Published online Sep 9, 2025. doi: 10.5492/wjccm.v14.i3.105235
Revised: March 18, 2025
Accepted: March 28, 2025
Published online: September 9, 2025
Processing time: 184 Days and 15.5 Hours
Acute kidney injury (AKI) is common in patients with liver failure, and for a significant subset it is severe enough to require kidney replacement therapy (KRT). Patients with liver failure have distinct clinical characteristics (e.g., cardio-circulatory dysfunction and a tendency to bleed) that mandate customization of their overall care including KRT. Herein, we provide an overview of AKI in liver failure, discuss the basic pathophysiology of hepatorenal syndrome, including the often-underemphasized role of the heart in its clinical manifestations, and the current therapies afforded to these patients. We also discuss the general aspects of KRT and how they apply to patients with liver failure (e.g., preference for con
Core Tip: Acute kidney injury (AKI) is common in patients with liver failure, and a significant subset requires kidney replacement therapy (KRT). These patients have distinct clinical characteristics and needs that mandate customization of their overall care, including KRT. This general overview is meant for critical care providers to familiarize themselves with the nuances of KRT in patients with concomitant liver failure and AKI.
- Citation: Belal AA, Santos Jr AH, Koratala A, Kazory A. Expanding the boundaries of kidney replacement therapy in patients with liver failure. World J Crit Care Med 2025; 14(3): 105235
- URL: https://www.wjgnet.com/2220-3141/full/v14/i3/105235.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v14.i3.105235
Acute kidney injury (AKI) is common in acute liver failure (ALF). In a retrospective analysis of data compiled by the United States ALF Study Group, approximately 70% of patients with ALF were found to have had an episode of AKI[1], with approximately 30% of those requiring kidney replacement therapy (KRT). ALF is defined by the presence of an acute onset of liver function deterioration, elevated international normalized ratio of 1.5 or greater, and hepatic encephalopathy (HE) in patients without known pre-existing liver disease[2]. There is regional variance, but overall, the ALF is relatively uncommon in the Western world, with a calculated incidence of around 1.13-1.61/100000 person-years[3-5]. Overall mortality for ALF is estimated on the order of 30%, with the estimated mortality without a liver transplant (LT) being as high as 50%, driven by multiple mechanisms, including shock and multi-organ failure[6,7]. As can be seen, a significant subset of these patients with ALF are critically ill. The presence of AKI, especially one severe enough to require KRT, portends worse short and long-term outcomes similar to other indications for intensive care[1,8].
A similar pattern is present in patients with cirrhosis as well, with an estimated incidence of AKI of 29% with a 6-fold increase of in-hospital mortality in cirrhotic patients with AKI[9,10]. The presence of AKI in patients with cirrhosis is associated with increased mortality when compared to an AKI on chronic kidney disease (CKD) in patients with cirrhosis[11]. Additionally, the severity of AKI in a decompensated cirrhotic prior to transplant contributes to the likelihood of renal recovery 4-6 weeks post-transplant, thus lending consideration for simultaneous liver-kidney transplantation[12]. The indications for KRT in these patients are fairly similar to the other critically ill patients, including uremic complications, fluid overload with oliguria or anuria, medically refractory hyperkalemia, or metabolic acidosis[13,14]. It should be noted however, that the presentation may be different due to additional factors in patients with cirrhosis, such as the common use of mineralocorticoid receptor antagonists, and the tendency for acidosis and intravascular volume depletion due to frequent diuretic and laxative use. Further considerations for KRT, which are distinct for patients with liver failure, include severe hyperammonemia with progressive HE[15]. Continuous renal replacement therapy (CRRT) is often the preferred modality of KRT in these patients due to their tendency for circulatory dysfunction and hemodynamic ins
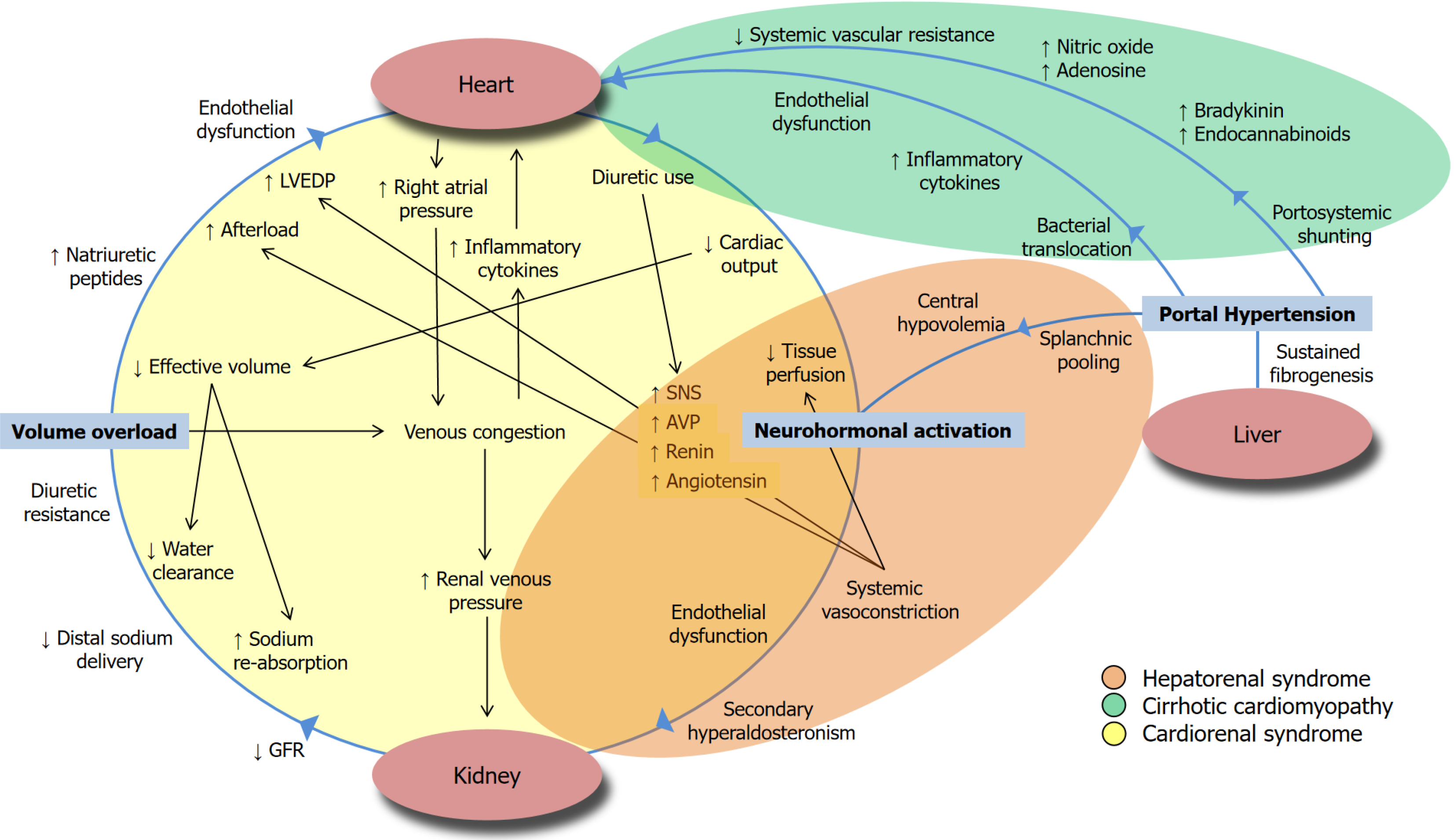
