Published online Mar 9, 2025. doi: 10.5492/wjccm.v14.i1.101499
Revised: October 22, 2024
Accepted: November 12, 2024
Published online: March 9, 2025
Processing time: 85 Days and 19.1 Hours
Infection is a public health problem and represents a spectrum of disease that can result in sepsis and septic shock. Sepsis is characterized by a dysregulated immune response to infection. Septic shock is the most severe form of sepsis which leads to distributive shock and high mortality rates. There have been significant advances in sepsis management mainly focusing on early identification and therapy. However, complicating matters is the lack of reliable diagnostic tools and the poor specificity and sensitivity of existing scoring tools i.e., systemic inflammatory response syndrome criteria, sequential organ failure assessment (SOFA), or quick SOFA. These limitations have underscored the modest progress in reducing sepsis-related mortality. This review will focus on novel therapeutics such as oxidative stress targets, cytokine modulation, endothelial cell modulation, etc., that are being conceptualized for the management of sepsis and septic shock.
Core Tip: The need for better diagnostic tests and treatments has been voiced for infectious diseases in general with a special emphasis on sepsis. The current state of sepsis treatment revolves around antibiotics and supportive measures. Under
- Citation: Jacob S, Jacob SA, Thoppil J. Targeting sepsis through inflammation and oxidative metabolism. World J Crit Care Med 2025; 14(1): 101499
- URL: https://www.wjgnet.com/2220-3141/full/v14/i1/101499.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v14.i1.101499
Sepsis is a clinical condition representing a spectrum of disease characterized by a dysregulated response to infection[1,2]. It is often the immediate cause of death in critically ill patients and one of the leading causes of morbidity and mortality in the world.3 Research has shown that early identification and timely care has had an impactful reduction in mortality[2-4]. However, sepsis is difficult to define as there is no singular consensus clinical or biochemical test to identify it. The Surviving Sepsis Campaign (SSC) was established to help create consensus strategies to help combat sepsis-related mortality[2]. While there have been successes with these guidelines, the overall mortality rates remain high partly due to the increasing incidence of sepsis worldwide. This may reflect our improved awareness of the importance of identifying sepsis.
The SSC has surveyed the literature and have periodically established guidelines highlighted by the quality of the available evidence backing their recommendations. The most recent guidelines advocate the use of hospital-based bundles with a focus on early identification and treatment with antibiotics, lactate-driven fluid resuscitation, early vasopressor support as needed, and corticosteroid use in refractory shock[1]. The mainstay of therapy remains antibiotics, which is efficacious in bacterial sepsis. However, viral forms of sepsis do not benefit from antibiotic therapy unless a secondary bacterial infection occurs. This was highlighted during the severe acute respiratory syndrome coronavirus-2 pandemic and identified deficiencies in our understanding and management of sepsis. Therefore, it becomes clear that different strategies for managing all forms of sepsis are needed. These strategies should be focused on sepsis pathophysiology which can range from managing inflammation, modulating immune responses, and/or modulating metabolism. This review will discuss the current understanding of sepsis and the role of immunomodulation and pharmacological treatments modulating inflammation in sepsis development and progression. It will explore the potential consequences of targeting these pathways as therapies for sepsis evaluation and treatments.
Advances in sepsis therapy have been limited by many failed therapeutic interventions to block particular pathways or augment immune processes[5]. It is clear that sepsis is an inflammatory disease marked by immune dysfunction; however immunomodulatory therapies trialed thus far have not had any success. This may be a reflection that monotherapy is not sufficient in the management of sepsis[6]. The following section will highlight various immune-modulating therapies and potential targets for the treatment of sepsis. This section highlights cytokine agonism and antagonism as potential therapeutic targets. As cytokines are a byproduct of a response to an inflammatory process, we must remain cognizant of how much modulating inflammation in this way results in a clinical benefit[7].
Interferons (IFNs) performs a variety of roles in sepsis and bacterial infections[8]. In order to combat bacterial and viral threats, IFNs have evolved to stimulate the adaptive immune system to avoid the propagation of infection by enhancing macrophage phagocytic and bactericidal activity. Additionally, IFNs trigger negative feedback loops designed to prevent excessive IFN-induced tissue damage and inflammation[9]. IFNs can also facilitate pro-inflammatory cytokine release and increase monocyte human leukocyte antigen-DR (HLA-DR) expression, thereby facilitating antigen presentation to T-cells. A hallmark of sepsis immunosuppression is the downregulation of HLA-DR expression[10]. For its role in regulating infection, IFN- can act as a potential therapeutic target for reversing immunosuppression in sepsis. Fu and Wang[11] found that in septic mice, using inhibitors of downstream effectors of IFN- signaling promoted sepsis-mediated immunosuppression and that IFN- supplementation reversed this sepsis immunosuppression. This was mediated by promoting the Warburg effect, cytokine secretion, and HLA-DR expression[11]. In simple terms, the Warburg effect is a phenomenon that shifts metabolism from oxidative phosphorylation to rapid aerobic glycolysis, seen typically in tumor cells and now there is growing evidence that this occurs in sepsis[11]. In a separate study, IFN- applied to septic patients with low monocytic HLA-DR expression restored the deficient HLA-DR expression and tumor necrosis factor-alpha (TNF-α) secretion, thus reversing the HLA-DR downregulation[12,13]. These studies illustrate the potential for the use of IFN- in the treatment of sepsis. To understand the complete therapeutic benefits and further establish guidelines, it is essential to conduct larger-scale studies to refine applications of IFN- in sepsis management.
Granulocyte-macrophage colony-stimulating factor (GM-CSF) has been shown to play an integral role in strengthening immune function by boosting the bactericidal abilities of macrophages, monocytes, and neutrophils during sepsis[12,14]. While having important systemic effects on the bone marrow, it also impacts myeloid cell adhesion and neutrophil migration[15]. In ex vivo whole blood cultures of severely septic patients, GM-CSF has been shown to stimulate the production of the monocytic HLA-DR antigen receptor molecule[14]. Similarly, in a controlled immunostimulatory trial of GM-CSF in septic patients, it was found that neutrophils and monocyte levels were increased and that monocytic HLA-DR expression increased rapidly. All patients receiving GM-CSF reached normal monocytic HLA-DR levels following the intervention interval. An increase in proinflammatory cytokine production [interleukin (IL)-6, TNF] and a decrease in anti-inflammatory cytokine production (IL-10) were also linked to the normalization of monocytic HLA-DR levels in septic patients. In addition, a randomized, double-blind clinical study observed a significant increase in mHLA-DR expression in all GM-CSF treatment groups compared to only 15.8% in the control group[15]. Working similarly to IFN-, these studies suggest that the production of HLA-DR, therefore, reverses the downregulation of HLA-DR, a key factor in sepsis immunosuppression. In a phase II trial utilizing GM-CSF in the treatment of septic patients with respiratory dysfunction, GM-CSF showed benefits in improving oxygenation index but was unable to improve 30 days mortality[16]. Also, in septic patients with abdominal infections, a placebo-controlled clinical trial of GM-CSF revealed that the drug could shorten the duration of antibiotic treatment and lessen infection-related complications, however, mortality rates were unaffected[17]. In another randomized trial of GM-CSF use in sepsis, it was found that GM-CSF treatment dramatically improved neutrophil phagocytosis and decreased rates of secondary infections[12,18]. These studies demonstrate that GM-CSF is a viable candidate to promote monocyte and neutrophilic function to combat sepsis immunosuppression.
IL-7 stimulates cell proliferation and suppresses apoptosis, which facilitates lymphocyte activation and survival[12,19]. Sepsis-induced lymphocyte dysfunction includes decreases in circulating CD4+ T cell number, decreased lymphocyte proliferation, increased coinhibitory receptor expression, decreased TCR diversity, and increased percentage of circulating T-regulatory cells[20]. Recombinant IL-7 has been shown in clinical trials to be safe and to have strong benefits on immunological reconstitution[21]. After the first 24 hours of the onset of sepsis, it has been known that T cells undergo apoptosis[22]. In a randomized, double-blind phase II clinical trial evaluating IL-7 therapy in septic shock, it was found IL-7 treatment significantly increased CD4+ and CD8+ T cell counts. It also demonstrated that therapy did not produce an excessive inflammatory cascade[12,23]. IL-7 has also been shown to repair lymphocytes in septic shock[19]. In an ex vivo study evaluating recombinant human IL-7, it was found that IL-7 levels were not significantly altered in sepsis, however, there were significant alterations in lymphocyte proliferation, IFN- production, and TCR expression. Furthermore, incubation with recombinant IL-7 increased IFN- production and promoted TCR diversity[20]. In murine models of sepsis, IL-7 was associated with significant improvement in T cell viability, trafficking, functionality, and survival[24]. These studies all support the hypothesis that IL-7 can improve lymphocyte deficiencies in sepsis.
