INTRODUCTION
Transgender persons
For most people, biological sex can be objectively determined by examining karyotype, internal and external genitalia, and secondary sex traits. As a result of these, the majority of persons can be readily categorized as male or female. In fact, observation of the genitals and, in ambiguous cases, the study of the sex chromosomes is routinely performed after delivery to classify the neonates into the respective sex (assigned sex at birth). Moreover, the observation of secondary sex characteristics occurs automatically and often subconsciously in everyday interactions and serves the categorization of people according to their perceived sex. However, gender identity is a person’s subjective sense of belonging to a gender type (masculine, feminine, a blend of both, or none) and cannot always be grasped by others. Gender expression is formulated through an individual’s exterior manifestations, such as name, attire, hairstyle, speech, mannerisms, and attitudes, and usually conforms to predetermined gender roles[1,2]. Whether these norms are driven by innate biological mechanisms or are socially constructed is a matter of debate.
Transgender persons are those whose gender identity does not correspond with their biological sex. Accordingly, transgender women are individuals who self-identify as female despite having been registered as males at birth, and transgender men are those with a male gender identity even though their natal sex was female. The term ‘transgender’ also includes people who do not fit into the traditional dipole of male/female[3]. The feeling of discomfort, if any, because of the discrepancy between gender identity and biological sex is known as gender dysphoria[4]. It is challenging to determine the precise prevalence of the transgender population due to the lack of specific data and variations in the evidence gathered by various studies. According to estimates, about 0.1%-4.5% of people identify as transgender to some degree. Nonetheless, the prevalence of persons receiving clinical care for gender transition is approximately 0.01%-0.03%[5].
Critical illness
Critical illness refers to severe health conditions that pose significant risks to life, necessitating intensive medical care, often in an intensive care unit (ICU). These conditions, which include sepsis, acute respiratory distress syndrome (ARDS), and severe trauma, often result in multi-organ failure and require complex interventions and continuous monitoring[6,7]. The pathophysiology of critical illness involves a dysregulated host response to stressors, leading to systemic inflammation, endothelial dysfunction, and impaired cellular metabolism. Sepsis, a life-threatening organ dysfunction caused by an abnormal response to infection, can lead to septic shock with profound circulatory and metabolic abnormalities. ARDS is characterized by rapid onset of widespread lung inflammation and severe hypoxemia, necessitating mechanical ventilation[7]. Severe trauma, such as traumatic brain injury and major fractures, demands complex surgical and medical management. Management of these conditions involves hemodynamic support with fluids and vasopressors, respiratory support via mechanical ventilation, renal support with dialysis, nutritional support, and prompt infection control with appropriate antimicrobials. The prognosis varies widely depending on the condition, patient age, comorbidities, and the timeliness of intervention, with some patients recovering fully and others experiencing long-term complications.
THE ENDOCRINOLOGY OF BEING TRANSGENDER
The decision to administer treatment for gender transition depends on the existence of persistent and well-documented gender dysphoria, the ability of the patient to give informed consent, the satisfactory control of underlying somatic or mental morbidities, and a minimum age[8]. A plan for continued care needs to be followed once the transgender state has been established and if physical transitioning is desired. In these cases, the goal of gender-affirming treatment is to align one’s physical attributes with the preferred gender identification and thereby reduce symptoms of gender dysphoria. Hormonal therapy and surgical sex reassignment are possible and appropriate interventions. In particular, the former aims to block the release of endogenous sex hormones and substitute them with those of the desired sex, whereas the latter intends to reconstruct genitalia and other sex traits. These medical procedures are considered to be safe and efficient[9].
Estrogens and testosterone-lowering medications, such as gonadotrophin-releasing hormone (GnRH) agonists or anti-androgens, are common components of transfeminine (male-to-female) hormonal therapy. The mainstay of transmasculine (female-to-male) hormonal therapy is the administration of testosterone. The target is to achieve and maintain sex hormone levels within the physiological range of the desired gender. Unfortunately, relevant large-scale prospective studies have not been conducted yet. Thus, existing guidelines are based primarily on extrapolation from data from the use of hormonal replacement therapy in hypogonadal men and postmenopausal women. In individuals with non-binary gender identities, regimens and dosages should be modified according to clinical goals and the recipient’s preferences. Of course, routine clinical monitoring of physical changes and laboratory screening for possible side effects are required. A typical follow-up is conducted every three months during the first year of treatment and semi-annually or annually afterward.