The criteria to diagnose AKI in patients with liver disease and cirrhosis, endorsed by the International Club of Ascites, is based on the Kidney Disease Improving Global Outcomes definition of a serum Cr increase by 0.3 mg/dL within 48 hours or an increase in Cr 50% above baseline Cr within 48 hours, or urine volume below 0.5 mL/kg/h for 6 hours[19]. In liver failure with portal hypertension, there is a special entity, hepatorenal syndrome (HRS) associated AKI (HRS-AKI), that is refractory to volume resuscitation and is due to reduced renal perfusion due to exaggerated splanchnic vasodilation with maladaptive hyperactivity of the neurohormonal axis[9,20]. This HRS-AKI can exist in addition to the common pre-renal, intra-renal, and post-renal etiologies[9,17]. The exact mechanism of this systemic vasodilation is not precise. Still, specific molecules such as the vasodilator nitric oxide (NO) and its distribution have been implicated in playing a key role in the development of portal hypertension, with it being high in the splanchnic circulation while being reduced in the intrahepatic microcirculation[21]. In the late stages of cirrhosis, the maintenance of arterial pressure is thought to be largely dependent on the activation of vasoconstrictor systems such as the renin-angiotensin-aldosterone system (RAAS) and the non-osmotic increased secretion of arginine vasopressin thus leading to ascites/edema and, due to subsequent intense intrarenal vasoconstriction and hypoperfusion, renal failure[22-24]. Recent data suggest that this circulatory dysfunction on display in HRS may overlap with a syndrome of cardiac dysfunction in cirrhosis that plays a role in kidney dysfunction in HRS[17,25]. In response to the reduction in systemic vascular resistance, there is an initial compensatory increase in cardiac output to maintain effective circulatory blood volume that eventually exhausts the cardiac reserve[17]. Cirrhotic cardiomyopathy is defined by cardiac dysfunction in cirrhosis suggested by electromechanical abnormalities, enlarged left atrium, and humoral changes such as an elevated brain natriuretic peptide[26]. This cardiomyopathy is defined by systolic dysfunction characterized by blunted responsiveness of cardiac output to volume and postural or pharmacological challenges that can often identified by advanced diagnostic techniques[27-29]. The systolic dysfunction is usually combined with impaired diastolic relaxation detected by echocardiography of E to A ratio less than one, prolonged deceleration time greater than 200 ms, and prolonged isovolumetric relaxation time greater than 80 ms[26,30]. As in HRS, the pathophysiology of cirrhotic cardiomyopathy is thought to be at least partly due to the pro-inflammatory state of cirrhosis and its corresponding increased activation of cellular signaling pathways due to increased circulating levels of c-reactive proteins and cytokines, such as tumor necrosis factor (TNF)-α and interleukin 6 (IL-6) alongside NO[24]. Based on animal models, these biomarkers could contribute to reduced cardiac contractility and abnormal function of the cardiac myofilaments[30,31]. This mechanism of liver failure-induced AKI is depicted gra
While in a recently published retrospective study of hospitalized patients with cirrhosis with AKI, the AKI etiology was not independently associated with an increased risk of 90-day mortality, it is crucial for a provider to distinguish AKI in liver failure as HRS-AKI and non-HRS-AKI given differences in its management[32]. Acute tubular necrosis was the most common etiology of non-HRS-AKI among patients hospitalized with cirrhosis[32,33]. As is often the case in me
There are specific implications to the management of HRS with this schema that HRS-AKI is borne out of maladaptive RAAS activation, inflammatory mediators involved in oxidative stress, and the knowledge that the heart plays a key role in HRS. For example, the use of albumin along with a vasoconstrictor has long been considered the cornerstone of therapy for HRS. However, in addition to volume expansion and resultant RAAS activation, albumin administration may also provide antioxidant and anti-inflammatory effects, modulating mediators like nuclear factor kappa-light-chain-enhancer activated B cells and NO synthase in cardiac tissue, thus improving cardiac contractility and enhancement of organ perfusion[17,35]. In addition to albumin, the use of terlipressin, the