IL-15 is crucial for the growth, homeostasis, and effector capacities of memory T cells, naïve CD8+ T cells, and NK T-lymphocytes, all of which are important for the removal of pathogens, making it an appealing therapeutic target in sepsis[25,26]. In addition to stimulating the production of mature NK cells in the bone marrow, IL-15 also potently activates and expands peripheral NK cells to carry out cytotoxic tasks and promotes the release of cytokines during bacterial and viral infections. In addition, it plays a significant role in the cytotoxicity, survival, and generation of CD8+ T lymphocytes. T cell exhaustion is an identified cause of sepsis-induced immunosuppression and is linked to secondary infection and has a poor prognosis, especially in the elderly. Although the pattern, effects, and mechanism of T cell exhaustion are still unclear, IL-15 has been shown to improve the survival rate of septic mice. It was revealed that IL-15 improved the survival rate of the mice and inhibited CD8+ T cell apoptosis. IL-15 also significantly increased macrophage and natural killer cell counts. Inoue et al[27] found that IL-15 had broad antiapoptotic effects and protected CD8 T, NK, DC, and intestinal epithelial cells from sepsis-induced apoptosis and augmented IFN-γ production in septic animals. IL-15 is a cytokine with potent survival and immunomodulatory effects on T lymphocytes that could be leveraged into therapy to combat sepsis-induced lymphocyte dysfunction.
IL-6 is considered to play an important role in the evolution of sepsis. It is an early indicator of an inflammatory state[28,29]. Numerous preclinical and clinical studies have demonstrated that IL-6 levels are a good indication of sepsis severity[30]. Vivas et al[28] found that serum levels of IL-6 levels correlated with severity in individuals with septic shock and bacterial sepsis. Declining levels of IL-6 correlated with improved survival at 48 hours following the pharmacotherapy of systemic infection[28]. A meta-analysis by Li et al[31] has also revealed that, after treatment in the ICU, the survival group’s levels of IL-6 were considerably lower than those at baseline, but not in the non-survival group. Researchers concluded that IL-6 variations between baseline and post-treatment levels in the intensive care unit (ICU) are indicators of a favorable prognosis for sepsis patients[31]. Additional studies have suggested that IL-6's primary function in sepsis is prognostic rather than diagnostic. According to Gentile et al[30], blood levels of IL-6 are high early in sepsis, identifying people who are at risk of developing severe sepsis. Hamilton et al[32] in a mendelian randomization study utilizing single nucleotide polymorphisms found that IL-6R blockade was causally associated with a reduced incidence of sepsis. Similarly, in a case series by Mella et al[33] found some benefit in the use of tocilizumab, an IL-6 receptor inhibitor, the treatment of coronavirus disease 2019 (COVID-19) pneumonia renal transplant patients. The REMAP-CAP study of tocilizumab or sarilumab vs standard care in critically ill COVID-19 patients found improved outcomes and when combined with high-dose steroids improved 90 days survival[7,34]. However, overall mortality was not affected with tocilizumab monotherapy and therefore the benefits remain unclear[7]. Overall, the evidence points to IL-6 to be prognostic with higher levels associated with poor outcomes. Further research needs to be done to determine if therapeutic blockade of the IL-6R or downregulation of IL-6 levels can improve outcomes in septic patients.
Transforming growth factor-β (TGF-β) regulates many cellular functions i.e., activation, proliferation, differentiation and migration of immune cells[35]. In sepsis, TGF-β may regulate inflammation by limiting the release of pro-inflammatory cytokine[36]. TGF- β has also been demonstrated to alter T-cell differentiation and suppress IL-2 production in sepsis[37]. Gauthier et al[35] has shown that TGF-β modulates glycolysis in macrophages, independent of inflammatory cytokine production, and influences survival in sepsis mice models. Further, Zheng et al[36] discovered that certain TGF-β1 gene polymorphisms that resulted in lower plasma TGF-β1 levels were associated with patients with a less severe sepsis phenotype, whereas gene polymorphisms resulting in higher plasma TGF-β1 levels were associated with a severe sepsis phenotype. Further research is required to assess the efficacy of TGF-β therapy in the treatment of sepsis.
Immunoglobulin therapy has shown a unique role in the regulation of immune and inflammatory responses. Immunoglobulin is a natural protein that is essential for neutralizing endotoxins and enhancing phagocytic ability[12]. Disulfide bonds and electrostatic forces hold together identical light and heavy chain pairs which constitute each immunoglobulin monomer. Within the heavy chain, there are five immunoglobulin isotypes: IgA, IgD, IgE, IgG, and IgM. IgA, IgG, and IgM are the three most significant classes within the human humoral immune system; IgA functions primarily in mucosal immunity, IgG’s key functions are complement activation, opsonization, and secondary antibody responses, and IgM’s main functions are primary antibody responses, and complement activation. A part of the Ig structure is the light chain which can be found in circulating levels and as a part of the whole Ig molecule. Interestingly, it has been noted that in septic patients there is a correlation with elevated free light chain as compared to healthy controls. Its significance is yet to be determined[38].
Polyvalent intravenous immunoglobulin (IVIG) therapy may be a tool for modulating both pro- and anti-inflammatory processes by regulating components of the immune system and the inflammatory response. IVIG therapy in septic patients can aid in (1) Pathogen detection and clearance; (2) Scavenging and suppressing upstream and downstream mediator gene transcription; and (3) Facilitating anti-apoptotic actions on immune cells[39]. This type of therapy may be ideal as it has been noted that mortality in sepsis is higher in patients with an IgG deficiency[40,41]. IVIG has been shown to neutralize exo- and endotoxins, enhance opsonization-based pathogen phagocytosis, and interact with complement components to minimize nonspecific activation[42]. Furthermore, in a systematic review and meta-analysis of 31 clinical trials, IVIG therapy was shown to reduce mortality, hospital length of stay, and APACHE II scores[39]. Limiting the use of IVIG in the management of sepsis is the cost and lack of large-scale clinical trials[39].
Thymosin alpha 1 (Tα1) has garnered attention for its immunomodulatory properties and potential efficacy in sepsis management. Tα1 is a peptide derived from the thymus gland, manifesting its immunomodulatory effects through various mechanisms[43], with the most notable one involving the enhancement of T-cell function[44]. Tα1 can facilitate T-cell proliferation, activation, and differentiation, thereby fortifying the immune response[44,45]. Additionally, Tα1 has been shown to regulate the maturation and functionality of dendritic cells[12].
There is evidence to support for a therapeutic role for Tα1 in sepsis management. According to a meta-analysis conducted by Gu et al[46] and Li et al[45], Tα1-based immunomodulatory therapy yielded a significant reduction in mortality compared to standard care. This outcome maintained consistency across all subgroup analyses by Gu et al[46]. Furthermore, Tα1-based immunomodulatory therapy demonstrated favorable effects on secondary endpoints, such as shortened duration of ICU stay and mechanical ventilation. It was also noted to increase levels of T lymphocyte subsets (CD3+, CD4+, and CD4+/CD8+), and decrease levels of inflammatory markers (TNF-α, IL-1β, and IL-6)[45]. Similarly, Liu et al[47] found in their systematic review there was a significant decrease in mortality events within the Tα1 group than the control group. Tα1 administration was also associated with reduced levels of inflammatory cytokines and improved clinical outcomes in patients with severe sepsis[47]. These findings necessitate careful examination, as there is a limited availability of studies with substantial sample sizes in the literature. To establish definitive clinical guidelines, further rigorously designed multicenter trials on a global scale are imperative.
