Published online Mar 9, 2024. doi: 10.5492/wjccm.v13.i1.86866
Peer-review started: July 11, 2023
First decision: August 10, 2023
Revised: August 25, 2023
Accepted: December 1, 2023
Article in press: December 1, 2023
Published online: March 9, 2024
Processing time: 237 Days and 20.9 Hours
Mucormycosis is a rare, rapidly progressive and often fatal fungal infection. The rarity of the condition lends itself to unfamiliarity, delayed treatment, and poor outcomes. Diagnosis of fungal infections early enough to enable appropriate treatment occurs in less than half of affected patients.
An 11-year-old girl with a history of 15% total body surface area scald burns involving both lower limbs progressed to develop angioinvasive mucormycosis. This further led to a thrombosis of the right external iliac artery and vein and rapidly progressive necrosis of surrounding soft tissues. She also had dextrocardia and patent foramen ovale. A right hip disarticulation and serial aggressive debridements were performed but she went on to develop systemic sepsis with multisystem involvement and succumbed to the infection. Pathology revealed mucor species with extensive vascular invasion.
This case highlights the importance of maintaining vigilance for mycotic infections and acting appropriately when there are signs of fulminant wound infection.
Core Tip: Mucor species are known spread rapidly across fascial tissue planes and cause vascular invasion leading to high mortality rates despite aggressive surgical debridement. There are only rare reports of mucormycosis in burn wounds and most surgeons are not well-versed with its early features. This can lead to delay in diagnosis and institution of appropriate medical and surgical care. We came across one such case at our center recently, which prompted us to conduct a review of available literature on incidence of mucormycosis in burn wounds and available guidelines for management.
- Citation: Parashar A, Singh C. Angioinvasive mucormycosis in burn intensive care units: A case report and review of literature. World J Crit Care Med 2024; 13(1): 86866
- URL: https://www.wjgnet.com/2220-3141/full/v13/i1/86866.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v13.i1.86866
Historically, mycotic infections in burn patients have been rare events. Burn wounds developing fungal infection should alarm the treating physician because of their association with high mortality rates, disabling amputations and prolonged hospital stay[1]. Because of the rarity of the condition, only 15%-40% of patients have been shown to be diagnosed early enough to ensure early appropriate treatment. Even in them, outcomes are poor and mortality remains high. Breach in the continuity of skin by trauma or burn injury may lead to colonization of the wound with fungi from surrounding environment, contaminated dressings etc. and this has been postulated to be the most common mechanism for cutaneous mucormycosis. Fungal infections, when occurring in burn wounds, tend to present in the second week or later following burn injury. The classic presentation is black deposits over the burn raw area appearing spontaneously in previously healing wounds[2]. Patients with larger surface area burns are at higher risk for acquiring such infections[3].
Mucor species are known to cause necrosis of adjacent soft tissues, spread rapidly across fascial tissue planes, cause vascular invasion and hematogenous dissemination, leading to mortality rates as high as 100% once disseminated infection has set in. Aggressive surgical debridement is advocated but even with that, survival may not be ensured in most of the victims. Considering there are only rare reports of mucormycosis in burn wounds[4,5], most treating surgeons are not well-versed with its early features. This leads to delay in diagnosis and institution of appropriate medical and surgical care. We came across one such case at our center recently, which prompted us to conduct a review of available literature on incidence of mucormycosis in burn wounds, its pathophysiology, and available guidelines for management. We hereby report our case and review relevant literature to raise awareness about this potentially fatal complication.
After Aspergillus, Mucorales fungi are the next common pathogens in patients with hematological malignancy, hematopoietic stem cell transplantation and solid organ transplantation[6,7]. Additionally, Mucorales infections are increasingly recognized in individuals with diabetes mellitus[8], after trauma or iatrogenic injury[9,10] and have been associated with outbreaks following natural disasters[11]. A review of the epidemiology, diagnosis, treatment and outcomes of mucormycosis (then zygomycosis) by Roden et al[9] has provided valuable insights into this important invasive fungal disease.
