Published online Sep 9, 2025. doi: 10.5492/wjccm.v14.i3.105428
Revised: March 15, 2025
Accepted: April 1, 2025
Published online: September 9, 2025
Processing time: 178 Days and 6.1 Hours
Many causal factors influence acute liver failure (ALF), including the primary underlying cause, age, and socioeconomic conditions. ALF outcomes depend on etiology, coagulopathy, bilirubin, age, and understanding of hepatic encepha
To evaluate the association between etiologies, clinical manifestations, and outcomes of adults admitted with ALF.
This institution-based, prospective cross-sectional study was conducted in the Department of Gastroenterology and Hepatology at Jinnah Postgraduate Medical Center, Karachi, from July 2019 to December 2022. A total of 102 patients dia
Mean age of the ALF cohort was 27.37 ± 6.60 years. Of the 102 patients, 71 (69.6%) were female, including 55 (77.5%) pregnant women with a mean gestational age of 34.56 ± 3.80 weeks. Regarding HE severity, 45 (44.1%) had grade III, and 13 (12.7%) had grade II. Among the patients admitted to the intensive care unit, 51 (72.9%) did not survive, while 14 (43.8%) recovered.
This study observed a high mortality rate among ALF patients in a tertiary care hospital. Hepatitis E virus infe
Core Tip: This study investigates the etiological factors, clinical manifestations, and outcomes of acute liver failure in a tertiary care hospital in Pakistan. Hepatitis E virus was the predominant cause, particularly in pregnant women, with high associated mortality. Jaundice, hepatic encephalopathy, sepsis, and fatigue were significant predictors of poor prognosis. Limited liver transplant facilities contribute to increased mortality, highlighting the urgent need for early referral, risk factor management, and improved supportive care to enhance patient survival.
- Citation: Butt N, Ali S, Khemani H, Mumtaz K. Acute liver failure etiology, clinical manifestation and outcomes in adults: Experience of tertiary care hospital in Karachi. World J Crit Care Med 2025; 14(3): 105428
- URL: https://www.wjgnet.com/2220-3141/full/v14/i3/105428.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v14.i3.105428
Acute liver failure (ALF) is a rare but intense clinical set of symptoms, characterized by abrupt and immense liver necrosis of previously healthy liver parenchyma, resulting in jaundice, coagulopathy international normalized ratio
ALF is influenced by many causal factors, with the primary underlying cause differing worldwide depending on age and geographical conditions[5]. It predominantly manifests in individuals in their 30 seconds with no prior health issues, posing distinct difficulties in terms of clinical treatment. The potential underlying causes may range from viral infections and drug-induced liver injury (DILI) to vascular and autoimmune hepatitis (AIH)[6]. Based on a study conducted in India, hepatitis A virus (HAV) and hepatitis E virus (HEV) are considered to be responsible for the global burden of ALF[7]. Furthermore, developing countries in Asia like India and Bangladesh, where HEV stands as a primary cause of ALF, have contributed substantial data that reinforces this assertion. A study conducted in India revealed that 44% of ALF cases were attributed to HEV as the primary underlying factor[8]. Likewise, a study from Bangladesh concluded that acute HEV infection is a predominant cause of a diverse spectrum of liver disorders, ranging from severe acute viral hepatitis to ALF, and decompensated liver cirrhosis[9]. In contrast, western countries attribute factors such as DILI to be the leading cause of ALF. In the United States, the ALF Study Group established that over 50% of ALF cases were Acetaminophen-induced, with hepatitis B virus (HBV) following the trend at 7%[10]. Other factors, infrequent yet profoundly devastating outcomes, include metabolic liver syndrome, AIH, seronegative hepatitis, and sepsis. Western research showed that 40%-80% of ALF patients, who succumb to dire outcomes of renal failure, are associated with acetaminophen use in 70% of such cases. Whereas in India, 10%, of total ALF cases reported, are those of renal failure[11].
