Published online Sep 9, 2024. doi: 10.5492/wjccm.v13.i3.95781
Revised: July 23, 2024
Accepted: August 9, 2024
Published online: September 9, 2024
Processing time: 133 Days and 21.1 Hours
The effect of intravenous bolus rates on patient outcomes is a complex and crucial aspect of critical care. Fluid challenges are commonly used in critically ill patients to manage their hemodynamic status, but there is limited information available on the specifics of when, how much, and at what rate fluids should be administered during these challenges. The aim of this review is to thoroughly examine the rela
Core Tip: This review aims to emphasize the importance of a meticulous and exact approach in medical settings, focusing on tailoring intravenous bolus rates to suit the specific needs of individual patients. This review seeks to provide valuable insights that can inform and optimize clinical practices in the critical care setting by maximizing desired outcomes through tailored strategies.
- Citation: Othman MI, Mustafa EM, Alfayoumi M, Khatib MY, Nashwan AJ. Impact of different intravenous bolus rates on fluid and electrolyte balance and mortality in critically ill patients. World J Crit Care Med 2024; 13(3): 95781
- URL: https://www.wjgnet.com/2220-3141/full/v13/i3/95781.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v13.i3.95781
Fluid management is crucial in managing critically ill patients, and involves flow and pressure variables, systemic shock responses, and various fluid types. The relationship between preload, stroke volume, and fluid administration is complex; the volume and rate of infusion have an impact on venous return. Precise fluid management is essential to prevent fluid overload in conditions such as septic shock. Crystalloids are the most common choice for fluid therapy, with balanced solutions possibly leading to an improved outcome[1]. Fluid management plays a crucial role in regulating hemodynamic parameters and tissue perfusion. It is difficult to precisely determine the critical oxygen delivery threshold to cells; however, several clinical indicators and parameters can function as surrogates, such as cardiac function's central role in therapy[1]. The daily fluid balance (FB) encompasses the cumulative intake and excretion of fluids over a 24-hour timeframe. On a daily basis, over 20% of patients in intensive care units (ICUs) require intravenous fluid resuscitation, with more than 30% receiving this type of therapy within the first day of admission to the ICU[2]. This encompasses all sources of fluid intake, including resuscitation and maintenance fluids, as well as different types of outputs like urine, ultrafiltration fluids, gastrointestinal losses, and third space losses. It is important to consider all these factors when pre
The fluid challenge is a widely used method for evaluating fluid responsiveness. It involves the administration of a fluid bolus and subsequent monitoring of the hemodynamic response. It is crucial to recognize that multiple fluid challenges have the potential to result in fluid overload. Thus, there is variation in the successful completion of fluid challenges in clinical settings. Fluid challenges are frequently utilized in patients who are critically ill, playing a crucial role in managing their hemodynamics. However, data regarding the indication, type, amount, and rate of fluid admi
This paper explores the importance of different intravenous fluid infusion rates for critically ill patients. It emphasizes the significance of immediately providing enough fluids to resuscitate patients and adopting a cautious strategy for subsequent fluid management, which can lead to better outcomes for patients. This review aims to look at the effects of different rates of intravenous fluid infusion in critically ill patients. This study seeks to enhance our understanding of the complicated dynamics of fluid and electrolyte management in critically ill patients through an analysis of extensive re
This review aimed to synthesize existing literature on the impact of different intravenous bolus rates on fluid and electrolyte balance, and mortality in critically ill patients. A narrative review approach was employed to summarize and analyze the findings from relevant studies.
From 2014 to 2024, a comprehensive literature review was conducted in electronic databases such as PubMed/MEDLINE, Embase, Scopus, and the Cochrane Library. The search approach used a combination of medical subject headings terms and keywords related to intravenous fluid management, bolus infusion, critically ill patients, fluid and electrolyte ba
Two reviewers separately conducted data extraction using a standardized form. The abstracts of discovered articles were reviewed based on the search strategies. Papers in English meeting the eligibility requirements were then obtained in full text. Furthermore, reference lists of selected papers were examined for further relevant studies. Multiple studies were found, but only a small number satisfied the requirements. Two reviewers were involved, with the second reviewer fo
Findings from the investigations were summarized and analyzed using a narrative synthesis approach. A full review was conducted on data regarding the effects of various intravenous bolus rates on fluid and electrolyte balance as well as mortality outcomes. The review focused on major results, trends, and areas of agreement or disagreement.