An 11-year-old girl presented to our center with 51-day-old post-burn raw areas over both lower limbs.
The patient had 15% total body surface area burns to begin with. She sustained the scald burn injuries by spillage of hot milk and was initially treated at several local hospitals where she received supportive care, intravenous antibiotics and the raw areas were managed with dressings. Since she had deep dermal wounds, there was no epithelization and she continued with local dressings at various peripheral medical centers. During the 50 d she was managed at three separate local hospitals and as the general condition continued to deteriorate, she was finally referred to our center on post-burn day 51.
She had dextrocardia with small patent foramen ovale. She also had a past history of left common femoral vein thrombosis in her neonatal period which was successfully treated but the underlying etiology was not determined.
Nothing significant.
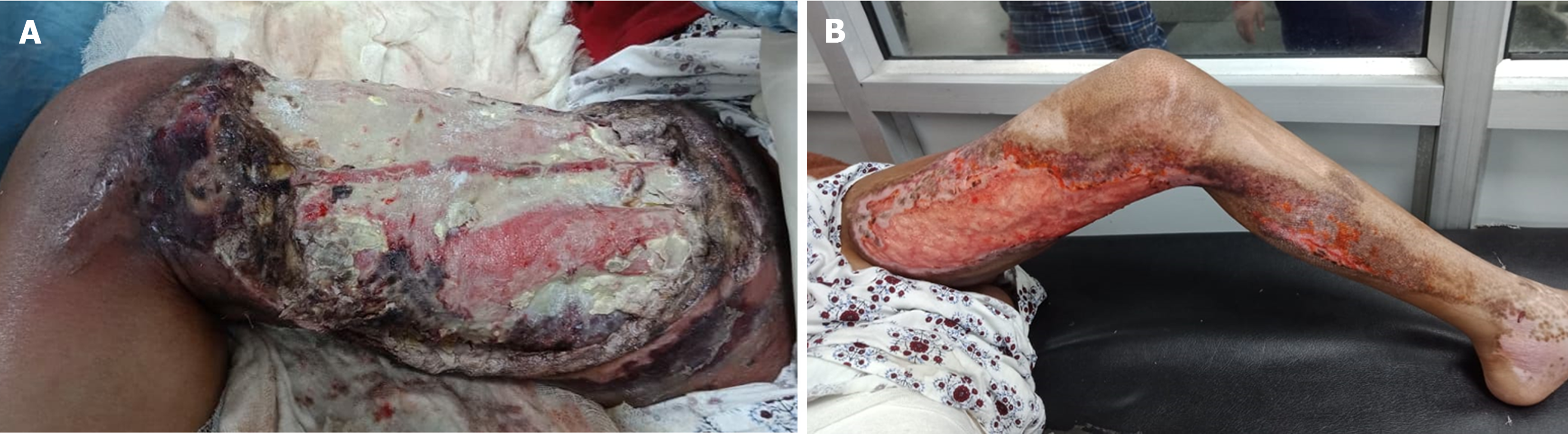
On presentation, she had systemic signs of inflammation, high fever, tachycardia and hypotension. Her general condition was poor with post-burn raw areas over the right thigh and groin and left thigh and leg. The right thigh had full thickness involvement over the anteromedial aspect with exposed thigh muscles There was slough and necrosis of surrounding soft tissues (Figure 1A). The left thigh and leg had partially healing raw areas with pale granulation tissue over the anteromedial thigh, extending to the left leg (Figure 1B).
Blood investigations were suggestive of anemia (hemoglobin: 8.1 g%), leukocytosis (total leukocyte count: 73 700) with shift to the left (91% neutrophils), thrombocytopenia (platelets: 7.14 × 105), hypoproteinemia (3.3 g/dL) and hypoalbuminemia (1.3 g/dL). Liver and kidney function tests were within normal limits. Wound swab on presentation revealed Gram-negative coccobacilli.