Apparently, most recipients of masculinizing and feminizing treatment are satisfied with the induced changes in physical features[10]. Existing evidence suggests that gender-affirming hormonal therapy (GAHT) reduces depressive symptoms and emotional distress in both transgender men and transgender women, contributing thereby to a better quality of life. This is presumably the result of either an improved body image or an enhancement of the individual’s sense of self-control and autonomy[11]. In addition, the disclosure of gender identity to the family and social environment seems to have a beneficial effect on the relief of depression and anxiety disorder[12].
Hormonal therapy for transgender men
The administration of testosterone is the appropriate treatment for transgender men and must be accompanied by laboratory monitoring of sex steroids’ serum levels and clinical evaluation of masculinizing manifestations. Before starting hormonal therapy, screening for conditions that may be aggravated by testosterone, such as polycythemia, sleep apnea, deep vein thrombosis, pulmonary embolism, and uncontrolled arterial hypertension, is required[13]. The goal of masculinizing treatment is to achieve the testosterone levels of cisgender men of the same age group while suppressing the endogenous sex hormones, mainly estradiol. Indeed, exogenous testosterone suppresses the hypothalamic-pituitary-gonadal axis and thus inhibits ovarian hormonal production, ovulation, and endometrial lining and apoptosis. The administration of testosterone is usually continued for life to avoid features of hypogonadism, such as vasomotor symptoms and osteoporosis. The selection of the formulation and dosage is based on the recipient’s age and preferences.
The initial masculinizing effects of testosterone therapy include cessation of menstruation, enlargement of the clitoris, and appearance of oily skin and facial acne. Deepening of the voice, male-type hair growth on the face and body, increase in muscle mass, and redistribution of body fat follow shortly thereafter. An increase in libido is also common. Vaginal and cervical atrophy, due to a lack of estrogens, can lead to vaginal dryness, itching, and dyspareunia, for which topical lubricants can be helpful. If amenorrhea is not achieved with testosterone alone, the addition of a progestogen may be considered. If menstruation persists, the progestogen can be replaced with a GnRH agonist. The addition of a progestogen or a GnRH agonist is not necessary if a hysteroophorectomy is performed. These changes develop over a period that begins in the first months of treatment and is completed more than four years later. Androgenetic alopecia can occur in the long-term in people with a genetic predisposition. Of course, height and bone structure remain unaltered, as does the percentage of subcutaneous fat, unless physical activity is increased[14].
Currently, there is no evidence of an increase in short-term and medium-term cardiovascular adverse outcomes in transgender men as a result of the masculinizing treatment. The therapy with testosterone is expected to cause a decrease in high density lipoprotein-cholesterol and an increase in triglycerides and low density lipoprotein-cholesterol[15]. Hematocrit rises during the first year, reaching the cis male reference range, but clinically significant erythrocytosis is rarely induced[16]. Thromboembolic events are also uncommon[17]. In general, the risk of developing polycythemia is greater when injectable testosterone preparations are used[18]. Insulin resistance or hepatotoxicity are not expected due to the discontinuation of the use of oral 17α-alkylated androgenic steroids[19].
Oncological data on transgender men are scarce and cannot lead to firm conclusions. It seems that the risk of developing endometrial cancer due to exposure to androgens is small[20]. In addition, transgender men have a lower risk of developing breast cancer compared to cisgender women[21]. If cervical or breast tissue is present, preventive follow-up with cervical smear examination and mammography, respectively, as performed in cis females, is recommended. Finally, although no reduction in bone mineral density is anticipated, screening for osteoporosis should be performed, especially in those discontinuing testosterone therapy, after gonadectomy, or in the presence of other risk factors for bone loss.
