Published online Sep 9, 2024. doi: 10.5492/wjccm.v13.i3.97205
Revised: July 16, 2024
Accepted: August 6, 2024
Published online: September 9, 2024
Processing time: 96 Days and 16 Hours
Neuromonitoring in medical intensive care units is challenging as most patients are unfit for invasive intracranial pressure (ICP) modalities or unstable to tran
To compile the existing evidence for understanding the scope of ONSD in mea
PubMed, Google Scholar and research citation analysis databases were searched for studies in adult patients with non-traumatic causes of raised ICP. Studies from 2010 to 2024 in English languages were included.
We found 37 articles relevant to our search. The cutoff for ONSD in predicting ICP varied from 4.1 to 6.3 mm. Most of the articles used cerebrospinal fluid open
ONSD is a useful tool for the diagnosis of raised ICP in non-traumatic neuro-critically ill patients and may also have a role in the prognostication of a subset of patients.
Core Tip: Neuromonitoring in critically ill patients is challenging as many patients are unfit for invasive intracranial pressure (ICP) monitoring or unstable to transport for imaging. Bedside ultrasonography based optic nerve sheath diameter (ONSD) has been proven to be a reliable option in patients with traumatic brain injury (TBI). However, it’s efficacy has not been extensively evaluated in neuro-medical patients. In this review, we analyzed data from 37 articles which had compared ONSD with other established modalities of measuring ICP in non-TBI patients. The analyzed data suggests that ONSD may be a useful tool to detect raised ICP and predict outcome in patients with acute ischemic stroke, intracerebral bleed and intracranial infection. However, further large-scale randomized trials are required, especially in patients with septic metabolic encephalopathy, dysnatremias and aneurysmal subarachnoid hemorrhage, before it is routinely employed in managing neuro-medical patients with elevated ICP.
- Citation: Bhide M, Juneja D, Singh O, Mohanty S. Optic nerve sheath diameters in nontraumatic brain injury: A scoping review and role in the intensive care unit. World J Crit Care Med 2024; 13(3): 97205
- URL: https://www.wjgnet.com/2220-3141/full/v13/i3/97205.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v13.i3.97205
A number of pathologies lead to increased intracranial pressure (ICP) in medical intensive care unit (ICUs). A new onset altered sensorium can be due to septic or metabolic encephalopathy, new intracranial vascular event, anoxic brain injury, newly diagnosed intracranial malignancy, seizures, posterior reversible leukoencephalopathy syndrome (PRES), intra
The review was conducted in accordance with the Joanna Briggs Institute methodology for scoping reviews[6].
The objective of this review was to describe the extent and type of evidence for monitoring ICP in non-TBI using US-ONSD. Our primary outcomes for the study were: To assess the strength of evidence of ONSD's accuracy in non-traumatic cases compared to other modalities of ICP monitoring, both invasive and noninvasive. Evaluate cut off for ONSD in non-traumatic increased ICP. Narrative review of the role of ONSD in medical ICUs.
Inclusion criteria: (1) Studies including patients over 18 years old with raised ICP on whom US-ONSD was performed; All the studies irrespective of the study site (emergency department, ICU, neurology or neurosurgery wards); (2) Studies which evaluated the predictive value of ONSD for determining the ICP using any of the comparator parameters to detect elevated ICP (intraventricular catheter, intraparenchymal monitor, CSF opening pressure on lumbar puncture (LP), radiological signs of cerebral oedema on CT/MRI) either as primary objective or secondary outcomes were included; (3) Studies on neurosurgical patients who did not have any history of trauma were also included; and (4) Studies in the English language.
Exclusion criteria: (1) Studies which included patients with TBI; (2) Studies with ill-defined aetiology of raised ICP; (3) Literature reviews, letters to editors, editorials and clinical opinions; and (4) Studies which compared ONSD to indirect evidence of raised ICP like papilloedema on fundoscopy or optic disc height (ODH) on ultrasonography, Glasgow Coma Score (GCS), automated pupillometry, transcranial doppler (TCD), or ophthalmic artery indices.
Concept: In patients with TBI, raised ICP is the norm. However, not all patients with altered sensorium in a medical ICU have increased ICP. Untreated intracranial hypertension (ICH) can lead to worse patient outcomes. The points of interest in our review included the primary pathology involving the central nervous system, methodological details, the diag
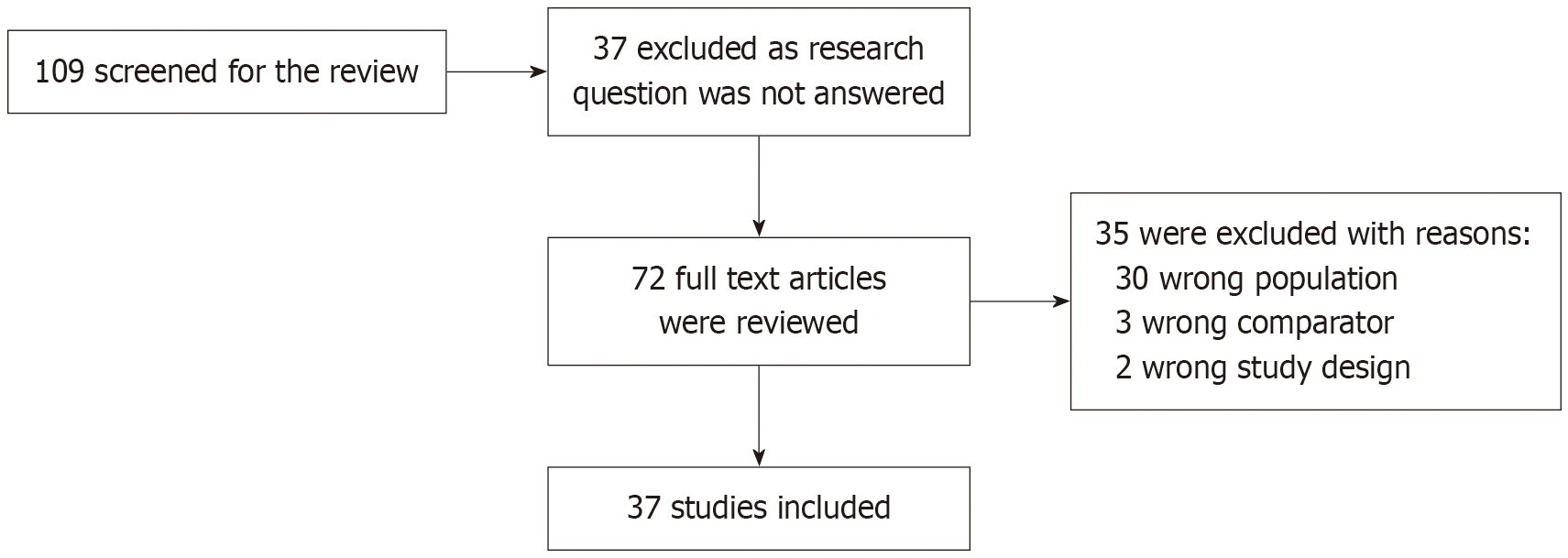
Search strategy: A search in PubMed, Google Scholar and Research citation analysis aimed to find studies published between January 2010 and March 2024. This expansive period was considered with an effort to integrate maximum number of studies. Only studies after 2010 were included to remain relevant to technological advances and updated clinical practices that have occurred in the last two decades. 109 studies were screened, 37 were excluded as they did not address the research question. Two reviewers reviewed 72 articles; 35 were excluded, as shown in Figure 1. A total of 37 articles which satisfied the inclusion and exclusion criteria and passed the review were included in the study.