No formal quality evaluation or risk of bias assessment was performed due to the review's nature as a narrative synthesis of current material. The interpretation of findings considered the level of evidence and limitations of the investigations.
Nine relevant papers were found during the literature review (Table 1). The studies examined the correlation between intravenous bolus rates, fluid and electrolyte balance, and mortality outcomes in critically ill patients. The results from the studies differed in various clinical contexts, such as acute kidney injury, traumatic brain injury, cancer, acute res
| Ref. | Research findings | Methodologies employed | Clinical approaches | Fluid types and bolus rates | Gaps in current research/limitations | Practical insights for healthcare professionals |
| Caltabeloti et al[5], 2014 | Early fluid loading worsens lung aeration without affecting oxygenation | Observational study with lung ultrasound | Early aggressive fluid loading in ARDS | Not specified | Limited by observational design, small sample size | Caution against early aggressive fluid loading in ARDS patients |
| Ukor et al[7], 2017 | Bolus infusion causes more pronounced hemodynamic changes compared to slower infusion | Experimental study with healthy volunteers | Comparison of bolus vs slow infusion | Intravenous crystalloid at different rates | Limited to healthy volunteers, not critically ill patients | Consider slower infusion rates for better hemodynamic stability |
| Kattan et al[8], 2020 | Identifies optimal targets for fluid resuscitation in septic shock | Review of existing studies and guidelines | Septic shock resuscitation strategies | Various fluids and administration rates | Variability in study designs and targets | Personalized resuscitation targets based on patient condition |
| Connor et al[9], 2021 | Crystalloid composition and administration rate impact resuscitation outcomes | Analysis of ICU patient data | Different rates and compositions of crystalloids | Crystalloids at various rates | Lack of large-scale randomized trials | Importance of considering fluid composition and rate in ICU resuscitation |
| Trejnowska et al[11], 2019 | Fluid balance is critical for outcomes in critically ill patients | Retrospective observational study | Monitoring and managing fluid balance | Not specified | Retrospective design, single-center study | Emphasizes meticulous fluid balance management |
| Barmparas et al[12], 2014 | Positive fluid balance is associated with worse outcomes in surgical patients | Prospective observational study | Monitoring fluid balance | Crystalloids and colloids | Single-center study, observational design | Avoid positive fluid balance to improve outcomes |
| Zampieri et al[15], 2021 | Slower bolus rates may improve mortality in critically ill patients | Randomized clinical trial | Comparison of slower and faster bolus rates | Intravenous fluids at different bolus rates | Potential variability in patient conditions | Slower bolus rates could be beneficial for critically ill patients |
| Shen et al[14], 2017 | Negative fluid balance is linked to lower mortality in critically ill patients | Retrospective cohort study | Monitoring fluid intake and outcomes | Not specified | Retrospective design, potential confounding factors | Targeting negative fluid balance may improve survival |
| Chen et al[13], 2020 | Early positive fluid balance was associated with higher 1-year mortality in critically ill cancer patients | Retrospective observational study | Emphasizes early fluid management in cancer patients | Not specified in the study | Retrospective design, single-center study, potential for confounding variables | Highlights the need for careful fluid management in critically ill cancer patients to improve long-term survival |
The studies emphasize the importance of precise FB monitoring and management to improve patient outcomes and reduce mortality rates in critically ill patients[6,7]. Another investigation was conducted to investigate the impact of FB and fluid intake on outcomes in critically ill patients. Examined data from the Multi-parameter Intelligent Monitoring in Intensive Care III Database, specifically looked at patients who attained negative FB 48 hours after ICU admission. The study revealed a correlation between slight negative FB and decreased hospital mortality and a connection between increased fluid intake and urine output and reduced mortality. However, an increase in negative FB was not associated with reduced mortality[8]. While some studies found no significant difference between slower and faster intravenous fluid bolus rates, others suggested that slower bolus rates may be correlated with reduced mortality[9]. A study was conducted to compare the impact of different intravenous fluid bolus rates on mortality in critically ill patients. The trial involved slower (333 mL/h) and faster (999 mL/h) rates. 10520 patients in 75 ICUs across Brazil were included in the study, which concluded that there was no statistically significant difference in 90-day mortality based on the infusion rates. Findings from the trial indicated that the rate of intravenous fluid bolus administration does not significantly affect mortality outcomes in critically ill patients who need fluid challenges.