synthetic vasopressin analog derived from the natural hormone lysine-vasopressin, has been extensively studied in HRS-AKI. The investigators have reported that it results in splanchnic vasoconstriction, shunting of blood to the systemic circulation, and reducing activation of RAAS and catecholamine release, thus blunting the release of arginine vasopressin and enhancing renal perfusion[36-38]. The recently published CONFIRM (A Multi-Center, Randomized, Placebo-Controlled Double-Blind Study to Confirm Efficacy and Safety of Terlipressin in Subjects With HRS Type 1) trial showed that terlipressin was able to reverse HRS-AKI[39]. Of note, there were significant respiratory events in the CONFIRM trial that may be modulated by optimizing patients’ intravascular volume status and avoiding excessive albumin administration[36,39]. It is within this context that point-of-care ultrasonography may play a role in the evaluation and management of cirrhotic patients with AKI[40]. It should be noted that with the increasing awareness of the key role of the heart in the development and progression of HRS (i.e. considering HRS as a hepatic form of cardiorenal syndrome), there may be implications for change in the current management of these patients[17]. For example, more recent guidelines have emphasized on the fact that not only albumin administration is not necessary in all cases of HRS-AKI, but it may be useful to use diuretics to decongest these patients[9,17].
There are several points that need to be taken into account when deciding to initiate KRT for patients with advanced liver disease and cirrhosis, particularly given that intradialytic hypotension, which is associated with increased mortality, can rapidly occur in these patients with baseline cardio-circulatory dysfunction[14,41]. Studies have established that continuous modalities offer advantages related to hemodynamic stability when compared against intermittent he
In addition to mitigation of intra-treatment hypotension, CRRT also provides more effective solute clearance as well as the possibility of correcting hyponatremia in a controlled fashion. This is important prior to a potential LT given that cirrhotic patients may be severely hyponatremic, and physiologic changes from the sudden increase in plasma osmolality due to rapid correction of hyponatremia after LT can exacerbate intracranial hypertension or precipitate pontine demyelination[16]. Additionally, patients with ALF are at increased risk of cerebral edema; iHD is known to increase intracranial pressure, leading to patients’ neurologic deterioration[47]. Though continuous, the instantaneous clearance of CRRT is lower than iHD. Therefore, to maintain similar efficiency as iHD, every effort must be made to optimize circuit uptime and reduce circuit clotting, hence leading to the need for anticoagulation[48]. Given the well-recognized pro
The conventional low dose of 25 mL/kg/h for CRRT is often utilized as a high dose CRRT of 40 mL/kg/h has not yet been established to confer superior benefits in terms of mortality reduction[8,52]. However, a higher dose of CRRT has been suggested to improve ammonia clearance[53]. Serum arterial ammonia is believed to be the main driver for cerebral edema in ALF, with higher levels associated with the onset of severe HE[6,54]. However, there is literature implicating serum endotoxin and inflammatory mediators (TNF, IL-6, IL-18) in HE[55]. For patients with AKI and ALF or cirrhosis managed with CRRT, our group tends to use higher clearances to potentially remove ammonia and other inflammatory mediators. A few hours prior to a LT, we lower serum potassium targets. This is due to the high concentration of pot
The optimal timing for initiation of KRT in patients with decompensated cirrhosis is not known. In general, early KRT initiation has not been associated with better outcomes in critically ill patients[57]. Decompensated cirrhotic patients with AKI requiring KRT that are transplant ineligible have dismal prognoses, experience very short-term survival, and require intensive end-of-life care[58]. The decision for KRT initiation for patients with decompensated cirrhosis with AKI where LT is not an option or unlikely should be made with shared decision-making with patients and families to permit goals of care discussions among multidisciplinary care teams[14,59].