We included 37 publications that answered our search question according to our inclusion and exclusion criteria. Table 1 outlines the characteristics of the reviewed studies. The most common method for detecting increased ICP was CSF opening pressure on LP (20 studies, 54.05%), followed by the presence of cerebral oedema on CT/MRI scans (14 studies, 37.83%) and intraventricular catheter (3 studies, 8.1%).
| Study characteristic | Number of studies, n = 37 |
| Year of publication | |
| 2013 | 1 (2.7) |
| 2014 | 3 (8.1) |
| 2015 | 5 (13.5) |
| 2016 | 3 (8.1) |
| 2017 | 4 (10.8) |
| 2018 | 2 (5.4) |
| 2019 | 4 (10.8) |
| 2020 | 1 (2.7) |
| 2021 | 3 (8.1) |
| 2022 | 2 (5.4) |
| 2023 | 7 (18.9) |
| 2024 | 2 (5.4) |
| Country of origin | |
| China | 8 (21.6) |
| India | 7 (18.9) |
| Turkey | 5 (13.5) |
| South Korea | 3 (8.1) |
| Iran | 2 (5.4) |
| Brazil | 2 (5.4) |
| Italy | 2 (5.4) |
| United States | 2 (5.4) |
| Uganda | 1 (2.7) |
| Spain | 1 (2.7) |
| Saudi Arabia | 1 (2.7) |
| Greece | 1 (2.7) |
| South Africa | 1 (2.7) |
| Germany | 1 (2.7) |
| Comparator parameter | |
| Intraventricular ICP monitoring | 3 (8.1) |
| CSF opening pressure for lumbar puncture | 20 (54) |
| MRI/CT scans | 14 (37.8) |
| Type of study | |
| Prospective observational study | 17 (45.9) |
| Prospective case control study | 12 (32.4) |
| Prospective cohort study | 4 (10.8) |
| Retrospective case study | 2 (5.4) |
| Case reports | 2 (5.4) |
Seventeen studies were prospective observational studies, 12 were prospective case–control, 4 were prospective cohort, and 2 were retrospective case series. Two case reports were also included. The details of the studies, sample size, study population, comparator measure, cut-off for ONSD with Area under receiver operating curve (AUROC), when available, and limitations of the study have been described in Table 2[1,7-42].
| Ref. | Country | Type of study | Number of participants | Patient characteristics | Comparator parameter and P value, r value | ONSD cut off, AUROC | Results | Limitations |
| Amini et al[7], 2013 | Iran | Descriptive prospective | 50 | Non-traumatic patients requiring lumbar puncture | CSF pressure on LP, (P = 0.05; r = 0.88) | 5.5, NA | The ONSD of greater than 5.5 mm predicted an ICP of ≥ 20 cmH2O with sensitivity and specificity of 100% (95%CI: 100-100) (P = 0.001) | Small sample size |
| Caffery et al[8], 2014 | United States | Prospective observational trial | 51 | Non-traumatic causes of raised ICP | Opening pressure on LP, r = 0.53 | > 5.0 mm, 0.69 | Sensitivity 0.75, specificity of 0.44 | Use of a convenience sample could introduce bias. Sample size was small with large confidence intervals. One physician with specialized training, patients were not matched for demographic variables such as age or sex |
| Nabeta et al[9], 2014 | Uganda | Prospective descriptive study | 57 | HIV positive, ART naïve adults suspected with meningitis | CSF opening pressure, P < 0.001 | 5 | Sensitivity 86% and specificity 63% for predicting a CSF ICP > 200 mm PPV was 77% and NPV was 75%. Also, in ONSD > 5mm had a RR of 2.39 for IICP > 200 cmH2O | Inter-operator variability, with training being essential |
| Shirodkar et al[10], 2014 | India | Prospective, observational case control study | 101, 60 study, 41 control | Non traumatic cause of increased ICP | Increased ICP on CT/MRI, P < 0.001 | 4.71, 0.986 | Sensitivity of 77.8% and specificity of 100% | Small size |
| Wang l et al[11], 2015 | China | Prospective observational cohort study | 279 | Non-traumatic cause of increased ICP | CSF opening pressure, P < 0.001 | 4.1, 0.965 | Sensitivity of 95% and a specificity of 92% | Average of 8 measurements of ONSD to decrease variability. May not be feasible practically |
| Du Toit et al[12], 2015 | South Africa | Prospective observational study | 76 | Meningitis | CSF opening pressure, Cohen’s kappa was 0.41 | 4.8, 0.73 | Sensitivity of 50% and specificity of 89.8% PPV of 54.8% and NPV of 88.3% PLR of 4.92 and NLR of 0.56 | The study was unable to establish inter-observer variability owing to the large number of operators and the small number of patients with increased ICP |
| Sangani et al[13], 2015 | India | Prospective observational study | 25 | Tubercular meningitis | CSF opening pressure, P < 0.001 | NA | Those patients with TBME had a mean ONSD of 5.81 mm | Small sample size |
| Singleton et al[14], 2015 | United States | Case report | 1 | Idiopathic ICH | CSF opening and closing pressure which was 36 cm H2O and 19.5 cm H2O after removal of 19 cc of CSF | NA | Pre-LP ONSD of left and right eye were 72 and 6.8 mm, respectively. Second study after 30 minutes left and right ONSD were 58 and 6.2 mm, respectively | |
| Karzamni et al[15], 2015 | Iran | Prospective case control study | 60, 30 cases and 30 controls | Intracranial SOL and ICH | Increased ICP on CT, P < 0.001 | 4.53 | Sensitivity and specificity of 100%. ONSD was the most sensitive and specific parameter, followed by RI, PI and EDV. ONSD correlated significantly with GCS (r = −0.40, P = 0.003) and ventricular shift on CT images (r = 0.37, P = 0.02) | Small size, lack of direct ICP measurement |
| Komut et al[16], 2016 | Turkey | Prospective case control study | 100 | Nontraumatic intracranial event in ED | Increased ICP on CT, P < 0.05 | 5.3, 0.728 | Sensitivity 70%, specificity 74% | Lack of direct ICP measurement |
| del Saz-Saucedo et al[17], 2016 | Spain | Prospective case control study | 30 | IIH | CSF opening pressure, P = 0.005 | 6.3 to predict CSF pressure of 25, 0.93 | Sensitivity 94.7%, specificity 90.9% and PLR of 10.4. After a therapeutic lumbar puncture an 87% of cases had a partial reduction of ONSD values | Small size |
| Salahudd et al[1], 2016 | Saudi Arabia | Prospective cohort study | 102 | Non traumatic raised ICP | Increased ICP on CT, P < 0.001 | 5.7, 0.785 | Sensitivity 84 % and specificity 71%. PLR = 2.89, NLR = 0.22 | Study did not include a detailed neurological exam or record any specific localizing neurologic signs, individual GCS |
| Jeon et al[18], 2017 | Korea | Prospective case control study | 62 | Nontraumatic cases requiring EVD placement | Opening pressure on EVD insertion, P < 0.01 | > 5.6, 0.936 | Sensitivity of 93.75% and a specificity of 86.67% for identifying increased ICP | To reduce selection bias, patients with severely increased ICP which required emergency surgical decompression before ICP monitor insertion. Study reflects increased ICP due to moderate hematoma in a Korean population |
| Gökcen et al[19], 2017 | Turkey | Retrospective comparative study | 191 | Acute ischemic stroke | Raised ICP on CT, P < 0.001 | Right ONSD 5.4, 0.941, Left ONSD 5.3, 0.922 | CVD subgroups were compared with the control group the highest ONSD was in TACI group and the lowest was in LACI group (P < 0.001) | Unequal number of cases in the subgroups and adjustment of baseline charecteristics not mentioned |
| Wang et al[20], 2017 | China | Prospective case control study | 316 | Nontraumatic increased ICP requiring LP | CSF opening pressure, r = 0.758, P < 0.001 | NA | Xing and Wang mathematical equation for predicted ICP = −111.92 + 77.36 × ONSD (Durbin-Watson value = 1.94) | Equation may underestimate the true ICP value in patients with extremely high ICP. The Bland-Altman analysis in this study suggested that any estimate might be deviate by as much as ± 80mmH2O |
