INTRODUCTION
Diagnosing acute kidney injury (AKI) in individuals with cirrhosis poses a challenge because of multiple potential causes with overlapping presentations. Type 1 Hepatorenal syndrome (HRS) stands as the most feared cause of AKI in these patients, carrying a grim prognosis. It remains a diagnosis of exclusion due to the absence of definitive diagnostic tools. Notably, guidelines emphasize the exclusion of volume depletion in cirrhotic patients with AKI, often resorting to empiric administration of albumin (1 g/kg) for two days along with diuretic withdrawal[1]. While the underlying rationale is to address volume depletion, frequently induced by factors like lactulose-induced diarrhea, infections, or over-diuresis, it's equally important to acknowledge that not all patients may be volume-depleted. Consequently, these universal recommendations might inadvertently harm some patients by causing fluid overload. This concern gains prominence in the contemporary medical landscape, where the adverse effects of fluid overload are being increasingly recognized in various clinical scenarios[2]. Even within the realm of cirrhosis, the detrimental aspects of excessive albumin administration are becoming apparent. For instance, in a randomized controlled trial involving patients with decompensated cirrhosis, the repeated daily infusion of intravenous albumin with a targeted serum level did not show an improvement in kidney function compared to standard care; rather, it increased the incidence of pulmonary edema and fluid overload[3]. This topic has garnered further attention, with findings from the CONFIRM trial indicating higher rates of respiratory complications in individuals who received both terlipressin and albumin[4]. The increased afterload induced by terlipressin, along with a potential direct effect on pulmonary vasculature is thought to contribute to this phenomenon with albumin-induced increased preload playing a crucial role. The limitations of conventional physical examination are well acknowledged in the assessment of fluid status. Laboratory data alone may not suffice to distinguish certain etiologies. For instance, low urine sodium can be observed in various conditions, including volume depletion, congestive nephropathy, iodinated contrast exposure, intra-abdominal hypertension (IAH), and HRS, as well as in some cases of tubular injury. While a thorough history aids to some extent, physicians often depend on their clinical judgment to guide management in these cases, influenced by their experience, pattern recognition skills, and biases. In recent times, point-of-care ultrasonography (POCUS) has emerged as a complement to the physical examination, demonstrating several advantages. These include improved diagnostic accuracy for facilitating appropriate management, decreased time to accurate diagnosis, diminished need for imaging studies like repeated chest radiographs, lowered healthcare costs, and enhanced patient satisfaction, as summarized in our prior reviews[5,6]. Fundamentally, POCUS is intended to address focused questions that clinicians have in mind after taking history, performing conventional clinical assessment, and reviewing available laboratory data. In this current review, I will provide an overview of how we utilize POCUS in evaluating patients with cirrhosis and AKI to unravel diagnostic possibilities.
POCUS IN AKI
AKI in cirrhosis presents a broad range of potential causes, encompassing hemodynamic factors (such as volume depletion, congestive nephropathy, IAH-induced hypoperfusion in the background of cirrhosis-related circulatory derangement or frank HRS), non-hemodynamic factors (like glomerulonephritis, cholemic nephropathy), or obstructive factors[7]. While it must be acknowledged that POCUS is not a panacea, its strategic use within the appropriate clinical context significantly assists in narrowing down the differential diagnosis. Initially considered limited to diagnosing hydronephrosis, the role of POCUS in AKI has evolved to encompass detailed hemodynamic assessment, which includes lung ultrasound, focused cardiac ultrasound (FoCUS), and venous Doppler[8]. It can be argued that POCUS performed by nephrologists or internists proves most valuable in evaluating hemodynamic AKI, followed by obstructive nephropathy, and is least impactful in intrinsic causes of AKI. In practical terms, POCUS is categorized into basic and advanced, depending on the training and skill set required to assess relevant sonographic parameters. Basic POCUS involves greyscale and color Doppler applications, while advanced POCUS incorporates spectral Doppler applications[5,9]. Spectral Doppler refers to the type of Doppler that allows quantitative and semi-quantitative assessment of blood flow. Whether performing basic or advanced POCUS, it is essential to consider the entire hemodynamic circuit and avoid relying solely on one organ.
BASIC POCUS
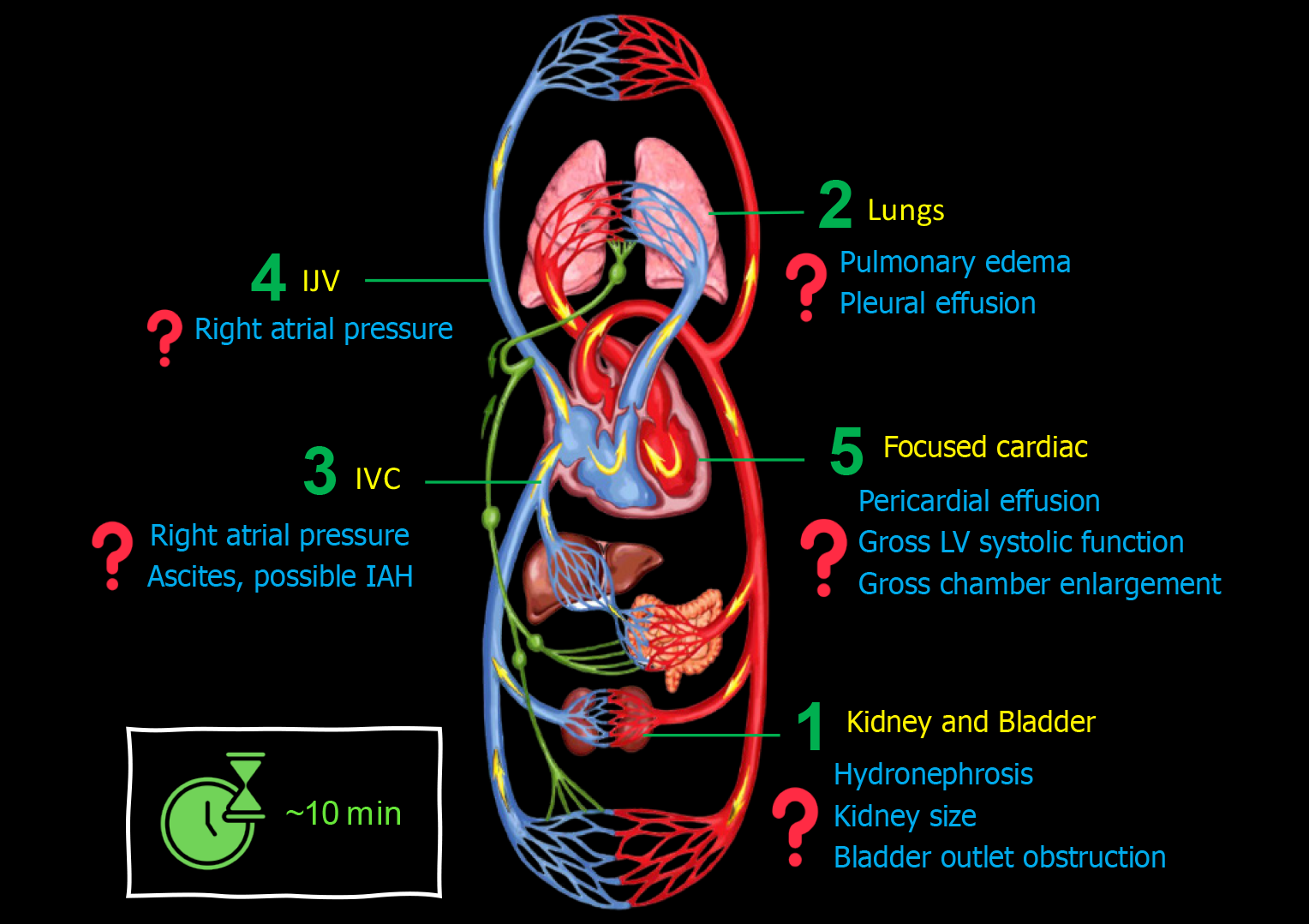
When consulting on a patient with cirrhosis and AKI, I perform a 5-point POCUS trying to answer pertinent focused questions Figure 1.
Figure 1 Basic point-of-care ultrasound evaluation in a patient with cirrhosis and acute kidney injury illustrating common focused clinical questions.
IJV: Internal jugular vein; IVC: Inferior vena cava.