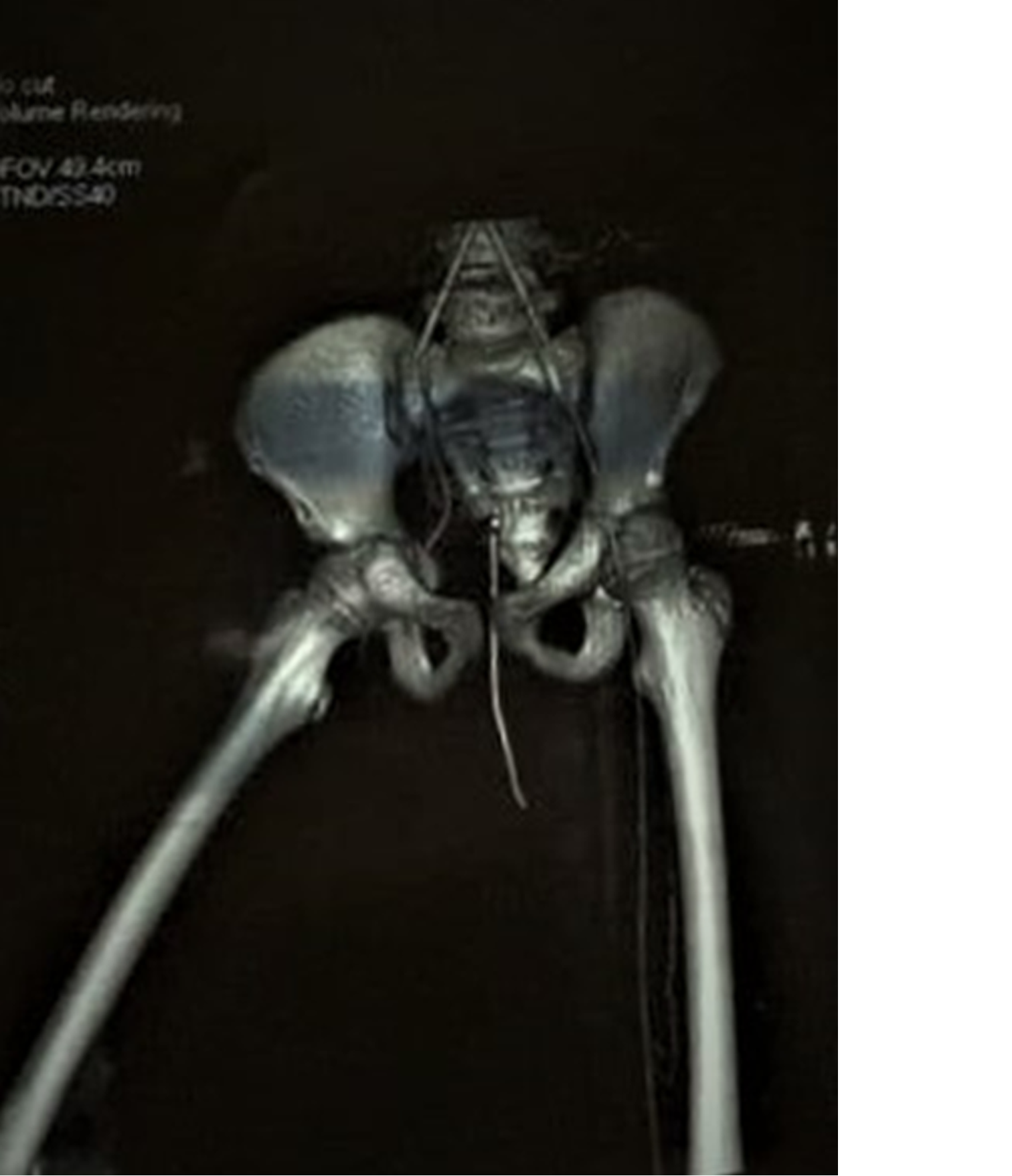
At presentation, chest X ray was suggestive of pleural effusion and abdominal ultrasonography revealed mild hepatomegaly. Postoperatively, computed tomography (CT) angiography was performed for bilateral lower limb vessels, which revealed acute thrombosis of the right external iliac artery and non-opacification of the right lower limb major vessels (Figure 2).