| Liu et al[21], 2017 | China | Prospective observational study | 110 | Non-traumatic increased ICP requiring LP | CSF opening pressure, P < 0.001 | 5.6, 0.861 | Sensitivity of 86.2% and specificity of 73.1% | 5%–15% of the cases were classified |
| Wang et al[22], 2018 | China | Prospective case and control study | 60 | Nontraumatic causes of IICP requiring LP | CSF opening pressure, P < 0.001. The ultrasonographic ONSD and ICP were measured on admission and follow-up | NA | ONSD was strongly correlated with ICP (r = 0.702, P < 0.001) | Small size, ONSD cut off not obtained |
| Canakci et al[24], 2018 | Turkey | Prospective case control study | 100 | Non-traumatic headache presenting to ER | Raised ICP on CT, P < 0.001 | 5.5, NA | ONSD value in the ipsilateral side with the lesion was significantly higher than the contralateral side (P < 0.001). Discharge, clinical hospitalization, referral, ICU stay, emergency surgery | ER based study including patients with nontraumatic headache not exclusively patients with clinical features of raised ICP. No AUROC calculated |
| Naldi et al[24], 2019 | Italy | Prospective case control study | 46 cases, 40 controls | Primary ICH | Increased ICP on CT, P < 0.01 | 5.6, 1.0 | Sensitivity 100%, Specificity 100% | ICP was presumed to be normal in control, limited predictive value of abnormal CT findings. Second CT scan was performed not on a given day, but depending on clinical conditions |
| Gupta et al[25], 2019 | India | Prospective observational study | 100 | Raised ICP requiring LP | CSF opening pressure, P < 0.001 | 6.3 to predict CSF pressure of > 20 cm of water | Sensitivity 77.3%, specificity 92.3%, PLR = 10.05, NLR = 0.25 | Did not include any condition causing a mass effect, malignant infarcts, ICH or obstructive hydrocephalus |
| Gupta et al[26], 2019 | India | Retrospective case series study | 100 | Raised ICP requiring LP | CSF opening pressure, P < 0.001 | 4.8 | Sensitivity of 85% and specificity of 88% | Single center, retrospective, small size |
| Wang et al[27], 2019 | China | Case reports | 2 | Venous sinus stenosis and venous sinus thrombosis | CSF opening pressure | NA | Case 1 A predicted ICP by ONSD was 346 mmH2O. and CSF opening pressure was 355 mmH2O. Case 2 ONSD was 5.95 mm with CSF opening pressure higher than 400 mmH2O | |
| Zoerle et al[28], 2020 | Italy | Prospective observational study | 20 | Aneurysmal SAH with EVD | Intraventricular ICP, P > 0.05 | NA | ONSD measurements were accurate, very similar to the diameters measured by MRI (the mean difference in the Bland–Altman plot was 0.08 mm, 95% limits of agreement: −1.13; + 1.23 mm). No clear relationship was detectable between the ICP and ONSD, and a linear regression model showed an angular coefficient very close to 0 (P > 0.05). US-ONSD and ICP values were in agreement after CSF drainage and shifts in ICP in a limited number of patients | Measured ICP in the ICU after the patients were stabilized, the aneurysm repaired, and large intracerebral hematomas surgically removed, with EVD and CSF drainage. As a consequence, the ICP values in our cohort were relatively low for the majority of cases |
| Sahu et al[29], 2021 | India | Prospective, double blinded observational study | 30 | Nontraumatic increased ICP | Direct intraventricular ICP, P = 0.01 | 5.5 to predict ICP > 20 mmHg, 0.904 | Sensitivity 100% and specificity 75%. The ONSD values predicting ICP at 25-, 30-, and 35-mm Hg were was 6.3 mm, 6.5 mm, and 6.7 mm, respectively | Small number of patients having ICP > 30 mm of Hg, appropriate ONSD values could not be predicted |
| Yildiz et al[30], 2021 | Turkey | Prospective, observational study | 82 | Acute ischaemic stroke | Increased ICP on CT, P < 0.05 | NA | ONSD on the 3rd day and 5th day was larger (> 5 mm) than on first day (P < 0.05). In the patients who received tPA right eye ONSD on the 5th day were significantly raised P < 0.05) | ONSD only after the symptoms started, and were also not measured during the decline periods and response to treatment |
| Kim et al[31], 2021 | South Korea | Prospective, observational study | 199 | Suspected raised ICP | Increased ICP on CT, P < 0.001 | 5.3, 0.903 | Sensitivity of 75.4%, specificity of 90.8%, PPV of 76.8%, and NPV of 90.2% | Single centre, 2 observers hence there can be variability |
| Qamar Akhtar et al[32], 2022 | India | Prospective case control study | 100 | Non traumatic emergencies with suspected raised ICP | Raised ICP on CT/MRI (P = 0.05; r = 0.88) | ≥ 6.3, 0.956 | Sensitivity of 100%, specificity of 89.2%, PPV of 83.3%, NPV of 100%, and diagnostic accuracy of 93% for detection of raised ICP by bedside USG ONSD measurement compared to CT/MRI brain | CT or MRI brain scan which is an indirect indicator of raised ICP, and use of a high ONSD mean value (mm) cut-off |
| Oliveira et al[33], 2022 | Brazil | Prospective observational study | 40 | Malignant MCA infarct requiring decompressive craniotomy | Increased ICP on CT, P: NA | 5.4 mm, ROC for, Right eye: 0.82, Left eye: 0.77 | Post craniectomy, there was a decrease in the mean value of 1.04mm in the right eye 086 mm in left. (P = 0.003) | Small size, CT unreliable for increased ICP. DC individualized is routinely adopted at this center, the neurosurgical team was allowed to perform surgery using individual interpretations of criteria, with controversial decisions on some patients |
| Roemer et al[34], 2022 | Germany | Prospective observational study | 23 | Increased ICH | CSF opening pressure, P = 0.9 | NA | No correlation between CSF opening pressure and ONSD was found | Small size, results could be biased by the ongoing treatment of the patients |
| Bhide et al[35], 2023 | India | Prospective observational study | 114 | Non-traumatic causes of raised ICP | Increased ICP on CT/MRI, P < 0.001 | 5.75, 0.844 | Sensitivity and specificity of 77.55% and 89.06%. PLR and NLR of 7.09 and 0.25 | Comparator used was CT or MRI brain scan which is an indirect indicator of raised ICP, and use of a high ONSD mean value (mm) cut-off |
| Yu et al[36], 2023 | China | Prospective observational study | 107 | Non traumatic increased ICP requiring LP | CSF opening pressure, P < 0.001 | 6.3 mm | 73% sensitivity and 83% specificity, ODH with ONSD showed the highest value under the receiver operating characteristic curve of 0.965 with a sensitivity of 93% and a specificity of 92% | Single lumbar puncture |
| Batur et al[37], 2023 | Turkey | Prospective case control study | 105 | Acute ischemic stroke | Features of raised ICP on MRI (P < 0.001) | 5.05, 0.978 | Sensitivity 96.8%, specificity 95.6%. The cut-off for need for treatment 4.95 mm with AUC of 0.807 (sensitivity = 71.4%, specificity = 79.6%) | Single-centered study. Although 30-day mortality rates were recorded, a detailed information about the outcome could be given by monitoring the neurological healing rate and time of the patients |
| Li et al[38], 2023 | China | Prospective observational study | 56 | Suspected encephalitis | CSF pressure, r = 0.769, P < 0.01 | NA | Both ODH and ONSD had the ability to predict ICP (P < 0.05), but with time factors, ONSD displayed a stronger ability to predict ICP than ODH | Single-center design and small sample size. Cut-off value with AUROC not calculated |
| de Moraes et al[39], 2023 | Brazil | Prospective observation study | 18 | Acute stroke (ischemic and hemorrhagic) | A 5-point visual scale for n raised ICP on CT and two parameters (time-to-peak and P2/P1 ratio) of a noninvasive ICP wave morphology monitor (r = 0.29) | 5.2, 0.69 | Sensitivity was 71.4%, the specificity was 70.4%, the PPV was 43.5%, and the NPV was 88.6% | Small size, assessment intervals varied, Non blinded, correlation modest to moderate strengths |