Kidney and urinary bladder
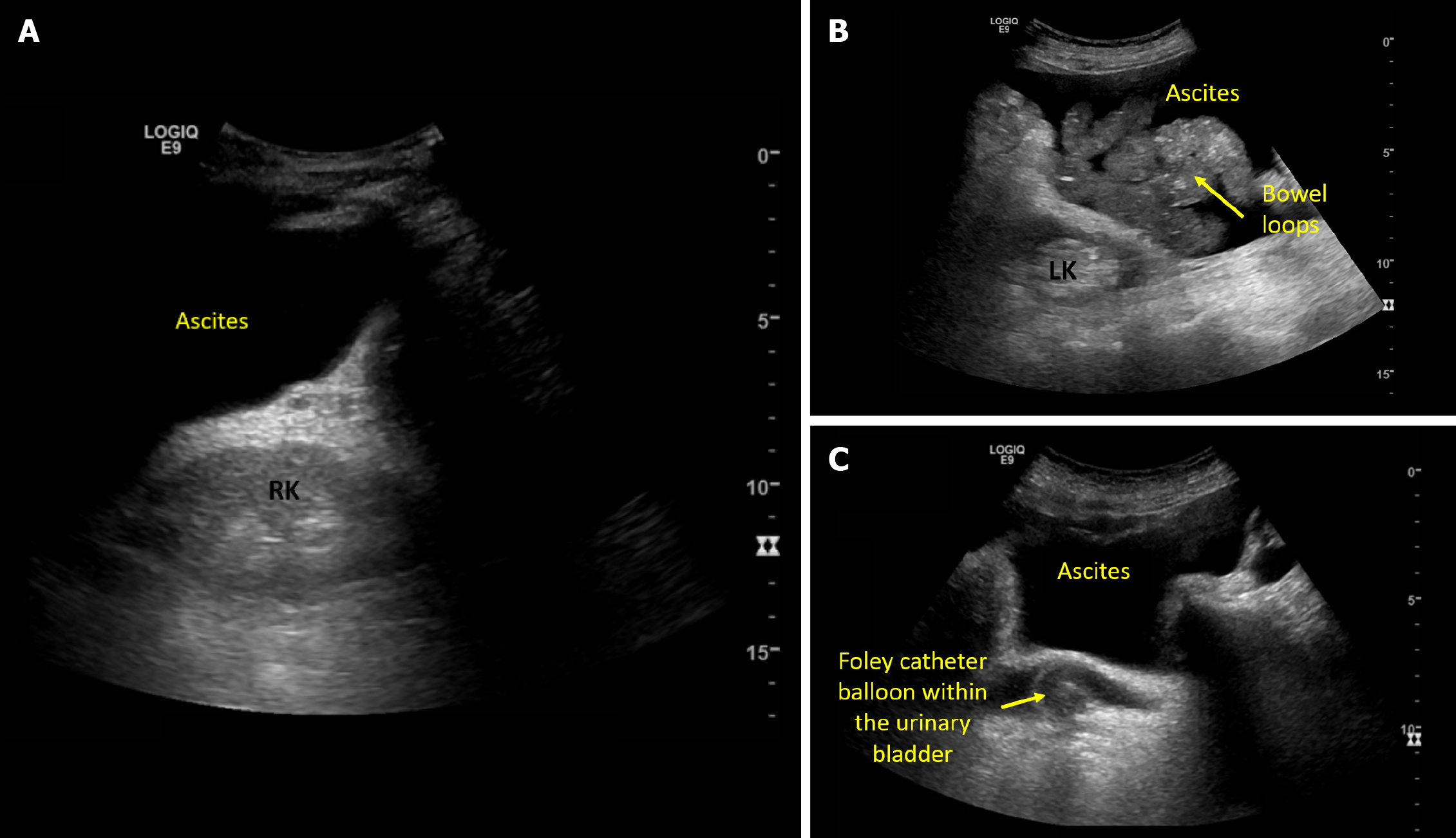
Obstructive nephropathy is a rare cause of AKI in cirrhosis, reported in instances of tense ascites and midodrine-induced increased vesical sphincter tone in susceptible patients[10,11]. Nonetheless, I consider excluding hydronephrosis as a part of the physical examination in any patient with AKI, as it requires only a minute or two, and relieving the source of obstruction may potentially reverse AKI. Having said that, if a radiology-performed kidney ultrasound is available from the day of consultation, I wouldn't repeat the scan but would simply review the images. Urinary bladder ultrasound is an integral component of renal system POCUS, allowing quick identification of bladder distension, obstructed or misplaced Foley catheters[12]. It is also helpful in cases of pelvic ascites to differentiate it from urine, as automated bladder scanners tend to mistake ascites for bladder[13]. Kidney size and cortical echogenicity are commonly used parameters to gauge the chronicity of kidney disease in patients without recent baseline serum creatinine. While POCUS assists in evaluating renal length and parenchymal thickness, the interpretability of cortical echogenicity becomes challenging in the presence of ascites because of acoustic enhancement. It is a property of fluids on ultrasound that creates a posterior hyperechoic (bright) area that makes the entire renal parenchyma appear bright in this scenario Figure 2. While assessing the kidneys, I also make a note of ascites, as it can be a contributing factor for IAH and subsequent AKI through renal hypoperfusion.
Figure 2 Ultrasound images demonstrating.
A: Acoustic enhancement from ascites making the right kidney appear bright; B: Left kidney adjacent to ascites and bowel loops; C: Pelvic ascites and a urinary bladder decompressed with Foley catheter.
Lung ultrasound
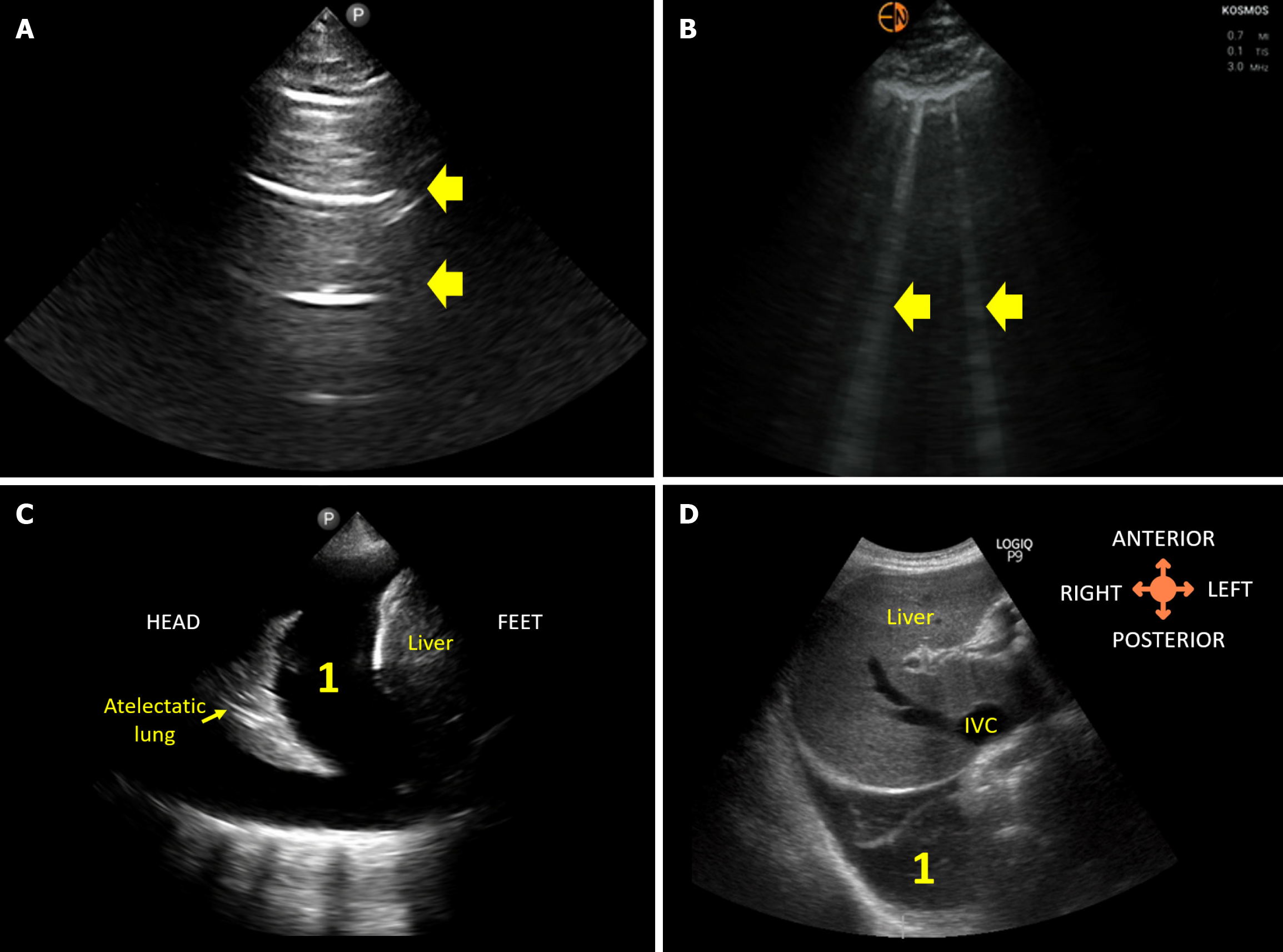
Given the high prevalence of diastolic dysfunction in cirrhosis patients and the elevated risk of pulmonary complications during therapy[3,4,14], it is prudent to assess the lungs for extravascular lung water, especially in patients receiving or likely to receive empiric intravenous fluids. AKI can further complicate the scenario by contributing to fluid retention in susceptible individuals. Unfortunately, the sensitivity of auscultation findings is low for detecting pulmonary congestion, as demonstrated in various clinical settings, including heart failure, end-stage kidney disease, and critically ill patients[15,16]. Obviously, it is not advisable to obtain repeated chest radiographs to monitor congestion, as this exposes patients to unnecessary radiation. In this context, lung POCUS emerges as a radiation-free option that surpasses auscultation in detecting extravascular lung water. The number of B-lines (vertical hyperechoic artifacts Figure 3 on lung POCUS correlates with pulmonary capillary wedge pressure and subclinical pulmonary congestion detected by POCUS holds prognostic significance[17-20]. Notably, POCUS outperforms even chest radiography in detecting cardiogenic pulmonary edema[21]. Lung POCUS is also effective in identifying pleural effusion, appearing as an anechoic (black) area in the dependent zones Figure 3. In one study, antero-posterior chest radiographs failed to recognize approximately 40% of effusions visible on ultrasound in patients with cirrhosis[22]. Phrases like "discontinue fluids if respiratory status worsens" or "oxygen saturation is OK, continue fluids" are commonly seen in the physicians’ notes. For patients considered for terlipressin therapy (with co-administration of albumin), the current recommendation is to avoid the drug if oxygen saturation is < 90%[23]. With a sensitive tool like POCUS available, it is regrettable that clinicians prefer to wait until patients become symptomatic or desaturate significantly before acting. While some may argue that performing continuous lung POCUS is impractical compared to monitoring oxygen saturation or waiting for the patient to complain of shortness of breath, in the modern day, multiple clinicians examine these patients (primary team, consultants). As such, performing lung POCUS once or twice a day is not a challenging task, especially in the era of nurse-performed and patient-performed lung POCUS[24,25]. It is a less convincing argument in my view.