Angioinvasive mucormycosis.
The patient was transferred to the burn care unit. Intravenous fluid resuscitation, titrated to adequate urine output and central venous pressure, was administered. Empirical antibiotic therapy based on the burn unit protocol at our center was started. Blood transfusions were given to improve the hemoglobin level. The wound surface slough was excised under intravenous sedation. The patient however continued to have regular fevers and hemodynamic instability. On day 3 of admission, the right lower limb turned pale with absent pinprick. She was moved to the operating room for debridement. Thorough debridement of necrotic muscles and soft tissue was performed and the tissue was sent for bacterial and fungal culture sensitivity. Intraoperative thromboses of the right femoral artery and veins were noted. However, the deeper layer of the muscles was viable with adequate bleeding. Intravenous infusion of heparin, 10 μg/kg/h, was started with activated partial thromboplastin time monitoring.
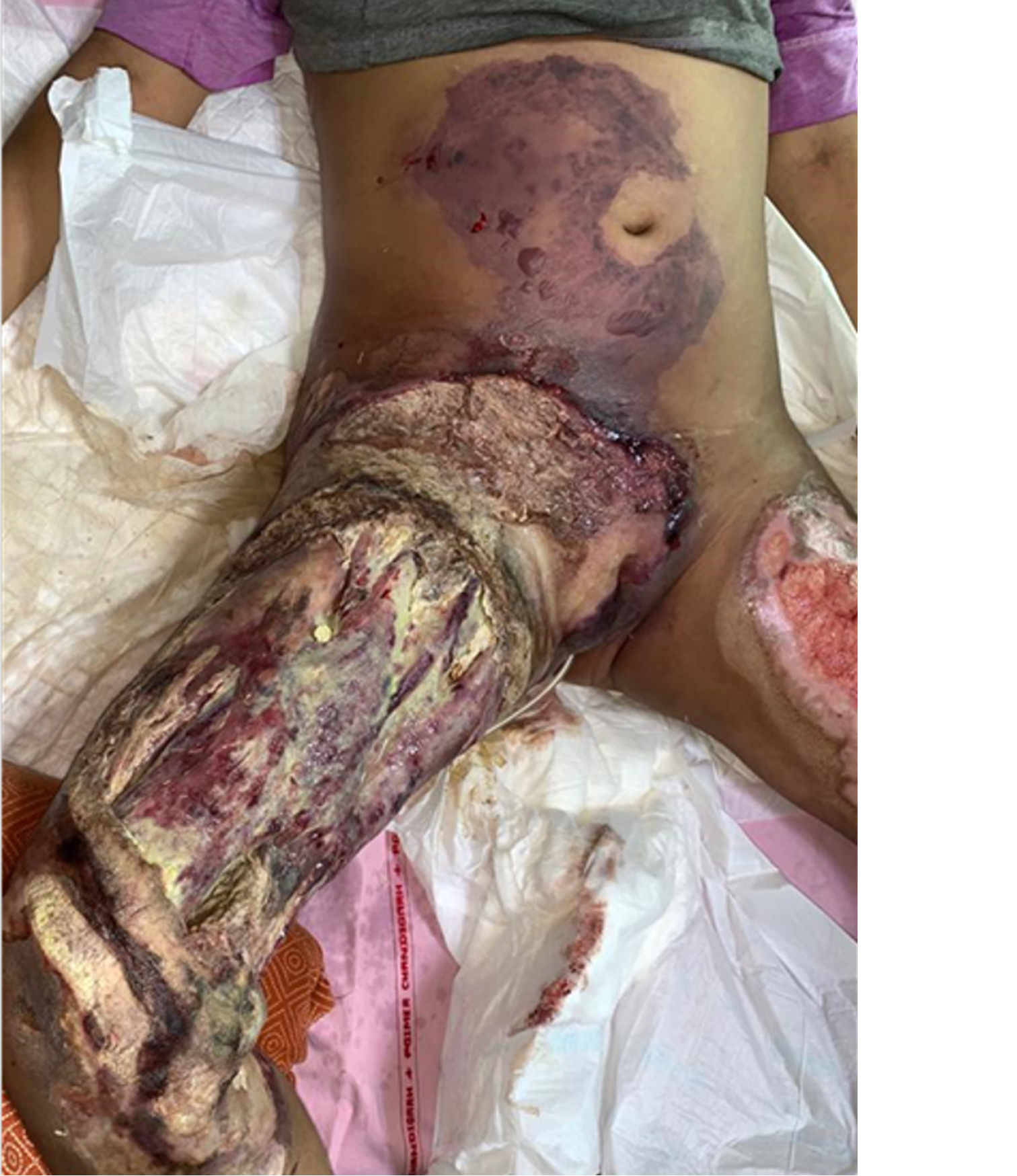
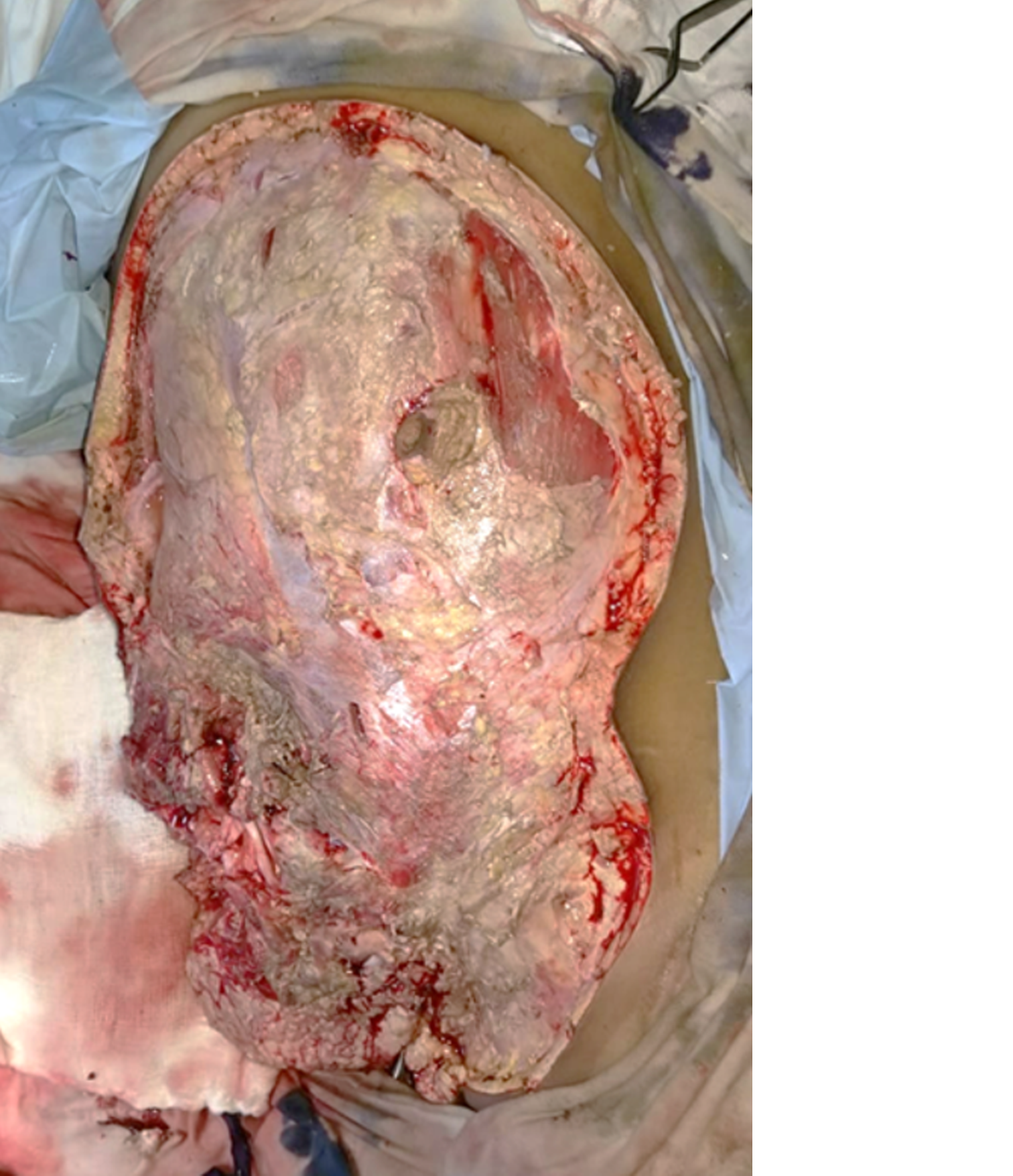
Intraoperative tissue biopsy showed growth of aseptate hyphae suggestive of Mucor species. The patient was started on intravenous liposomal amphotericin B (5 mg/kg). Despite all these measures, soft tissue necrosis rapidly progressed to involve the anterior abdominal