| Cheng et al[40], 2023 | China | Prospective cohort study | 223 | Non-traumatic causes of raised ICP requiring LP | CSF opening pressure, P < 0.001 | 5.47, 0.933 | ICP values were strongly correlated with ONSD, ONSD, and ONSD/ETD. ONSD and OND combined model predicted ICP = 139.394 × ONSD-112.428 × OND267.461 prediction accuracy was the highest. (ICC = 0.88) | Underestimated the ICP in very high cases, the maximum limit of our ICP values was 330 mmH2O, and values greater than 330 mmH2O were counted as 330 mmH2O |
| Bakola et al[41], 2024 | Greece | Prospective center case-control study | 31 case and 34 controls | Idiopathic ICH | CSF opening pressure on LP, (r = 0.716, P < 0.001) | 5.15, 0.914 | Sensitivity and specificity of TOS for diagnosis of IIH were 85% (95%CI: 66%-95%) and 90% (95%CI: 76%-98%), respectively. PPV 83% (95%CI: 74%-96%), NPV 94% (95%CI: 83-98%) | Subsequent measurements to estimate the potential treatment response using TOS were not part of our study protocol. |
| Kim et al[42], 2024 | Korea | Retrospective analysis of prospectively gathered data ONSD measurements were conducted using a handheld ultrasonography device during the course of endovascular treatment | 126 | Aneurysmal SAH | CSF opening pressure on LP, (P < 0.001), the association between ONSD and ICP was validated through the application of a linear regression machine learning model. The correlation between ICP and various factors was explored through the modeling | 5.45, 0.90, SHAP 5.58 | Sensitivity 92.50, specificity 78.00, PPV 82.70 NPV 90.20 | Small size, single center |
In our scoping review of 37 studies, US-ONSD was found to be fairly predictive of ICP. Barring the 2 case reports, 30 studies calculated the p-value for the association between US-ONSD and ICP. Twenty-eight studies showed a statistical significance between US-ONSD and ICP with P < 0.05. A prospective observational study by Zoerle et al[28] included 20 patients with aneurysmal subarachnoid hemorrhage (SAH) who underwent extra-ventricular drain (EVD) insertion and intraventricular ICP monitoring. An average of 4 measurements were taken for 15-day period for each patient. This study did not find significant correlation between US-ONSD and ICP (P > 0.5). They further conducted a dynamic test in 10 patients where ONSD values were correlated with ICP before and after CSF drainage. The correlation was inconsistent with 2 patients (20%) having completely different trends for US-ONSD and ICP[28]. Another study conducted by Roemer et al[34] in 23 patients with idiopathic ICH also did not show a significant correlation between US-ONSD and ICP (P = 0.9)[34]. Both these studies had a small sample size; hence, in the face of positive data from multiple other studies, these results must be interpreted cautiously.
Even though ONSD has been extensively used in trauma patients, there are still concerns regarding the procedure of ONSD and its clinical applications in non-traumatic patients. Hence, we have expanded on the concerns and grey areas regarding ONSD in medical ICU, outlined in Table 3.
| Related issues | |
| How to measure ONSD? | |
| (1) | A scan or B scan? What is blooming effect? |
| (2) | CLOSED protocol? |
| (3) | Transverse or horizontal? |
| (4) | How many values before obtaining a mean? |
| (5) | ONSD or OND or ratio? |
| What is the body of evidence in various subset of patients? | |
| (1) | Acute ischemic stroke and CVST |
| (2) | Acute hemorrhagic stroke |
| (3) | Hydrocephalus |
| (4) | Idiopathic intracranial hemorrhage |
| (5) | Meningitis |
| (6) | Septic metabolic encephalopathy |
| Can ONSD be used as a management tool? | |
| Can ONSD be a reliable outcome measure? | |
| (1) | Post cardiac arrest |
| (2) | Dysnatremia |
The ONS is an anatomical continuum of the meninges. Hence, the ICP variation is reflected in the ONS, resulting in dilatation and increased ONSD. The point of measurement of ONSD lies 3mm behind the optic disc as this is where the sheath is most sensitive to ICP changes[43,44]. Most of the studies have followed this landmark for obtaining the ONSD.
A single cutoff for ONSD has yet to be defined to diagnose raised ICP in neuro-medical patients. 27 studies specified the cutoff for ONSD by ROC to vary from 4.1 to 6.3 mm. A systematic review of idiopathic ICH patients reported similar concerns. Still, they noted that a cutoff of 5.15 mm had the best accuracy with the area under the curve (AUC) of 0.914 to predict idiopathic ICH[41].
A study by Wang et al[20] included 316 neurology patients who underwent LP. The group was divided using a 7: 3 ratio into modelling and test groups. The modelling group was studied for the patient's baseline characteristics, age, sex, body mass index (BMI), mean and diastolic blood pressure, and they were found not significantly associated with raised ICP. The authors then derived Xing and Wang's mathematical equation to predict ICP using US-ONSD values. Predicted ICP was calculated as = −111.92 + 77.36 × ONSD. This was further validated in the test group (n = 94), where a significant correlation was found between the observed and predicted ICP (r = 0.76, P < 0.001). The mean difference between mea
Various studies describe different thresholds for the cutoff for ONSD. The blooming effect partly explains this. When the diameter is measured by decreasing the gain, it will appear larger, and increasing the gain will appear smaller. This blooming effect is the error attributed to non-standardized gain and sensitivity settings[45]. The differences between the A and B scans are summarized in Table 4. The role of A scan is limited by its availability in the ICU. If A scan is proven superior, future research implications would be developing hand-held A scan devices to accurately measure ONSD.
| A scan | B scan |
| Amplitude modulation scan | Brightness modulation scan |
| 8 mHertz frequency with small non focused probe | 10 mHertz with a larger focused probe |
| One-dimensional image of spikes of varying amplitudes along a baseline | Two-dimensional image |
| Provides quantitative information: Ex length of eyeball before surgery. | Provides topographical information |
| Basis of ocular biometry | Evaluation of ocular pathology |
| No blooming effect | Blooming effect while measuring ONSD |
| Not available as bedside equipment | Part of point-of-care ultrasonography, easily available |
Another way of overcoming the blooming effect would be using a Color Doppler to delineate the optic disc. CLOSED protocol expands to Color Doppler–Low power examination–Optic disk clarity–Safety (short examination dura
The central retinal artery and vein runs through the center of the optic nerve.
The ophthalmic artery runs along the medial border of the optic nerve and limits the optic sheath. The dicrotic notch on Doppler waves can further confirm the ophthalmic artery.
Once the course of the optic nerve is defined, the sheath diameter must be measured 3 mm behind the optic disc. The same authors used the CLOSED protocol to be validated in patients with idiopathic normal pressure hydrocephalus. ONSD calculated by CLOSED protocol had a lower scatter, and the values were lower than those calculated by US-ONSD or MRI scan[46]. ONSD calculated by CLOSED protocol needs to be validated against ICP measurement by standard techniques, using tests of diagnostic accuracy.