Figure 3 Lung ultrasound images demonstrating.
A: Normal A-lines; B: B-lines (vertical artifacts) suggestive of extravascular lung water; C: 1Pleural effusion visualized from the conventional lateral view; D: 1Right pleural effusion visualized from the subcostal view.
Inferior vena cava ultrasound
In echocardiography, the sonography of the inferior vena cava (IVC) is a standard practice for estimating right atrial pressure (RAP) since it functions as a conduit linked to the right atrium. Elevated RAP becomes crucial in the context of AKI as it indicates fluid intolerance, signifying a heightened risk of congestive nephropathy. Simplistically, fluid intolerance refers to a state where administering fluids may potentially cause harm due to volume overload[26]. Congestive nephropathy refers to renal dysfunction observed in the presence of venous congestion, resulting from elevated RAP, and subsequently reducing renal perfusion[27]. The underlying pathophysiology necessitates understanding that grossly, renal perfusion pressure is the difference between the inflow pressure mean arterial pressure (MAP) and outflow pressure (RAP or intra-abdominal pressure when elevated). Consequently, even when the MAP is maintained, elevated RAP leads to a decrease in perfusion pressure, causing interstitial edema, inflammation, increased intra-tubular pressure, and a reduction in the glomerular filtration gradient, ultimately contributing to worsening AKI. In a study involving 127 patients ‘clinically’ diagnosed with HRS, over 60% exhibited both elevated RAP and pulmonary capillary wedge pressure, underscoring the inadequacy of conventional clinical parameters in accurately determining volume status[28]. Notably, diuretic therapy improved creatinine levels in this subgroup of patients, providing a compelling argument for the presence of congestive nephropathy.
Coming back to the IVC POCUS, in spontaneously breathing patients, an IVC diameter of < 2.1 cm with a collapse of > 50% during a sniff suggests a RAP between 0-5 mmHg. On the other hand, an IVC > 2.1 cm with a collapse < 50% during a sniff indicates a RAP of 10-20 mmHg. If the vessel is dilated but well-collapsible, or vice versa, it suggests an intermediate RAP (5-10 mmHg)[29]. In the context of cirrhosis, an observational study by Velez et al[30] reignited interest in IVC POCUS. Within their cohort of 53 patients ‘clinically’ diagnosed with HRS, IVC ultrasound significantly altered the management in more than 60% of the cases by revealing potentially treatable volume disorders (such as volume depletion, overload, or possible IAH), once again emphasizing the limitations of conventional physical examination in assessing volume status.
While IVC POCUS is popular among novice users due to its perceived technical simplicity, potential pitfalls exist. These include the risk of confusing the vessel with the aorta, unawareness that the aforementioned cutoffs are not applicable in mechanically ventilated patients, placing excessive emphasis on percent collapsibility without recognizing that the strength of breath varies among hospitalized, sick patients, and succumbing to the cylinder effect, where the two-dimensional ultrasound beam bisects the three-dimensional vessel at the periphery, resulting in a falsely smaller diameter when imaged in long axis alone.
Furthermore, in patients with cirrhosis, there are specific considerations regarding IVC POCUS. Hepatic fibrosis, resulting in increased stiffness, can impede pressure-dependent alterations in venous diameter[31]. Additionally, IVC dilation may be secondary to factors unrelated to RAP, such as portosystemic collaterals draining into the IVC, constriction from an enlarged caudate lobe, or the extension of hepatic vein thromboses or hepatocellular carcinoma into the IVC[32]. It is also notable that IAH caused by tense ascites can compress the IVC, creating a false impression of low RAP when the RAP might actually be elevated[33]. I want to underscore that IVC POCUS should not be relied upon for distinguishing between volume depletion and a euvolemic state, despite seemingly convincing findings from studies utilizing arbitrary cutoffs. Those with substantial experience in POCUS would agree that it is common to observe small, completely collapsible IVCs in clinically stable patients who are euvolemic with a steady creatinine level.
Internal jugular vein ultrasound
If the IVC is challenging to access or appears unreliable, it is advisable to assess the internal jugular vein (IJV) for estimating RAP. In patients with cirrhosis, IJV exhibits a stronger correlation with RAP compared to IVC because variations in IJV diameter are not influenced by liver stiffness and IAH[34]. However, one must exercise caution due to potential caveats in IJV POCUS, including errors due to inappropriate head angle, excessive transducer pressure on the vein, inaccessibility due to previous thrombosis, variations in right atrial depth (contrary to the traditional assumption of 5 cm in everybody), and the diverse range of techniques found in the literature (e.g., height of the venous column, respiratory variation in diameter, cross-sectional area, response to Valsalva, etc.) detailed in our recent review article[35].
FoCUS
FoCUS is an integral element of bedside hemodynamic evaluation in patients with AKI[2]. For instance, a plethoric IVC could be due to various factors such as pericardial effusion, pulmonary embolism, right ventricular (RV) failure from chronic pulmonary hypertension, biventricular failure, tricuspid regurgitation (TR), among others. The appropriate management, aimed at optimizing hemodynamics and consequently renal perfusion, varies depending on the underlying cause. Just through meticulous examination of greyscale and color Doppler images, one can rapidly assess left ventricular (LV) systolic function, ascertain the presence or absence of pericardial effusion, identify significant valvular lesions (such as mitral regurgitation leading to pulmonary edema), and observe gross chamber enlargement (for example, RV dilation with potential pressure effect on the LV, left atrial dilation in diastolic dysfunction etc.). In a meta-analysis, FoCUS exhibited a substantial improvement in sensitivity compared to auscultation for diagnosing LV dysfunction and aortic or mitral valve disease (84% vs 43% and 71% vs 46%, respectively)[36].