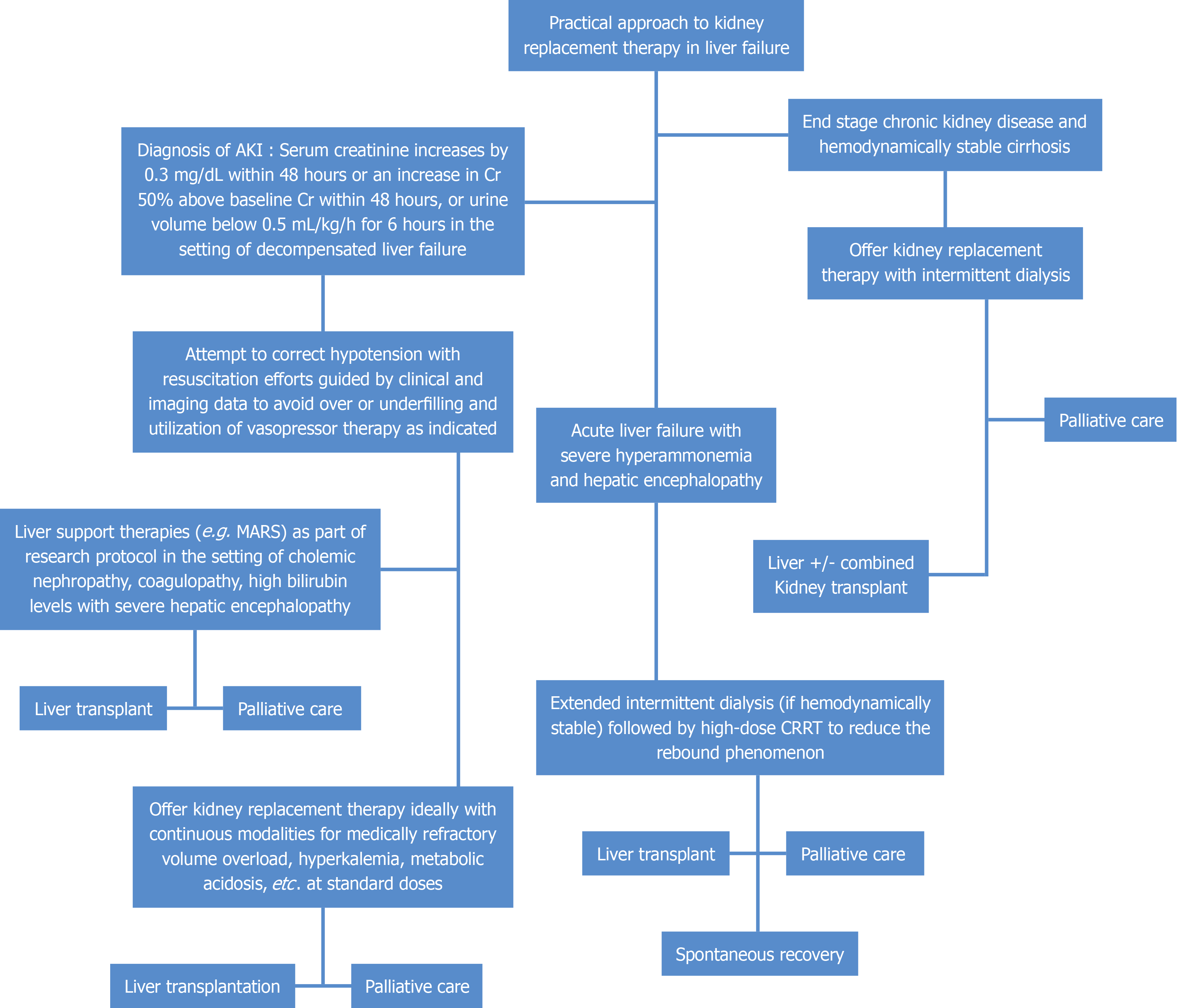
While higher levels of blood ammonia have been implicated in HE in ALF by causing cytotoxic cerebral edema and neuronal oxidative stress[60], inconsistencies in the direct correlation between ammonia concentration and severity of HE in cirrhosis suggest it may not be the sole responsible factor[61]. Rather, it is suspected that inflammation, infection, and ammonia have a complex synergistic relationship in bringing about HE, so there is insufficient data supporting KRT use in chronic liver dysfunction for hyperammonemia[62]. There is a dearth of large cohort studies on adults with chronic liver failure who received KRT for hyperammonemia, with much of the data on the use of KRT for elevated ammonia based on case studies and studies on neonates with inborn errors of metabolism and urea cycle disorders[62]. iHD is the modality that affords the most rapid removal of ammonia, given that it is a small molecule that is not significantly protein-bound. However, there is a significant rebound effect within a few hours of treatment discontinuation, and therefore CRRT has been used in this setting as, in addition to its advantage in patients with hemodynamic instability, it allows continuous removal of ammonia[63,64]. For this reason, it is recommended that iHD be considered as the initial KRT of choice for stable patients with severe hyperammonemia with concern for immediate cerebral herniation with a plan to transition patients to CRRT to prevent a rebound in ammonia level and use of CRRT in hemodynamically unstable patients with hyperammonemia[65-67]. In our institution, for hemodynamically stable patients with ALF and severe hyperammonemia, we perform an extended iHD followed by high-dose CRRT that is meant to reduce the rebound phenomenon. Figure 2 offers a practical approach to choosing modes of KRT in the setting of liver failure.