Therefore, etiological factor identification is of extreme significance as it influences the final prognosis and facilitates the management of ALF. Intensive supportive treatment, such as fluid replenishment, and timely intervention is crucial to ensure the individual’s survival. In cases of severe coagulopathy and coma, supportive treatment proves to be ineffective and liver transplantation (LT) remains the mainstay to achieving prolonged survival[7,8,12]. Prior commu
In Pakistan, there are limited statistics on ALF, along with inadequate availability of LT facilities. The need to explore the underlying causes of ALF and simultaneously strive to achieve a proficient management plan in a developing country like Pakistan is essential as the burden of such patients is relatively higher than that officially reported. Consequently, the study aimed to check the association between the etiology and imminent challenges, clinical manifestation, associated mortality, and possible treatment approaches in ALF, tertiary hospitals. Thereby determining appropriate measures required to reduce morbidity and mortality of such patients in Pakistan.
This prospective, cross-sectional, institution-based study was conducted in the Department of Gastroenterology and Hepatology at Jinnah Postgraduate Medical Center (JPMC), Karachi, from July 2019 to December 2022.
A consecutive sampling technique was employed, and 102 patients were recruited from those who visited JPMC, the gastroenterology and hepatology department. Sample size was determined using OpenEpi sample size calculator, considering an expected prevalence of ALF, a 95% confidence interval, and a 5% margin of error[12]. Patients aged ≥ 15 years who met the criteria for ALF[13] were included. ALF was diagnosed in jaundiced patients who developed encephalopathy within eight weeks of jaundice onset and had evidence of coagulopathy, that is, prothrombin time (PT) > 20 seconds beyond control, along with impaired liver function, indicated by total serum bilirubin > 1.5 mg/dL and alanine aminotransferase (ALT) > 40 IU/L. Encephalopathy severity was classified into four grades based on the West Haven criteria[14]. Data collection was conducted using a structured questionnaire administered by trained medical personnel. This questionnaire covered demographic characteristics (age, gender, occupation, residence, ethnicity, and socioeconomic status), clinical presentation, laboratory findings, and patient outcomes (recovery or death). Liver function tests (LFT) and INR were documented. Additionally, ELISA testing was performed to detect viral markers for hepatitis A to E (anti-HAV IgM, HBsAg, HBc IgM, anti-HCV antibody, and anti-HEV IgM). To ensure data accuracy, double-entry verification was conducted, and inconsistencies were resolved through cross-checking. The study adhered to ethical principles outlined in the Declaration of Helsinki and received approval from the Institutional Review Board of JPMC (Reference No: F.2-81/2019-GENIL/35813/JPMC). Informed consent was obtained from all adult participants. For minors (< 18 years old), consent was obtained from their parents or legal guardians. Patient confidentiality was strictly maintained, and identifying information was removed from the data set.
The study's dependent variable was patient outcome (recovery or death), while independent variables included age, gender, socioeconomic status, residence, ethnicity, grade of encephalopathy, ALF etiology, LFTs, INR, and the presence of sepsis. Data entry was performed using Microsoft Excel for accuracy, and statistical analysis was conducted using SPSS version 26.0. Descriptive analysis was applied to categorical variables such as gender, socioeconomic status, residence, ethnicity, encephalopathy grade, ALF etiology, and patient outcome, while continuous variables were presented as mean ± SD. The χ2 test was used to determine associations between categorical variables, and a P value < 0.05 was considered statistically significant.
For all patients diagnosed with ALF, the mean age was 27.37 ± 6.60 years. The minimum age was 15 years and the maximum age was 45 years and 71 (69.6%) out of 102 were females.
Of which 55 (77.5%) were pregnant with the mean of gestational age was 34.56 ± 3.80 weeks. According to marital status, 78 (76.4%) patients were married and most of patients 56 (54.9%) were Urdu-speaking, 57 (55.9%) were urban residents and 59 (57.8%) had lower socioeconomic status. In terms of occupation, most of the females 67 (65.7%) were housewives, 18 (17.6%) were shopkeepers and 18 (17.6%) were students. 25 (24.5%) patients were addicted to chewable tobacco and 77 (75.5%) patients did not have any drug habit as mentioned in Table 1.