In cirrhosis, the activation of vasodilators, primarily nitric oxide, induces systemic vasodilation, leading to decreased systemic vascular resistance. Additionally, arteriovenous, and portosystemic shunts contribute to this phenomenon. Initial hypoperfusion is compensated through the renin-angiotensin-aldosterone system and antidiuretic hormone-mediated volume expansion, and adrenergic activation, resulting in increased cardiac output via enhanced preload and chronotropism[37-39]. However, if the ensuing cardiac output proves inadequate for metabolic demands and volume expansion surpasses the limits of the Frank-Starling mechanism, high-output cardiac failure may occur, causing elevated filling pressures and venous congestion. In this context, it is noteworthy that a 'hyperdynamic LV' is not specific to volume depletion; it can indicate hypovolemia, a euvolemic state, or a high cardiac output state, much like a small collapsible IVC. A recent study in patients with sepsis and septic shock supports this idea, revealing significantly higher LV ejection fraction (64% vs 56%, P < 0.001) and stroke volume (72 vs 48 mL, P < 0.001), coupled with lower arterial elastance and systemic vascular resistance in patients with cirrhosis compared to those without cirrhosis. This implies that volume depletion is not the primary factor driving a hyperdynamic heart in these patients[40].
In essence, basic POCUS enables us to rule out urinary obstruction, consider IAH as a possibility, and assess fluid tolerance. For a proficient POCUS user, this evaluation typically spans around 10 min, including tasks like adjusting bed height and cleaning off the gel. An added benefit is the ability to engage with the patient during the procedure, providing education on the findings and gathering relevant history. Even if a less experienced individual takes slightly longer, it remains reasonable, especially considering that not every patient on the nephrology rounding list requires POCUS.
ADVANCED POCUS
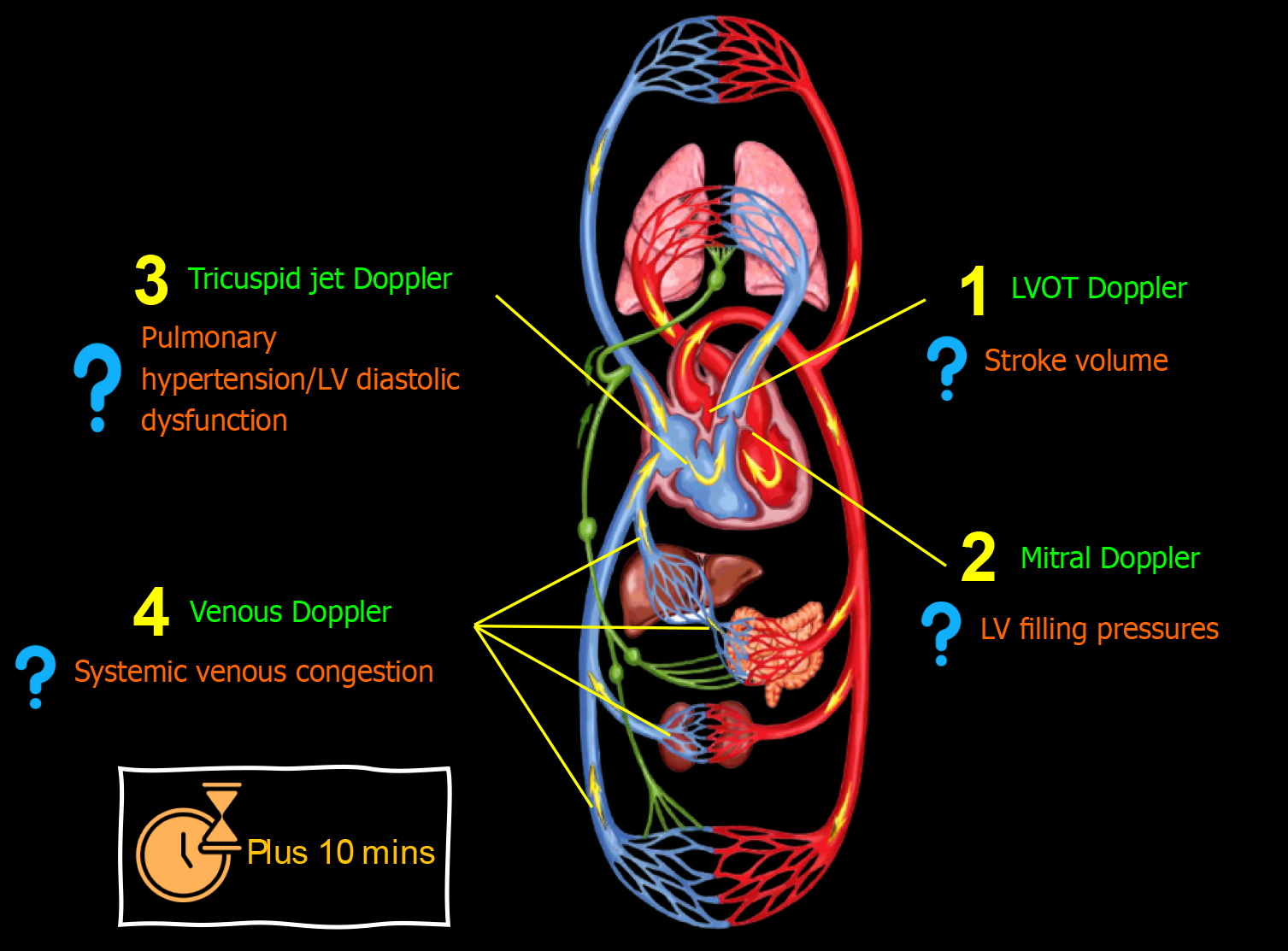
In certain cases, evaluating additional Doppler parameters may be essential, either during the initial consultation or follow-up, and whether through a combined or 1-2 selected parameters Figure 4. Although advanced POCUS demands extra training and expertise, nephrologists should aim to acquire proficiency in these techniques. This is crucial because we are not the primary point of contact for patients when they first present to the hospital, during which binary findings like 'high RAP vs low RAP' might be more relevant. When assessing patients after multiple therapeutic interventions, addressing more nuanced, focused questions becomes imperative. Following are some common parameters I evaluate.
Figure 4 Advanced (using spectral Doppler) point-of-care ultrasound evaluation in a patient with cirrhosis and acute kidney injury illustrating common focused clinical questions.
Plus 10 min indicates typical time taken in addition to basic point-of-care ultrasonography. LV: Left ventricular.
Stroke volume and cardiac output
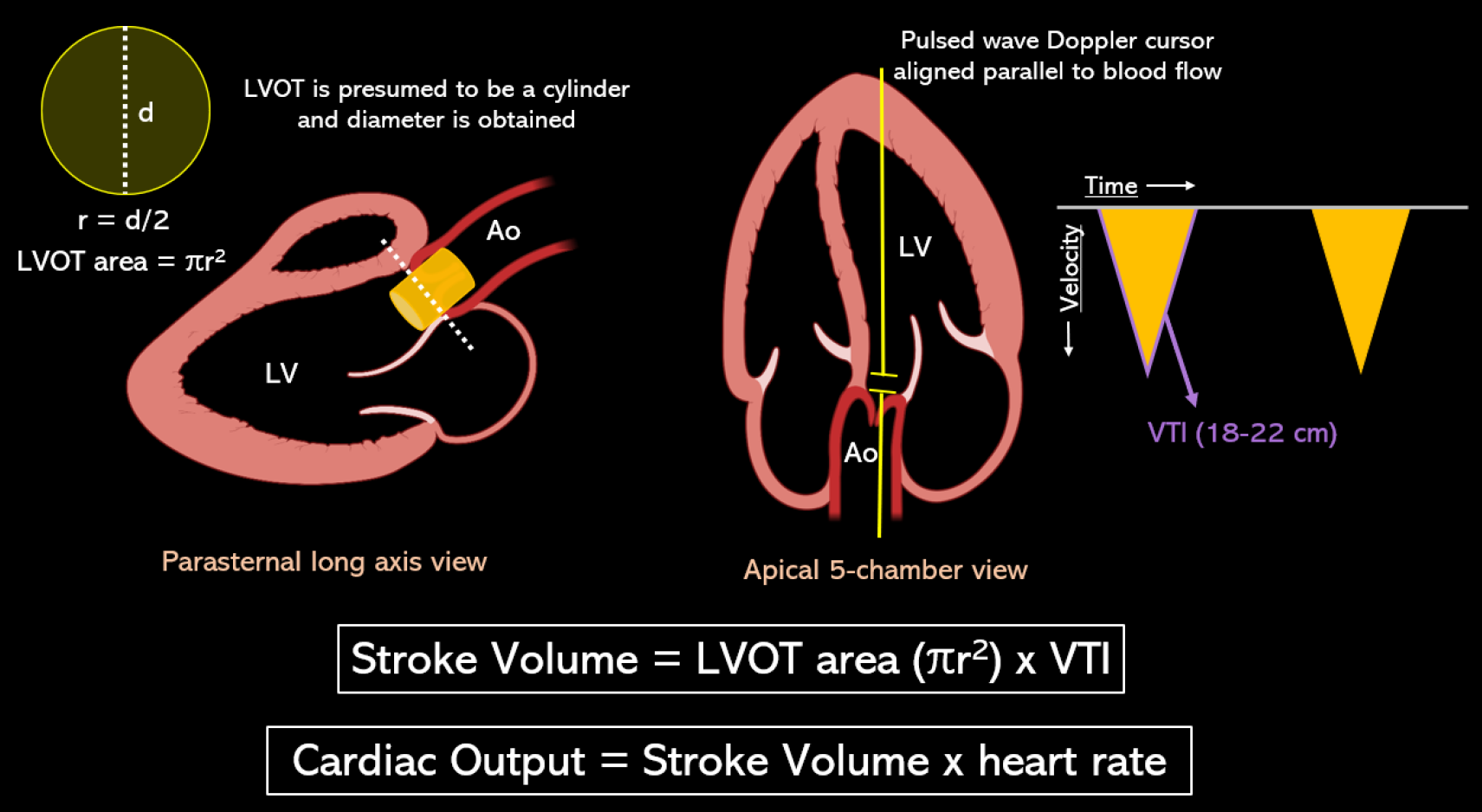
This holds significance in two scenarios: When evaluating fluid responsiveness and when attempting to differentiate volume depletion from a high cardiac output state, thereby determining whether a patient would benefit more from intravenous fluids or vasopressors such as norepinephrine and terlipressin. Figure 5 depicts POCUS-guided measurement of stroke volume and cardiac output using LV outflow tract (LVOT) Doppler-derived velocity time integral (VTI). LVOT VTI (normal: approximately 18-22 cm for heart rates 55-95 bpm)[41] serves as a surrogate for stroke volume, assuming a constant LVOT area for an individual. If needed to calculate precise cardiac index, I usually refer to the prior echo report for LVOT diameter instead of remeasuring it since it’s only the VTI that is dynamic. This approach not only saves time but also reduces the likelihood of errors. Minor discrepancies in measuring the LVOT diameter can result in inaccuracies in calculating the area of the circle, as the radius is squared.