While a complete review of the liver support therapies is beyond the scope of this review, it suffices to mention that it is a form of extracorporeal therapy in which albumin is used within the Molecular Adsorbent Recycling System (MARS)[65]. Briefly, it is a nonbiologic liver support system that reduces levels of protein-bound and water-soluble toxins such as ammonia, aromatic amino acids, and several inflammatory cytokines[68,69]. MARS is composed of 2 separate dialysis circuits, with the first containing human serum albumin that is in contact with the patient’s blood and allows toxins to pass through a semipermeable membrane. Then, this toxin-laden albumin solution is sent through another dialyzer using a conventional CRRT machine to remove water-soluble toxins with counter-current bicarbonate-based dialysate, and the protein-bound toxins are removed by adsorbent cartridges containing an anion exchanger and activated charcoal[68,70]. There also exists the fractionated plasma separation and adsorption (Prometheus) system[68]. This Prometheus system is also beyond the scope of this review, so briefly speaking, it is a type of albumin dialysis that utilizes a 250-kilo Dalton semipermeable membrane that generates an albumin-containing plasma-like solution that is then adsorbed on two albumin-detoxifying columns upon which hemodialysis is performed[71].
AKI and the need for KRT in liver failure portends a poor prognosis for patients. CRRT is often preferred given the advantages of cardiovascular stability and the possibility of gradual fluid extraction. Hyperammonemia is an emerging non-renal indication for initiation of KRT in this population. Although KRT in this setting is biologically relevant and its role in the management of hyperammonemia in children has been well studied, there remains a relative dearth of high-quality large studies in adults with liver failure to determine the optimal timing of initiation, dose, and the impact on the outcomes. Based on the data available it appears that high-dose CRRT provides more effective ammonia clearance than standard dose. However there does not yet appear to be literature to support improved mortality with high-dose CRRT. Regenerative medicine and gene therapy are among emerging fields that have been expanding exponentially to include several pathologic circumstances including AKI. Whether they will have a role in this specific form of AKI remains to be elucidated by future studies.
| 1. | Tujios SR, Hynan LS, Vazquez MA, Larson AM, Seremba E, Sanders CM, Lee WM; Acute Liver Failure Study Group. Risk factors and outcomes of acute kidney injury in patients with acute liver failure. Clin Gastroenterol Hepatol. 2015;13:352-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 2. | O'Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet. 1993;342:273-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 419] [Article Influence: 12.7] [Reference Citation Analysis (2)] |
| 3. | Weiler N, Schlotmann A, Schnitzbauer AA, Zeuzem S, Welker MW. The Epidemiology of Acute Liver Failure. Dtsch Arztebl Int. 2020;117:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Kulkarni AV, Gustot T, Reddy KR. Liver transplantation for acute liver failure and acute-on-chronic liver failure. Am J Transplant. 2024;24:1950-1962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 5. | Goldberg DS, Forde KA, Carbonari DM, Lewis JD, Leidl KB, Reddy KR, Haynes K, Roy J, Sha D, Marks AR, Schneider JL, Strom BL, Corley DA, Lo Re V 3rd. Population-representative incidence of drug-induced acute liver failure based on an analysis of an integrated health care system. Gastroenterology. 2015;148:1353-61.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (1)] |
| 6. | Dong V, Robinson AM, Dionne JC, Cardoso FS, Rewa OG, Karvellas CJ. Continuous renal replacement therapy and survival in acute liver failure: A systematic review and meta-analysis. J Crit Care. 2024;81:154513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 7. | Reuben A, Tillman H, Fontana RJ, Davern T, McGuire B, Stravitz RT, Durkalski V, Larson AM, Liou I, Fix O, Schilsky M, McCashland T, Hay JE, Murray N, Shaikh OS, Ganger D, Zaman A, Han SB, Chung RT, Smith A, Brown R, Crippin J, Harrison ME, Koch D, Munoz S, Reddy KR, Rossaro L, Satyanarayana R, Hassanein T, Hanje AJ, Olson J, Subramanian R, Karvellas C, Hameed B, Sherker AH, Robuck P, Lee WM. Outcomes in Adults With Acute Liver Failure Between 1998 and 2013: An Observational Cohort Study. Ann Intern Med. 2016;164:724-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 311] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 8. | Sharma B, Bhateja A, Sharma R, Chauhan A, Bodh V. Acute kidney injury in acute liver failure: A narrative review. Indian J Gastroenterol. 2024;43:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Nadim MK, Forni LG, Ostermann M; ADQI 29/ICA Expert Panel. Hepatorenal syndrome in the intensive care unit. Intensive Care Med. 2024;50:978-981. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Tariq R, Hadi Y, Chahal K, Reddy S, Salameh H, Singal AK. Incidence, Mortality and Predictors of Acute Kidney Injury in Patients with Cirrhosis: A Systematic Review and Meta-analysis. J Clin Transl Hepatol. 2020;8:135-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 11. | St Hillien SA, Robinson JE, Ouyang T, Patidar KR, Belcher JM, Cullaro G, Regner KR, Chung RT, Ufere N, Velez JCQ, Neyra JA, Asrani SK, Wadei H, Teixeira JP, Saly DL, Levitsky J, Orman E, Sawinski D, Dageforde LA, Allegretti AS; HRS-HARMONY Consortium. Acute Kidney Injury in Patients with Cirrhosis and Chronic Kidney Disease: Results from the HRS-HARMONY Consortium. Clin Gastroenterol Hepatol. 2024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Chancharoenthana W, Leelahavanichkul A. Acute kidney injury spectrum in patients with chronic liver disease: Where do we stand? World J Gastroenterol. 2019;25:3684-3703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (4)] |