Mesenchymal stem cells (MSCs) possess unique immunomodulatory and regenerative properties that make them promising candidates for sepsis therapy. MSCs possess the capacity to differentiate into various cell lineages rendering them potentially advantageous for therapeutic intervention in sepsis[48]. Limited clinical trials have explored the utilization of MSCs as a novel therapy for septic shock[49]. Alp et al[50], undertook a study to examine the effectiveness of MSC therapy in 30 patients with concurrent septic shock and severe neutropenia. MSCs were administered at a dose of 106/kg, and patients were followed up for 28 days. Results indicated that MSC treatment improved short-term survival rates; however, it did not prevent mortality[50]. These findings suggest that early MSC administration during the initial stages of septic shock may improve short-term survival in neutropenic patients, yet it may not offer long-term protection against mortality due to sepsis-related organ dysfunction. An ongoing study in Canada has started a phase II RCT on Umbilical Cord Cellular Immunotherapy for Septic Shock (UC-CISS II). Participants are randomized to receive either 300 million cryopreserved, allogeneic, umbilical cord-derived MSCs or placebo. The primary outcome of UC-CISS II focuses on intermediate measures of clinical efficacy, while secondary outcomes include biomarkers, safety assessments, clinical outcome measures, and a health economic analysis (NCT05969275). This ongoing study may provide crucial insights into the therapeutic potential of MSCs in septic shock management. While there is promise, there are also challenges to ubiquitous utilization. One such challenge will be the availability and accessibility of frozen allogeneic MSCs. Another concern is that the freezing/thawing of MSCs may result in diminished efficacy. Some studies[51,52] suggested that frozen MSCs may exhibit diminished immunomodulatory properties compared to its fresh counterparts.
The Programed cell death-1 (PD1)/programmed cell death ligand-1 (PD-L1) pathway is integral to immune cell regulation and maintenance of immune cell equilibrium. In typical conditions, PD-1 on T-cells interacts with PD-L1 on antigen-presenting cells, inducing T-cell exhaustion and suppressing immune responses to prevent tissue damage[53]. However, during sepsis, this regulatory mechanism becomes disrupted, leading to immune dysfunction and compromised pathogen clearance. Targeting the PD-1/PD-L1 pathway presents a promising strategy to rejuvenate immune function and enhance antimicrobial defenses in septic patients by counteracting T-cell exhaustion and bolstering immune responses[54].
Numerous investigations, both in animal models and human trials, have shed light on modulating the PD-1/PD-L1 pathway in the context of sepsis. Liu et al[55] demonstrated that the blockade of the PD-1/PD-L1 pathway improved survival and attenuated organ dysfunction in multiple murine models of sepsis. Zhang et al[54] found that the administration of anti-PD-L1 antibodies to septic mice was found to diminish lymphocyte apoptosis and increase levels of pro-inflammatory cytokines TNF-α and IL-6, while decreasing anti-inflammatory IL-10 levels. Furthermore, there was a substantial increase in survival rate[54]. Additionally, clinical trials assessing PD-1/PD-L1 checkpoint inhibitors, such as nivolumab, have demonstrated encouraging outcomes in improving the clinical status of patients experiencing immune dysregulation due to sepsis. In a phase I/II study conducted by Watanabe et al[56], Japanese patients with immunosuppressive sepsis received a single intravenous infusion of either 480 or 960 mg of nivolumab. Following nivolumab treatment, there was an increase in absolute lymphocyte counts and monocyte HLA-DR expression levels. These findings support the potential use of PD-1/PD-L1 modulation as a therapeutic approach for sepsis. Additional research, particularly larger-scale clinical trials, is essential to fully comprehend the therapeutic benefits and refine the clinical application of PD-1/PD-L1 pathway inhibitors in the management of sepsis.
Corticosteroid therapy is a promising adjunctive treatment in sepsis, targeting the dysregulated host response and systemic inflammation. It acts by inhibiting pro-inflammatory cytokines, suppressing immune response, and modulating vascular permeability[57]. A meta-analysis conducted by Zhang et al[58] examined the efficacy and safety of various corticosteroids in sepsis. Findings suggested that methylprednisolone and dexamethasone could potentially reduce short-term mortality compared to placebo. Hydrocortisone and hydrocortisone plus fludrocortisone were also found to be superior to placebo in the duration of the resolution of shock[58]. Furthermore, Pitre et al[59] conducted a systematic review focusing on corticosteroid use in septic patients, examining mortality, shock reversal, ICU length of stay, and adverse events. They found that corticosteroids might decrease mortality and increase shock reversal rates[58,59].
In 2024, the Society of Critical Care Medicine empaneled a diverse cohort of intensivists, endocrinologists, pulmonologists, nurses and pharmacists to create guidelines on the use of corticosteroids in sepsis and septic shock. While the evidence was scarce, the panel felt that corticosteroids offered small to moderate desirable effects in patients with septic shock. Specifically, it was noted that corticosteroids resulted in a reduction in organ dysfunction and shock reversal. Despite the small relative effect there is a potential for the translation to large absolute effects given the high prevalence and mortality of septic shock globally[60].
Norepinephrine (NE) has a multi-faceted role in sepsis and there is growing evidence to support that NE interacts with immune cells directly and alters their signaling patterns and metabolic pathways, particularly those that regulate ROS production[61]. It begs the questions of whether NE has a larger role in sepsis than just improving peripheral vascular resistance. The potential for NE dysregulation to contribute to sepsis pathology is worth considering with implications for early introduction of NE in septic patients as therapy, which has had some success in early trials[61-63]. Understanding these pathophysiological mechanisms may pave the way for new diagnostic strategies and therapeutic approaches.
The standard practice typically involves initiating vasopressor therapy only after addressing the hypovolemic aspect of circulatory failure, even in severe patients[64]. Nonetheless, there's growing acknowledgment that in certain scenarios, it may be prudent to commence NE therapy earlier, alongside fluid resuscitation measures. Permpikul et al[63] conducted a randomized controlled study comparing the early administration of NE alone with its concurrent administration alongside a placebo. Shock control within the first 6 hours, the primary outcome, was more frequently achieved in the "early NE" group compared to the other group. This study stands out as one of the few to compare early NE administration directly to a placebo[63]. Ospina-Tascón et al[62] employed a propensity score to investigate the potential influence of initiating norepinepherine early, even prior to completing initial fluid loading, on clinical outcomes in septic shock. Their analysis revealed that early administration of NE, occurring within 1 hour and 3 hours after shock diagnosis, respectively, was associated with a reduction in both the volume of fluid administered and day-28 mortality[62]. Ospina-Tascón et al[62] identified a key limitation is the dependence on individualized considerations, specifically, preload responsiveness. In essence, the efficacy of this approach hinges on personalized care.
Beta blockers, primarily known for their cardiovascular effects in conditions like hypertension and heart failure, have shown promise in modulating the immune response and improving outcomes in sepsis. The first clinical findings on the effects of beta-blocker therapy in patients with septic shock was investigated by Morelli et al[65]. A phase-2 randomized trial was conducted, assessing the use of esmolol in septic shock patients to achieve a target heart rate of 80–94 beats per minute. The trial showed a significant reduction in 28-day mortality with esmolol compared to placebo, alongside increased stroke volume index and decreased fluid volume and noradrenaline requirements[65]. Although the trial lacked power for morbidity or mortality outcomes, it highlighted the potential benefits of esmolol in septic shock management.
Furthermore, Ge et al[66] aimed to investigate the impact of β-blocker therapy on mortality among patients with sepsis. The findings revealed that β-blockers were associated with reduced 28- and 90-day mortality rates, particularly with long-acting β-blocker therapy. Conversely, short-acting β-blocker treatment, such as esmolol, did not demonstrate a mortality reduction in septic patients[66]. These results underscore the potential benefit of long-acting β-blockers in improving survival outcomes for individuals with sepsis and septic shock.
The utilization of beta blockers in sepsis presents a subject of ongoing discussion within the medical community. This discussion is largely fueled by apprehensions surrounding potential adverse outcomes, including hypotension and the obscuring of sepsis-associated tachycardia[66]. Moreover, there is a pressing need for additional research aimed at delineating the most appropriate dosage, timing, and patient criteria for beta-blocker therapy. Existing preclinical and clinical evidence supports the safety of initiating β-blockade at a low dosage in terms of hemodynamic effects[67].
Pharmacological treatments modulating inflammation in sepsis have not resulted in meaningful success in reducing mortality[3,4]. There is evidence to suggest a role in the involvement of oxidative stress in the mechanisms of sepsis and septic shock, thereby laying a foundation for the potential of adjuvant antioxidant therapy. Glycocalyx, Vitamin C, and N-acetylcysteine all have roles in reducing oxidative stress, and their impact in sepsis is summarized in the subsequent section.