wall and perineum over the next 8 h (Figure 3). The patient continued to have high fever and systemic sepsis. Consent for amputation was obtained and right hip disarticulation with external iliac ligation and aggressive debridement of the anterior abdominal wall and perineum were performed (Figure 4).
Postoperatively, in view of severe acidosis, the patient was kept on mechanical ventilation and also required inotropic support. Hemoglobin further fell to 6.8 g% and multiple transfusions were given. The wound condition continued to deteriorate rapidly, and the patient was managed with bedside debridement under sedation because her general condition was considered unfit for anesthesia. The blood oxygenation failed to improve, and metabolic acidosis persisted despite mechanical ventilation. Chest X ray revealed bilateral lung infiltrates. She arrested on day 7 of admission at our center and could not be revived. The cause of death was deemed to be angioinvasive cutaneous mucormycosis infection of the burn wound with hematogenous dissemination and secondary pulmonary invasion leading to systemic sepsis and respiratory failure.
Zygomycetes were first reported as a cause of human disease in 1885 by Paltauf[12] but it remained a rare diagnosis with fatal consequences for a long time. The last decade though, has seen their emergence as increasingly important pathogens. This rise in incidence of infection is seen in specific population groups, such as solid organ transplant recipients, diabetics, and patients on deferoxamine therapy[13]. Although still encountered less frequently than other fungal infections such as candidiasis or aspergillosis, these organisms are special because of their disproportionately high propensity to cause life-threatening infections even in patients with no underlying immunodeficiencies or immunosuppressive therapy. Roden et al[9] conducted a large scale review of all cases of zygomycosis reported in English literature since 1885 and studied a total of 929 cases. They reported an increasing trend in the incidence of these infections and found that 19% of cases had no underlying predisposing condition. Only 1.2% of the cases were reported to be associated with burn injuries. The mortality rate was 64% in this subgroup. Among the others, 44 patients (25%) had associated penetrating trauma and 32 (18%) had undergone surgery. Mortality was lower in these groups at 23% and 38%, respectively. In contrast, the larger majority of patients (81%, n = 753) had associated underlying conditions such as diabetes mellitus (36%), malignancy (17%), solid organ or bone marrow transplantation (12%), desferoxamine therapy (6%), injecting drug use (5%), renal failure (4%), or HIV infection (2%).