Hormonal therapy for transgender women
Oral or transdermal estradiol is the treatment of choice for transgender women. The dosage should be adjusted to maintain serum estradiol at the same levels as those of premenopausal cisgender women during the follicular phase of the menstrual cycle. The strategy involves administering either low-dose oral estrogens or transdermal or injectable estrogen formulations that escape initial hepatic metabolism to drastically reduce the risk of thrombosis. The use of 17β-estradiol is the most common therapeutic approach. A change in pharmaceutical form can be made if the clinical response is insufficient, if side effects occur, or according to the recipient’s preferences. Oral conjugated estrogens should be avoided because their serum levels cannot be measured with routine blood tests, and therefore, the risk of overdosing is possible. Ethinylestradiol is also not preferred because it is highly thrombogenic[14]. There is no consensus on the necessity of stopping hormonal therapy at a certain age. The usual practice is to reduce the dosage and prescribe transdermal preparations to middle-aged individuals.
The usual doses of estrogens do not cause sufficient suppression of the hypothalamic-pituitary-testicular axis. Therefore, adjunctive therapy to reduce endogenous testosterone is usually required unless a bilateral orchiectomy has been performed. GnRH agonists are very effective in suppressing testosterone secretion, but they are expensive. For this reason, anti-androgens can be alternatively used to suppress testosterone secretion and activity. Cyproterone acetate, a progestogen with anti-androgenic properties, is commonly used. Spironolactone is a potassium-sparing diuretic with an antagonistic effect on the androgen receptor and estrogenic activity. It can be used if other options are limited[9,14]. The use of progesterone remains controversial and is generally avoided in routine clinical practice.
Before starting hormonal therapy, the presence of conditions that may be aggravated by the administered medications, including thromboembolic disease, breast cancer, prolactinoma, coronary artery disease, cerebrovascular disease, cholelithiasis, and hypertriglyceridemia, should be investigated. Necessary laboratory monitoring includes the regular measurement of serum testosterone and estradiol. In addition, prolactin levels need to be determined both before starting treatment and on a regular basis. As the main clinical findings of prolactinomas (hypogonadism, decreased sexual desire, and sometimes gynecomastia) are not readily distinguishable in transgender women, pituitary imaging should be performed in patients with persistently elevated prolactin values despite stable or decreased estrogen levels. However, it should be considered that some transgender women take psychiatric medications that can potentially cause hyperprolactinemia. Regular monitoring of body weight, blood pressure, and blood sugar is also important. In the case of treatment with spironolactone, blood electrolytes should be measured frequently[13,14].
Feminizing treatment causes breast development. However, less than 20% of transgender women reach the breast size of an adult cis woman after two years of treatment. Specifically, the breast volume increases by approximately 72-100 cm3[22], and most recipients usually reach Tanner stage 2 or 3[23]. Consequently, many individuals resort to plastic surgery for a better aesthetic result. Testicular volume is reduced by approximately 40% after one year of treatment[13]. There is also a reduction in muscle mass and a redistribution of body fat with an increase in subcutaneous adipose tissue. The feminizing treatment also leads to decreased facial and body hair and reduced skin oiliness. However, complete hair removal usually requires laser treatment or other hair removal methods. Prior effects of endogenous androgens on the skeleton and voice, including the laryngeal prominence, are not reversed. Lack of sexual desire and erectile dysfunction are usually observed from the first months after initiating hormonal therapy[14].
Side effects due to estrogen and anti-androgen therapy are rare. However, surveillance for the occurrence of cardiovascular events is a primary task. Indeed, there is a generally higher incidence of thromboembolic events in transgender women compared to cis men and cis women. The incidence of myocardial infarction is higher among transgender women compared to that of cis women, but similar to that of cis men[24]. An increase in triglycerides may also occur[25]. Cessation of smoking is essential to reduce the risk of venous thrombosis and cardiovascular complications. Although transgender females often have low bone density prior to the initiation of gender-affirming treatment, pharmacologic estrogen is expected to optimize skeletal health[26]. The incidence of hormone-sensitive cancers appears to be low[27].