| Variables | n (%) |
| Age (years), mean ± SD | 27.37 ± 6.60 |
| Gender | |
| Male | 31(30.4) |
| Female | 71(69.6) |
| Pregnant | 55 (77.5) |
| Non- Pregnant | 16 (32.5) |
| Gestational age of pregnant (weeks), mean ± SD | 34.56 ± 3.80 |
| Marital status | |
| Married | 78 (76.5) |
| Unmarried | 24 (23.5) |
| Ethnicity | |
| Urdu | 56 (54.9) |
| Sindhi | 36 (35.3) |
| Pathan | 10 (9.8) |
| Residence | |
| Rural | 45 (44.1) |
| Urban | 57 (55.9) |
| Socio-economic status | |
| Lower class | 59 (57.8) |
| Middle class | 43 (42.2) |
| Occupation | |
| Housewife | 67 (65.7) |
| Student | 18 (17.6) |
| Shopkeeper | 17 (16.7) |
| Habits | |
| Chewable tobacco | 25 (24.5) |
| None | 77 (75.5) |
The average duration of hospital stay was 4.80 ± 3.39 days. The mean PT was 11.77 ± 1.75 seconds and the normalization ratio was 1.96 ± 0.59. According to LFT, the median and interquartile range of ALT was [3600 (2060-8655)] IU/L, aspartate aminotransferase was [200 (100-267)] IU/L, mean ± SD of gamma-glutamyl transferase was 104.62 ± 46.90 IU/L, alkaline phosphate was 719.51 ± 32.90 IU/L, total bilirubin was 15.41 ± 7.13 mg/dL, direct bilirubin was 7.66 ± 3.12 mg/dL and albumin was 3.45 ± 0.50 g/L as showed in Table 2.
| Variable | Mean ± SD |
| Duration of stay (days) | 4.80 ± 3.39 |
| Prothrombin time (seconds) | 11.77 ± 1.75 |
| International normalization ratio | 1.96 ± 0.59 |
| Alanine aminotransferase (IU/L) [median (IQR)] | [3600 (2060-8655)] |
| Aspartate aminotransferase (IU/L) [median (IQR)] | [200 (100-267)] |
| Gamma-glutamyl transferase (IU/L) | 104.62 ± 46.90 |
| Alkaline phosphatase (IU/L) | 719.51 ± 327.90 |
| Total bilirubin (mg/dL) | 15.41 ± 7.13 |
| Direct bilirubin (mg/dL) | 7.66 ± 3.12 |
| Albumin (g/L) | 3.45 ± 0.50 |
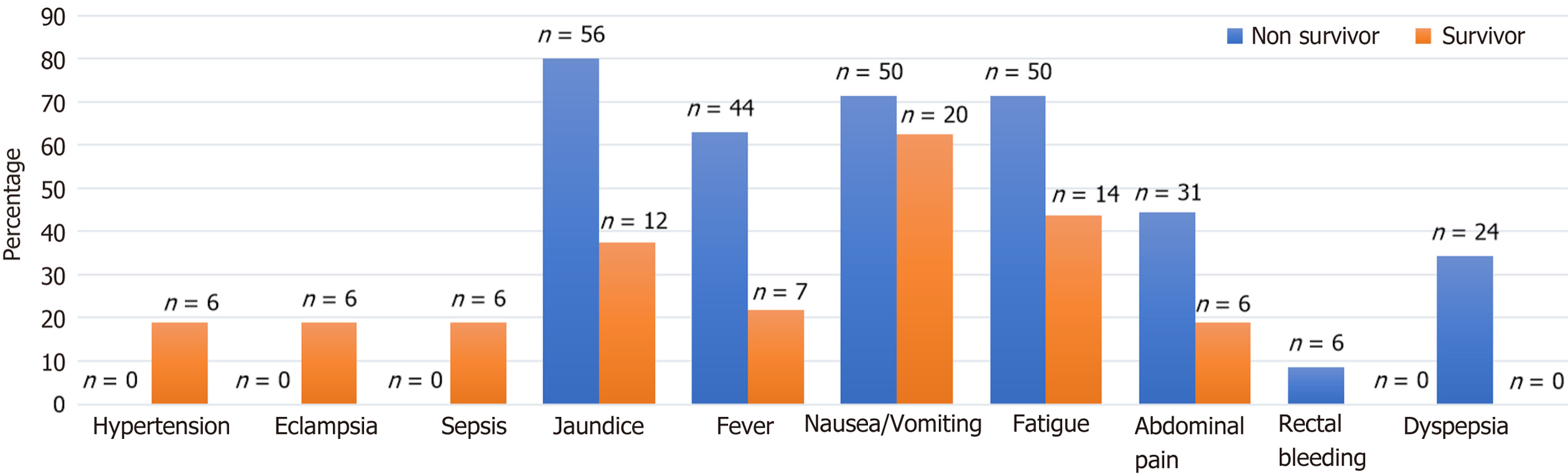
Table 3 and Figure 1 showed the association between various clinical manifestations and patient outcomes (mortality vs survival) in individuals diagnosed with ALF. Among the 102 patients, 70 (68.6%) did not survive, while 32 (31.4%) recovered. Hypertension, eclampsia, and sepsis were exclusively observed in surviving patients (18.8%, P = 0.001), whereas none of the deceased patients had these conditions. Jaundice was significantly more common in patients who died (80% vs 37.5%, P = 0.000). Similarly, fever was more frequent among non-survivors (62.9% vs 21.9%, P = 0.000). Fatigue (71.4% vs 43.8%, P = 0.009) and abdominal pain (44.3% vs 18.8%, P = 0.015) were also significantly