The term mucormycosis has been interchangeably used with the term zygomycosis. It is used to describe infections caused by fungi belonging to Zygomycota, a former phylum that has now become obsolete after revision of nomenclature of the kingdom Fungi[14,15]. Now, mucormycosis is used for infections caused by fungi belonging to the order Mucorales, which includes species belonging to the following genera, Rhizopus, Mucor, Rhizomucor, Lichtheimia, Saksenaea, Cunninghamella, and Apophysomyces. Among these, various reviews have reported Rhizopus to be the most common causative pathogen (47%) followed by Mucor (14%–18%)[16]. Causative pathogens also vary by geographical region. Lichthemia infections are largely reported in Europe (23% vs 7%) whereas Saksenaea spp. have been reported in isolates from North and South America, India and Australia[16].
These infections occur in patients with disrupted cutaneous barriers, as a result of either traumatic implantation of soil as in road side accidents, burn injuries, contaminated dressings maceration of skin by a moist surface[17-20], or even via direct access through intravenous catheters or subcutaneous injections (e.g. insulin injections in diabetics)[21-23]. In addition, it has been shown that Rhizopus spp. utilize deferoxamine as a siderophore leading to increased pathogenesis in patients on deferoxamine therapy[24,25].
Based on sites of involvement, mucormycosis may be grossly divided into six clinical categories, namely rhino–orbital–cerebral (ROC), pulmonary, cutaneous, gastrointestinal, disseminated, and miscellaneous. Of these, ROC mucormycosis is the most commonly noted (34%), followed by cutaneous (22%), pulmonary (20%) and disseminated (13%) mucormycosis[26]. Different underlying conditions predispose to specific sites of involvement; for example, ROC mucormycosis is significantly more common in patients with diabetes mellitus (51% vs 23%). Cutaneous mucormycosis is more commonly observed in immunocompetent patients with a history of trauma (69% vs 11%), and pulmonary mucormycosis is more prevalent in patients with a history of solid organ transplantation and those with neutropenia. Disseminated infection is more frequently seen in patients with underlying hematological malignancy[26].
The skin is reported to be the primary site of involvement in 14%–22% cases of mucormycosis overall[9] and in 27% of cases among children[27,28]. Most of these patients do not have associated neutropenia or underlying predisposing conditions. Instead, disruption of the normal protective cutaneous barrier is present in virtually all cases, followed by contamination with fungal spores. It is found to be associated with major penetrating trauma in 34% of cases, postsurgical in 33%, after-burn raw areas in 11%, and minor trauma such as cuts and grazes (during gardening etc.) in 4%[29,30]. In another review by Jeong et al[16], eight of 851 cases were attributed to the use of contaminated dressings, intravenous access sites, or needles[16]. Additionally, Roden et al[9] observed female sex and HIV infection to be independent risk factors for cutaneous involvement[9]. In diabetics, cutaneous lesions may arise at subcutaneous insulin injection or catheter insertion sites[22,23]. In cutaneous involvement, the infection may remain limited to the skin or involve the underlying deeper structures, muscles, fascia and even bone. This may lead to necrotizing fasciitis, which has a mortality approaching 80%[31-33]. In 20% of cases it may undergo hematogenous dissemination from the skin to other noncontiguous organs.
Extensive angioinvasion leading to vascular thrombosis and tissue necrosis is a hallmark of mucormycosis on histopathology[26]. The pathogen achieves this by invading and damaging the endothelial cells lining the blood vessels, thereby achieving the ability of hematogenous dissemination from the primary site of infection to other target organs (central nervous system, lungs etc.). Incidence of dissemination is noted to be the highest in neutropenic patients with pulmonary mucormycosis. Burn patients are particularly prone to cutaneous disease. After disseminated mucormycosis sets in, it has a high mortality rate approaching 94%–100%[14]. Diagnosing disseminated disease is often difficult because patients are usually already severely ill with multisystem involvement and blood cultures turn out to be negative for growth. This diagnosis must be considered if there is evidence of infarction in multiple organs[26].