Studies indicate that positive or negative FB can affect the long-term survival of critically ill patients. Excess fluid leads to higher mortality rates, whereas inadequate fluid intake decreases short-term mortality but increases long-term mor
In a randomized, double-blind trial, researchers found no significant difference in outcomes between balanced mul
The existing literature on the impact of intravenous bolus rates on fluid and electrolyte balance in critically ill patients has a few limitations. There are several factors that contribute to the complexity of this topic. These factors include variations in study populations, differences in protocols for administering fluids, variations in the types of fluids used, differences in short-term and long-term outcomes, variations in research methods, a lack of patient-centered approaches, insufficient reporting of adverse effects, limited comparative studies, regional and institutional differences, and a tendency to focus on single-center studies. These factors pose challenges in generalizing findings across various patient groups and establishing optimal practices. In addition, it is important to consider regional and institutional differences which may in
The limitations affect data interpretation, highlighting the necessity for additional research to overcome these constraints and offer stronger evidence. A rapid infusion of 20 mL/kg over 30 minutes had various effects on gas exchange and cardiovascular parameters. It caused a small but significant reduction in forced expiratory flow and elevated systolic blood pressure by about 9%[13]. However, there were no significant changes in forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC), heart rate, or lung volume. The FEV1/FVC ratio indicates how much air you can forcefully exhale. Spirometry, a test for diagnosing or monitoring lung conditions, mea
These findings highlight the significance of managing FB in critically ill patients, as both positive and negative balances can greatly impact long-term survival outcomes. It is crucial to carefully manage fluid administration to prevent excess accumulation and ensure proper resuscitation for optimal patient outcomes in critical care settings[2,21]. The randomized clinical trial conducted has limitations, including not being analyzed as an intention to treat, a discrepancy between expected and observed mortality rates, and patients receiving small amounts of fluid.