associated with higher mortality. In contrast, nausea/vomiting (P = 0.491) and rectal bleeding (P = 0.173) did not show a statistically significant association with survival or death. Dyspepsia was only reported among non-survivors (34.3%, P = 0.000), while all surviving patients (100%) did not have dyspepsia. These findings indicate that sepsis, jaundice, fever, fatigue, and dyspepsia were significantly associated with increased mortality, while hypertension, eclampsia, and sepsis were more prevalent among survivors.
| Clinical manifestations | Outcome, n (%) | P value | |
| Died (n = 70) | Survived (n = 32) | ||
| Hypertension | 0.001a | ||
| Yes | - | 6 (18.8) | |
| No | 70 (100) | 26 (81.3) | |
| Eclampsia | 0.001a | ||
| Yes | - | 6 (18.8) | |
| No | 70 (100) | 26 (81.3) | |
| Sepsis | 0.001a | ||
| Yes | - | 6 (18.8) | |
| No | 70 (100) | 26 (81.3) | |
| Jaundice | 0.000a | ||
| Yes | 56 (80) | 12 (37.5) | |
| No | 14 (20) | 20 (62.5) | |
| Fever | 0.000a | ||
| Yes | 44 (62.9) | 7 (21.9) | |
| No | 26 (37.1) | 25 (78.1) | |
| Nausea/vomiting | 0.491 | ||
| Yes | 50 (71.4) | 20 (62.5) | |
| No | 20 (28.6) | 12 (37.5) | |
| Fatigue | 0.009a | ||
| Yes | 50 (71.4) | 14 (43.8) | |
| No | 20 (28.6) | 18 (56.3) | |
| Abdominal pain | 0.015a | ||
| Yes | 31 (44.3) | 6 (18.8) | |
| No | 39 (55.7) | 26 (81.3) | |
| Rectal bleeding | 0.173 | ||
| Yes | 6 (8.6) | - | |
| No | 64 (91.4) | 32 (100) | |
| Dyspepsia | 0.000a | ||
| Yes | 24 (34.3) | - | |
| No | 46 (65.7) | 32 (100) | |
AIH was only observed in surviving patients (18.8%, P = 0.001), whereas none of the deceased patients had AIH. HAV infection was significantly associated with mortality, with 27.1% of non-survivors testing reactive for anti-HAV IgM, while none of the survivors had a reactive result (P = 0.002). HEV infection was also strongly associated with survival, as all surviving patients (100%) tested positive for HEV IgM, whereas only 72.9% of deceased patients had a reactive result (P = 0.000). In contrast, HBsAg (P = 0.173), HBc IgM (P = 0.95), and hepatitis C virus antibody (anti-HCV Ab) (P = 0.105) did not show a statistically significant association with mortality or survival. These findings indicate that AIH and HEV IgM positivity were more common in survivors, while HAV infection was more prevalent in non-survivors. Other viral markers, including HBsAg, HBc IgM, and anti-HCV Ab, did not demonstrate a significant impact on patient outcomes as shown in Table 4.