ENDOCRINE ALTERATIONS IN CRITICALLY ILL PATIENTS
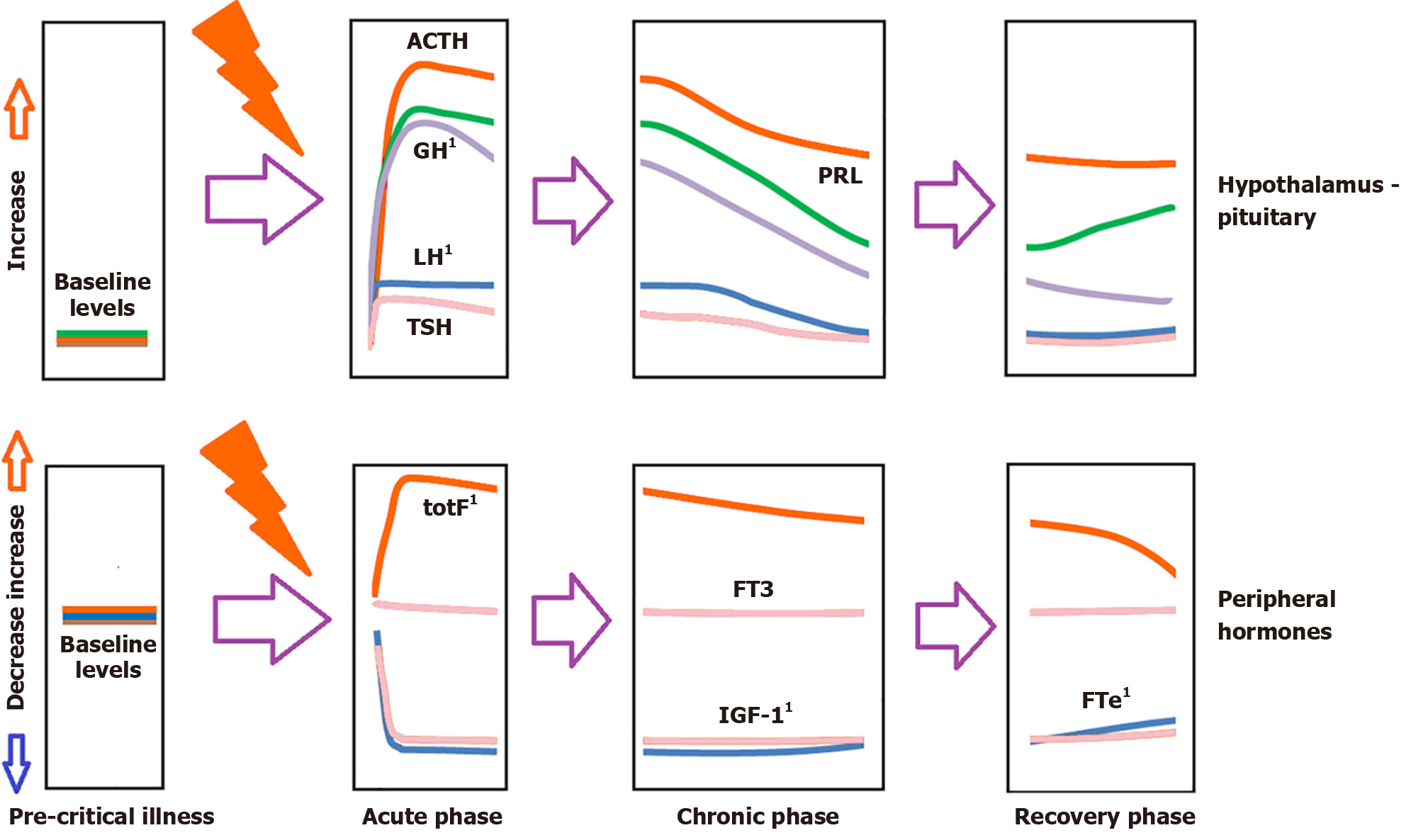
Critical illness induces significant stress in patients, leading to an immediate rise in pituitary stress hormones, including adrenocorticotropin (ACTH), growth hormone (GH), and prolactin (Figure 1)[28].
Figure 1 Schematic hormonal changes in plasma over time in critical illness.
1Denote measurements that may be altered/affected by gender-affirming hormone therapy, please see the article’s text for details. ACTH: Adrenocortocotropin; FTe: Free testosterone; FT3: Free triiodothyronine; GH: Growth hormone; IGF-1: Insulin-like growth factor 1; LH: Luteinizing hormone; PRL: Prolactin; TSH: Thyrotropin; TotF: Total cortisol.