The optic nerve is thickest, about 3 mm behind the retina, followed by a curved path posteriorly and medially, as shown by MRI and cadaveric studies. A transverse measurement shows the optic nerve as a pyramidal structure that widens posteriorly. This widening could be due to an artefact from the lamina cribrosa or an ONS. This artefact may look significantly wider in patients with elevated ICP. A coronal view is achieved by placing the probe on the temporal aspect of the eyeball, directing it nasally and posteriorly. This produces a circular cross-section of the ONS, which is more accurate than transverse or axial orientation in healthy volunteers[47].
A blinded observational study in two tertiary teaching hospitals by Agrawal et al[48] included 20 adults with increased ICP expected to receive invasive intracranial monitoring. Axial and coronal measurements were taken and the highest and average values were recorded. The coronal values showed less variability between each eye as compared to axial measurements (0.5 mm vs 1mm; P = 0.03). For predicting ICP, axial ONSD was shown to have higher accuracy. Also, a cutoff of highest axial measurement greater than 6.2 mm in either eye or mean axial measurement of 5.6 mm had a sensitivity of 100% in predicting high ICP over the following 24 hours[48].
Horizontal diameter is more frequently used to measure ONSD due to the ease of use. Studies that used both transverse and sagittal diameters calculated the mean. There was no significant difference between the readings taken transversely or sagittaly[48]. In our review, most authors took 2–4 readings before taking the mean. Few studies went up to 8 readings in each section. Multiple readings, however, are concerned with taking more time, especially when the patient is critically ill and needs immediate intervention. However, most studies documented the time taken for the study to under a minute.
Both ONSD and OND are measured 3 mm behind the globe. Eyeball transverse diameter (ETD) is measured horizontally till maximum diameter is obtained. Ratios that can be used are ONSD/OND and ONSD/ETD, which have been found to correlate with poorer outcomes in comatose patients[49]. However, the utility of these newer indices is yet to be researched in predicting increased ICP.
Acute ischemic stroke (AIS) can lead to raised ICP in two ways. Firstly, when large hemispheric infarctions have surrounding cerebral oedema significant enough to cause elevated ICP. These malignant ischemic strokes are usually secondary to occlusion of the internal carotid artery or M1 segment of the middle cerebral artery (MCA). These patients usually have progressive worsening over 36 to 48 hours, requiring decompressive craniotomy (DC). The second cause of raised ICP in ischemic strokes could be hemorrhagic transformation of the stroke. The deterioration is often sudden and unpredictable in these patients. A study of 40 patients with ischemic stroke showed an increase in ONSD on day 5 as compared to day 1 of stroke. A cutoff of 5.4 mm was found to be predictable of raised ICP. This study also showed a significant decrease in the ONSD post-DC (P = 0.003). The ONSD trend was also higher in patients who died compared to the survivors in both surgical and non-surgical groups[33]. Thus, ONSD could be helpful in prognosticating patients in malignant MCA infarcts.
In a study of 82 patients with AIS, 22 patients were administered recombinant tissue plasminogen activator (rtPA) treatment. The values of ONSD in the right eye on the 5th day were significantly higher in thrombolysis patients than those of patients who did not receive rtPA (P < 0.05). However, no statistical difference occurred between 24, 36, and 48 hours of ONSD. Additionally, in patients who underwent DC, there was no correlation between ONSD and decom
The utility of ONSD in AIS can be questioned in two aspects. Firstly, which eye would be more sensitive to predict the increased ICP. Oliveira et al[33] found a simultaneous increase in ONSD in both eyes in patients with severely increased ICP. Furthermore, when the injured hemisphere was left, the left ONSD had higher sensitivity; when the right hemis
Secondly, the site of ischemic stroke may be a confounding factor in the utility of ONSD. Cerebral hemispheric infarcts are more likely to influence the ONS than infratentorial lesions. Two studies expanded on this by first dividing the strokes using Oxfordshire Community Stroke Project classification on the basis of their maximum neuro deficit into total anterior circulation infarct (TACI), partial anterior circulation infarct (PACI), posterior circulation infarct (POCI), or lacunar infarct (LACI). Gökcen et al[19] found that ONSD was higher in all the subgroups of strokes than in the cohorts. Also, the TACI group had the highest ONSD values, and LACI was found to have the lowest values. Right and left ONSD had a cutoff of 5.4 and 5.3 mm, respectively, to predict the presence of cerebrovascular disease[19]. Similar findings were also seen in a study by Batur et al[37] with TACI having higher ONSDs than the others (TACI: 5.27 mm; PACI: 4.73 mm; POCI: 4.77 mm; and LACI: 4.64 mm, P < 0.001). They also found that the patients with TACI had an increased need for ICU admission. Raised ONSD also significantly correlated with mortality in this study (P < 0.001)[37].
We also found one case report of a patient with cerebral venous sinus thrombosis (CVST) who had raised ONSD of 5.95 mm on presentation with a CSF opening pressure of 400 cmH2O. Further, follow up of patient after treatment showed a concurrent decline in ONSD and CSF opening pressure[27]. Another case reported a 32-year-old female who underwent a caesarian section, following which she developed a post-dural puncture headache. The patient initially had low ICP syndrome. After a progressive increase in symptoms, a repeat radiological examination indicated CVST. ONSD showed raised values of 6.0 and 6.2 mm, leading to the suspicion of raised ICP. An ophthalmologic examination revealed papi
A study in ICH patients showed higher median binocular ONSD, resistive index and retinal venous pulsation values compared to the control group. Median binocular ONSD cut-off of ≥ 5.6 mm showed a higher accuracy for the detection of increased ICP with AUROC of 1.0. At the onset of ICH, ONSD also showed a good correlation with haemorrhage volume (r = 0.677, P = 0.0002). As with AIS, both right and left ONSD eye were found to have good accuracy (0.92, 95%CI: 0.87–0.96), suggesting a small side-to-side variation in a study of multimodal ICP monitoring in ICH patients. The cut-off for predicting increased ICP was 5.2 mm with an AUROC of 0.69. However, the correlation between the mean ONSD value and the mean ICP was found to be weak (r = 0.29)[24].
A large study of 529 patients with ICH showed that the ONSD in patients with poor outcomes was significantly higher than in survivors (P < 0.001). There was a significant correlation between hematoma volume and ONSD measurements
In another large study of 126 patients of aneurysmal SAH undergoing endovascular coiling, raised ONSD correlated significantly with an ICP > 20 cmH2O (5.9 mm vs 4.8 mm, P = 0.00). Similar findings were seen at an ICP cut-off of 25 cmH2O. A linear regression model was used to generate a line of best fit. It used a predictive equation of ICP (cmH2O) = 79.70 × ONSD (cm) − 21.60. The coefficient of determination was 0.54 as per this analysis[42].
However, other studies have found ONSD to be a weak predictor of increased ICP in aSAH. A study by Zoerle et al[28] included patients with aneurysmal SAH who had an EVD system placed. There was no clear correlation between the ICP and US-ONSD and a linear regression model also concurred with a lack of statistical significance (P > 0.05)[28].
Intracranial infections are a common cause of raised ICP in medical ICUs. Nabeta et al[9] conducted a study on 98 human immunodeficiency virus (HIV) infected patients with suspected meningitis. They found a moderate correlation between increased ICP and US-ONSD (ρ = 0.44, P < 0.001) and reported that ONSD > 5 mm was likely to have an elevated ICP (> 200 mmH2O). Eighty-one percent of these patients had cryptococcal meningitis. A restricted analysis of this subset of patients showed a significant correlation of ONSD with ICP (P < 0.001) with a cut-off of 5.4 mm. There was no statistical correlation between fungal burden by quantitative CSF culture and ONSD. Hence, evidence of increased ONSD, along with relevant clinical features can be a trigger for therapeutic CSF drainage[9].
