Published online Mar 9, 2024. doi: 10.5492/wjccm.v13.i1.90617
Peer-review started: December 8, 2023
First decision: December 19, 2023
Revised: December 28, 2023
Accepted: January 22, 2024
Article in press: January 22, 2024
Published online: March 9, 2024
Processing time: 87 Days and 8.7 Hours
The increase in severe traumatic brain injury (sTBI) incidence is a worldwide phenomenon, resulting in a heavy disease burden in the public health systems, specifically in emerging countries. The shock index (SI) is a physiological parameter that indicates cardiovascular status and has been used as a tool to assess the presence and severity of shock, which is increased in sTBI. Considering the high mortality of sTBI, scrutinizing the predictive potential of SI and its variants is vital.
To describe the predictive potential of SI and its variants in sTBI.
This study included 71 patients (61 men and 10 women) divided into two groups: Survival (S; n = 49) and Non-survival (NS; n = 22). The responses of blood pressure and heart rate (HR) were collected at admission and 48 h after admission. The SI, reverse SI (rSI), rSI multiplied by the Glasgow Coma Score (rSIG), and Age multiplied SI (AgeSI) were calculated. Group comparisons included Shapiro-Wilk tests, and independent samples t-tests. For predictive analysis, logistic regression, receiver operator curves (ROC) curves, and area under the curve (AUC) measurements were performed.
No significant differences between groups were identified for SI, rSI, or rSIG. The AgeSI was significantly higher in NS patients at 48 h following admission (S: 26.32 ± 14.2, and NS: 37.27 ± 17.8; P = 0.016). Both the logistic regression and the AUC following ROC curve analysis showed that only AgeSI at 48 h was capable of predicting sTBI outcomes.
Although an altered balance between HR and blood pressure can provide insights into the adequacy of oxygen delivery to tissues and the overall cardiac function, only the AgeSI was a viable outcome-predictive tool in sTBI, warranting future research in different cohorts.
Core Tip: Patients who suffer severe head trauma are also affected by altered balance between heart rate and blood pressure which influences oxygen delivery to tissues and the overall cardiac function. Although previous studies indicated that shock index (SI) and its variants could predict the outcomes following traumatic brain injury (TBI) the studies were conducted in patients with different severities of injury. Therefore, when evaluating patients who suffered a severe TBI (sTBI), the SI and its variants are not a viable outcome-predictive tool in sTBI, due to similar responses in both surviving and non-surviving patients. However, the Age multiplied SI was a viable outcome-predictive tool in sTBI, warranting future research in different cohorts.
- Citation: Carteri RB, Padilha M, de Quadros SS, Cardoso EK, Grellert M. Shock index and its variants as predictors of mortality in severe traumatic brain injury. World J Crit Care Med 2024; 13(1): 90617
- URL: https://www.wjgnet.com/2220-3141/full/v13/i1/90617.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v13.i1.90617
Presently recognized as a significant public health issue, traumatic brain injury (TBI) commonly results in persistent neurological dysfunction[1,2]. TBI is defined as an alteration in normal brain function resulting from biomechanical forces, caused by rapid acceleration or deceleration of the brain due to motorcycle or automobile accidents; impact resulting from the brain's collision due to falls, motorcycle and automobile accidents, or contact sports; changes in pres
TBI is classified as mild, moderate, or severe, and it can lead to premature death, cognitive alterations, and neuropsychiatric impairments, often compromising the quality of life of surviving individuals[1,5]. This classification is a combination of various criteria, with the Glasgow Coma Scale (GCS) being the most commonly used tool[6]. The severity level holds prognostic value but does not necessarily predict the patient's final level of functioning. The pathophy
Hence, to the best of our knowledge, there are no studies that assess the role of SI and its variants as a predictor tool of mortality in severe TBI (sTBI) patients without multiple central injuries. The findings of this study can guide future clinical procedures to ensure a positive impact on the prognosis and quality of life of this population. Therefore, this study aims to describe the predictive potential of SI and its variants as an outcome-predictive tool in sTBI patients.
This was a prospective observational study by convenience sampling conducted between January 2019 and December of 2022 at the Pronto-Socorro Hospital, a trauma reference center at Porto Alegre, RS, Brazil.