During the acute phase of critical illness (days 1 to 7), free triiodothyronine primarily decreases, whereas in the chronic phase (after 7 days), reductions in both thyrotropin and free thyroxine are observed. In the acute phase of critical illness, there is a marked increase in GH accompanied by a simultaneous decrease in insulin-like growth factor 1 (IGF-1) levels. In the chronic phase of critical illness (after 7 days), there is central suppression of the growth axis, characterized by a relative decrease in both GH and IGF-1.
In both acute and chronic phases, testosterone levels are significantly reduced. In the chronic phase, a central suppression of the hypothalamus-pituitary-gonadal (HPG) axis is noted, accompanied by a decrease in luteinizing hormone (LH)[29-31].
Prolactin levels rise during the acute phase and fall in the chronic phase.
There is a significant increase in ACTH and cortisol during the acute phase. In the chronic phase, ACTH levels decrease relative to baseline, while cortisol levels stabilize[32]. In critically ill patients, the hypothalamic-pituitary-adrenal (HPA) axis is particularly noteworthy. Evidence suggests that the observed increase in cortisol is due to reduced cortisol breakdown rather than increased cortisol production[33]. In critically ill patients, the expression of glucocorticoid receptors decreases over time[34,35]. Additionally, pro-opiomelanocortin (the precursor of ACTH) increases, potentially stimulating the adrenal cortex to produce cortisol.
The acute reduction in binding proteins [thyroid-binding globulin (TBG), corticosteroid-binding globulin, sex hormone-binding globulin (SHBG), insulin-like growth factor binding protein] in critically ill patients impacts both the measurement of total hormones (cortisol, triiodothyronine, thyroxine, IGF-1, sex steroids) and the action and clearance of their free forms.
Approximately one-quarter of critically ill patients without a prior history of diabetes experience stress hyperglycemia[36]. This condition is linked to activation of the HPA axis, increased insulin resistance, and the release of proinflammatory cytokines[37].
The Glasgow Coma Scale (GCS) assesses a patient's level of consciousness based on eye, verbal, and motor responses, providing a score from 3 to 15. The Acute Physiology and Chronic Health Evaluation II (APACHE II) score predicts ICU mortality by evaluating acute physiology, age, and chronic health conditions, with scores ranging from 0 to 71. The Sequential Organ Failure Assessment score tracks a patient's status during ICU stay by measuring the function of six organ systems, with scores from 0 to 24, to assess organ failure severity. Most studies have focused on cortisol or thyroid hormones. Cortisol is linked to APACHE II scores and GCS scores, with the latter also correlating with thyroid hormone levels[37,38]. The prognostic value of adrenal androgens, prolactin, or gonadotropins has been studied to a lesser extent. Prolactin levels have been correlated with the GCS. LH is associated with APACHE II scores, but follicle-stimulating hormone (FSH) is not. Adrenal androgens may also have prognostic value in septic shock[38]. In terms of prognosis, some endocrine parameters correlate with scores from critical illness assessment tools. However, there are limitations to consider, particularly regarding the HPA axis and cortisol levels. Furthermore, regarding prognosis, relatively elevated estradiol levels are linked to a worse prognosis[30,31]. In the chronic phase of critical illness, low ACTH levels, often partly due to corticosteroid administration, are also associated with a poor prognosis. Additionally, while initially promising, the cosyntropin test for assessing adrenocortical reserve in ICU patients may be less reliable than previously believed, due to the increased distribution volume of cortisol[33].
CRITICALLY ILL TRANSGENDER PERSONS: ENDOCRINE CONSIDERATIONS
The main goal of cross-sex therapy is to transform the physiology of the reproductive system by altering the secretion patterns of sex hormones. Estrogen in GAHT typically suppresses LH levels, which in turn reduces testosterone production, often increases SHBG, which binds to and reduces the availability of free testosterone and lowers FSH levels. Testosterone in GAHT suppresses LH and FSH levels, decreasing the production of endogenous estrogen and can reduce SHBG levels, increasing the availability of free testosterone. GnRH agonists are used in GAHT to suppress endogenous hormone production. They suppress the release of LH and FSH from the pituitary gland, decreasing the production of testosterone in transgender women and estrogen in transgender men. Although the hormones of the HPG axis are not apparently essential to survival, their measurement has been considered to assist in evaluating prognosis.