Figure 5 Stroke volume is the product of the cross-sectional area of left ventricular outflow tract in cm2 and velocity time integral in cm.
Left ventricular outflow tract (LVOT) area is derived from the LVOT diameter in zoomed parasternal view using the formula πr2 assuming it to be a circle, and LVOT velocity time integral is obtained by tracing the envelope of the pulsed wave Doppler spectrum of systolic flow in the apical 5-chamber view. LVOT: Left ventricular outflow tract; VTI: Velocity time integral. Citation: Argaiz ER, Romero-Gonzalez G, Rola P, Spiegel R, Haycock KH, Koratala A. Bedside Ultrasound in the Management of Cardiorenal Syndromes: An Updated Review. Cardiorenal Med 2023; 13: 372-384. Copyright ©The Silverchair Publisher 2023. Published by Karger Publishers.
Regarding fluid responsiveness, it essentially refers to an increase in stroke volume after a fluid challenge. Passive leg raise is a simple bedside method of predicting fluid responsiveness, which transfers up to 300-400 mL of blood into central circulation acting as an autogenous fluid bolus. LVOT VTI is measured before and one minute after the leg raise; an increase by 10% suggests fluid responsiveness[42]. Practically, assessing fluid responsiveness is less critical than fluid tolerance because it does not dictate management. In other words, fluid responsiveness is a physiologic state and not an indication for intravenous fluid administration. I generally perform passive leg raise test when no clear indication for intravenous fluids exists in the clinical context, but another physician recommends them for any reason. If there's no fluid responsiveness, we can agree that the risks of fluids outweigh the benefits. It's noteworthy that a single reading of transduced central venous pressure or IVC ultrasound is an unreliable tool for assessing fluid responsiveness despite multiple studies exploring this relationship[43].
Estimating cardiac output also assists in advising other physicians that in cases of high-output cardiac 'states,' increasing preload with additional fluid is futile. This could result in iatrogenic fluid overload since these patients are prone to elevated cardiac filling pressures (leading to high-output cardiac 'failure')[44]. Furthermore, recent findings highlight that seemingly fluid-tolerant individuals may not necessarily be fluid-responsive[45]. On a note of caution, inability of the POCUS user to obtain an optimal cardiac view (apical 5-chamber or 3-chamber with a near-parallel Doppler angle) can introduce a significant source of error in estimating stroke volume and cardiac output.
LV filling pressures
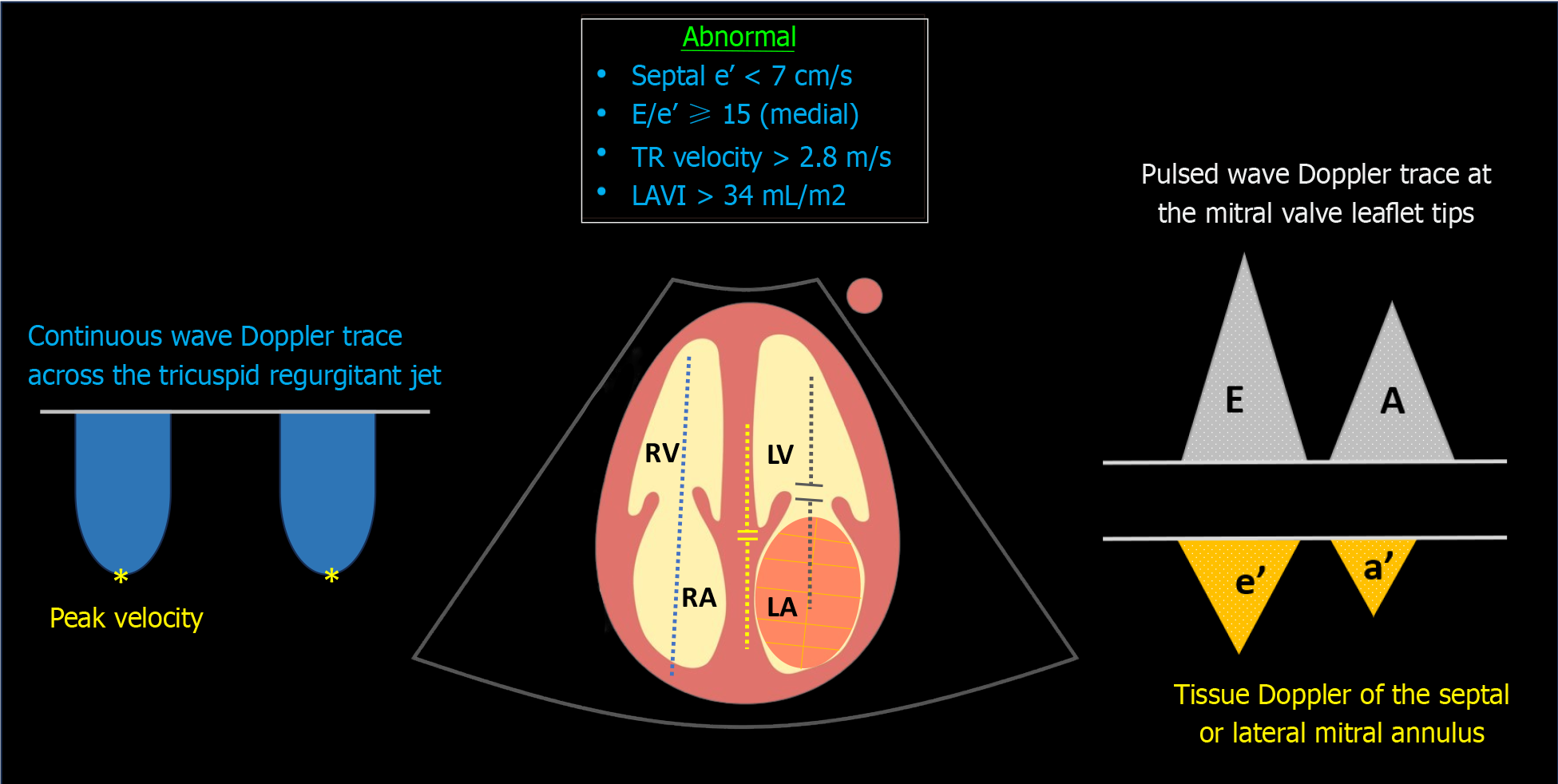
Clinicians often prioritize LV systolic function when considering fluid therapy; however, neglecting the backward pressures or cardiac filling pressures due to diastolic dysfunction can result in iatrogenic fluid overload. Diastolic dysfunction is proven to be the most sensitive parameter in diagnosing cirrhotic cardiomyopathy, affecting individuals early on. In a systematic review, diastolic dysfunction was identified in 44.6% of Child-Pugh class A patients, 62% of class B, and 63.3% of class C patients[46]. The Cirrhotic Cardiomyopathy Consortium defines advanced diastolic dysfunction by meeting three or more of the following criteria: (1) Septal e′ velocity < 7 cm/s; (2) E/e’ ratio ≥ 15; (3) left atrial volume index (LAVI) > 34 mL/m2; (4) TR jet peak velocity > 2.8 m/s[47] Figure 6.