| Viral serology | Outcome, n (%) | P value | |
| Died (n = 70) | Survived (n = 32) | ||
| AIH | 0.001a | ||
| Yes | - | 6 (18.8) | |
| No | 70 (100) | 26 (81.3) | |
| Anti-HAV IgM | 0.002a | ||
| Reactive | 19 (27.1) | - | |
| Non-Reactive | 51 (72.9) | 32 (100) | |
| HBs Ag | 0.173 | ||
| Reactive | 6 (8.6) | - | |
| Non-Reactive | 64 (91.4) | 32 (100) | |
| HBc IgM | 0.95 | ||
| Reactive | 7 (10) | - | |
| Non-reactive | 63 (90) | 32 (100) | |
| HEV IgM | 0.000a | ||
| Reactive | 51 (72.9) | 32 (100) | |
| Non-Reactive | 19 (27.1) | - | |
| Anti-HCV Ab | 0.105 | ||
| Reactive | 6 (8.6) | 7 (21.9) | |
| Non-reactive | 64 (91.4) | 25 (78.1) | |
Table 5 examined the relationship between ultrasound findings, HE grading, pregnancy status, and intensive care unit (ICU) admission with mortality and survival in ALF patients. Ultrasound findings (liver parenchymal changes, hepatomegaly, and hypoechoic liver) were not significantly associated with mortality (P = 0.582). However, higher HE grades
| Variables | Outcome, n (%) | P value | |
| Died (n = 70) | Survived (n = 32) | ||
| Ultrasonography findings | 0.582 | ||
| Liver parenchymal changes | 12 (17.1) | 7 (21.9) | |
| Hepatomegaly | 25 (35.7) | 7 (21.9) | |
| Hypoechoic liver | 12 (17.1) | 6 (18.8) | |
| None | 21 (30.0) | 12 (37.5) | |
| Encephalopathy grades | 0.000a | ||
| Grade 0 | - | - | |
| Grade I | - | - | |
| Grade II | 13 (18.6) | 18 (56.3) | |
| Grade III | 45 (64.3) | 14 (43.8) | |
| Pregnancy | 0.000a | ||
| Pregnant | 23 (32.8) | 32 (100) | |
| Non-pregnant | 47 (67.2) | - | |
| ICU need | 45 (64.3) | ||
| Yes | 45 (64.3) | 14 (43.8) | |
| No | 45 (64.3) | 18 (56.2) | |
This study examined to assess etiological factors, clinical manifestations, and results in patients suffering ALF. The myriads of manifestations include jaundice, abdominal pain, fever, fatigue, malaise, nausea, and anorexia. A study reported manifestations such as hepatomegaly, liver parenchymal changes, hypoechoic liver, nausea/vomiting, jaundice, fatigue, fever, and abdominal pain. The progression itself is characterized by severe coagulopathy followed by encepha
According to the present study, concerned risk factors denote that HEV was positive in 77 (75.5%) cases. In Pakistan, HEV affects the adult population. Recovery is the norm once infected, except in the late trimester of pregnancy, when up to 30% maternal or fetal death is observed, particularly during epidemics. HEV, like HAV, is widespread in Pakistan due to drinking contaminated water[17]. In cities, the primary water supply can become polluted due to leaks from nearby sewage pipes. On the other hand, in rural regions, water sources such as wells, streams, canals, rivers, and ponds are tainted by the direct dumping of sewage into those locations[17]. The present study showed that out of 55 (77.5%) pregnant females, HEV IgM-positive was 49 (89.1%), with a mean gestational age of 34.56 ± 3.80 weeks. According to a study, HEV infection leading to ALF and high mortality is a common feature in Indian women during the second and third trimesters of pregnancy[18].
Pregnancy has all the earmarks of being a possible risk factor for viral replication and extremely low immune status of Indian/Asian pregnant ladies. It is recommended that reduced cell resistance (showed by a diminishing in CD4, an expansion in CD8 cell counts, and brought down CD4/CD8 cell proportion) and an elevated degree of steroid hormones that impact viral replication during pregnancy give off an impression of being the conceivable reasons behind seriousness of the disease[19]. The virus, although otherwise self-limiting, is responsible for morbid outcomes when present in pregnancy, particularly in the second and third trimesters, ranging from several obstetric complications to even death, reported in 30%-100% of patients. The devastating outcomes are associated with longer jaundice-to-encephalopathy duration in pregnant females[13].