Nonetheless, GAHT can have effects on various endocrine axes beyond its primary targets. As noted above, the integrity of HPA is highly important during critical illness. However, GAHT involves the administration of hormones like estrogen, anti-androgens, and testosterone, which can influence the HPA axis in several ways. Estrogens can modulate the activity of the HPA axis, potentially increasing total cortisol levels in transgender women[39]. It is uncertain though, whether such an effect can affect adrenal reserves in a potential period of stress. Additionally, testosterone can alter the feedback mechanisms of the HPA axis, by inhibiting Corticotropin-releasing hormone secretion, affecting cortisol production and adrenal response[40]. Furthermore, testosterone might increase IGF-1 levels independently of GH[41] or might raise GH concentrations by enhancing the response to GH-releasing hormone[42]. Estrogen has a more variable effect, but it might increase IGF-1 levels in some cases. Moreover, it can increase the levels of TBG, potentially affecting total thyroid hormone concentration without necessarily altering free hormone levels or thyroid function.
In general, the impact of GAHT on the renal function and electrolyte homeostasis of transgender individuals is far-reaching. Indeed, creatinine levels decrease in transgender women undergoing GAHT, whereas in transgender men taking GAHT, creatinine levels can increase[43]. This should be taken into consideration by treating physicians. Spironolactone, a potassium-sparing diuretic and anti-androgen agent, primarily used for heart failure or liver disease, is also employed in chronic anti-androgen therapy for gender-affirming purposes. However, the dosing in GAHT is significantly higher, ranging from 100 mg to 400 mg daily, compared to the 25 mg to 100 mg daily used for heart failure or liver disease. At these higher dosages, spironolactone can interfere with adrenal function, leading to an increase in potassium levels, reduction in volume status, or exacerbation of acute kidney injury during illness, although these effects have not been formally studied[44,45]. Active monitoring of potassium and renal function is crucial in the acute setting, with dosage adjustments made as necessary. If treating physicians opt to discontinue spironolactone therapy, tapering the dosage is not required.
For transgender individuals on estrogen therapy, the greatest concern is the elevated risk of venous thromboembolism (VTE). The risk varies with the formulation of estrogen and additional risk factors like tobacco use or a personal history of VTE. Studies have found that transgender women have a higher incidence of VTE compared to cisgender individuals, with the risk increasing over time[44]. Thrombogenic estrogens, such as ethinylestradiol, should be avoided due to higher VTE risk, although some individuals may still use these formulations from nonprescription sources. Transdermal estrogen appears to confer a lower risk of venous thrombosis, possibly by avoiding first-pass liver metabolism. There is no evidence supporting routine cessation of estrogen therapy in the hospital setting or around the time of surgery, and interruption is typically based on opinion rather than evidence. For VTE prophylaxis, commonly used risk models do not account for hormone therapy in transgender individuals, making it unclear if transgender women should receive additional risk points. Estrogen therapy may also interact with other drugs, such as antiretrovirals and antiepileptics, potentially altering drug efficacy. Indeed, estrogen is metabolized by cytochrome P4503A4. Several antiepileptics induce this cytochrome, leading to enhanced metabolism of the estrogenic agents[46]. Similar mechanisms modulate the interaction between antiretrovirals and exogenous estrogen[47]. Monitoring estrogen levels and adjusting doses might be necessary, although this approach has not been formally studied. If estrogen therapy must be discontinued, tapering can help manage vasomotor symptoms, but abrupt cessation is generally well-tolerated. Further studies are needed to clarify coagulability, cardiovascular, and cerebrovascular risks in transgender individuals on gender-affirming estrogen therapy.