This study followed the ethical precepts, guidelines, and norms established in Resolution No. 466 of 2012 of the National Health Council, and was carried out only after approval by the Health Research and Ethics Committee of the Municipal Health Secretariat Office of Porto Alegre (CEP SMSPA; registration number: 3.912.623). Patients were identified through registration numbers, which only serves to validate the individuality of the information. The sample was determined in a non-probabilistic way for convenience, selected through the inclusion and exclusion criteria described below, without any discrimination in the selection of individuals or exposure to unnecessary risks. Patients admitted to the adult trauma intensive care units (ICUs) aged 18 years or older who required enteral or parenteral nutritional therapy were included. The following were excluded from the study: Patients with a GCS score of 9 to 15; patients who were diagnosed with cervical, thoracic or abdominal trauma; patients who received only oral diet, and those with incomplete medical records or records due to lack of data. Of 342 patients admitted to the trauma ICU during the explored period, 71 patients were included in this study.
The study was carried out in the adult trauma ICU of the Hospital de Pronto Socorro de Porto Alegre, with retrospective data, covering the period from January 2019 to December 2022. Data collection was carried out using the institutional Hospital Information System, which includes the complete electronic medical record of the patient. The collected variables were: GCS score, injury description, age, sex, days of fasting, body mass, estimated height, blood pressure, and HR parameters. Body mass index (BMI = Body mass/Height2) was calculated to classify the patients according to the criteria of the World Health Organization[14]. The SI, rSI, and rSIG were calculated as the ratio of HR to systolic blood pressure (SBP) (SI = HR/SBP), the ratio of SBP to HR (rSI = SBP/HR), the score of rSI × GCS, and age multiplied SI (AgeSI = Age × SI) respectively.
The general description of the selected data is available through simple and relative frequencies. The normality of distributions of all variables were evaluated using the Shapiro-Wilk test. Student's t test for independent or the Pearson’s Chi-Square test was used to compare data between groups. Spearman’s rho was used to evaluate the correlation between different variables. To evaluate the predictive potential of SI, rSI, rSIG, and AgeSI we used logistic regression, where regression coefficients (B) were obtained for each variable. When the Wald test values were significant, the odds ratio was calculated to indicate the percentage changes (Exp(B) – 100). Also, receiver operator curves (ROC) analysis was performed. Significant correlations and differences were considered where P < 0.05. All data were analyzed using the Statistical Package for Social Sciences 26.0 statistical program.