The endothelial glycocalyx, an intricate network comprising glycoproteins and proteoglycans lining the luminal surface of blood vessels, is pivotal for maintaining vascular integrity and functionality. However, in sepsis, the systemic inflammatory response triggers the degradation of the glycocalyx. This degradation compromises endothelial barrier function and promotes neutrophil adherence and microvascular thrombosis. Consequently, preserving the integrity of the glycocalyx emerges as an innovative therapeutic approach in the management of sepsis[68]. Several molecules are currently under investigation as potential therapeutics for protecting the glycocalyx. Schmidt et al[69] investigated the mechanisms underlying glycocalyx loss in sepsis and its role in facilitating neutrophil adhesion in the pulmonary circulation. It was hypothesized that sepsis triggers the activation of heparinase, an endogenous enzyme specific to heparin sulfate, leading to degradation of the pulmonary endothelial glycocalyx and promoting neutrophil adherence, thereby contributing to inflammatory lung injury[69]. These findings suggest potential novel therapeutic strategies to mitigate inflammatory lung injury onset during sepsis. Sphingosine-1-phosphate (S1P) emerges as another promising molecule currently under investigation for its potential to safeguard the glycocalyx. S1P, a sphingolipid, is believed to enhance glycocalyx integrity by inhibiting syndecan-1 shedding[70]. This effect is mediated through the activation of the S1P1 receptor, which in turn suppresses the activity of matrix metalloproteinases responsible for syndecan-1 ectodomain shedding[71]. Notably, Coldewey et al[72] indicated that serum S1P levels are diminished in patients with sepsis and septic shock, and this reduction correlates with the severity of sepsis. There remains a necessity for further studies aimed at implementing therapeutic interventions targeted toward glycocalyx degradation in septic conditions.
Vitamin C is an enzymatic cofactor with antioxidant function due to its function acting as an electron donor in oxidative reactions. It can reduce lipid peroxidation and carbonylation, O2–, H2O2, and hypochlorite ion levels, and maintain glutathione (GSH) and vitamin E levels. It can also elevate the levels of the enzymes that facilitate these reactions such as peroxidases, which has been shown to be reduced in sepsis[73]. Vitamin C has also been shown to inhibit the expression of mRNA for the inducible nitric oxide synthase, which leads to the overproduction of nitric oxide and is overexpressed in multi-organ failure in sepsis[73]. It is because of these characteristics that make it a potential therapeutic candidate for the treatment of sepsis and septic shock. There is evidence to suggest that oral vitamin C may reduce the incidence and duration of respiratory infections and intravenous vitamin C has been shown to reduce mortality, ICU and hospital stays, and time on mechanical ventilation for severe respiratory infections[74]. In a review published by Fowler[75], it is postulated that the studies of the molecular pharmacology of vitamin C indicate that it is a potential anti-inflammatory agent that can prevent acute injury of the lungs and other body organs[75]. In a phase 1 trial by Fowler et al[76], it was found that Vitamin C significantly reduced proinflammatory biomarkers in a safe and well-tolerated manner. Subsequently, Marik et al[77] found that the combination of intravenous vitamin C, moderate-dose hydrocortisone, and thiamine appeared to have a marked effect in lowering hospital mortality of patients with severe sepsis and septic shock. At present, there is still much controversy regarding the use of Vitamin C in treating septic shock. In a systematic review with a meta-analysis that included 16 randomized clinical trials, it was concluded that Vitamin C does not help improve clinical outcomes in patients[78]. Furthermore, dosing strategies of vitamin C in critically ill patients are still uncertain as there are concerns for renal injury and many of the studies thus far have been single-center studies with small sample sizes[77]. Larger scale trials are needed for further scrutiny.
N-acetyl cysteine (NAC) is one of the most widely used antioxidants in the context of clinical studies and animal and cell culture experiments; it is also the mainstay of therapy for acetaminophen overdose. It has been shown to lower endogenous oxidant levels and to protect cells against an oxidative insult. It remains unclear as to the molecular mechanisms behind its antioxidative effects[79]. Oliva et al[80] have found in a retrospective case-control study that NAC can reduce mortality in septic shock in patients infected with carbapenem-resistant Klebsiella pneumoniae and Acinetobacter baumannii. In a rat model, NAC was shown to reduce organ damage and ROS accumulation induced by the shock caused by endotoxin[81]. In a randomized clinical trial evaluating vitamin C, vitamin E, NAC, and melatonin, it was found that all the antioxidants used as adjuvant therapy in septic shock decreased multi-organ failure, oxidative stress markers, and sequential organ failure assessment (SOFA) scores. NAC in particular decreased SOFA scores by 42%, increased GSH levels, and decreased proinflammatory cytokine levels[73]. In a randomized clinical trial by Spapen et al[82], it was observed that NAC improves oxygenation and lung compliance in patients with early diagnosed septic shock. Furthermore, the NAC-treated group had a shorter stay in the ICU[82]. In contrast, a separate clinical trial by Molnár et al[83] found that NAC did not show any statistical improvement in measured outcomes in patients with severe sepsis. Further, large-scale trials are needed to evaluate the efficacy of NAC in the treatment of sepsis and septic shock.
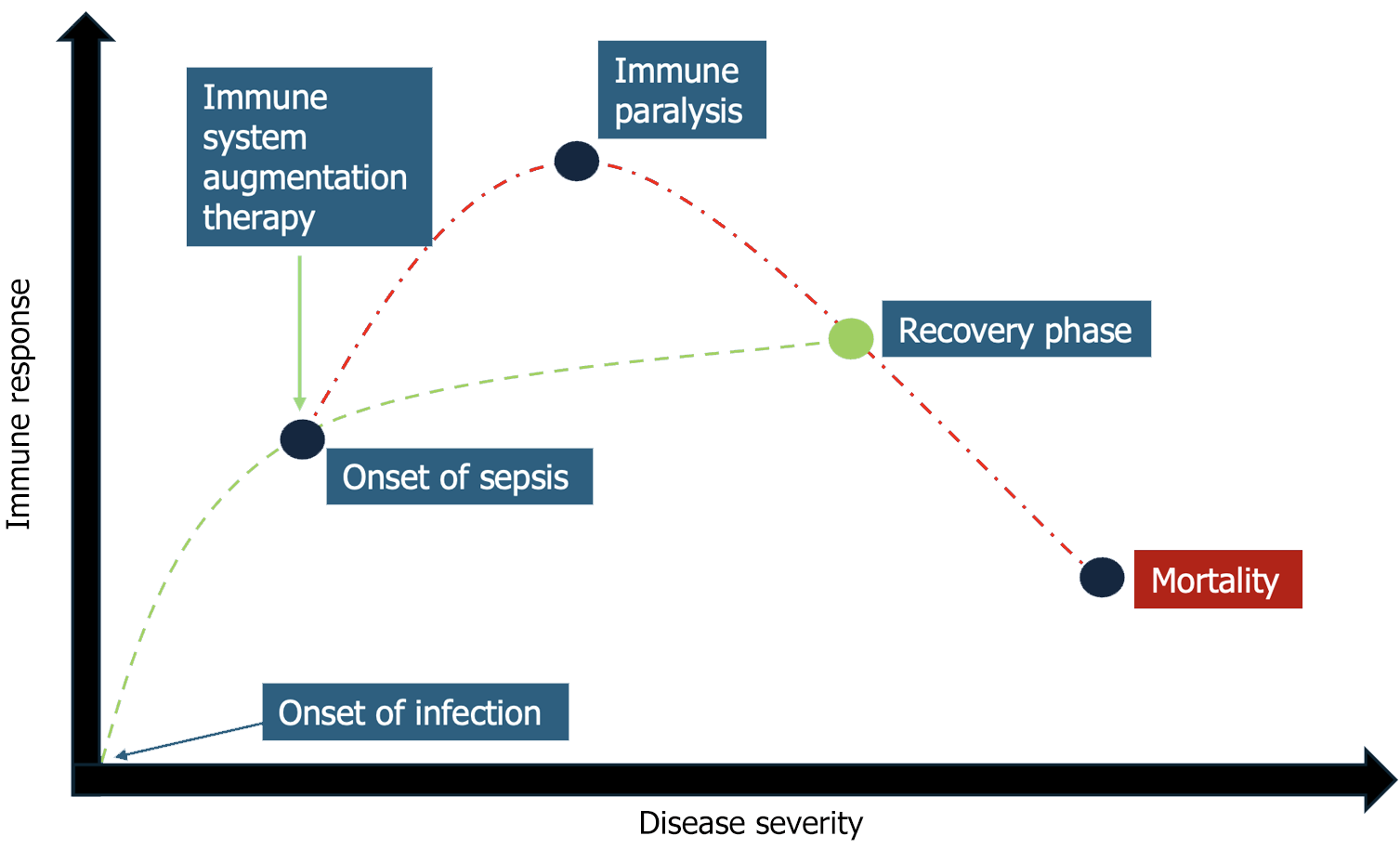
Infections are typically a self-limiting event. However, a proportion of these can develop into a systemic process i.e., sepsis. Sepsis can then progress into organ dysfunction due to a dysregulated host response as delineated in the background portion of this proposal. The complex differential responses of the immune system necessitate careful clinical monitoring to avoid organ dysfunction and damage. The scope of sepsis research, thus far, has been aimed at lowering pathogen burden and supportive care i.e., antipyretics, fluid administration, and antibiotics/anti-virals[84]. Far less time has been spent on identifying regenerative responses of the immune system, which allow the host to retain cell and organ function while fighting infection. Rather than focusing on pathogen burden, perhaps, research focuses should be aimed at augmenting the immune system with its own innate responses to infection (Figure 1). The time-critical measures that have thus far been the mainstay of sepsis therapy have hindered research into regenerative and immune augmenting therapies aimed at limiting dysregulated host responses[84].