In a prospective case study of 56 patients with encephalitis, ONSD and CSF opening pressure were measured on admission and after 2 weeks. As per the etiology of encephalitis, 19 patients had viral, 16 had tuberculous, 9 had pyogenic and 22 had autoimmune or other non-infective encephalitis. There was a moderate correlation between ONSD and ICP on admission (r = 0.769; P < 0.01) and on follow up at 2 weeks and 1 month. ONSD also showed a better predictability of ICP as compared to ODH[38].
Another prospective, observational study included 25 patients with suspected tubercular meningoencephalitis (TBME) who underwent MRI followed by LP. In this study, there was a significant difference between ONSD in TBME patients as compared to the control group (5.81 mm vs 4.37 mm, P < 0.001)[13].
In a study of a patient suspected of idiopathic ICH by Bakola et al[41], ONSD significantly correlated with ICP measured using CSF opening pressure (r = 0.716, P < 0.001). For predicting idiopathic ICH, the optimal ONSD cutoff value was 5.15 mm, with an AUC of 0.914. The authors then conducted a systematic review which included 14 studies and 415 patients of idiopathic ICH. The meta-analysis of pooled patient data from 8 studies which studied relation between ONSD and CSF opening pressure found a moderate correlation between ONSD and idiopathic ICH (r = 0.44; P for Cochran Q < 0.02). The cutoff values used in each study ranged from 4.8 to 6.3 mm, while the optimal cutoff point for idiopathic ICH discrimination was detected at 5.0 mm with AUC of 0.878[41].
We found 3 studies apart from those included in the systematic review and meta-analysis. In a study of 23 patients with idiopathic ICH, no correlation was found between CSF opening pressure and ONSD[34]. Another case control study of 30 patients found a positive but moderate (Spearman's rho = 0.500) correlation between the values of ONSD and CSF opening pressure (P = 0.005). The study also found a significant decrease in ONSD post-therapeutic LP[17]. A 25-year-old patient with an idiopathic ICH case report followed real-time ONSD changes. When measured before the LP, the left and right eye ONSD were 7.2 and 6.8 mm. The opening pressure was 36 cmH2O, and the closing pressure was 19.5 cmH2O after removal of 19 cc of clear, colorless CSF. The post-LP ultrasound was performed after 30 minutes, showing an ONSD of 5.8 and 6.2 mm in the left and right eyes, respectively[14].
This is a known complication of sepsis, with the risk factors being uremia, hypoglycemia, hyperglycemia, hypercapnia, hypernatremia, elderly age and higher acute physiology and chronic health evaluation score. A prospective observational study included 123 patients with sepsis, out of which 58 developed sepsis associated encephalopathy (SAE). ONSD was measured on admission (ONSDo) and alternate days. The highest ONSD was documented during illness and was re
A study by Yang et al[53] included 90 patients with sepsis, for whom 142 ONSD measurements were carried out. ONSD was higher in SAE group but did not correlate with patient outcomes[53]. Another prospective case series of 10 patients with septic shock requiring mechanical ventilation and sedation took multiple ONSD values during the ICU stay. A value > 5.7 mm was taken as a cut-off for raised ONSD. Forty nine out of 80 measurements (62%) were raised. There was no correlation between ONSDs and C-reactive protein, highest daily lactate or sequential organ failure assessment score during the study period[54].
A case report of aneurysmal SAH showed that a combination of TCD and ONSD was used as a trigger for the evaluation of vasospasm. This helped in the early ventriculoperitoneal shunt and patient management[55].
Neuro-prognostication of post cardiac arrest patients is imperative for forming a plan of care. However, most patients may not be stable to be shifted for CT or MRI scans. Other tests like electroencephalography, somatosensory evoked potential and pupillary reflex may be confounded by metabolic derangements and sedatives.
A retrospective study of 86 adult patients with cardiac arrest underwent radiological imaging to assess for markers of poor outcomes post cardiopulmonary resuscitation. However, no correlation was found between ONSD and poor outcomes[56]. Another study, which combined ONSD and grey-white matter differentiation on CT scan, showed better sensitivity for predicting outcomes in these patients[57].
In a prospective longitudinal cohort study, which included 100 adult patients with cardiac arrest, ONSD was measured daily for 3 consecutive days or until awakening or death, by trained personnel in the first 3 days after cardiac arrest, or until decease or awakening. Good inter observer variability was found with intraclass correlation coefficient for offline and real-time measurements (inter-observer reliability) was 0.872[58].
A prospective observational study of 54 patients with serum sodium below 135 mEq/L presenting to the emergency department was conducted to test the efficacy of ONSD as a guide to correct hyponatremia. ONSD was documented on admission and on discharge. The change in ONSD did not correlate with the variation in sodium levels[59].
On the contrary, in a prospective cohort study that included 65 patients (35 with hypernatremia and 30 with hyponatremia) and 14 healthy volunteers (control group), ONSD values were found to be higher in the hypernatremia and hyponatremia groups comparing to the control group (P < 0.001). The right and left ONSD detected hypernatremia with 91.4% and 88.6% sensitivity and 92.9% and 85.7% specificity, respectively. For hyponatremia, sensitivity was 83.3% and 93.0%, and specificity was 92.9% and 86.0% for right and left ONSD, respectively. Furthermore, in patients with hypernatremia, ONSD was found to be an independent predictor of mortality. An increase of one mm ONSD in the right eye increased the probability of mortality by 6.21, and a similar rise in ONSD in the left eye increased the likelihood of mortality by 4.21[60].
ONSD was found to have a significant association with raised ICP in most of the studies in non-traumatic, neuro-critically ill patients. In a patient with altered sensorium, it can be used as a bedside screening tool for raised ICP. This can lead to early diagnosis of a significant intracranial event. However, the cutoffs still need to be defined, and larger RCTs will be required to determine this value. Due to the heterogenicity of the patient population in a medical ICU, disease-wise studies are pertinent. The quantitative equation for predicting ICP using US-ONSD opens up promising possibilities for neuromonitoring, as ONSD could also be a handy outcome measure tool in select patient subgroups.
Firstly, as this was a scoping review, no statistical analysis was done. Secondly, we excluded all the studies in a mixed population of traumatic and non-traumatic cases to avoid trauma being the confounding factor. These consisted of 30 out of 109 studies (27.5%) reviewed, indicating a large data set left unassessed. We included all the causes of non-traumatic increased ICP to improve the generalizability of medical ICUs. The subset population with AIS, ICH, hydrocephalus, meningitis and septic encephalopathy will require relevant RCTs, followed by systematic review and meta-analysis before ONSD can be incorporated to change the clinical practices or management guidelines.