Table 1 provides the characteristics of the 72 patients included in this study, which were allocated in two distinct groups: Survival (S; n = 49) and non-survival (NS; n = 22). Analysis of the variables indicated that the groups were significantly different regarding mean age (S: 40.51 ± 17.4, and NS: 50.73 ± 14.6; P = 0.013), number of days in hospital (S: 28.76 ± 14.6, and NS: 14.36 ± 16.8; P = 0.001). No differences were observed for the other variables, except for the presence of COPD in the NS group (P = 0.032).
| Survival (n = 49) | Non-survival (n = 22) | P value1 | |||
| Age (years), mean ± SD | 40.51 | 17.4 | 50.73 | 14.6 | 0.013 |
| Days in MV, mean ± SD | 28.76 | 14.6 | 14.36 | 16.8 | 0.001 |
| Fasted days, mean ± SD | 13.78 | 8.7 | 7.68 | 6.4 | 0.002 |
| Days in hospital, mean ± SD | 28.76 | 14.6 | 14.36 | 16.8 | 0.001 |
| P value2 | |||||
| Sex, n (%) | 0.161 | ||||
| Male, n (%) | 44 | 89.8% | 17 | 77.3% | |
| Female, n (%) | 5 | 10.2% | 5 | 22.7% | |
| Injury type, n (%) | 0.607 | ||||
| Closed | 35 | 71.4% | 17 | 77.3% | |
| Open | 14 | 28.6% | 5 | 22.7% | |
| Injury cause, n (%) | 0.408 | ||||
| Fall | 13 | 26.5% | 10 | 45.5% | |
| Transit accident | 18 | 36.7% | 4 | 18.2% | |
| Assault | 13 | 26.5% | 6 | 27.3% | |
| Gunshot | 4 | 8.2% | 1 | 4.5% | |
| Other | 1 | 2.0% | 1 | 4.5% | |
| Associated injuries, n (%) | 0.658 | ||||
| None | 36 | 73.5% | 19 | 86.4% | |
| Thoracic | 4 | 8.2% | 1 | 4.5% | |
| Arms | 1 | 2.0% | 0 | 0.0% | |
| Legs | 5 | 10.2% | 2 | 9.1% | |
| Spine | 3 | 6.1% | 0 | 0.0% | |
| Craniotomy procedure, n (%) | 0.822 | ||||
| No | 34 | 77.3% | 15 | 68.2% | |
| Yes | 14 | 31.8% | 7 | 31.8% | |
| Body mass index (kg/cm²), n (%) | 0.761 | ||||
| Underweight | 4 | 8.2% | 2 | 13.6% | |
| Eutrophic | 25 | 51.0% | 0 | 54.5% | |
| Overweight | 14 | 28.6% | 2 | 18.2% | |
| Grade I Obese | 6 | 12.2% | 2 | 13.6% | |
| Comorbidities, n (%) | |||||
| COPD | 0 | 0 | 2 | 9.1% | 0.032 |
| Asma | 1 | 0.02 | 0 | 0.0% | 0.513 |
| T2DM | 1 | 0.02 | 2 | 9.1% | 0.172 |
| SAH | 4 | 8.2% | 2 | 9.1% | 0.897 |
| EVA | 1 | 0.02 | 1 | 4.5% | 0.555 |
| AD | 2 | 4.1% | 1 | 4.5% | 0.928 |
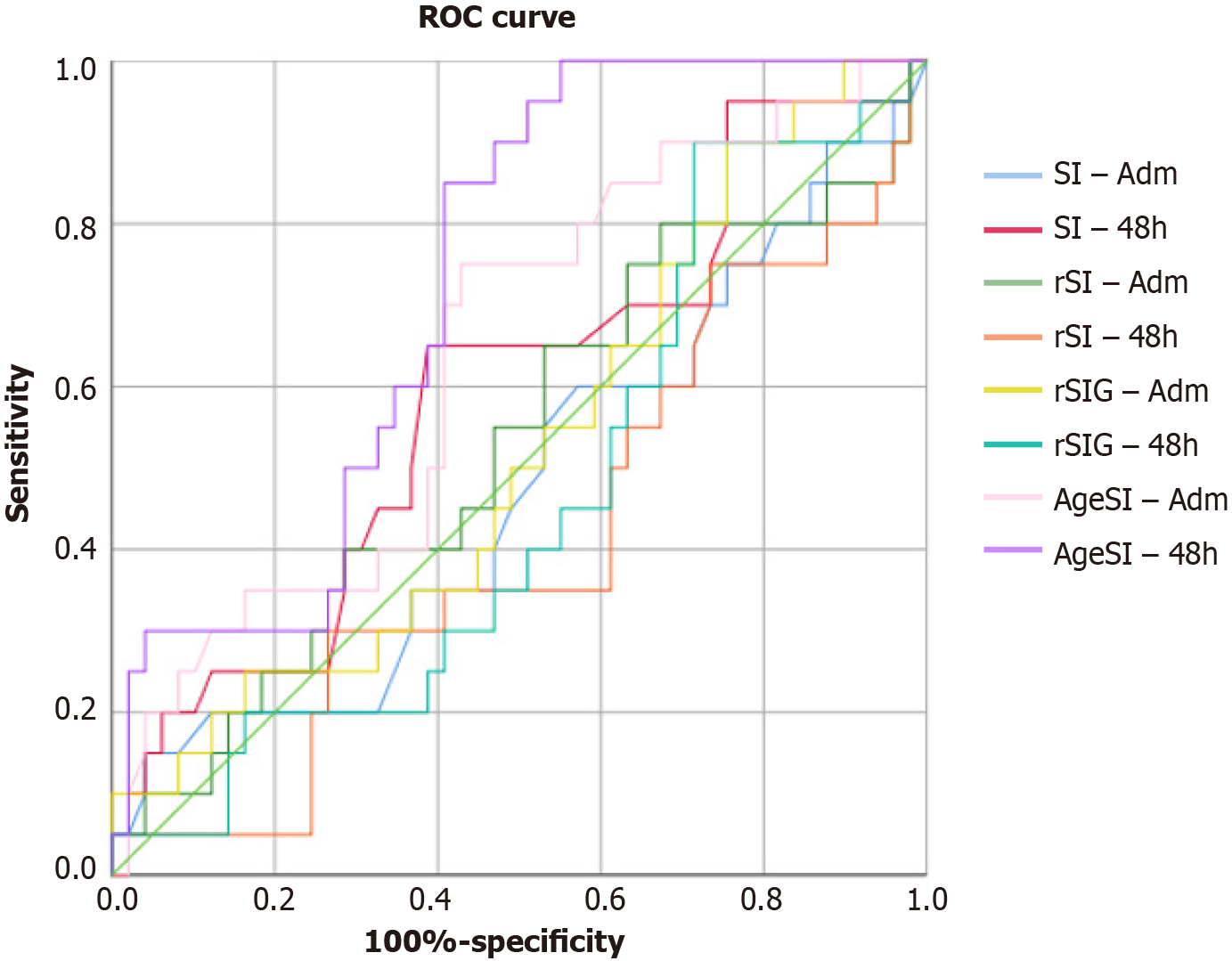