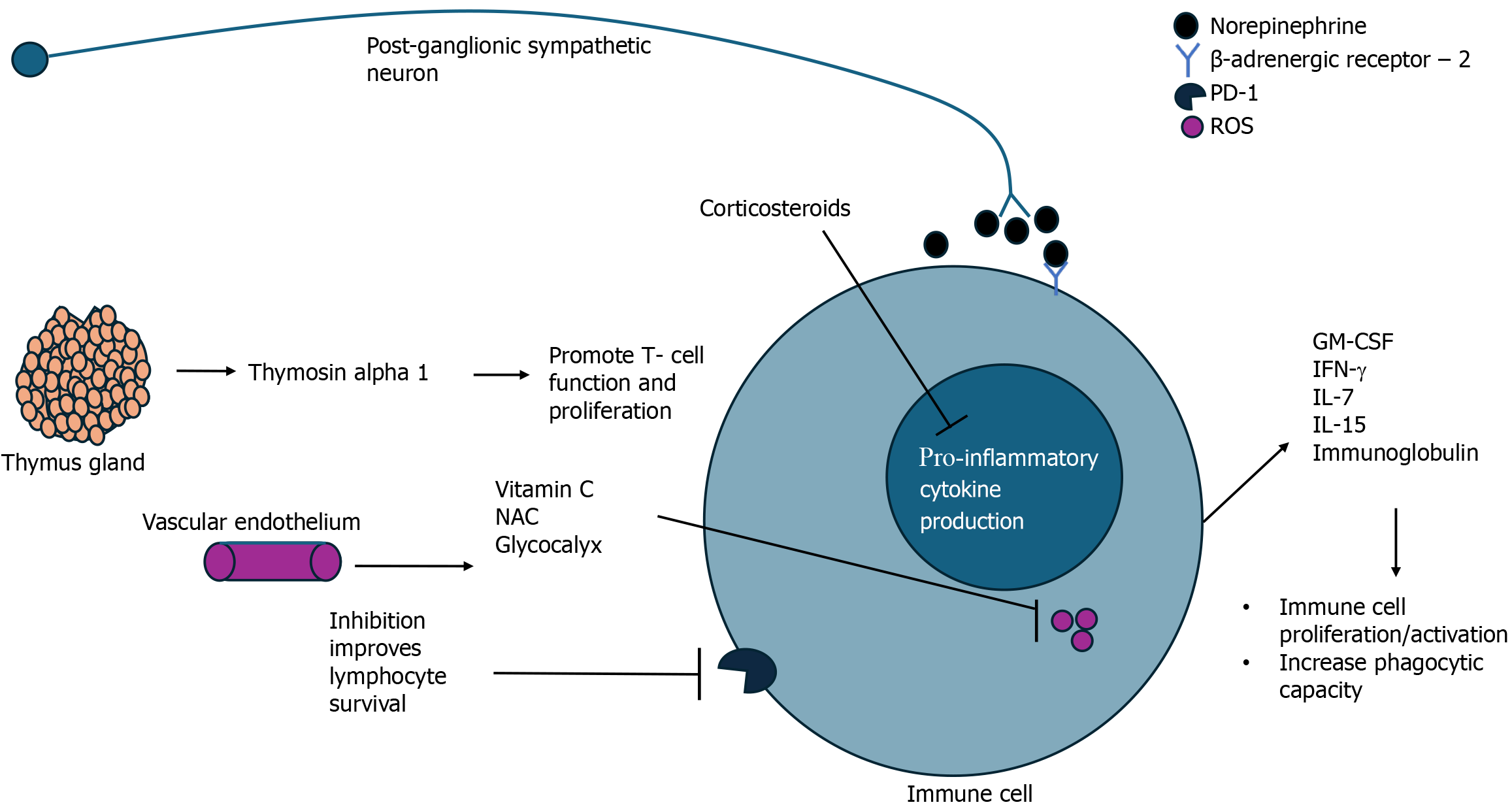
This review has highlighted the evolving landscape of sepsis research and the promise of novel therapeutics targeting either inflammations, oxidative metabolism, or both. Table 1 provides a summary of all the presented targets in this review. Modulation of inflammation and immune responses by utilizing cytokine therapy has shown the ability to avoid sepsis-based immunosuppression, enhance immune responses to infection, and improve outcomes (Figure 2). Harnessing the body’s own defenses and providing exogenous immune support may be a key to unlocking success in the treatment of sepsis. In that same line of reasoning, corticosteroids have re-emerged into the limelight of sepsis management. The most recent guidelines by the Society of Critical Medicine suggests that given the small but measurable benefits of corticosteroid use in septic shock, it is recommended for the reversal of organ dysfunction and shock in these patients[60]. NE, another endogenous molecule, has been deemed the vasopressor of choice in sepsis management; recent evidence has highlighted the potential of NE in modulating immune responses as a mediator of the sympathetic nervous system[61]. Targeting oxidative metabolism as a means of immune augmentation in sepsis is a novel approach to sepsis treatment has largely gone unnoticed due to the lack of clear definitions and understanding of sepsis pathophysiology. Antioxidant therapies such as Vitamin C, NAC, and glycocalyx protection have all shown potential in mitigative oxidative damage and reducing inflammation thereby improving patient outcomes.
| Target | Agonism/antagonism | Proposed mechanism |
| IFN- | Agonism | Reversal of sepsis-based immunosuppression by promotion of Warburg effect, inflammatory cytokine production and HLA-DR expression |
| GM-CSF | Agonism | Promotions of neutrophil and monocyte phagocytic function in sepsis |
| IL-7 | Agonism | Promotions of T-cell activation and suppression of T-cell apoptosis in sepsis |
| IL-15 | Agonism | Promotion of T-cell function and survival in sepsis |
| IL-6 | Antagonism | Unclear; associated with anti-inflammatory cytokine production |
| TGF-β | Agonism | Promotion of T-cell and macrophage activation, proliferation and differentiation |
| Immunoglobulin | N/A | Neutralize toxins, enhance opsonization, and interact with complement components to minimize nonspecific activation |
| Thymosin α1 | Agonism | Enhance T-cell function |
| Mesenchymal stem cells | N/A | Potential to differentiate to different immune cell lineages, modulating to specific host need |
| PD1/PD-L1 | Antagonism | Promoted T-cell survival and function |
| Corticosteroids | N/A | Suppression of the immune response, pro-inflammatory cytokine production and modulation of vascular permeability |
| Norepinephrine | N/A | Unclear; mediates sympathetic nervous system response to infection by immune cell modulation; improve vascular permeability |
| Beta blockers | N/A | Unclear |
| Glycocalyx | N/A | Maintain vascular permeability and function to promote leukocyte adhesion and function |
| Vitamin C | N/A | Reduction of inflammation by modulating pro-inflammatory cytokine release |
| NAC | N/A | Reduction of inflammation by modulating pro-inflammatory cytokine release |
The current state of sepsis treatment revolves around antibiotics and supportive measures. However, when viral-mediated sepsis occurs, we are left with supportive measures alone. Understanding sepsis pathophysiology is crucial to identifying effective therapies to support the immune system, thereby preventing disease progression and organ dysfunction. Discovering novel strategies for immune augmentation may be the key to successfully treating sepsis. Moving forward, it is imperative to continue investigating the mechanisms of action of these therapeutics, conducting rigorous large-scale clinical trials, and refining treatment strategies to enhance sepsis outcomes. By addressing the intricate pathophysiology of sepsis and leveraging innovative treatment approaches, we can strive towards reducing mortality rates, improving patient care, and alleviating the burden of sepsis-related morbidity worldwide.