The management of fluids in critically ill patients presents several challenges, such as optimizing FB, mitigating the risk of fluid overload, accounting for diverse patient populations, navigating complex clinical decision-making processes, and appropriately evaluating response to fluid administration[21]. Due to the dynamic nature of the patient's condition, re
Excessive administration of fluids can result in unfavorable consequences, including the development of pulmonary edema and heart failure. When making treatment options, clinicians must consider many parameters, such as hemody
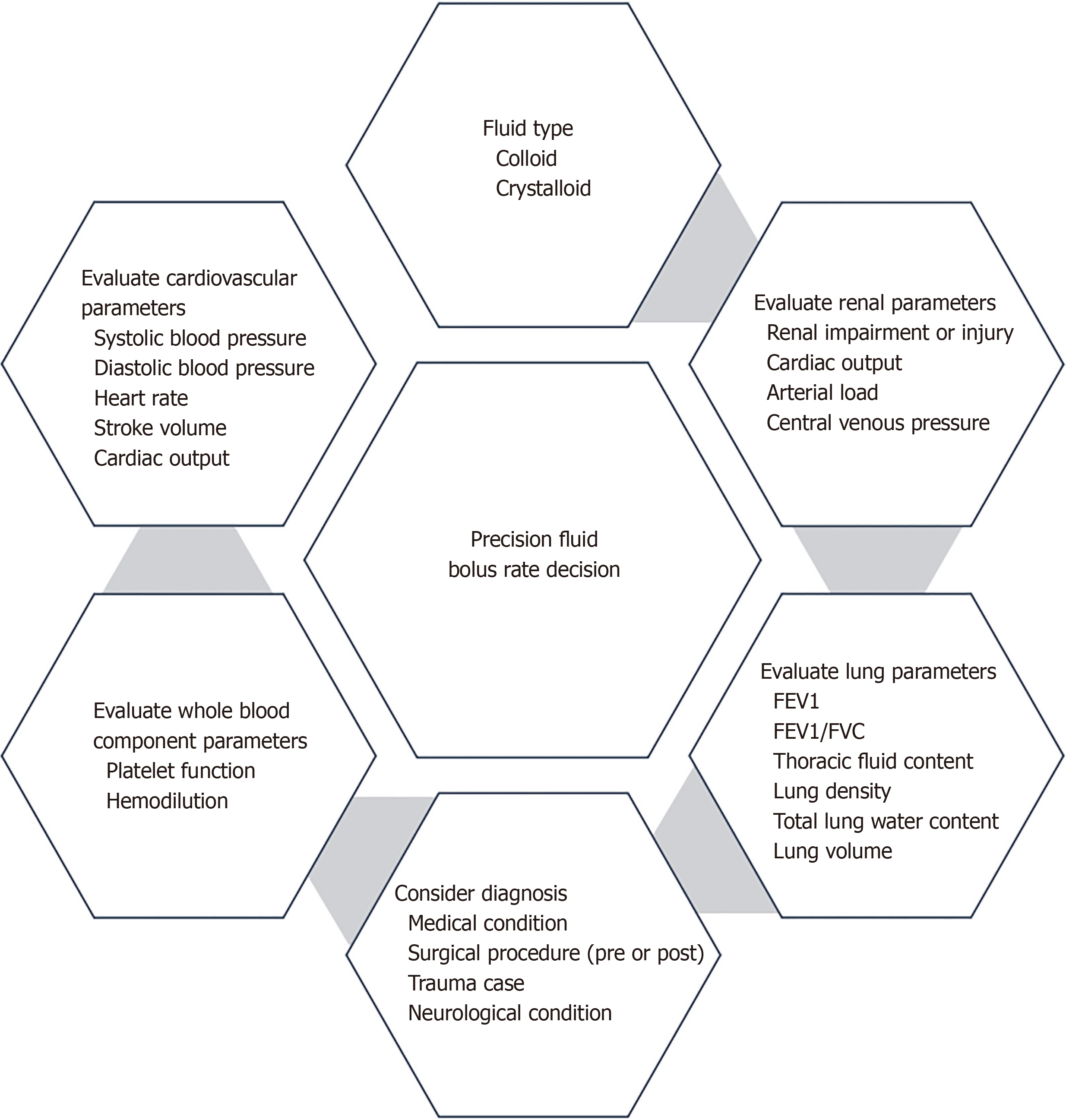
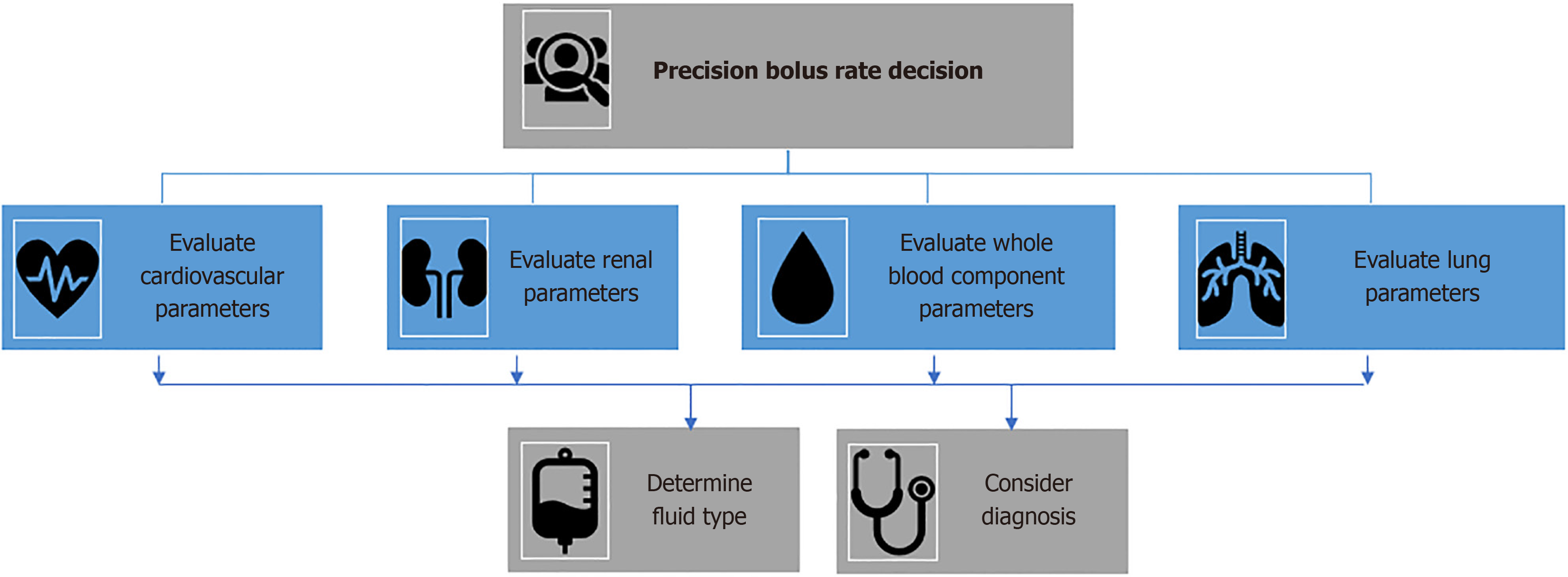
Precision medicine and genomics can improve fluid management for critically ill patients. By customizing treatments according to individual genetic profiles and characteristics, we can effectively address the challenges in this area[23]. Advancements in genomics have opened possibilities for a personalized approach to critical care, which could sig
One approach to addressing the difficulties associated with fluid management in critically ill patients is by imple
Implementing precision medicine for fluid management in critically ill patients presents several challenges that need to be addressed to optimize patient care. The implementation of precision medicine in the ICU is complex due to the re
This paper discusses how various bolus rates affect FB in critically ill patients, highlighting the necessity for additional research given the complexity and variations among individual patients. Various factors, including patient characteristics, the clinical context, and the timing and volume of fluid administration, can influence the impact of IV fluid bolus intervention on mortality. Research should be conducted on precision medicine and genomics to optimize fluid bolus rate administration in critically ill patients by considering individual patient characteristics and genetic factors. Additional large-scale randomized controlled trials with stratified analyses are necessary to establish the optimal fluid infusion rates and their impact on mortality, organ dysfunction, and other clinical outcomes in critically ill patients. Managing fluid levels in critically ill patients is a complex task that calls for personalized treatment in parallel to prescribing medication tailored to the patient's unique requirements and tolerances.