Table 2 presents the data regarding blood pressure, HR, and different SI. The HR and the SI at 48 h after admission significantly differed between S and NS patients (P = 0.036, and P = 0.03, respectively). No differences were observed for the other variables, including the different SI, except for the AgeSI. The AgeSI was significantly higher in NS patients at 48 h following admission (S: 26.32 ± 14.2, and NS: 37.27 ± 17.8; P = 0.016). The logistic regression and area under the receiver operating characteristic curve (AUROC) results are shown in Table 3. When evaluating the significance and the odds ratio to explore further the relationship of different SI with survival odds, no relationship was identified. In patients with sTBI (Figure 1), the AUROC analysis indicated that the predictive accuracy of SI and its variants were insignificant, except for AgeSI at 48 h, where the AUROC curve for predicting mortality was 0.727.
| Survival (n = 49) | Non-survival (n = 22) | P value | |||
| SBP-24 h (mmHg) | 135.59 | 36.5 | 138.95 | 40.1 | 0.739 |
| DBP-24 h (mmHg) | 81.27 | 23.5 | 85.38 | 25.2 | 0.526 |
| HR-24 h (bpm) | 88.22 | 25.4 | 88.68 | 29.0 | 0.949 |
| SBP-48 h (mmHg) | 131.47 | 27.6 | 127.20 | 25.0 | 0.536 |
| DBP-48 h (mmHg) | 67.77 | 12.1 | 72.33 | 15.0 | 0.257 |
| HR-48 h (bpm) | 82.61 | 18.5 | 93.95 | 19.9 | 0.036 |
| SI-adm | 0.70 | 0.3 | 0.69 | 0.3 | 0.901 |
| SI-48 h | 0.65 | 0.2 | 0.79 | 0.3 | 0.03 |
| rSI-adm | 1.70 | 0.8 | 1.78 | 1.0 | 0.742 |
| rSI-48 h | 1.66 | 0.5 | 1.44 | 0.5 | 0.106 |
| rSIG-adm | 10.45 | 5.9 | 11.02 | 7.7 | 0.758 |
| rSIG-48 h | 10.26 | 4.8 | 9.29 | 4.8 | 0.452 |
| AgeSIG-adm | 28.02 | 16.8 | 34.40 | 17.1 | 0.152 |
| AgeSIG-48 h | 26.32 | 14.2 | 37.27 | 17.8 | 0.016 |
| Sig. | Exp(B) | 95%CI for EXP(B) | Odds ratio (%) | AUC | P value | ||
| Inferior | Superior | ||||||
| SI-adm | 0.895 | 0.885 | 0.144 | 5.444 | -11.5 | 0.487 | 0.864 |
| SI-48 h | 0.129 | 7.592 | 0.554 | 104.036 | 659.2 | 0.606 | 0.176 |
| rSI-adm | 0.727 | 1.107 | 0.626 | 1.956 | 10.7 | 0.517 | 0.832 |
| rSI-48 h | 0.194 | 0.436 | 0.125 | 1.527 | -56.4 | 0.395 | 0.180 |
| rSIG-adm | 0.652 | 1.018 | 0.942 | 1.101 | 1.8 | 0.537 | 0.637 |
| rSIG-48 h | 0.641 | 0.973 | 0.867 | 1.092 | -2.7 | 0.473 | 0.727 |
| AgeSIG-adm | 0.153 | 1.022 | 0.992 | 1.052 | 2.2 | 0.639 | 0.071 |
| AgeSIG-48 h | 0.015 | 1.044 | 1.008 | 1.082 | 4.4 | 0.727 | 0.003 |
The present study evaluated the role of SI as a variable to predict the outcomes of sTBI patients coinfected patients. Notably, the different SI were not predictors of outcomes for severe head injury patients, despite the significantly different HR and SI responses at 48 h following admission between S and NS patients. However, the AgeSI could be a useful tool to predict mortality, showing statistical difference among surviving and non-surviving sTBI patients, and significant predictive value.
