and microcirculation in severe acute pancreatitis: a randomized experimental trial. Crit Care. 2013;17:R281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Freise H, Lauer S, Konietzny E, Hinkelmann J, Minin E, Van Aken HK, Lerch MM, Sielenkaemper AW, Fischer LG. Hepatic effects of thoracic epidural analgesia in experimental severe acute pancreatitis. Anesthesiology. 2009;111:1249-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Freise H, Daudel F, Grosserichter C, Lauer S, Hinkelmann J, Van Aken HK, Sielenkaemper AW, Westphal M, Fischer LG. Thoracic epidural anesthesia reverses sepsis-induced hepatic hyperperfusion and reduces leukocyte adhesion in septic rats. Crit Care. 2009;13:R116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Lundberg J, Lundberg D, Norgren L, Ribbe E, Thörne J, Werner O. Intestinal hemodynamics during laparotomy: effects of thoracic epidural anesthesia and dopamine in humans. Anesth Analg. 1990;71:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Gould TH, Grace K, Thorne G, Thomas M. Effect of thoracic epidural anaesthesia on colonic blood flow. Br J Anaesth. 2002;89:446-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Meierhenrich R, Wagner F, Schütz W, Rockemann M, Steffen P, Senftleben U, Gauss A. The effects of thoracic epidural anesthesia on hepatic blood flow in patients under general anesthesia. Anesth Analg. 2009;108:1331-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Michelet P, Roch A, D’Journo XB, Blayac D, Barrau K, Papazian L, Thomas P, Auffray JP. Effect of thoracic epidural analgesia on gastric blood flow after oesophagectomy. Acta Anaesthesiol Scand. 2007;51:587-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (3)] |
| 23. | Tanaka N, Nagata N, Hamakawa T, Takasaki M. The effect of dopamine on hepatic blood flow in patients undergoing epidural anesthesia. Anesth Analg. 1997;85:286-290. [PubMed] |
| 24. | Trepenaitis D, Pundzius J, Macas A. The influence of thoracic epidural anesthesia on liver hemodynamics in patients under general anesthesia. Medicina (Kaunas). 2010;46:465-471. [PubMed] |
| 25. | Väisänen O, Parviainen I, Ruokonen E, Hippeläinen M, Berg E, Hendolin H, Takala J. Epidural analgesia with bupivacaine does not improve splanchnic tissue perfusion after aortic reconstruction surgery. Br J Anaesth. 1998;81:893-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Spackman DR, McLeod AD, Prineas SN, Leach RM, Reynolds F. Effect of epidural blockade on indicators of splanchnic perfusion and gut function in critically ill patients with peritonitis: a randomised comparison of epidural bupivacaine with systemic morphine. Intensive Care Med. 2000;26:1638-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Kortgen A, Silomon M, Pape-Becker C, Buchinger H, Grundmann U, Bauer M. Thoracic but not lumbar epidural anaesthesia increases liver blood flow after major abdominal surgery. Eur J Anaesthesiol. 2009;26:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Hopf HB, Schlaghecke R, Peters J. Sympathetic neural blockade by thoracic epidural anesthesia suppresses renin release in response to arterial hypotension. Anesthesiology. 1994;80:992-99; discussion 992-99;. [PubMed] |
| 29. | Taniguchi M, Kasaba T, Takasaki M. Epidural anesthesia enhances sympathetic nerve activity in the unanesthetized segments in cats. Anesth Analg. 1997;84:391-397. [PubMed] |
| 30. | Bohlen HG. Integration of intestinal structure, function, and microvascular regulation. Microcirculation. 1998;5:27-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Vollmar B, Menger MD. Intestinal ischemia/reperfusion: microcirculatory pathology and functional consequences. Langenbecks Arch Surg. 2011;396:13-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 32. | Ince C, Sinaasappel M. Microcirculatory oxygenation and shunting in sepsis and shock. Crit Care Med. 1999;27:1369-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 341] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 33. | Lamontagne F, Meade M, Ondiveeran HK, Lesur O, Fox-Robichaud AE. Nitric oxide donors in sepsis: a systematic review of clinical and in vivo preclinical data. Shock. 2008;30:653-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Schäper J, Wagner A, Enigk F, Brell B, Mousa SA, Habazettl H, Schäfer M. Regional sympathetic blockade attenuates activation of intestinal macrophages and reduces gut barrier failure. Anesthesiology. 2013;118:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Cuthbertson CM, Christophi C. Disturbances of the microcirculation in acute pancreatitis. Br J Surg. 2006;93:518-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 224] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 36. | Klar E, Schratt W, Foitzik T, Buhr H, Herfarth C, Messmer K. Impact of microcirculatory flow pattern changes on the development of acute edematous and necrotizing pancreatitis in rabbit pancreas. Dig Dis Sci. 1994;39:2639-2644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Schröder T, Kivisaari L, Standertskjöld-Nordenstam CG, Somer K, Lehtola A, Puolakkainen P, Karonen SL, Kivilaakso E, Lempinen M. Pancreatic blood flow and contrast enhancement in computed tomography during experimental pancreatitis. Eur Surg Res. 1985;17:286-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Zhou ZG, Chen YD. Influencing factors of pancreatic microcirculatory impairment in acute panceatitis. World J Gastroenterol. 2002;8:406-412. [PubMed] |
| 39. | Bernhardt A, Kortgen A, Niesel HCh, Goertz A. Using epidural anesthesia in patients with acute pancreatitis--prospective study of 121 patients. Anaesthesiol Reanim. 2002;27:16-22. [PubMed] |
| 40. | Jakob SM. Splanchnic blood flow in low-flow states. Anesth Analg. 2003;96:1129-1138, table of contents. [PubMed] |
| 41. | Asfar P, De Backer D, Meier-Hellmann A, Radermacher P, Sakka SG. Clinical review: influence of vasoactive and other therapies on intestinal and hepatic circulations in patients with septic shock. Crit Care. 2004;8:170-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Hiltebrand LB, Krejci V, Banic A, Erni D, Wheatley AM, Sigurdsson GH. Dynamic study of the distribution of microcirculatory blood flow in multiple splanchnic organs in septic shock. Crit Care Med. 2000;28:3233-3241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 43. | Albuszies G, Radermacher P, Vogt J, Wachter U, Weber S, Schoaff M, Georgieff M, Barth E. Effect of increased cardiac output on hepatic and intestinal microcirculatory blood flow, oxygenation, and metabolism in hyperdynamic murine septic shock. Crit Care Med. 2005;33:2332-2338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | La Mura V, Pasarín M, Rodriguez-Vilarrupla A, García-Pagán JC, Bosch J, Abraldes JG. Liver sinusoidal endothelial dysfunction after LPS administration: a role for inducible-nitric oxide synthase. J Hepatol. 2014;61:1321-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 45. | Spapen H. Liver perfusion in sepsis, septic shock, and multiorgan failure. Anat Rec (Hoboken). 2008;291:714-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 46. | Richards ER, Kabir SI, McNaught CE, MacFie J. Effect of thoracic epidural anaesthesia on splanchnic blood flow. Br J Surg. 2013;100:316-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Eipel C, Abshagen K, Vollmar B. Regulation of hepatic blood flow: the hepatic arterial buffer response revisited. World J Gastroenterol. 2010;16:6046-6057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 339] [Cited by in RCA: 388] [Article Influence: 24.3] [Reference Citation Analysis (2)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Yang ZJ S- Editor: Ji FF L- Editor: A E- Editor: Wu HL