Figure 6 Sonographic parameters used in the assessment of diastolic dysfunction.
Blue dotted line denotes continuous wave Doppler across the tricuspid valve; yellow dotted line indicates tissue Doppler of the medial mitral annulus; grey dotted line represents transmitral pulsed wave Doppler. TR: Tricuspid regurgitation; LAVI: Left atrial volume index; LV: Left ventricle; LA: Left atrium; RV: Right ventricle; RA: Right atrium.
In brief, the Doppler trace at the mitral valve tips displays E (early diastolic) and A (late diastolic) waves corresponding to flow during the rapid filling period and atrial systole, respectively. Tissue Doppler imaging of the septal or lateral mitral annulus provides a similar trace but below the baseline, showing e' and a' waves. These waves reflect ‘tissue’ (not blood) movement or myocardial relaxation capacity, with septal measurements slightly lower than those of the lateral annular measurements. LAVI is calculated by tracing the left atrial border in two different echocardiographic views at end-systole. In point-of-care settings, we typically estimate left atrial size visually instead of actually measuring it, as precise measurement can be time-consuming. TR jet peak velocity is evaluated using continuous wave Doppler across the tricuspid valve, aligning with the regurgitant jet. It's important to avoid excessive reliance on these parameters, particularly in isolation. For instance, transmitral flow is influenced by cardiac preload variations. While this characteristic poses a challenge in diagnosing diastolic dysfunction, the E/A ratio is dynamic and can be monitored before and after diuretic therapy. For a more in-depth exploration of this concept, I recommend referring to the excellent review by Nagueh[48]. Although left atrial size holds prognostic significance, it can also be elevated in high-output states (common in cirrhosis) without diastolic dysfunction[46]. Regarding TR velocity, it cannot distinguish between pre- and post-capillary pulmonary hypertension, and thus, it does not always reflect LV diastolic function but could be a valuable adjunct in diagnosing Porto-pulmonary hypertension. It can also be influenced by positive pressure ventilation.
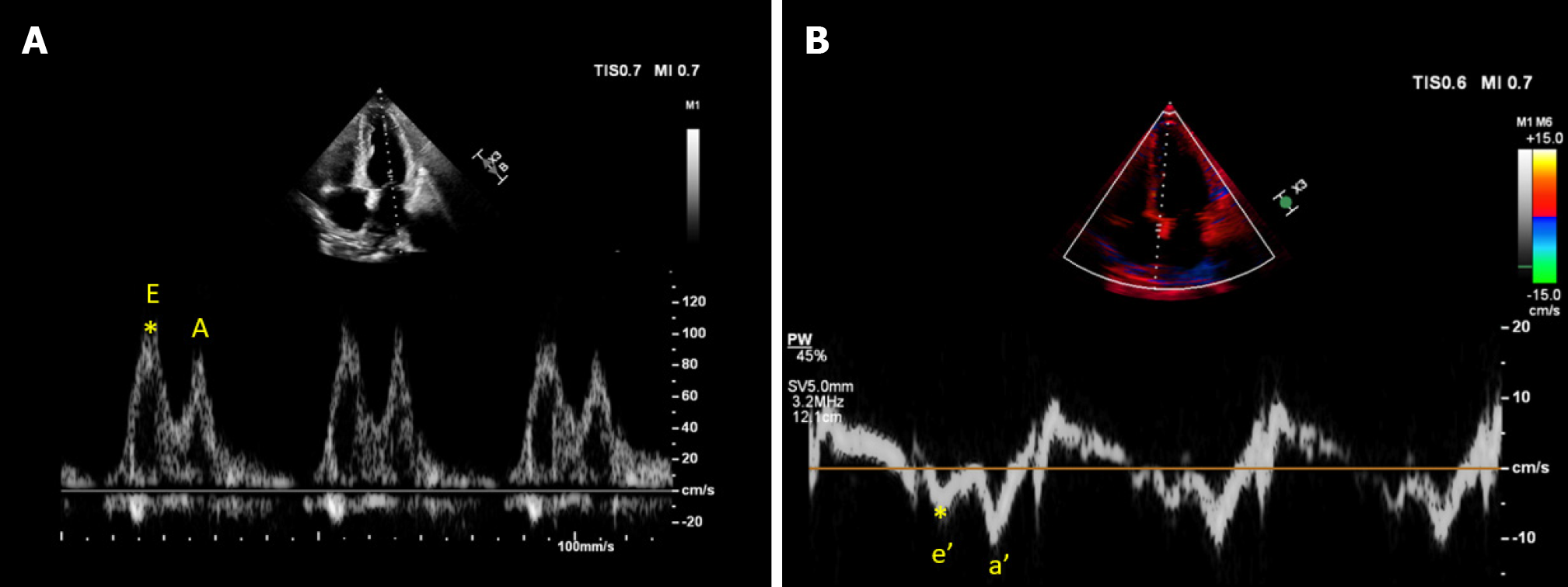
In contrast, e' (and E/e' ratio) serves as a relatively preload-independent marker of diastolic function, indicating myocardial relaxation status Figure 7. Therefore, e' is particularly important in patients at risk for volume overload. This parameter has also demonstrated reliability in predicting elevated LV filling pressures in mechanically ventilated patients. For instance, in a study of critically ill general intensive care unit (ICU) patients, those with preserved LV ejection fraction showed that lateral e' < 8 cm/s was the best predictor of elevated pulmonary capillary wedge pressure[49]. Moreover, in a recent study, an e' value less than 7 cm/s was found to be associated with terlipressin nonresponse in patients with cirrhosis and AKI, potentially indicating the severity of cirrhotic cardiomyopathy and a reduced capacity to withstand increased afterload[50]. Another noteworthy aspect of this study is that in this cohort, central venous pressure measured 9.8 mmHg at ICU admission and 11.2 mmHg after 24 h, making volume depletion an improbable cause of AKI. Additionally, the lung ultrasound scores were elevated at ICU admission, suggesting increased extravascular lung water, signifying fluid intolerance.
Figure 7 Cardiac ultrasound images from a patient with cirrhosis demonstrating.
A: Transmitral pulsed wave Doppler early diastolic wave (E) velocity approximately 100 cm/s; B: Tissue Doppler early diastolic velocity (e’) of the medial mitral annulus approximately 6 cm/s yielding an elevated E/e’ ratio of 16.6.
I typically perform diastolic function assessment in cases where there's suspicion of high-output cardiac failure or disagreements regarding ongoing fluid administration. Additionally, these parameters help distinguish between cardiogenic and pneumogenic pulmonary edema, considering that lung B-lines may occur in conditions like pulmonary fibrosis and pneumonia. Similar to LVOT VTI, inaccurate measurements can stem from poor acoustic windows and suboptimal Doppler angles. POCUS practitioners should be adept at recognizing when the views are insufficient for accurate interpretation.