| 1. | Messina A, Bakker J, Chew M, De Backer D, Hamzaoui O, Hernandez G, Myatra SN, Monnet X, Ostermann M, Pinsky M, Teboul JL, Cecconi M. Pathophysiology of fluid administration in critically ill patients. Intensive Care Med Exp. 2022;10:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 2. | Finfer S, Myburgh J, Bellomo R. Intravenous fluid therapy in critically ill adults. Nat Rev Nephrol. 2018;14:541-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 150] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 3. | Van Regenmortel N, De Weerdt T, Van Craenenbroeck AH, Roelant E, Verbrugghe W, Dams K, Malbrain MLNG, Van den Wyngaert T, Jorens PG. Effect of isotonic versus hypotonic maintenance fluid therapy on urine output, fluid balance, and electrolyte homeostasis: a crossover study in fasting adult volunteers. Br J Anaesth. 2017;118:892-900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Maes T, Meuwissen A, Diltoer M, Nguyen DN, La Meir M, Wise R, Spapen H, Malbrain MLNG, De Waele E. Impact of maintenance, resuscitation and unintended fluid therapy on global fluid load after elective coronary artery bypass surgery. J Crit Care. 2019;49:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Caltabeloti F, Monsel A, Arbelot C, Brisson H, Lu Q, Gu WJ, Zhou GJ, Auler JO, Rouby JJ. Early fluid loading in acute respiratory distress syndrome with septic shock deteriorates lung aeration without impairing arterial oxygenation: a lung ultrasound observational study. Crit Care. 2014;18:R91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (2)] |
| 6. | Hoesterey D, Das N, Janssens W, Buhr RG, Martinez FJ, Cooper CB, Tashkin DP, Barjaktarevic I. Spirometric indices of early airflow impairment in individuals at risk of developing COPD: Spirometry beyond FEV(1)/FVC. Respir Med. 2019;156:58-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Ukor IF, Hilton AK, Bailey MJ, Bellomo R. The haemodynamic effects of bolus versus slower infusion of intravenous crystalloid in healthy volunteers. J Crit Care. 2017;41:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 8. | Kattan E, Castro R, Vera M, Hernández G. Optimal target in septic shock resuscitation. Ann Transl Med. 2020;8:789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Connor MJ Jr, Coopersmith CM. Does Crystalloid Composition or Rate of Fluid Administration Make a Difference When Resuscitating Patients in the ICU? JAMA. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Boyd CJ, Claus MA, Raisis AL, Hosgood G, Sharp CR, Smart L. Hypocoagulability and Platelet Dysfunction Are Exacerbated by Synthetic Colloids in a Canine Hemorrhagic Shock Model. Front Vet Sci. 2018;5:279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Trejnowska E, Skoczyński S, Armatowicz P, Knapik M, Kurdyś P, Ślusarz K, Tarczyńska-Słomian M, Knapik P. The importance of fluid balance in critically ill patients: a retrospective observational study. Kardiol Pol. 2019;77:1147-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Barmparas G, Liou D, Lee D, Fierro N, Bloom M, Ley E, Salim A, Bukur M. Impact of positive fluid balance on critically ill surgical patients: a prospective observational study. J Crit Care. 2014;29:936-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Chen YC, Zheng ZR, Wang CY, Chao WC. Impact of Early Fluid Balance on 1-Year Mortality in Critically Ill Patients With Cancer: A Retrospective Study in Central Taiwan. Cancer Control. 