Testosterone therapy in transgender men can lead to thrombogenic erythrocytosis, raising the risk of venous blood clots due to high blood viscosity. This concern led the United States Food and Drug Administration (FDA) to issue a warning about testosterone products in 2014[44]. However, a 2017 systematic review and meta-analysis found that there was insufficient data to accurately assess the VTE risk associated with testosterone use in transgender men[48]. Nonetheless, polycythemia is indeed an unwanted side effect of gender-affirming treatment in transgender men. The direct effects of androgen to erythropoiesis include the stimulation of erythropoietin and the suppression of hepcidin (a regulatory peptide of iron absorption and transport). These two mechanisms reset the regulation of the secretion of erythropoietin at a higher physiologic level of hemoglobin[49]. Higher hemoglobin and hematocrit levels may also derive from the constricting effect of testosterone on the renal microvasculature which in turn promotes the secretion of erythropoietin[50]. It is reasonable to lower the dosage of testosterone during critical illness, while phlebotomy can be performed at hematocrit values above 56% in hypoxic conditions, such as chronic lung disease and cyanotic heart disease (along with oxygen therapy)[51].
Testosterone and estrogen are involved in the metabolism of carbohydrates both through their physiological effects on skeletal muscle, liver, adipose tissue, and immune cells and due to the resultant changes in body fat amount and distribution. It is not clear how cross-sex treatment affects glucose tolerance. Exogenous testosterone and insulin sensitivity in transgender men might be positively, neutrally, or even negatively correlated, while feminizing hormonal therapy for transgender women mostly increases resistance to insulin[52]. Stress hyperglycemia is the elevation of blood glucose as part of the body's response to stress; it is often noted in critically ill patients[53]. This occurs due to increased levels of cortisol, glucagon, and epinephrine, which can also induce insulin resistance, further raising blood glucose levels. However, there is no empirical evidence to suggest that stress hyperglycemia is more prevalent among persons under gender-affirming treatment.
The administration of GAHT entails the immunological modulation of treated persons. This domain has not been studied, with the exception of a few–very limited in size–studies. Estrogen, for instance, has been shown to decrease interleukin-6 (IL-6), a proinflammatory cytokine that accurately reflects the severity of the inflammatory response[54,55], whereas testosterone has been shown to increase IL-6[56]. Studies have identified a strong link between elevated IL-6 levels and a higher risk of multiple organ dysfunction and/or mortality in critically ill patients, including those with sepsis[55]. As a result, IL-6 is widely used as both a diagnostic and prognostic biomarker in critical illness[55]. At present, the immunological alterations in critically ill transgender persons have not been evaluated.
CONCLUSION
In conclusion, critically ill transgender individuals receiving GAHT present unique endocrine challenges that necessitate vigilant management. Estrogen therapy in transgender women reduces creatinine levels and suppresses LH and FSH, while testosterone therapy in transgender men increases creatinine levels and suppresses endogenous estrogen production. Spironolactone, used at higher doses for GAHT, can impact potassium and renal function, requiring close monitoring. Elevated risk of VTE is a significant concern with estrogen therapy, with risks varying by estrogen formulation and patient risk factors. The optimal VTE prophylaxis protocol for estrogen-treated patients should be the object of future studies. Despite FDA concerns about testosterone-related thrombogenic erythrocytosis, relevant data on VTE risk remains inconclusive. Additionally, GAHT affects the HPA axis, with estrogens potentially increasing cortisol levels, and testosterone altering adrenal response. Management of these patients should include careful monitoring of hormone levels, renal function, and potential drug interactions, while recognizing that routine cessation of hormonal therapy is generally unsupported by evidence. Nevertheless, the task of defining a standard monitoring frequency of laboratory parameters is not easy and largely depends on the severity of the clinical status. Daily measurement of electrolytes as well as measurement of the involved hormones (especially cortisol) at the initial assessment as well as every three weeks until sufficient recovery is achieved is a reasonable strategy, but it needs to be validated with evidence from relevant clinical research. Further research is also needed to elucidate the coagulability, immunological, cardiovascular, and cerebrovascular risks in this population, ensuring optimized care for critically ill transgender individuals.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country of origin: Greece
Peer-review report’s classification
Scientific Quality: Grade A
Novelty: Grade A
Creativity or Innovation: Grade B
Scientific Significance: Grade A
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
P-Reviewer: Lampridis S S-Editor: Luo ML L-Editor: Webster JR P-Editor: Li X