| 1. | Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, Mcintyre L, Ostermann M, Prescott HC, Schorr C, Simpson S, Wiersinga WJ, Alshamsi F, Angus DC, Arabi Y, Azevedo L, Beale R, Beilman G, Belley-Cote E, Burry L, Cecconi M, Centofanti J, Coz Yataco A, De Waele J, Dellinger RP, Doi K, Du B, Estenssoro E, Ferrer R, Gomersall C, Hodgson C, Hylander Møller M, Iwashyna T, Jacob S, Kleinpell R, Klompas M, Koh Y, Kumar A, Kwizera A, Lobo S, Masur H, McGloughlin S, Mehta S, Mehta Y, Mer M, Nunnally M, Oczkowski S, Osborn T, Papathanassoglou E, Perner A, Puskarich M, Roberts J, Schweickert W, Seckel M, Sevransky J, Sprung CL, Welte T, Zimmerman J, Levy M. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit Care Med. 2021;49:e1063-e1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 1705] [Article Influence: 341.0] [Reference Citation Analysis (2)] |
| 2. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 18855] [Article Influence: 1885.5] [Reference Citation Analysis (4)] |
| 3. | Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, Fleischmann-Struzek C, Machado FR, Reinhart KK, Rowan K, Seymour CW, Watson RS, West TE, Marinho F, Hay SI, Lozano R, Lopez AD, Angus DC, Murray CJL, Naghavi M. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2870] [Cited by in RCA: 4959] [Article Influence: 826.5] [Reference Citation Analysis (7)] |
| 4. | Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 858] [Cited by in RCA: 1055] [Article Influence: 81.2] [Reference Citation Analysis (0)] |
| 5. | Marshall JC. Why have clinical trials in sepsis failed? Trends Mol Med. 2014;20:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 583] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 6. | Delano MJ, Ward PA. The immune system's role in sepsis progression, resolution, and long-term outcome. Immunol Rev. 2016;274:330-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 602] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 7. | McElvaney OJ, Curley GF, Rose-John S, McElvaney NG. Interleukin-6: obstacles to targeting a complex cytokine in critical illness. Lancet Respir Med. 2021;9:643-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 180] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 8. | Romero CR, Herzig DS, Etogo A, Nunez J, Mahmoudizad R, Fang G, Murphey ED, Toliver-Kinsky T, Sherwood ER. The role of interferon-γ in the pathogenesis of acute intra-abdominal sepsis. J Leukoc Biol. 2010;88:725-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Fenton SE, Saleiro D, Platanias LC. Type I and II Interferons in the Anti-Tumor Immune Response. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 10. | Leijte GP, Rimmelé T, Kox M, Bruse N, Monard C, Gossez M, Monneret G, Pickkers P, Venet F. Monocytic HLA-DR expression kinetics in septic shock patients with different pathogens, sites of infection and adverse outcomes. Crit Care. 2020;24:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 11. | Fu XZ, Wang Y. Interferon-γ regulates immunosuppression in septic mice by promoting the Warburg effect through the PI3K/AKT/mTOR pathway. Mol Med. 2023;29:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Liu D, Huang SY, Sun JH, Zhang HC, Cai QL, Gao C, Li L, Cao J, Xu F, Zhou Y, Guan CX, Jin SW, Deng J, Fang XM, Jiang JX, Zeng L. Sepsis-induced immunosuppression: mechanisms, diagnosis and current treatment options. Mil Med Res. 2022;9:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 309] [Article Influence: 77.3] [Reference Citation Analysis (1)] |
| 13. | Döcke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk HD, Kox W. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med. 1997;3:678-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 859] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 14. | Mathias B, Szpila BE, Moore FA, Efron PA, Moldawer LL. A Review of GM-CSF Therapy in Sepsis. Medicine (Baltimore). 2015;94:e2044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, Weber-Carstens S, Hasper D, Keh D, Zuckermann H, Reinke P, Volk HD. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med. 2009;180:640-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 490] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 16. | Presneill JJ, Harris T, Stewart AG, Cade JF, Wilson JW. A randomized phase II trial of granulocyte-macrophage colony-stimulating factor therapy in severe sepsis with respiratory dysfunction. Am J Respir Crit Care Med. 2002;166:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 155] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Orozco H, Arch J, Medina-Franco H, Pantoja JP, González QH, Vilatoba M, Hinojosa C, Vargas-Vorackova F, Sifuentes-Osornio J. Molgramostim (GM-CSF) associated with antibiotic treatment in nontraumatic abdominal sepsis: a randomized, double-blind, placebo-controlled clinical trial. Arch Surg. 2006;141:150-3; discussion 154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Pinder EM, Rostron AJ, Hellyer TP, Ruchaud-Sparagano MH, Scott J, Macfarlane JG, Wiscombe S, Widdrington JD, Roy AI, Linnett VC, Baudouin SV, Wright SE, Chadwick T, Fouweather T, Juss JK, Chilvers ER, Bowett SA, Parker J, McAuley DF, Conway Morris A, Simpson AJ. Randomised controlled trial of GM-CSF in critically ill patients with impaired neutrophil phagocytosis. Thorax. 2018;73:918-925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Francois B, Jeannet R, Daix T, Walton AH, Shotwell MS, Unsinger J, Monneret G, Rimmelé T, Blood T, Morre M, Gregoire A, Mayo GA, Blood J, Durum SK, Sherwood ER, Hotchkiss RS. Interleukin-7 restores lymphocytes in septic shock: the IRIS-7 randomized clinical trial. JCI Insight. 2018;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 302] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 20. | Venet F, Foray AP, Villars-Méchin A, Malcus C, Poitevin-Later F, Lepape A, Monneret G. IL-7 restores lymphocyte functions in septic patients. J Immunol. 2012;189:5073-5081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 21. | Chen D, Tang TX, Deng H, Yang XP, Tang ZH. Interleukin-7 Biology and Its Effects on Immune Cells: Mediator of Generation, Differentiation, Survival, and Homeostasis. Front Immunol. 2021;12:747324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 176] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 22. | Kasten KR, Prakash PS, Unsinger J, Goetzman HS, England LG, Cave CM, Seitz AP, Mazuski CN, Zhou TT, Morre M, Hotchkiss RS, Hildeman DA, Caldwell CC. Interleukin-7 (IL-7) treatment accelerates neutrophil recruitment through gamma delta T-cell IL-17 production in a murine model of sepsis. Infect Immun. 2010;78:4714-4722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Daix T, Mathonnet A, Brakenridge S, Dequin PF, Mira JP, Berbille F, Morre M, Jeannet R, Blood T, Unsinger J, Blood J, Walton A, Moldawer LL, Hotchkiss R, François B. Intravenously administered interleukin-7 to reverse lymphopenia in patients with septic shock: a double-blind, randomized, placebo-controlled trial. Ann Intensive Care. 2023;13:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 52] [Reference Citation Analysis (0)] |
| 24. | Unsinger J, McGlynn M, Kasten KR, Hoekzema AS, Watanabe E, Muenzer JT, McDonough JS, Tschoep J, Ferguson TA, McDunn JE, Morre M, Hildeman DA, Caldwell CC, Hotchkiss RS. IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J Immunol. 2010;184:3768-3779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 247] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 25. | Guo Y, Luan L, Patil NK, Wang J, Bohannon JK, Rabacal W, Fensterheim BA, Hernandez A, Sherwood ER. IL-15 Enables Septic Shock by Maintaining NK Cell Integrity and Function. J Immunol. 2017;198:1320-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Saito M, Inoue S, Yamashita K, Kakeji Y, Fukumoto T, Kotani J. IL-15 Improves Aging-Induced Persistent T Cell Exhaustion in Mouse Models of Repeated Sepsis. Shock. 2020;53:228-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Inoue S, Unsinger J, Davis CG, Muenzer JT, Ferguson TA, Chang K, Osborne DF, Clark AT, Coopersmith CM, McDunn JE, Hotchkiss RS. IL-15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis. J Immunol. 2010;184:1401-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 28. | Vivas MC, Villamarin Guerrero HF, Tascon AJ, Valderrama-Aguirre A. Plasma interleukin-6 levels correlate with survival in patients with bacterial sepsis and septic shock. Interv Med Appl Sci. 2021;11:224-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 29. | Riedemann NC, Neff TA, Guo RF, Bernacki KD, Laudes IJ, Sarma JV, Lambris JD, Ward PA. Protective effects of IL-6 blockade in sepsis are linked to reduced C5a receptor expression. J Immunol. 