2020;27:1073274820920733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Shen Y, Huang X, Zhang W. Association between fluid intake and mortality in critically ill patients with negative fluid balance: a retrospective cohort study. Crit Care. 2017;21:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Zampieri FG, Machado FR, Biondi RS, Freitas FGR, Veiga VC, Figueiredo RC, Lovato WJ, Amêndola CP, Assunção MSC, Serpa-Neto A, Paranhos JLR, Andrade J, Godoy MMG, Romano E, Dal Pizzol F, Silva EB, Silva MML, Machado MCV, Malbouisson LMS, Manoel ALO, Thompson MM, Figueiredo LM, Soares RM, Miranda TA, de Lima LM, Santucci EV, Corrêa TD, Azevedo LCP, Kellum JA, Damiani LP, Silva NB, Cavalcanti AB; BaSICS investigators and the BRICNet members. Effect of Slower vs Faster Intravenous Fluid Bolus Rates on Mortality in Critically Ill Patients: The BaSICS Randomized Clinical Trial. JAMA. 2021;326:830-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 16. | Balakumar V, Murugan R, Sileanu FE, Palevsky P, Clermont G, Kellum JA. Both Positive and Negative Fluid Balance May Be Associated With Reduced Long-Term Survival in the Critically Ill. Crit Care Med. 2017;45:e749-e757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 17. | Ben Aicha Y, Ben Souissi A, Kamoun S, Koubaji S, Haddad F, Mebazaa MS. Fluid balance and mortality in critically ILL patients. Intensive Care Med Exp. 2015;3 Suppl 1:A532. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 18. | Finfer S, Micallef S, Hammond N, Navarra L, Bellomo R, Billot L, Delaney A, Gallagher M, Gattas D, Li Q, Mackle D, Mysore J, Saxena M, Taylor C, Young P, Myburgh J; PLUS Study Investigators and the Australian New Zealand Intensive Care Society Clinical Trials Group. Balanced Multielectrolyte Solution versus Saline in Critically Ill Adults. N Engl J Med. 2022;386:815-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 194] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 19. | Vincent JL. Fluid management in the critically ill. Kidney Int. 2019;96:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 20. | Othman MI, Nashwan AJ, Alfayoumi M, Khatib M, Abujaber AA. Plasma-Lyte-148 Versus Normal Saline 0.9% in Diabetic Ketoacidosis Management: A Review. Cureus. 2023;15:e41079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 21. | Malbrain MLNG, Langer T, Annane D, Gattinoni L, Elbers P, Hahn RG, De Laet I, Minini A, Wong A, Ince C, Muckart D, Mythen M, Caironi P, Van Regenmortel N. Intravenous fluid therapy in the perioperative and critical care setting: Executive summary of the International Fluid Academy (IFA). Ann Intensive Care. 2020;10:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 179] [Article Influence: 29.8] [Reference Citation Analysis (1)] |
| 22. | Besen BA, Gobatto AL, Melro LM, Maciel AT, Park M. Fluid and electrolyte overload in critically ill patients: An overview. World J Crit Care Med. 2015;4:116-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 23. | Bauer SR, Gellatly RM, Erstad BL. Precision fluid and vasoactive drug therapy for critically ill patients. Pharmacotherapy. 2023;43:1182-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Golubev AM. Personalized Critical Care Medicine (Review). Obŝaâ reanimatologiâ. 2022;18:45-54. [DOI] [Full Text] |
| 25. | Rogers AJ, Meyer NJ. Precision Medicine in Critical Illness: Sepsis and Acute Respiratory Distress Syndrome. In: Gomez J, Himes B, Kaminski N, editors. Precision in Pulmonary, Critical Care, and Sleep Medicine. Respiratory Medicine. Cham: Humana, 2020. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Seymour CW, Gomez H, Chang CH, Clermont G, Kellum JA, Kennedy J, Yende S, Angus DC. Precision medicine for all? Challenges and opportunities for a precision medicine approach to critical illness. Crit Care. 2017;21:257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 27. | Méndez Hernández R, Ramasco Rueda F. Biomarkers as Prognostic Predictors and Therapeutic Guide in Critically Ill Patients: Clinical Evidence. J Pers Med. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 28. | Maslove DM, Lamontagne F, Marshall JC, Heyland DK. A path to precision in the ICU. Crit Care. 2017;21:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (2)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/