Whereas, HAV was positive in 19 (18.6%) cases as per this research. HAV infection typically leads to an acute hepatitis that is self-limiting in most individuals. However, in rare cases, it can result in severe liver failure. The phenomenon of HAV-related liver failure is indeed thought to arise from an excessive immune response rather than direct viral cytotoxicity. According to research, in 79 patients with acute hepatitis A, age, gender, and drug toxicity (paracetamol) were identified as potential contributing factors to mortality[20].
In this study, HBsAg was reactive in 6 (5.9%) cases. HBV-actuated ALF might be because of acute hepatitis yet may likewise be because of HBV reactivation in constant carriers of HBV. HBV reactivation can be unconstrained or auxiliary to chemotherapy or because of escaped mutant HBV in patients on nucleotide analogs. HBV ALF is serious and brings about death or transplantation in 80% of people[21].
Pregnant women infected with HEV may develop severe hepatitis, particularly during the third trimester, leading to a high risk of death. Therefore, it is important to prioritize screening for and monitoring HEV infection early in pregnancy. Pregnant women should also be educated about the potential impact of HEV on the unborn child and advised to steer clear of consuming contaminated food and water to prevent potential HEV exposure. The development of an HEV vaccine shows promise in reducing HEV-related mortality in pregnant women[20].
The current study is limited by its single-center design, small sample size, lack of long-term follow-up, absence of liver transplant data, potential selection bias, and limited generalizability to broader populations or different healthcare settings. However, the study proposed that HEV infection transmission was much greater in individuals with liver failure despair. Jaundice, sickness/retching, weakness, and fever. By addressing the risk factors associated with poor outcomes, the mortality rate in a non-liver transplant region was much higher. As a result, patients with liver failure should be transmitted as soon as possible to a transplant center to ensure that complications.
This study highlights a higher mortality rate in adults by revealing an association between clinical manifestations, viral serology, and mortality in patients with ALF. It concludes that survival rate revealed that anti-HEV IgM, HE, sepsis, jaundice, fatigue, abdominal pain, and dyspepsia were significantly associated with mortality rate. Moreover, the mortality rate was significantly higher which can be reduced somewhat in non-liver transplant areas by managing risk factors but are generally observed with a worse outcome. Consequently, patients with liver failure should be shifted to a transplant center as quickly as possible, to ensure proper management of complications.
The authors thank Getz Pharma's Medical Affairs Department for their support with the publication process.
| 1. | Trautwein C, Koch A. Mechanisms of Acute Liver Failure. In: Gershwin ME, Vierling JM, Tanaka A, Manns MP, editors. Liver Immunology. Cham: Springer, 2020: 471–490. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Acharya SK. Overview of acute liver failure in India. Indian J Gastroenterol. 2024;43:296-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Martí-carvajal AJ, Gluud C, Gluud LL, Pavlov CS, Mauro E, Monge Martín D, Liu JP, Nicola S, Comunián-carrasco G, Martí-amarista CE. Liver support systems for adults with acute liver failure. Cochrane Database Syst Rev. 2022;2022. [RCA] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Strong AJ, Smith SE, Whittington DJ, Meldrum BS, Parsons AA, Krupinski J, Hunter AJ, Patel S, Robertson C. Factors influencing the frequency of fluorescence transients as markers of peri-infarct depolarizations in focal cerebral ischemia. Stroke. 2000;31:214-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Jindal A, Sarin SK. Epidemiology of liver failure in Asia-Pacific region. Liver Int. 2022;42:2093-2109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Andrade RJ, Aithal GP, de Boer YS, Liberal R, Gerbes A, Regev A, Terziroli Beretta-Piccoli B, Schramm C, Kleiner DE, De Martin E, Kullak-Ublick GA, Stirnimann G, Devarbhavi H, Vierling JM, Manns MP, Sebode M, Londoño MC, Avigan M, Robles-Diaz M, García-Cortes M, Atallah E, Heneghan M, Chalasani N, Trivedi PJ, Hayashi PH, Taubert R, Fontana RJ, Weber S, Oo YH, Zen Y, Licata A, Lucena MI, Mieli-Vergani G, Vergani D, Björnsson ES; IAIHG and EASL DHILI Consortium. Nomenclature, diagnosis and management of drug-induced autoimmune-like hepatitis (DI-ALH): An expert opinion meeting report. J Hepatol. 