| 1. | Salahuddin N, Mohamed A, Alharbi N, Ansari H, Zaza KJ, Marashly Q, Hussain I, Solaiman O, Wetterberg TV, Maghrabi K. The incidence of increased ICP in ICU patients with non-traumatic coma as diagnosed by ONSD and CT: a prospective cohort study. BMC Anesthesiol. 2016;16:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Ohle R, McIsaac SM, Woo MY, Perry JJ. Sonography of the Optic Nerve Sheath Diameter for Detection of Raised Intracranial Pressure Compared to Computed Tomography: A Systematic Review and Meta-analysis. J Ultrasound Med. 2015;34:1285-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 148] [Article Influence: 13.5] [Reference Citation Analysis (1)] |
| 3. | Tayal VS, Neulander M, Norton HJ, Foster T, Saunders T, Blaivas M. Emergency department sonographic measurement of optic nerve sheath diameter to detect findings of increased intracranial pressure in adult head injury patients. Ann Emerg Med. 2007;49:508-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 298] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 4. | Ballantyne SA, O'Neill G, Hamilton R, Hollman AS. Observer variation in the sonographic measurement of optic nerve sheath diameter in normal adults. Eur J Ultrasound. 2002;15:145-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 174] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Carney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GW, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, Rubiano AM, Shutter L, Tasker RC, Vavilala MS, Wilberger J, Wright DW, Ghajar J. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery. 2017;80:6-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1786] [Cited by in RCA: 2389] [Article Influence: 265.4] [Reference Citation Analysis (1)] |
| 6. | Pollock D, Peters MDJ, Khalil H, McInerney P, Alexander L, Tricco AC, Evans C, de Moraes ÉB, Godfrey CM, Pieper D, Saran A, Stern C, Munn Z. Recommendations for the extraction, analysis, and presentation of results in scoping reviews. JBI Evid Synth. 2023;21:520-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 828] [Article Influence: 276.0] [Reference Citation Analysis (0)] |
| 7. | Amini A, Kariman H, Arhami Dolatabadi A, Hatamabadi HR, Derakhshanfar H, Mansouri B, Safari S, Eqtesadi R. Use of the sonographic diameter of optic nerve sheath to estimate intracranial pressure. Am J Emerg Med. 2013;31:236-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 8. | Caffery TS, Perret JN, Musso MW, Jones GN. Optic nerve sheath diameter and lumbar puncture opening pressure in nontrauma patients suspected of elevated intracranial pressure. Am J Emerg Med. 2014;32:1513-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Nabeta HW, Bahr NC, Rhein J, Fossland N, Kiragga AN, Meya DB, Dunlop SJ, Boulware DR. Accuracy of noninvasive intraocular pressure or optic nerve sheath diameter measurements for predicting elevated intracranial pressure in cryptococcal meningitis. Open Forum Infect Dis. 2014;1:ofu093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Shirodkar CG, Rao SM, Mutkule DP, Harde YR, Venkategowda PM, Mahesh MU. Optic nerve sheath diameter as a marker for evaluation and prognostication of intracranial pressure in Indian patients: An observational study. Indian J Crit Care Med. 2014;18:728-734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Wang L, Feng L, Yao Y, Wang Y, Chen Y, Feng J, Xing Y. Optimal optic nerve sheath diameter threshold for the identification of elevated opening pressure on lumbar puncture in a Chinese population. PLoS One. 2015;10:e0117939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Du Toit GJ, Hurter D, Nel M. How accurate is ultrasound of the optic nerve sheath diameter performed by inexperienced operators to exclude raised intracranial pressure? S Afr j radiol. 2015;19. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Sangani SV, Parikh S. Can sonographic measurement of optic nerve sheath diameter be used to detect raised intracranial pressure in patients with tuberculous meningitis? A prospective observational study. Indian J Radiol Imaging. 2015;25:173-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Singleton J, Dagan A, Edlow JA, Hoffmann B. Real-time optic nerve sheath diameter reduction measured with bedside ultrasound after therapeutic lumbar puncture in a patient with idiopathic intracranial hypertension. Am J Emerg Med. 2015;33:860.e5-860.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Karami M, Shirazinejad S, Shaygannejad V, Shirazinejad Z. Transocular Doppler and optic nerve sheath diameter monitoring to detect intracranial hypertension. Adv Biomed Res. 2015;4:231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 16. | Komut E, Kozacı N, Sönmez BM, Yılmaz F, Komut S, Yıldırım ZN, Beydilli İ, Yel C. Bedside sonographic measurement of optic nerve sheath diameter as a predictor of intracranial pressure in ED. Am J Emerg Med. 2016;34:963-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | del Saz-Saucedo P, Redondo-González O, Mateu-Mateu Á, Huertas-Arroyo R, García-Ruiz R, Botia-Paniagua E. Sonographic assessment of the optic nerve sheath diameter in the diagnosis of idiopathic intracranial hypertension. J Neurol Sci. 2016;361:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Jeon JP, Lee SU, Kim SE, Kang SH, Yang JS, Choi HJ, Cho YJ, Ban SP, Byoun HS, Kim YS. Correlation of optic nerve sheath diameter with directly measured intracranial pressure in Korean adults using bedside ultrasonography. PLoS One. 2017;12:e0183170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 19. | Gökcen E, Caltekin İ, Savrun A, Korkmaz H, Savrun ŞT, Yıldırım G. Alterations in optic nerve sheath diameter according to cerebrovascular disease sub-groups. Am J Emerg Med. 2017;35:1607-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Wang LJ, Yao Y, Feng LS, Wang YZ, Zheng NN, Feng JC, Xing YQ. Noninvasive and quantitative intracranial pressure estimation using ultrasonographic measurement of optic nerve sheath diameter. Sci Rep. 2017;7:42063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Liu D, Li Z, Zhang X, Zhao L, Jia J, Sun F, Wang Y, Ma D, Wei W. Assessment of intracranial pressure with ultrasonographic retrobulbar optic nerve sheath diameter measurement. BMC Neurol. 2017;17:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Wang LJ, Chen LM, Chen Y, Bao LY, Zheng NN, Wang YZ, Xing YQ. Ultrasonography Assessments of Optic Nerve Sheath Diameter as a Noninvasive and Dynamic Method of Detecting Changes in Intracranial Pressure. JAMA Ophthalmol. 2018;136:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 23. | Canakci Y, Koksal O, Durak VA. The value of bedside ocular ultrasound assessment of optic nerve sheath diameter in the detection of increased intracranial pressure in patients presenting to the emergency room with headache. Niger J Clin Pract. 2018;21:778-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Naldi A, Pivetta E, Coppo L, Cantello R, Comi C, Stecco A, Cerrato P, Lesmeister M, Lochner P. Ultrasonography Monitoring of Optic Nerve Sheath Diameter and Retinal Vessels in Patients with Cerebral Hemorrhage. J Neuroimaging. 2019;29:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Gupta S, Pachisia A. Ultrasound-measured optic nerve sheath diameter correlates well with cerebrospinal fluid pressure. Neurol India. 2019;67:772-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 26. | Gupta P, Mahajan V, Gupta A. Correlation of Ultrasonographic Optic Nerve Sheath Diameter with Direct Measurement of Intracranial Pressure. Int J Med Sci Curr Res. 2019;2:292-296. |