2003;170:503-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 253] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 30. | Gentile LF, Cuenca AG, Vanzant EL, Efron PA, McKinley B, Moore F, Moldawer LL. Is there value in plasma cytokine measurements in patients with severe trauma and sepsis? Methods. 2013;61:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Li XY, Liu M, Fu YJ, Jiang YJ, Zhang ZN. Alterations in levels of cytokine following treatment to predict outcome of sepsis: A meta-analysis. Cytokine. 2023;161:156056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | Hamilton FW, Thomas M, Arnold D, Palmer T, Moran E, Mentzer AJ, Maskell N, Baillie K, Summers C, Hingorani A, MacGowan A, Khandaker GM, Mitchell R, Davey Smith G, Ghazal P, Timpson NJ. Therapeutic potential of IL6R blockade for the treatment of sepsis and sepsis-related death: A Mendelian randomisation study. PLoS Med. 2023;20:e1004174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 89] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 33. | Mella A, Mingozzi S, Gallo E, Lavacca A, Rossetti M, Clari R, Randone O, Maffei S, Salomone M, Imperiale D, Biancone L. Case series of six kidney transplanted patients with COVID-19 pneumonia treated with tocilizumab. Transpl Infect Dis. 2020;22:e13348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | REMAP-CAP Investigators; Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, Annane D, Beane A, van Bentum-Puijk W, Berry LR, Bhimani Z, Bonten MJM, Bradbury CA, Brunkhorst FM, Buzgau A, Cheng AC, Detry MA, Duffy EJ, Estcourt LJ, Fitzgerald M, Goossens H, Haniffa R, Higgins AM, Hills TE, Horvat CM, Lamontagne F, Lawler PR, Leavis HL, Linstrum KM, Litton E, Lorenzi E, Marshall JC, Mayr FB, McAuley DF, McGlothlin A, McGuinness SP, McVerry BJ, Montgomery SK, Morpeth SC, Murthy S, Orr K, Parke RL, Parker JC, Patanwala AE, Pettilä V, Rademaker E, Santos MS, Saunders CT, Seymour CW, Shankar-Hari M, Sligl WI, Turgeon AF, Turner AM, van de Veerdonk FL, Zarychanski R, Green C, Lewis RJ, Angus DC, McArthur CJ, Berry S, Webb SA, Derde LPG. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N Engl J Med. 2021;384:1491-1502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1355] [Cited by in RCA: 1369] [Article Influence: 273.8] [Reference Citation Analysis (11)] |
| 35. | Gauthier T, Yao C, Dowdy T, Jin W, Lim YJ, Patiño LC, Liu N, Ohlemacher SI, Bynum A, Kazmi R, Bewley CA, Mitrovic M, Martin D, Morell RJ, Eckhaus M, Larion M, Tussiwand R, O'Shea JJ, Chen W. TGF-β uncouples glycolysis and inflammation in macrophages and controls survival during sepsis. Sci Signal. 2023;16:eade0385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 36. | Zheng R, Fu Z, Zhao Z. Association of Transforming Growth Factor β1 Gene Polymorphisms and Inflammatory Factor Levels with Susceptibility to Sepsis. Genet Test Mol Biomarkers. 2021;25:187-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 37. | Ahmad S, Choudhry MA, Shankar R, Sayeed MM. Transforming growth factor-beta negatively modulates T-cell responses in sepsis. FEBS Lett. 1997;402:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Shankar-Hari M, Singer M, Spencer J. Can Concurrent Abnormalities in Free Light Chains and Immunoglobulin Concentrations Identify a Target Population for Immunoglobulin Trials in Sepsis? Crit Care Med. 2017;45:1829-1836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Pan B, Sun P, Pei R, Lin F, Cao H. Efficacy of IVIG therapy for patients with sepsis: a systematic review and meta-analysis. J Transl Med. 2023;21:765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 40. | Dietz S, Lautenschläger C, Müller-Werdan U, Pilz G, Fraunberger P, Päsler M, Ebelt H, Walli AK, Werdan K, Nuding S. Serum IgG levels and mortality in patients with severe sepsis and septic shock : The SBITS data. Med Klin Intensivmed Notfmed. 2017;112:462-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Akatsuka M, Tatsumi H, Sonoda T, Masuda Y. Low immunoglobulin G level is associated with poor outcomes in patients with sepsis and septic shock. J Microbiol Immunol Infect. 2021;54:728-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Jarczak D, Kluge S, Nierhaus A. Use of Intravenous Immunoglobulins in Sepsis Therapy-A Clinical View. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 43. | Lynch HE, Goldberg GL, Chidgey A, Van den Brink MR, Boyd R, Sempowski GD. Thymic involution and immune reconstitution. Trends Immunol. 2009;30:366-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 405] [Cited by in RCA: 379] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 44. | Pei F, Guan X, Wu J. Thymosin alpha 1 treatment for patients with sepsis. Expert Opin Biol Ther. 2018;18:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 45. | Li C, Bo L, Liu Q, Jin F. Thymosin alpha1 based immunomodulatory therapy for sepsis: a systematic review and meta-analysis. Int J Infect Dis. 2015;33:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 46. | Gu WJ, Gu XP, Ma ZL. Thymosin α1-Based Immunomodulatory Therapy for Sepsis: A Meta-Analysis with Trial Sequential Analysis of Randomized Controlled Trials. J Anesth Perioper Med. 2018;5:125-135. [DOI] [Full Text] |
| 47. | Liu F, Wang HM, Wang T, Zhang YM, Zhu X. The efficacy of thymosin α1 as immunomodulatory treatment for sepsis: a systematic review of randomized controlled trials. BMC Infect Dis. 2016;16:488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 48. | Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A; International Society for Cellular Therapy. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1345] [Cited by in RCA: 1397] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 49. | Cóndor JM, Rodrigues CE, Sousa Moreira Rd, Canale D, Volpini RA, Shimizu MH, Camara NO, Noronha Ide L, Andrade L. Treatment With Human Wharton's Jelly-Derived Mesenchymal Stem Cells Attenuates Sepsis-Induced Kidney Injury, Liver Injury, and Endothelial Dysfunction. Stem Cells Transl Med. 2016;5:1048-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 50. | Alp E, Gonen ZB, Gundogan K, Esmaoglu A, Kaynar L, Cetin A, Karakukcu M, Cetin M, Kalin G, Doganay M. The Effect of Mesenchymal Stromal Cells on the Mortality of Patients with Sepsis and Septic Shock: A Promising Therapy. Emerg Med Int. 2022;2022:9222379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 51. | Moll G, Alm JJ, Davies LC, von Bahr L, Heldring N, Stenbeck-Funke L, Hamad OA, Hinsch R, Ignatowicz L, Locke M, Lönnies H, Lambris JD, Teramura Y, Nilsson-Ekdahl K, Nilsson B, Le Blanc K. Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells. 2014;32:2430-2442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 291] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 52. | Bárcia RN, Santos JM, Teixeira M, Filipe M, Pereira ARS, Ministro A, Água-Doce A, Carvalheiro M, Gaspar MM, Miranda JP, Graça L, Simões S, Santos SCR, Cruz P, Cruz H. Umbilical cord tissue-derived mesenchymal stromal cells maintain immunomodulatory and angiogenic potencies after cryopreservation and subsequent thawing. Cytotherapy. 2017;19:360-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 53. | Gianchecchi E, Delfino DV, Fierabracci A. Recent insights into the role of the PD-1/PD-L1 pathway in immunological tolerance and autoimmunity. Autoimmun Rev. 2013;12:1091-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 219] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 54. | Zhang T, Yu-Jing L, Ma T. Role of regulation of PD-1 and PD-L1 expression in sepsis. Front Immunol. 2023;14:1029438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 55. | Liu Q, Li CS. Programmed Cell Death-1/Programmed Death-ligand 1 Pathway: A New Target for Sepsis. Chin Med J (Engl). 2017;130:986-992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 56. | Watanabe E, Nishida O, Kakihana Y, Odani M, Okamura T, Harada T, Oda S. Pharmacokinetics, Pharmacodynamics, and Safety of Nivolumab in Patients With Sepsis-Induced Immunosuppression: A Multicenter, Open-Label Phase 1/2 Study. Shock. 