Right-sided congestion
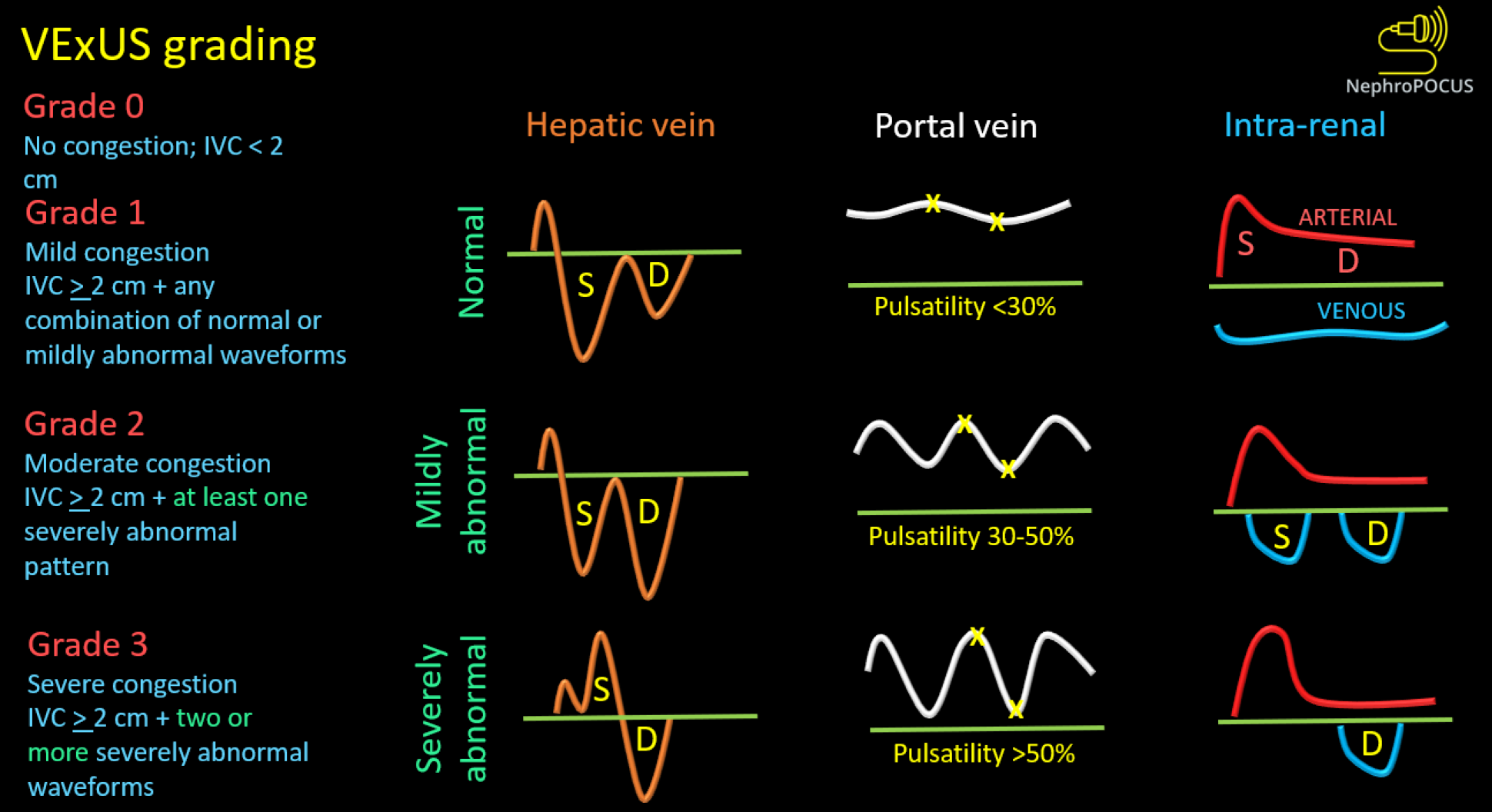
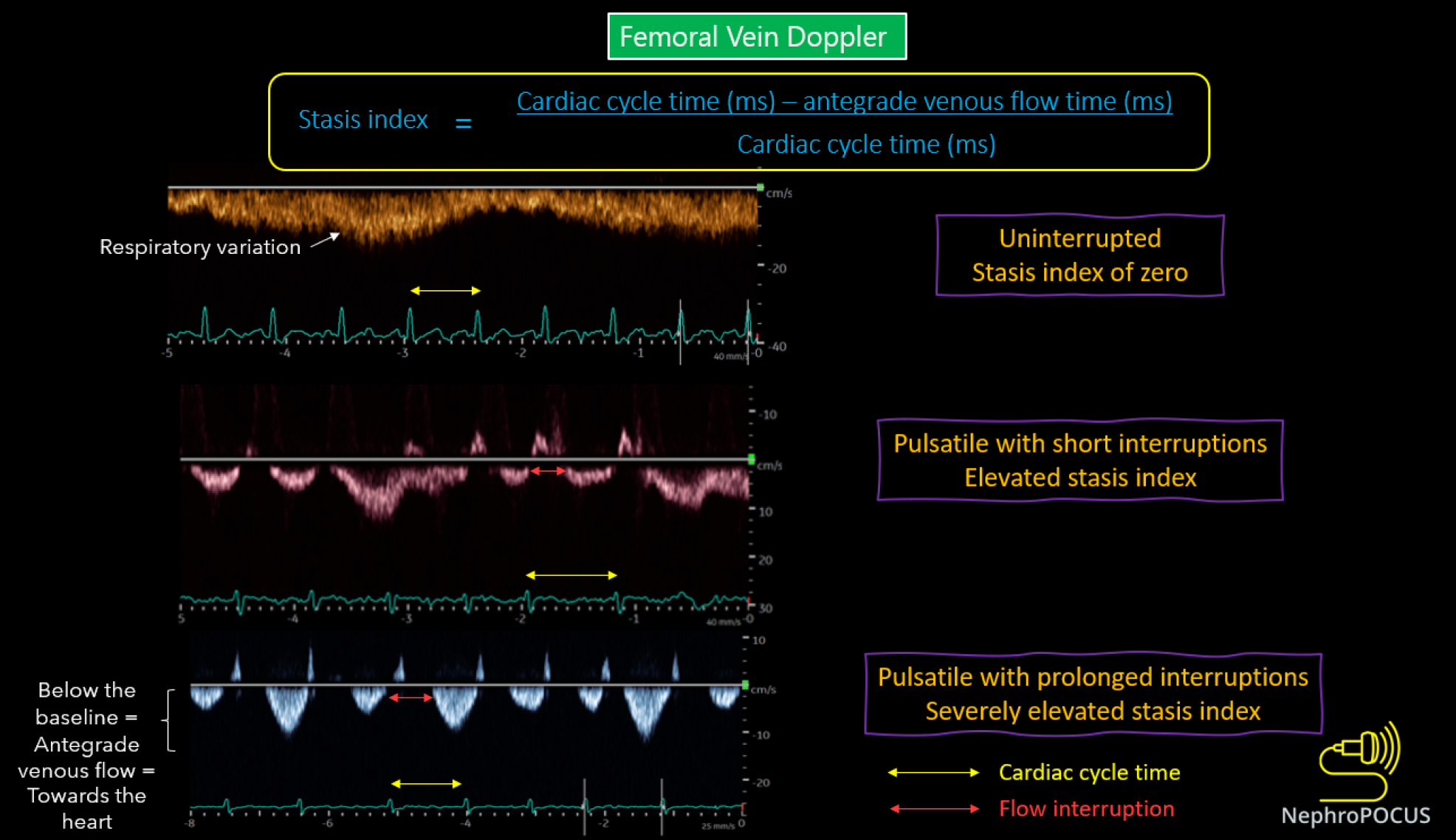
We previously discussed the association between elevated RAP and congestive nephropathy. While IVC and IJV POCUS assist in estimating RAP, they fall short in demonstrating its impact on venous circulation and consequent congestive organ injury. In this regard, venous excess ultrasound (VExUS) has gained traction as a valuable bedside tool for quantifying venous congestion and monitoring the efficacy of decongestive therapy due to its dynamic Doppler waveforms. Figure 8 illustrates the VExUS grading system; in-depth discussions about the individual waveforms (hepatic, portal, and intrarenal) can be found elsewhere[51,52]. In cirrhosis, hepatic and portal vein waveforms may be unreliable, emphasizing the importance of renal parenchymal waveform assessment to establish congestive nephropathy. Femoral vein Doppler is emerging as a complement to VExUS and, being easily accessible, may be employed in cirrhotic patients alongside renal parenchymal vein assessment[53]. Figure 9 depicts femoral Doppler waveform changes with increasing congestion severity. However, femoral vein Doppler is less sensitive to detecting elevated RAP, and a normal waveform should not dismiss the possibility of congestion. Nevertheless, further research is warranted to establish the precise role of VExUS in cirrhosis. Figure 10 showcases sonographic findings from a cirrhotic patient with fluid overload, highlighting significantly elevated LVOT VTI, a plethoric IVC, and pulsatile intrarenal and femoral vein waveforms with flow interruptions.
Figure 8 Venous excess ultrasound grading system.
When the diameter of inferior vena cava is ≥ 2 cm, three grades of congestion are defined based on the severity of abnormalities on hepatic, portal, and intrarenal venous Doppler. Hepatic vein Doppler is considered mildly abnormal when the systolic (S) wave is smaller than the diastolic (D) wave, but still below the baseline; it is considered severely abnormal when the S-wave is reversed. Portal vein Doppler is considered mildly abnormal when the pulsatility is 30% to 50%, and severely abnormal when it is ≥ 50%. Asterisks represent points of pulsatility measurement. Renal parenchymal vein Doppler is mildly abnormal when it is pulsatile with distinct S and D components, and severely abnormal when it is monophasic with D-only pattern. Reused from NephroPOCUS.com with permission (https://nephropocus.com/about/).