| 13. | Anand AC, Nandi B, Acharya SK, Arora A, Babu S, Batra Y, Chawla YK, Chowdhury A, Chaoudhuri A, Eapen EC, Devarbhavi H, Dhiman RK, Datta Gupta S, Duseja A, Jothimani D, Kapoor D, Kar P, Khuroo MS, Kumar A, Madan K, Mallick B, Maiwall R, Mohan N, Nagral A, Nath P, Panigrahi SC, Pawar A, Philips CA, Prahraj D, Puri P, Rastogi A, Saraswat VA, Saigal S, Shalimar, Shukla A, Singh SP, Verghese T, Wadhawan M; INASL Task-Force on Acute Liver Failure. Indian National Association for the Study of Liver Consensus Statement on Acute Liver Failure (Part-2): Management of Acute Liver Failure. J Clin Exp Hepatol. 2020;10:477-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Pelusio C, Endres P, Neyra JA, Allegretti AS. Renal Replacement Therapy in Cirrhosis: A Contemporary Review. Adv Kidney Dis Health. 2024;31:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Cardoso FS, Gottfried M, Tujios S, Olson JC, Karvellas CJ; US Acute Liver Failure Study Group. Continuous renal replacement therapy is associated with reduced serum ammonia levels and mortality in acute liver failure. Hepatology. 2018;67:711-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 169] [Article Influence: 21.1] [Reference Citation Analysis (6)] |
| 16. | Davenport A. Continuous renal replacement therapies in patients with liver disease. Semin Dial. 2009;22:169-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Koratala A, Verbrugge F, Kazory A. Hepato-Cardio-Renal Syndrome. Adv Kidney Dis Health. 2024;31:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 18. | Lekakis V, Wong F, Gkoufa A, Papatheodoridis GV, Cholongitas E. Mortality of Acute Kidney Injury in Cirrhosis: A Systematic Review and Meta-Analysis of Over 5 Million Patients Across Different Clinical Settings. Aliment Pharmacol Ther. 2025;61:420-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 19. | Angeli P, Gines P, Wong F, Bernardi M, Boyer TD, Gerbes A, Moreau R, Jalan R, Sarin SK, Piano S, Moore K, Lee SS, Durand F, Salerno F, Caraceni P, Kim WR, Arroyo V, Garcia-Tsao G; International Club of Ascites. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut. 2015;64:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 407] [Article Influence: 37.0] [Reference Citation Analysis (1)] |
| 20. | Nadim MK, Garcia-Tsao G. Acute Kidney Injury in Patients with Cirrhosis. N Engl J Med. 2023;388:733-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 111] [Reference Citation Analysis (0)] |
| 21. | Bosch J, Abraldes JG, Fernández M, García-Pagán JC. Hepatic endothelial dysfunction and abnormal angiogenesis: new targets in the treatment of portal hypertension. J Hepatol. 2010;53:558-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 155] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 22. | Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361:1279-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 547] [Article Influence: 32.2] [Reference Citation Analysis (1)] |
| 23. | Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodés J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1131] [Cited by in RCA: 1032] [Article Influence: 27.2] [Reference Citation Analysis (2)] |
| 24. | Khemichian S, Francoz C, Durand F, Karvellas CJ, Nadim MK. Hepatorenal Syndrome. Crit Care Clin. 2021;37:321-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Kazory A, Ronco C. Hepatorenal Syndrome or Hepatocardiorenal Syndrome: Revisiting Basic Concepts in View of Emerging Data. Cardiorenal Med. 2019;9:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (2)] |
| 26. | Møller S, Lee SS. Cirrhotic cardiomyopathy. J Hepatol. 2018;69:958-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Kazankov K, Holland-Fischer P, Andersen NH, Torp P, Sloth E, Aagaard NK, Vilstrup H. Resting myocardial dysfunction in cirrhosis quantified by tissue Doppler imaging. Liver Int. 2011;31:534-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Laffi G, Barletta G, La Villa G, Del Bene R, Riccardi D, Ticali P, Melani L, Fantini F, Gentilini P. Altered cardiovascular responsiveness to active tilting in nonalcoholic cirrhosis. Gastroenterology. 1997;113:891-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Nesbitt GC, Mankad S. Strain and strain rate imaging in cardiomyopathy. Echocardiography. 2009;26:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Wiese S, Hove JD, Bendtsen F, Møller S. Cirrhotic cardiomyopathy: pathogenesis and clinical relevance. Nat Rev Gastroenterol Hepatol. 2014;11:177-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 184] [Article Influence: 15.3] [Reference Citation Analysis (1)] |