2020;53:686-694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 57. | Annane D, Renault A, Brun-Buisson C, Megarbane B, Quenot JP, Siami S, Cariou A, Forceville X, Schwebel C, Martin C, Timsit JF, Misset B, Ali Benali M, Colin G, Souweine B, Asehnoune K, Mercier E, Chimot L, Charpentier C, François B, Boulain T, Petitpas F, Constantin JM, Dhonneur G, Baudin F, Combes A, Bohé J, Loriferne JF, Amathieu R, Cook F, Slama M, Leroy O, Capellier G, Dargent A, Hissem T, Maxime V, Bellissant E; CRICS-TRIGGERSEP Network. Hydrocortisone plus Fludrocortisone for Adults with Septic Shock. N Engl J Med. 2018;378:809-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 652] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 58. | Zhang S, Chang W, Xie J, Wu Z, Yang Y, Qiu H. The Efficacy, Safety, and Optimal Regimen of Corticosteroids in Sepsis: A Bayesian Network Meta-Analysis. Crit Care Explor. 2020;2:e0094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 59. | Pitre T, Drover K, Chaudhuri D, Zeraaktkar D, Menon K, Gershengorn HB, Jayaprakash N, Spencer-Segal JL, Pastores SM, Nei AM, Annane D, Rochwerg B. Corticosteroids in Sepsis and Septic Shock: A Systematic Review, Pairwise, and Dose-Response Meta-Analysis. Crit Care Explor. 2024;6:e1000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 60. | Chaudhuri D, Nei AM, Rochwerg B, Balk RA, Asehnoune K, Cadena R, Carcillo JA, Correa R, Drover K, Esper AM, Gershengorn HB, Hammond NE, Jayaprakash N, Menon K, Nazer L, Pitre T, Qasim ZA, Russell JA, Santos AP, Sarwal A, Spencer-Segal J, Tilouche N, Annane D, Pastores SM. 2024 Focused Update: Guidelines on Use of Corticosteroids in Sepsis, Acute Respiratory Distress Syndrome, and Community-Acquired Pneumonia. Crit Care Med. 2024;52:e219-e233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 139] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 61. | Thoppil J, Mehta P, Bartels B, Sharma D, Farrar JD. Impact of norepinephrine on immunity and oxidative metabolism in sepsis. Front Immunol. 2023;14:1271098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 62. | Ospina-Tascón GA, Hernandez G, Alvarez I, Calderón-Tapia LE, Manzano-Nunez R, Sánchez-Ortiz AI, Quiñones E, Ruiz-Yucuma JE, Aldana JL, Teboul JL, Cavalcanti AB, De Backer D, Bakker J. Effects of very early start of norepinephrine in patients with septic shock: a propensity score-based analysis. Crit Care. 2020;24:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 63. | Permpikul C, Tongyoo S, Viarasilpa T, Trainarongsakul T, Chakorn T, Udompanturak S. Early Use of Norepinephrine in Septic Shock Resuscitation (CENSER). A Randomized Trial. Am J Respir Crit Care Med. 2019;199:1097-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 213] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 64. | Monnet X, Lai C, Ospina-Tascon G, De Backer D. Evidence for a personalized early start of norepinephrine in septic shock. Crit Care. 2023;27:322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 65. | Morelli A, Ertmer C, Westphal M, Rehberg S, Kampmeier T, Ligges S, Orecchioni A, D'Egidio A, D'Ippoliti F, Raffone C, Venditti M, Guarracino F, Girardis M, Tritapepe L, Pietropaoli P, Mebazaa A, Singer M. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA. 2013;310:1683-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 551] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 66. | Ge CL, Zhang LN, Ai YH, Chen W, Ye ZW, Zou Y, Peng QY. Effect of β-blockers on mortality in patients with sepsis: A propensity-score matched analysis. Front Cell Infect Microbiol. 2023;13:1121444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 67. | Lescroart M, Pequignot B, Kimmoun A, Klein T, Levy B. Beta-blockers in septic shock: What is new? J Intensive Med. 2022;2:150-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 68. | Sullivan RC, Rockstrom MD, Schmidt EP, Hippensteel JA. Endothelial glycocalyx degradation during sepsis: Causes and consequences. Matrix Biol Plus. 2021;12:100094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 69. | Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, Zemans RL, Bowman JC, Koyanagi DE, Yunt ZX, Smith LP, Cheng SS, Overdier KH, Thompson KR, Geraci MW, Douglas IS, Pearse DB, Tuder RM. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. 2012;18:1217-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 672] [Article Influence: 48.0] [Reference Citation Analysis (1)] |
| 70. | Uchimido R, Schmidt EP, Shapiro NI. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Crit Care. 2019;23:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 444] [Article Influence: 63.4] [Reference Citation Analysis (1)] |
| 71. | Zeng Y, Adamson RH, Curry FR, Tarbell JM. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. Am J Physiol Heart Circ Physiol. 2014;306:H363-H372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 212] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 72. | Coldewey SM, Benetti E, Collino M, Pfeilschifter J, Sponholz C, Bauer M, Huwiler A, Thiemermann C. Elevation of serum sphingosine-1-phosphate attenuates impaired cardiac function in experimental sepsis. Sci Rep. 2016;6:27594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 73. | Aisa-Álvarez A, Pérez-Torres I, Guarner-Lans V, Manzano-Pech L, Cruz-Soto R, Márquez-Velasco R, Casarez-Alvarado S, Franco-Granillo J, Núñez-Martínez ME, Soto ME. Randomized Clinical Trial of Antioxidant Therapy Patients with Septic Shock and Organ Dysfunction in the ICU: SOFA Score Reduction by Improvement of the Enzymatic and Non-Enzymatic Antioxidant System. Cells. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 74. | Holford P, Carr AC, Jovic TH, Ali SR, Whitaker IS, Marik PE, Smith AD. Vitamin C-An Adjunctive Therapy for Respiratory Infection, Sepsis and COVID-19. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 75. | Fowler AA 3rd. Vitamin C: Rationale for Its Use in Sepsis-Induced Acute Respiratory Distress Syndrome (ARDS). Antioxidants (Basel). 2024;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 76. | Fowler AA 3rd, Syed AA, Knowlson S, Sculthorpe R, Farthing D, DeWilde C, Farthing CA, Larus TL, Martin E, Brophy DF, Gupta S; Medical Respiratory Intensive Care Unit Nursing, Fisher BJ, Natarajan R. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. 2014;12:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 410] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 77. | Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest. 2017;151:1229-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 726] [Cited by in RCA: 657] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 78. | Chen CY, Chiu CT, Lee HS, Lai CC. The impact of vitamin C-containing treatment on the mortality of patients with sepsis: A systematic review and meta-analysis of randomized controlled trials. J Infect Public Health. 2022;15:1514-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 79. | Ezeriņa D, Takano Y, Hanaoka K, Urano Y, Dick TP. N-Acetyl Cysteine Functions as a Fast-Acting Antioxidant by Triggering Intracellular H(2)S and Sulfane Sulfur Production. Cell Chem Biol. 2018;25:447-459.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 304] [Article Influence: 38.0] [Reference Citation Analysis (6)] |
| 80. | Oliva A, Bianchi A, Russo A, Ceccarelli G, Cancelli F, Aloj F, Alunni Fegatelli D, Mastroianni CM, Venditti M. Effect of N-Acetylcysteine Administration on 30-Day Mortality in Critically Ill Patients with Septic Shock Caused by Carbapenem-Resistant Klebsiella pneumoniae and Acinetobacter baumannii: A Retrospective Case-Control Study. Antibiotics (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 81. | Hsu BG, Lee RP, Yang FL, Harn HJ, Chen HI. Post-treatment with N-acetylcysteine ameliorates endotoxin shock-induced organ damage in conscious rats. Life Sci. 2006;79:2010-2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 82. | Spapen H, Zhang H, Demanet C, Vleminckx W, Vincent JL, Huyghens L. Does N-acetyl-L-cysteine influence cytokine response during early human septic shock? Chest. 1998;113:1616-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 83. | Molnár Z, Shearer E, Lowe D. N-Acetylcysteine treatment to prevent the progression of multisystem organ failure: a prospective, randomized, placebo-controlled study. Crit Care Med. 1999;27:1100-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 84. | Bauer M, Wetzker R. The cellular basis of organ failure in sepsis-signaling during damage and repair processes. Med Klin Intensivmed Notfmed. 2020;115:4-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/