| 27. | Wang LJ, Chen HX, Tong L, Chen LM, Dong YN, Xing YQ. Ultrasonographic optic nerve sheath diameter monitoring of elevated intracranial pressure: two case reports. Ann Transl Med. 2020;8:20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Zoerle T, Caccioppola A, D'Angelo E, Carbonara M, Conte G, Avignone S, Zanier ER, Birg T, Ortolano F, Triulzi F, Stocchetti N. Optic Nerve Sheath Diameter is not Related to Intracranial Pressure in Subarachnoid Hemorrhage Patients. Neurocrit Care. 2020;33:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Sahu S, Panda N, Swain A, Mathew P, Singla N, Gupta S, Jangra K, Bhardwaj A, Bhagat H. Optic Nerve Sheath Diameter: Correlation With Intra-Ventricular Intracranial Measurements in Predicting Dysfunctional Intracranial Compliance. Cureus. 2021;13:e13008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Yildiz G, Acar N, Cevik AA, Ozdemir AO, Metintas S, Kaplan D, Ozakin E, Yıldız CG, Ayyildiz A. The evaluation of intracranial pressure evaluation by optic nerve sheath diameter measurement on bedside ultrasonography after ischemic stroke. Clin Neurol Neurosurg. 2021;209:106914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 31. | Kim DY, Kim SY, Hong DY, Sung BY, Lee S, Paik JH, Jung HM. Comparison of ultrasonography and computed tomography for measuring optic nerve sheath diameter for the detection of elevated intracranial pressure. Clin Neurol Neurosurg. 2021;204:106609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Qamar Akhtar M, Goud R, Mishra P, Reddy S, Argey S. Ocular Ultrasound to detect raised intracranial pressure in non-traumatic emergencies in Emergency Department. J Emerg Med, Trauma Acute Care. 2022;2022. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 33. | Oliveira BDD, Lima FO, Homem HDC, Figueirêdo AA, Freire VMB, Maia Carvalho FM. Optic Nerve Sheath Diameter Detects Intracranial Hypertension in Acute Malignant Middle Cerebral Artery Infarction. J Stroke Cerebrovasc Dis. 2022;31:106276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Roemer SN, Friedrich EB, Kettner M, Rauzi M, Schub P, Kulikovski J, Janitschke D, Stögbauer J, Lochner P. Transorbital sonography and MRI reliability to assess optic nerve sheath diameter in idiopathic intracranial hypertension. J Neuroimaging. 2023;33:375-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 35. | Bhide M, Singh O, Juneja D, Goel A. Bedside ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure in nontraumatic neuro-critically ill patients. World J Crit Care Med. 2023;12:10-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Yu ZY, Xing YQ, Li C, Wang SB, Song XN, Wang CC, Wang LJ. Ultrasonic optic disc height combined with the optic nerve sheath diameter as a promising non-invasive marker of elevated intracranial pressure. Front Physiol. 2023;14:957758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 37. | Batur A, Karaca MA, Arslan V, Boz M, Ibrahimov Z, Erbil B, Onur MR. Prognostic role of optic nerve sheath diameter in stroke in emergency department, A case control study. Niger J Clin Pract. 2023;26:863-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 38. | Li C, Wang CC, Meng Y, Fan JY, Zhang J, Wang LJ. Ultrasonic optic nerve sheath diameter could improve the prognosis of acute ischemic stroke in the intensive care unit. Front Pharmacol. 2022;13:1077131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 39. | de Moraes FM, Adissy ENB, Rocha E, Barros FCD, Freitas FGR, Miranda M, Valiente RA, de Andrade JBC, Chaddad-Neto FEA, Silva GS. Multimodal monitoring intracranial pressure by invasive and noninvasive means. Sci Rep. 2023;13:18404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 40. | Cheng M, Liu T, Guo G, Hui Z, Zhang L, Hu J, Zhang J, Fang Y. Comparison of models for predicting intracranial pressure by optic nerve sheath diameter in combination with other indicators: A study based on ultrasound measurements. 2023 Preprint. [DOI] [Full Text] |
| 41. | Bakola E, Palaiodimou L, Eleftheriou A, Foska K, Pikouli A, Stefanatou M, Chondrogianni M, Velonakis G, Andreadou E, Papadopoulou M, Karapanayiotides T, Krogias C, Arvaniti C, Tsivgoulis G. Transorbital sonography in idiopathic intracranial hypertension: Single-center study, systematic review and meta-analysis. J Neuroimaging. 2024;34:108-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Kim KH, Kang HK, Koo HW. Prediction of Intracranial Pressure in Patients with an Aneurysmal Subarachnoid Hemorrhage Using Optic Nerve Sheath Diameter via Explainable Predictive Modeling. J Clin Med. 2024;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 43. | Blaivas M, Theodoro D, Sierzenski PR. Elevated intracranial pressure detected by bedside emergency ultrasonography of the optic nerve sheath. Acad Emerg Med. 2003;10:376-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 164] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 44. | Dubourg J, Javouhey E, Geeraerts T, Messerer M, Kassai B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: a systematic review and meta-analysis. Intensive Care Med. 2011;37:1059-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 389] [Article Influence: 25.9] [Reference Citation Analysis (1)] |
| 45. | Aspide R, Bertolini G, Albini Riccioli L, Mazzatenta D, Palandri G, Biasucci DG. A Proposal for a New Protocol for Sonographic Assessment of the Optic Nerve Sheath Diameter: The CLOSED Protocol. Neurocrit Care. 2020;32:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 46. | Aspide R, Bertolini G, Belotti LMB, Albini Riccioli L, Toni F, Mazzatenta D, Palandri G, Vetrugno L, Biasucci DG. The CLOSED protocol to assess optic nerve sheath diameter using color-Doppler: a comparison study in a cohort of idiopathic normal pressure hydrocephalus patients. Ultrasound J. 2022;14:43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 47. | Blehar DJ, Gaspari RJ, Montoya A, Calderon R. Correlation of visual axis and coronal axis measurements of the optic nerve sheath diameter. J Ultrasound Med. 2008;27:407-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Agrawal A, Cheng R, Tang J, Madhok DY. Comparison of Two Techniques to Measure Optic Nerve Sheath Diameter in Patients at Risk for Increased Intracranial Pressure. Crit Care Med. 2019;47:e495-e501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 49. | Zhu S, Cheng C, Zhao D, Zhao Y, Liu X, Zhang J. Clinical Value of Optic Nerve Sheath Diameter Assessment in Prognosis of Comatose Patients with Supratentorial Lesions. 2021 Preprint. [DOI] [Full Text] |
| 50. | Wang P, Zhou X, Sheng F, Wang X, Shi C, Feng W. Ultrasonic optic nerve sheath diameter can be used as a diagnostic measure after accidental dural puncture during cesarean section: a case report. BMC Anesthesiol. 2024;24:35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Xu H, Li Y, Liu J, Chen Z, Chen Q, Xiang Y, Zhang M, He W, Zhuang Y, Yang Y, Chen W, Chen Y. Dilated Optic Nerve Sheath Diameter Predicts Poor Outcome in Acute Spontaneous Intracerebral Hemorrhage. Cerebrovasc Dis. 2022;51:199-206. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 52. | Luo Y, Yang H, Zhou M, Yang W, Zhang W, Li QQ. Elevated Intracranial Pressure Level Is a Risk Factor for Sepsis-associated Encephalopathy: A Prospective Cohort Study. In Vivo. 2023;37:2585-2596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 53. | Yang Z, Qin C, Zhang S, Liu S, Sun T. Bedside ultrasound measurement of optic nerve sheath diameter in patients with sepsis: a prospective observational study. Crit Care. 2020;24:235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Czempik PF, Gąsiorek J, Bąk A, Krzych ŁJ. Ultrasonic Assessment of Optic Nerve Sheath Diameter in Patients at Risk of Sepsis-Associated Brain Dysfunction: A Preliminary Report. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 55. | Tarimah K, Bisri DY. The Role of ONSD-TCD in Transforming the Approach in Critical Care Management of Acute Spontaneous High-Grade Subarachnoid Hemorhagic: A Case Report. Migr Lett. 2023;21:679-692. |
| 56. | Kim JG, Kim W, Shin H, Lim TH, Jang BH, Cho Y, Choi KS, Na MK, Ahn C, Lee J. Optic Nerve Sheath Diameter for Predicting Outcomes in Post-Cardiac Arrest Syndrome: An Updated Systematic Review and Meta-Analysis. J Pers Med. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 57. | Chae MK, Ko E, Lee JH, Lee TR, Yoon H, Hwang SY, Cha WC, Shin TG, Sim MS, Jo IJ, Song KJ, Rhee JE, Jeong YK. Better prognostic value with combined optic nerve sheath diameter and grey-to-white matter ratio on initial brain computed tomography in post-cardiac arrest patients. Resuscitation. 2016;104:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 58. | Verhulst MMLH, Visser IM, Keijzer HM, de Kruijf NLM, Peters EJG, Wilbers T, Peelen RV, Hofmeijer J, Blans MJ. Additional predictive value of optic nerve sheath diameter for neurological prognosis after cardiac arrest: a prospective cohort study. Ultrasound J. 2023;15:46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 59. | Uttanganakam S, Hansda U, Sahoo S, Shaji IM, Guru S, Topno N, Sahoo NK. Sonographic Optic Nerve Sheath Diameter as a Guide for Correction of Hyponatremia in the Emergency Department: A Cross-sectional Study. Indian J Crit Care Med. 2023;27:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Kuru BK, Baydın A, Ocak M, Aksoy İ. Diagnostic and prognostic efficacy of optic nerve sheath diameter in patients with dysnatremia. Med Ultrason. 2024;26:147-152. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/