The rationale behind the SI is rooted in the understanding that an altered balance between HR and blood pressure can provide insights into the adequacy of oxygen delivery to tissues and the overall cardiac function[15]. Therefore, these physiological responses are directly implicated in survival of TBI patients, due to the relationship with the extent of both primary and secondary damage mechanisms, including restriction of flow in the long pituitary portal vessels after injury[16]. The predictive value of the SI in determining mortality in critically ill patients (including TBI patients) has been a subject of investigation in recent studies. Notably, studies such as those conducted by Cannon et al[17] and McNab et al[18] have contributed to our understanding of the prognostic significance of the SI in this population. Cannon et al[17] conducted a retrospective analysis of TBI patients, elucidating the association between an elevated SI and increased mortality. Their findings underscored the utility of the SI as an early prognostic marker, with increased values indicative of higher mortality risk. The study highlighted the clinical relevance of SI assessment in identifying TBI patients at heightened risk of adverse outcomes[17].
Building upon this foundational work, McNab et al[18] conducted a prospective study to further investigate the predictive capabilities of the SI in severe TBI patients. Their results affirmed a significant association between an elevated SI on admission and increased mortality, emphasizing the potential utility of this simple yet informative metric in risk stratification and early intervention[18]. In an earlier investigation, Rady et al[19] explored the predictive value of the SI in a broader trauma population, including TBI cases. Their prospective study demonstrated the sensitivity of the SI in identifying patients at risk of adverse outcomes. Although not specific to TBI, the results provided insights into the potential applicability of the SI as a valuable tool for early prognostication[19].
Recently, Wu et al[12] contributed to the literature by conducting a retrospective analysis focusing on the SI and reverse SI (rSI) multiplied by GCS as a predictor of mortality in 2438 patients with isolated head injury. Like the present study, the patients who died were significantly older that those who survived. However, the analysis included patients with different levels of TBI, as indicated by significant differences in the GCS. The study affirmed the independent association between an elevated SI and mortality, indicating that the rSI is superior to SI as a predictor of mortality in TBI, with comparable predictive power to both the Trauma and Injury Severity Score and Revised Trauma Score, further supporting its potential role in risk stratification for TBI patients. Comparatively, in the present study we investigated sTBI patients, which are more prone to have a higher SI score due to the nature of the injury mechanisms. Thus, no differences were identified for SI and its variants among S and NS patients. Interpreting traditional vital signs and the SI proves challenging when applied to the elderly population. Advanced age is associated with lower HR responses and elevated systolic blood pressures, leading to an escalation in false-negative values and influencing SI outcomes with increasing age. To address this issue, previous research suggested that SI multiplied by age (AgeSI) is a better predictor of mortality following traumatic injury of an elderly patient, we also included this variant in the analysis[20,21]. In the present study, AgeSI showed tendency to significance at admission, and was significantly different at 48 h following admission, showing significant predictive value. Our findings those of Kim et al[22], showing that the predictive power of the AgeSI for in-hospital mortality was higher in geriatric trauma patients. Therefore, AgeSI is a viable predictive tool in sTBI which is supported by previous research validating AgeSI index[23,24].
This study is subject to several limitations. Firstly, it relied on a retrospective analysis. Secondly, the exact time profile from injury occurrence to mortality was not measured. While the SI proves effective in predicting short-term mortality, the lack of a precise timeline from injury to mortality, due to database constraints, limits the comprehensive predictive capacity of the SI assessment. Rather than presenting an exact time profile, our evaluation focused on the SI's predictive efficacy for mortality during the emergency department stay and the overall in-hospital period, respectively. Thirdly, the database did not furnish information regarding the use of anti-hypertensive medications (such as beta blockers), introducing a potential factor that may impact the validity of SI assessment. Also, the data regarding previous comorbidities rely on the information given by the patients or their caregivers and may present inconsistencies. As for strengths, we highlight the investigation in sTBI patients, the study's originality, and the importance of this study evaluating the SI and its variants, an important tool for prognosis in the clinical treatment of critical patients.