| 31. | van Obbergh L, Vallieres Y, Blaise G. Cardiac modifications occurring in the ascitic rat with biliary cirrhosis are nitric oxide related. J Hepatol. 1996;24:747-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Cama-Olivares A, Ouyang T, Takeuchi T, St Hillien SA, Robinson JE, Chung RT, Cullaro G, Karvellas CJ, Levitsky J, Orman ES, Patidar KR, Regner KR, Saly DL, Sawinski D, Sharma P, Teixeira JP, Ufere NN, Velez JCQ, Wadei HM, Wahid N, Allegretti AS, Neyra JA, Belcher JM; HRS-HARMONY Consortium. Association of Hepatorenal Syndrome-Acute Kidney Injury with Mortality in Patients with Cirrhosis Requiring Renal Replacement Therapy: Results from the HRS-HARMONY Consortium. Kidney360. 2025;6:247-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Ma AT, Solé C, Juanola A, Escudé L, Napoleone L, Avitabile E, Pérez-Guasch M, Carol M, Pompili E, Gratacós-Ginés J, Soria A, Rubio AB, Cervera M, Moreta MJ, Morales-Ruiz M, Solà E, Poch E, Fabrellas N, Graupera I, Pose E, Ginès P. Prospective validation of the EASL management algorithm for acute kidney injury in cirrhosis. J Hepatol. 2024;81:441-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 34. | European Association for the Study of the Liver. ; Clinical practice guidelines panel, Wendon, J; Panel members, Cordoba J, Dhawan A, Larsen FS, Manns M, Samuel D, Simpson KJ, Yaron I; EASL Governing Board representative, Bernardi M. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66:1047-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 673] [Article Influence: 74.8] [Reference Citation Analysis (1)] |
| 35. | Bortoluzzi A, Ceolotto G, Gola E, Sticca A, Bova S, Morando F, Piano S, Fasolato S, Rosi S, Gatta A, Angeli P. Positive cardiac inotropic effect of albumin infusion in rodents with cirrhosis and ascites: molecular mechanisms. Hepatology. 2013;57:266-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 36. | Belcher JM, Parada XV, Simonetto DA, Juncos LA, Karakala N, Wadei HM, Sharma P, Regner KR, Nadim MK, Garcia-Tsao G, Velez JCQ, Parikh SM, Chung RT, Allegretti AS; HRS-HARMONY Study Investigators. Terlipressin and the Treatment of Hepatorenal Syndrome: How the CONFIRM Trial Moves the Story Forward. Am J Kidney Dis. 2022;79:737-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 37. | Colson PH, Virsolvy A, Gaudard P, Charrabi A, Corbani M, Manière MJ, Richard S, Guillon G. Terlipressin, a vasoactive prodrug recommended in hepatorenal syndrome, is an agonist of human V1, V2 and V1B receptors: Implications for its safety profile. Pharmacol Res. 2016;113:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Francoz C, Durand F, Kahn JA, Genyk YS, Nadim MK. Hepatorenal Syndrome. Clin J Am Soc Nephrol. 2019;14:774-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 39. | Wong F, Pappas SC, Curry MP, Reddy KR, Rubin RA, Porayko MK, Gonzalez SA, Mumtaz K, Lim N, Simonetto DA, Sharma P, Sanyal AJ, Mayo MJ, Frederick RT, Escalante S, Jamil K; CONFIRM Study Investigators. Terlipressin plus Albumin for the Treatment of Type 1 Hepatorenal Syndrome. N Engl J Med. 2021;384:818-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 332] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 40. | Koratala A, Reisinger N. Point of Care Ultrasound in Cirrhosis-Associated Acute Kidney Injury: Beyond Inferior Vena Cava. Kidney360. 2022;3:1965-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 41. | Durand F, Kellum JA, Nadim MK. Fluid resuscitation in patients with cirrhosis and sepsis: A multidisciplinary perspective. J Hepatol. 2023;79:240-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 42. | Wong LP, Blackley MP, Andreoni KA, Chin H, Falk RJ, Klemmer PJ. Survival of liver transplant candidates with acute renal failure receiving renal replacement therapy. Kidney Int. 2005;68:362-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 43. | Fernández J, Blasi A, Hidalgo E, Karvellas CJ. Bridging the critically ill patient with acute to chronic liver failure to liver transplantation. Am J Transplant. 2024;24:1348-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 44. | Davenport A. Ultrafiltration in diuretic-resistant volume overload in nephrotic syndrome and patients with ascites due to chronic liver disease. Cardiology. 2001;96:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Ali H, Begum Ozturk N, Herdan NE, Luu H, Simsek C, Kazancioglu R, Gurakar A. Current status of simultaneous liver-kidney transplantation. Hepatol Forum. 2024;5:207-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 46. | Prudhomme T, Mesnard B, Branchereau J, Roumiguié M, Maulat C, Muscari F, Kamar N, Soulié M, Gamé X, Sallusto F, Timsit MO, Drouin S. Simultaneous liver-kidney transplantation: future perspective. World J Urol. 2024;42:489. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 47. | Davenport A. Neurogenic pulmonary oedema post-haemodialysis. NDT Plus. 2008;1:41-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 48. | Agarwal B, Shaw S, Shankar Hari M, Burroughs AK, Davenport A. Continuous renal replacement therapy (CRRT) in patients with liver disease: is circuit life different? J Hepatol. 2009;51:504-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 49. | Rabbani A, Dalili N, Ashrafi S, Hassanzadeh K, Aliabbar S, Nikeghbalian S, Malekhoseini SA, Nadiri A. Continuous Renal Replacement Therapy with Low Dose Systemic Heparin in Liver Transplant Recipients. Iran J Kidney Dis. 2021;15:229-234. [PubMed] |