Figure 9 Changes in femoral vein Doppler correlate with escalating congestion.
In normal state, the waveform appears continuous or minimally pulsatile, with dips aligning with atrial contraction at the end of each diastole. With worsening congestion, the duration of flow interruptions rises within each cardiac cycle (indicated by an increased stasis index). Employing simultaneous electrocardiogram tracing aids in accurately identifying the duration of the cardiac cycle. Reused from NephroPOCUS.com with permission (https://nephropocus.com/about/).
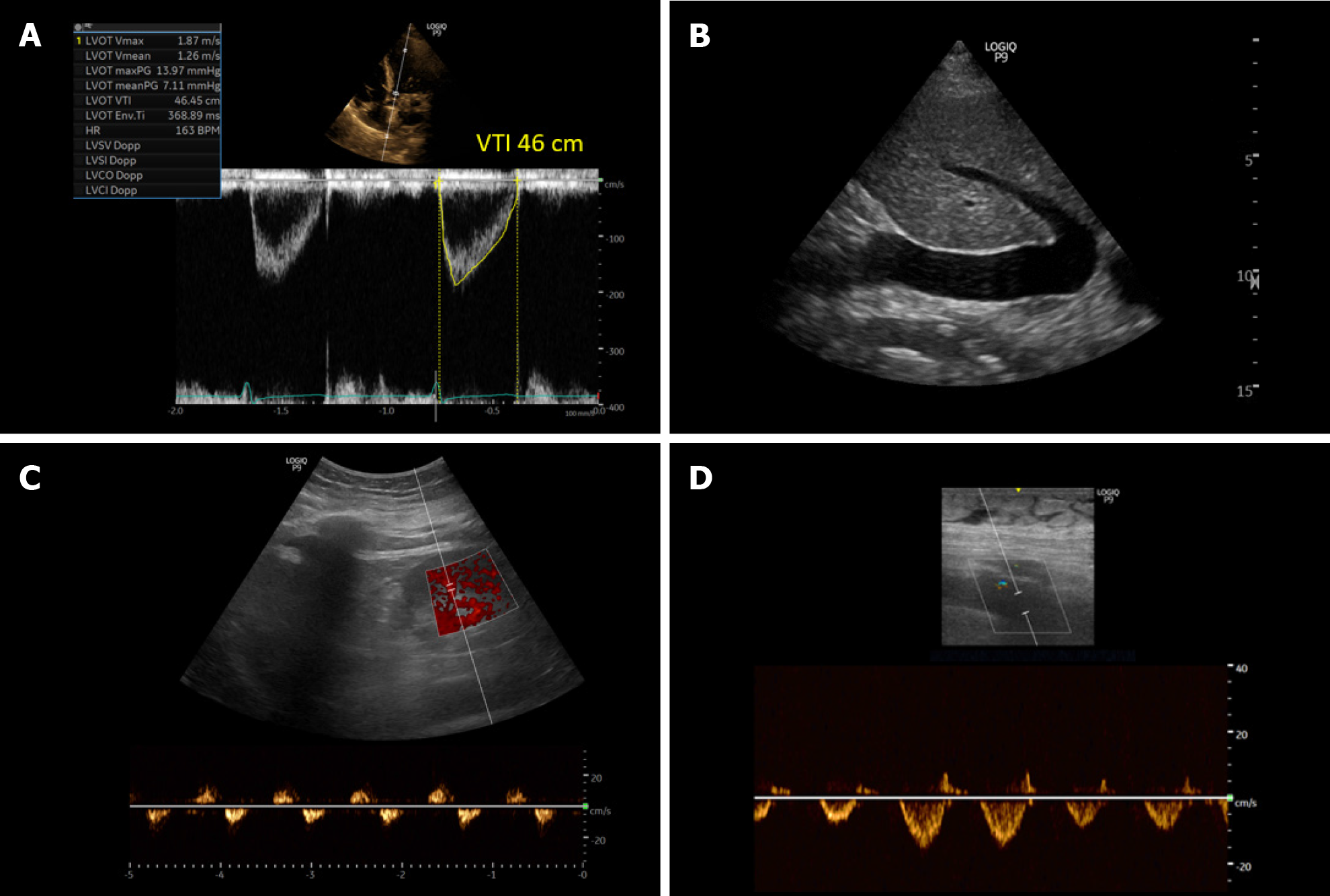
Figure 10 Ultrasound images obtained from a patient with cirrhosis and fluid overload demonstrating.
A: An elevated left ventricular outflow tract velocity time integral signifying very high stroke volume (high cardiac output); B: A plethoric inferior vena cava suggestive of elevated right atrial pressure; C and D: Significantly pulsatile renal parenchymal vein and femoral vein Doppler with flow interruptions.
Performing these additional Doppler assessments requires an extra 10 minutes on top of the initial evaluation. I strongly recommend simultaneous electrocardiogram (ECG) use to prevent misinterpretation of Doppler waveforms, especially VExUS. Most cart-based ultrasound machines offer ECG capability with a separately sold specialized module, which is quick to set up by placing just the limb leads. While some may question the value of POCUS in the presence of a recent echocardiogram, it's essential to acknowledge the dynamic nature of most parameters, which can change with therapy. It's not practical to repeat a comprehensive echocardiogram when the focused clinical question can be addressed bedside in a few minutes. Generally, I would refrain from performing FoCUS if a formal echocardiogram is available from the day of consultation. However, I might still do IVC POCUS, as cardiac sonographers often do not employ a right lateral approach and may report the vessel as not visible from the subxiphoid window, a common occurrence in patients with ascites.
CONCLUSION
In summary, POCUS aids in simplifying the diagnosis of AKI in cirrhosis by assisting in identifying various hemodynamic and structural factors. It is crucial to recognize that a diagnostic tool alone cannot alter outcomes unless linked to a treatment with a positive impact. One would not expect improved prognosis in heart failure solely from detecting lung crackles, but often unfair expectations are placed on POCUS, which is also a diagnostic tool like stethoscope, albeit a better one. Evidence-based medicine should not be confused with outcome-based medicine; not every medical intervention is solely about mortality. Refraining from diagnosing due to a lack of mortality-improving treatments is regressive, especially when non-invasive, cost-effective tools like POCUS are available. If POCUS enhances diagnostic confidence and prevents unnecessary therapies, thus averting potential patient discomfort (e.g., shortness of breath from fluid overload), it represents a significant improvement. In the modern medical landscape, justifying blind fluid administration under the pretext of POCUS not improving mortality is lamentable. Intravenous fluids are drugs, requiring justification and clear indications. We should always bear in mind the principle of "primum non nocere" and strive to avoid an intervention unless there is a clear and sound rationale. Physicians are meant to apply pathophysiology at the bedside, not mindlessly follow guidelines and blanket recommendations. Moreover, critics contend that POCUS is not universally accessible and should not be glorified. Contrary to prevalent beliefs, POCUS proves to be particularly advantageous in low-income settings, where sophisticated imaging tools may be scarce. This facilitates timely management and prevents potential deterioration of patients due to inappropriate therapies. The emergence of inexpensive handheld ultrasound devices further enhances the accessibility and relevance of POCUS in such settings.
However, there is a flipside to consider. POCUS remains reliant on the operator's skills, much like other facets of medicine such as history taking and clinical judgment. Its effectiveness hinges on the operator's proficiency in acquiring, interpreting, and integrating images into the management plan. In theory, POCUS is akin to a component of the physical examination, like auscultation. Nevertheless, the reality is that many medical schools do not provide sufficient exposure to POCUS compared to the physical exam, leaving medical graduates less competent in this aspect. Consequently, oversight and quality checks are imperative. Furthermore, the adage" when you have a hammer, everything looks like a nail" applies to overenthusiastic POCUS users. Just because POCUS enhances the diagnosis of hemodynamic kidney injury doesn't mean we can overlook other causes of AKI in cirrhosis, such as acute interstitial nephritis and glomerulonephritis, which POCUS cannot identify. Likewise, excessive dependence on specific POCUS parameters, whether due to their perceived technical simplicity (such as lung ultrasound) or an overestimation of one's skills (claiming expertise in hemodynamics solely based on performing IVC ultrasound) and overrating the equipment's capabilities (relying on suboptimal quality images to rule out significant pathology) may pose a risk of potential harm to patients.
While the diagnostic efficacy of POCUS is well-established, future research endeavors should focus on incorporating multi-organ POCUS into the individualized care of medically complex patient populations. This approach is more constructive than simply questioning its effectiveness or engaging in futile study questions such as, "Will placing the probe on the body improve mortality?"