In conclusion, only AgeSI was a viable predictor of mortality following severe head injury. Therefore, future studies should continue to search for cost-effective clinical tools that can predict survival and other outcomes in sTBI patients, considering the cohort-specific characteristics.
Patients who suffer severe head trauma are also affected by altered balance between heart rate (HR) and blood pressure which influences oxygen delivery to tissues and the overall cardiac function. Although previous studies indicated that shock index (SI) and its variants could predict the outcomes following traumatic brain injury (TBI) the studies were conducted in patients with different severities of injury.
To the best of our knowledge, there are no studies that assess the role of SI and its variants as a predictor tool of mortality in severe TBI (sTBI) patients without multiple central injuries. The findings of this study can guide future clinical procedures to ensure a positive impact on the prognosis and quality of life of this population.
This study aims to describe the predictive potential of SI and its variants as an outcome-predictive tool in sTBI patients.
This was a prospective observational study conducted at the Pronto-Socorro Hospital, a trauma reference center at Porto Alegre, RS, Brazil, including 71 patients were included in this study. The study included retrospective data, covering the period from January 2019 to December 2022. The collected variables were: Glasgow Coma Scale (GCS) score, injury description, age, sex, days of fasting, body mass, estimated height, blood pressure, and HR parameters. Body mass index (BMI = body mass/Height2) was calculated to classify the patients according to the criteria of the World Health Organization. The SI, reverse SI (rSI), and rSI multiplied by the Glasgow Coma Score (rSIG) were calculated as the ratio of HR to systolic blood pressure (SBP) (SI = HR/SBP), ratio of SBP to HR (rSI = SBP/HR), the score of rSI × GCS, and age multiplied SI (AgeSI = Age × SI) respectively. Group comparisons included Shapiro-Wilk tests and independent samples t-tests. For predictive analysis, logistic regression, receiver operator curves (ROC) curves, and area under the curve (AUC) measurements were performed.
No significant differences between groups were identified for SI, rSI, or rSIG. The AgeSI was significantly higher in non-survival (NS) patients at 48 h following admission (Survival: 26.32 ± 14.2, and NS: 37.27 ± 17.8; P = 0.016). Both the logistic regression and the AUC following ROC curve analysis showed that only AgeSI at 48 h was capable of predicting sTBI outcomes. For AgeSI at 48 h, the AUROC curve for predicting mortality was 0.727.
Patients who suffer severe head trauma are also affected by altered balance between HR and blood pressure which influences oxygen delivery to tissues and the overall cardiac function. Although previous studies indicated that SI and its variants could predict the outcomes following TBI the studies were conducted in patients with different severities of injury. Therefore, when evaluating patients who suffered a sTBI, the SI and its variants are not a viable outcome-predictive tool in sTBI, due to similar responses in both surviving and non-surviving patients. However, the AgeSI was a viable outcome-predictive tool in sTBI, warranting future research in different cohorts.
Future studies should evaluate the AgeSI as an outcome-predictive tool in sTBI.