| 50. | Kovvuru K, Velez JCQ. Kidney Replacement Therapy in Patients with Acute Liver Failure and End-Stage Cirrhosis Awaiting Liver Transplantation. Clin Liver Dis. 2022;26:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Zhang W, Bai M, Yu Y, Li L, Zhao L, Sun S, Chen X. Safety and efficacy of regional citrate anticoagulation for continuous renal replacement therapy in liver failure patients: a systematic review and meta-analysis. Crit Care. 2019;23:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 52. | Khan R, Koppe S. Modern Management of Acute Liver Failure. Gastroenterol Clin North Am. 2018;47:313-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 53. | Slack AJ, Auzinger G, Willars C, Dew T, Musto R, Corsilli D, Sherwood R, Wendon JA, Bernal W. Ammonia clearance with haemofiltration in adults with liver disease. Liver Int. 2014;34:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 54. | Bernal W, Hall C, Karvellas CJ, Auzinger G, Sizer E, Wendon J. Arterial ammonia and clinical risk factors for encephalopathy and intracranial hypertension in acute liver failure. Hepatology. 2007;46:1844-1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 315] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 55. | Jain L, Sharma BC, Sharma P, Srivastava S, Agrawal A, Sarin SK. Serum endotoxin and inflammatory mediators in patients with cirrhosis and hepatic encephalopathy. Dig Liver Dis. 2012;44:1027-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 56. | Siniscalchi A, Gamberini L, Laici C, Bardi T, Ercolani G, Lorenzini L, Faenza S. Post reperfusion syndrome during liver transplantation: From pathophysiology to therapy and preventive strategies. World J Gastroenterol. 2016;22:1551-1569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 57. | STARRT-AKI Investigators; Canadian Critical Care Trials Group; Australian and New Zealand Intensive Care Society Clinical Trials Group; United Kingdom Critical Care Research Group; Canadian Nephrology Trials Network; Irish Critical Care Trials Group, Bagshaw SM, Wald R, Adhikari NKJ, Bellomo R, da Costa BR, Dreyfuss D, Du B, Gallagher MP, Gaudry S, Hoste EA, Lamontagne F, Joannidis M, Landoni G, Liu KD, McAuley DF, McGuinness SP, Neyra JA, Nichol AD, Ostermann M, Palevsky PM, Pettilä V, Quenot JP, Qiu H, Rochwerg B, Schneider AG, Smith OM, Thomé F, Thorpe KE, Vaara S, Weir M, Wang AY, Young P, Zarbock A. Timing of Initiation of Renal-Replacement Therapy in Acute Kidney Injury. N Engl J Med. 2020;383:240-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 460] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 58. | Diaz PM, Saly DL, Horick N, Petrosyan R, Gitto Z, Indriolo T, Li L, Kahn-Boesel O, Donlan J, Robinson B, Dow L, Liu A, El-Jawahri A, Parada XV, Combs S, Teixeira J, Chung R, Allegretti AS, Ufere NN. Prognosis of Transplant-Ineligible Patients with Cirrhosis and Acute Kidney Injury Who Initiate Renal Replacement Therapy. Dig Dis Sci. 2024;69:3710-3720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 59. | Bansal AD, Patel AA. Dialysis initiation for patients with decompensated cirrhosis when liver transplant is unlikely. Curr Opin Nephrol Hypertens. 2024;33:212-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 60. | Jackson C, Carlin K, Blondet N, Jordan I, Yalon L, Healey PJ, Symons JM, Menon S. Continuous renal replacement therapy and therapeutic plasma exchange in pediatric liver failure. Eur J Pediatr. 2024;183:3289-3297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 61. | Aldridge DR, Tranah EJ, Shawcross DL. Pathogenesis of hepatic encephalopathy: role of ammonia and systemic inflammation. J Clin Exp Hepatol. 2015;5:S7-S20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 237] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 62. | Kamboj M, Kazory A. Expanding the Boundaries of Combined Renal Replacement Therapy for Non-Renal Indications. Blood Purif. 2019;47:69-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 63. | Seethapathy H, Fenves AZ. Pathophysiology and Management of Hyperammonemia in Organ Transplant Patients. Am J Kidney Dis. 2019;74:390-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 64. | Cordoba J, Blei AT, Mujais S. Determinants of ammonia clearance by hemodialysis. Artif Organs. 1996;20:800-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 65. | Larsen FS, Saliba F. Liver support systems and liver transplantation in acute liver failure. Liver Int. 2025;45:e15633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 66. | Warrillow S, Fisher C, Tibballs H, Bailey M, McArthur C, Lawson-Smith P, Prasad B, Anstey M, Venkatesh B, Dashwood G, Walsham J, Holt A, Wiersema U, Gattas D, Zoeller M, García Álvarez M, Bellomo R; Australasian Management of Acute Liver Failure Investigators (AMALFI). Continuous renal replacement therapy and its impact on hyperammonaemia in acute liver failure. Crit Care Resusc. 2020;22:158-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 67. | Gupta S, Fenves AZ, Hootkins R. The Role of RRT in Hyperammonemic Patients. Clin J Am Soc Nephrol. 2016;11:1872-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 68. | Tsipotis E, Shuja A, Jaber BL. Albumin Dialysis for Liver Failure: A Systematic Review. Adv Chronic Kidney Dis. 2015;22:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 69. | Ronco C, Reis T. Continuous renal replacement therapy and extended indications. Semin Dial. 2021;34:550-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 70. | Struecker B, Raschzok N, Sauer IM. Liver support strategies: cutting-edge technologies. Nat Rev Gastroenterol Hepatol. 2014;11:166-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 71. | Rifai K, Ernst T, Kretschmer U, Bahr MJ, Schneider A, Hafer C, Haller H, Manns MP, Fliser D. Prometheus--a new extracorporeal system for the treatment of liver failure. J Hepatol. 2003;39:984-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 171] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/