2023;79:853-866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 101] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 7. | Palewar MS, Joshi S, Choudhary G, Das R, Sadafale A, Karyakarte R. Prevalence of Hepatitis A virus (HAV) and Hepatitis E virus (HEV) in patients presenting with acute viral hepatitis: A 3-year retrospective study at a tertiary care Hospital in Western India. J Family Med Prim Care. 2022;11:2437-2441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 8. | Kumar S, Atam V, Sonkar SK, Kumar A, Gupta H, Chaudhary SC. Study of Aetiology, Complications, Prognostic Factors and Mortality Predictors in Acute Liver Failure in a Tertiary Care Hospital of North India. Int J Curr Res Rev. 2020;12:03-09. [DOI] [Full Text] |
| 9. | Stravitz RT, Fontana RJ, Karvellas C, Durkalski V, McGuire B, Rule JA, Tujios S, Lee WM; Acute Liver Failure Study Group. Future directions in acute liver failure. Hepatology. 2023;78:1266-1289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 10. | Garg K, Jain AK, Nimje GR, Kajal K. Perioperative care in acute liver failure: An anaesthesiologist perspective in the operating theatre. Indian J Gastroenterol. 2024;43:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Bernal W, Karvellas C, Saliba F, Saner FH, Meersseman P. Intensive care management of acute-on-chronic liver failure. J Hepatol. 2021;75 Suppl 1:S163-S177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 12. | Anand AC, Nandi B, Acharya SK, Arora A, Babu S, Batra Y, Chawla YK, Chowdhury A, Chaoudhuri A, Eapen EC, Devarbhavi H, Dhiman R, Datta Gupta S, Duseja A, Jothimani D, Kapoor D, Kar P, Khuroo MS, Kumar A, Madan K, Mallick B, Maiwall R, Mohan N, Nagral A, Nath P, Panigrahi SC, Pawar A, Philips CA, Prahraj D, Puri P, Rastogi A, Saraswat VA, Saigal S, Shalimar, Shukla A, Singh SP, Verghese T, Wadhawan M; INASL Task-Force on Acute Liver Failure. Indian National Association for the Study of the Liver Consensus Statement on Acute Liver Failure (Part 1): Epidemiology, Pathogenesis, Presentation and Prognosis. J Clin Exp Hepatol. 2020;10:339-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 13. | Häussinger D, Dhiman RK, Felipo V, Görg B, Jalan R, Kircheis G, Merli M, Montagnese S, Romero-Gomez M, Schnitzler A, Taylor-Robinson SD, Vilstrup H. Hepatic encephalopathy. Nat Rev Dis Primers. 2022;8:43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 14. | Glass DM, Al-khafaji A. The Clinical Spectrum and Manifestations of Acute and Acute on Chronic Liver Failure. Liver Fail. 2020. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Lao TT. Drug-induced liver injury in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2020;68:32-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Shata MTM, Hetta HF, Sharma Y, Sherman KE. Viral hepatitis in pregnancy. J Viral Hepat. 2022;29:844-861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 17. | Wu T, Ge Y, Zhao K, Zhu X, Chen Y, Wu B, Zhu F, Zhu B, Cui L. A reverse-transcription recombinase-aided amplification assay for the rapid detection of N gene of severe acute respiratory syndrome coronavirus 2(SARS-CoV-2). Virology. 2020;549:1-4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Sanyal AJ, Van Natta ML, Clark J, Neuschwander-Tetri BA, Diehl A, Dasarathy S, Loomba R, Chalasani N, Kowdley K, Hameed B, Wilson LA, Yates KP, Belt P, Lazo M, Kleiner DE, Behling C, Tonascia J; NASH Clinical Research Network (CRN). Prospective Study of Outcomes in Adults with Nonalcoholic Fatty Liver Disease. N Engl J Med. 2021;385:1559-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 832] [Article Influence: 166.4] [Reference Citation Analysis (0)] |
| 19. | Teschke R. Acetaminophen syn. Paracetamol: Acute Liver Injury and Acute on Chronic Liver Failure with Case Analysis and Causality Assessment Using RUCAM. Liver Fail. 2020. [DOI] [Full Text] |
| 20. | Biswas S, Kumar R, Acharya SK, Shalimar. Prognostic Scores in Acute Liver Failure Due to Viral Hepatitis. Diagnostics (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Wu C, Wu X, Xia J. Hepatitis E virus infection during pregnancy. Virol J. 2020;17:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/