| 1. | Meaney DF, Morrison B, Dale Bass C. The mechanics of traumatic brain injury: a review of what we know and what we need to know for reducing its societal burden. J Biomech Eng. 2014;136:021008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 2. | Rosenfeld JV, Maas AI, Bragge P, Morganti-Kossmann MC, Manley GT, Gruen RL. Early management of severe traumatic brain injury. Lancet. 2012;380:1088-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 397] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 3. | Blennow K, Hardy J, Zetterberg H. The neuropathology and neurobiology of traumatic brain injury. Neuron. 2012;76:886-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 488] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 4. | Tamsin G, Martin S. Cardiovascular complications of brain injury. Contin Educ Anaesth Crit Care Pain. 2011;12:67-71. [RCA] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Levin HS, Diaz-Arrastia RR. Diagnosis, prognosis, and clinical management of mild traumatic brain injury. Lancet Neurol. 2015;14:506-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 382] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 6. | Multidisciplinary Postacute Rehabilitation for Moderate to Severe Traumatic Brain Injury in Adults [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2012 Jun- . [PubMed] |
| 7. | Roozenbeek B, Maas AI, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol. 2013;9:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 822] [Cited by in RCA: 965] [Article Influence: 74.2] [Reference Citation Analysis (0)] |
| 8. | Dash PK, Zhao J, Hergenroeder G, Moore AN. Biomarkers for the diagnosis, prognosis, and evaluation of treatment efficacy for traumatic brain injury. Neurotherapeutics. 2010;7:100-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 152] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Hemphill MA, Dauth S, Yu CJ, Dabiri BE, Parker KK. Traumatic brain injury and the neuronal microenvironment: a potential role for neuropathological mechanotransduction. Neuron. 2015;85:1177-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 10. | Prasad Hrishi A, Ruby Lionel K, Prathapadas U. Head Rules Over the Heart: Cardiac Manifestations of Cerebral Disorders. Indian J Crit Care Med. 2019;23:329-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. |
Mutschler M, Nienaber U, Münzberg M, Wölfl C, Schoechl H, Paffrath T, Bouillon B, Maegele M, The Shock Index revisited - a fast guide to transfusion requirement? A retrospective analysis on 21, 853 patients derived from the TraumaRegister DGU.
|
| 12. | Wu SC, Rau CS, Kuo SCH, Chien PC, Hsieh HY, Hsieh CH. The Reverse Shock Index Multiplied by Glasgow Coma Scale Score (rSIG) and Prediction of Mortality Outcome in Adult Trauma Patients: A Cross-Sectional Analysis Based on Registered Trauma Data. Int J Environ Res Public Health. 2018;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Jung E, Ryu HH, Heo BG. The reverse shock index multiplied by Glasgow coma scale (rSIG) is predictive of mortality in trauma patients according to age. Brain Inj. 2023;37:430-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 14. | Marfell-Jones M, Olds T, Stewart A, Carter JEL. International Standards for Anthropometric Assessment. Potchefstroom: North-West University, 2006: 168. |
| 15. | King RW, Plewa MC, Buderer NM, Knotts FB. Shock index as a marker for significant injury in trauma patients. Acad Emerg Med. 1996;3:1041-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 134] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Klose M, Feldt-Rasmussen U. Hypopituitarism in Traumatic Brain Injury-A Critical Note. J Clin Med. 2015;4:1480-1497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Cannon CM, Braxton CC, Kling-Smith M, Mahnken JD, Carlton E, Moncure M. Utility of the shock index in predicting mortality in traumatically injured patients. J Trauma. 2009;67:1426-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 18. | McNab A, Burns B, Bhullar I, Chesire D, Kerwin A. A prehospital shock index for trauma correlates with measures of hospital resource use and mortality. Surgery. 2012;152:473-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Rady MY, Smithline HA, Blake H, Nowak R, Rivers E. A comparison of the shock index and conventional vital signs to identify acute, critical illness in the emergency department. Ann Emerg Med. 1994;24:685-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 238] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 20. | Zarzaur BL, Croce MA, Fischer PE, Magnotti LJ, Fabian TC. New vitals after injury: shock index for the young and age x shock index for the old. J Surg Res. 2008;147:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 134] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | McNab A, Burns B, Bhullar I, Chesire D, Kerwin A. An analysis of shock index as a correlate for outcomes in trauma by age group. Surgery. 2013;154:384-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Kim SY, Hong KJ, Shin SD, Ro YS, Ahn KO, Kim YJ, Lee EJ. Validation of the Shock Index, Modified Shock Index, and Age Shock Index for Predicting Mortality of Geriatric Trauma Patients in Emergency Departments. J Korean Med Sci. 2016;31:2026-2032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Bruijns SR, Guly HR, Bouamra O, Lecky F, Lee WA. The value of traditional vital signs, shock index, and age-based markers in predicting trauma mortality. J Trauma Acute Care Surg. 2013;74:1432-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 24. | Juárez San Juan V, Juárez San Juan P, Castillo Acosta S, Rodríguez Mata C, Ortiz López D, Freixinet Gilart JL. Shock index combined with age and the Glasgow Coma Scale during the initial care of polytraumatized patients as a predictor of mortality. Emergencias. 2021;33:427-432. [PubMed] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ong H, Malaysia S-Editor: Fan JR L-Editor: A P-Editor: Zhao YQ