Reported independent risk predictors for development of invasive, disseminated zygomycosis are: Burns, premature neonate, deferoxamine use, diabetes and HIV infection[9]. Nevertheless, isolated cutaneous mucormycosis (without dissemination) has a favorable prognosis and a low mortality if aggressive surgical debridement is done promptly[26].
Suspected mucormycosis is an emergency and requires rapid action. In cutaneous involvement, tissue samples must be sent for analysis as follows[34]. (1) Direct microscopy with fluorescence (calcofluor white) and histopathology with special stains (like hematoxylin–eosin, periodic acid–Schiff or Grocott methenamine silver. To confirm the diagnosis, aseptate/pauci-septate, nonpigmented hyphae, 6–16 μm wide, ribbon like with irregular branching pattern must be demonstrated. In addition, surrounding tissues show evidence of angioinvasion, vessel occlusion, perineural invasion, coagulative necrosis, and polymorphonuclear infiltration. (2) Culture performed on routine media at 30 and 37°C. Cotton white or greyish black colonies. (3) Molecular identification and immunohistochemical staining with specific primary reagents.
CT scans of the chest, sinuses, cranium, abdomen or other parts involved must be performed. Halo and reverse halo signs and pleural effusion are noted in chest CT in cases with pulmonary involvement. On CT angiography, vascular occlusion sign defined as interrupted vessel at the border of a focal lesion may be seen. Given the limitations of imaging studies, diagnosing mucormycosis almost always requires histopathological evidence of fungal invasion of the tissues. In addition, serology for galactomannan and 1,3-β-D-glucan may be performed[34]. Identification to the genus and species level is strongly recommended for improved epidemiological understanding of mucormycosis and antifungal susceptibility testing[35,36]. Species identification requires the use of molecular techniques for DNA detection, which may also yield faster results as compared to culturing the organism. However, their clinical utility is currently limited by lack of technique standardization and clinical validation[37]. Large-scale clinical studies are needed to evaluate the role of molecular approaches as the primary diagnostic modality of mucormycosis[38].
Before the introduction of amphotericin B in the 1960s, reported overall mortality from the infection was as high as 85%. The introduction of amphotericin B administered systemically is the first line of treatment for the infection and has reduced mortality to 40%–60%. In combination with aggressive surgical therapy, this is seen to decrease to 30%. Overall, four factors are deemed critical for achieving cure in mucormycosis[26]; namely, early diagnosis, treating the underlying predisposing factors, antifungal therapy, and aggressive surgical debridement. The significance of delay in diagnosis of mucormycosis may be underscored by the fact that several autopsy series have reported that up to 50% of cases are diagnosed postmortem[39-41]. Small, localized lesions, diagnosed early can often be surgically excised before they spread to cause extensive disease or disseminate[42]; while delayed diagnosis has been shown to result in dramatically worse outcomes (83% vs 43% survival)[43]. Unfortunately, so far there are no serum or molecular tests to allow rapid diagnosis of the entity. Thus, the treating physician must maintain a high index of clinical suspicion and aggressively pursue diagnostic biopsy in suspected cases for improved outcomes.
Mucoraceous fungi are resistant to most antifungals and amphotericin B is the most active drug, against most isolates. Amphotericin B may be administered as amphotericin B deoxycholate, liposomal amphotericin B or amphoterecin B lipid complex. Other investigational/adjunctive therapies with variable efficacy include triazoles like, itraconazole, ketoconazole, posaconazole, isavuconazole, caspofungin, hyperbaric oxygen, iron chelation, cytokine therapy such as interferon-λ, and granulocyte colony-stimulating factor, which may enhance phagocytic activity against the pathogen[26]. A major obstacle for clinicians to choose among the current available antifungal agents in treating mucormycosis is the lack of available randomized clinical trials. The 2016 recommendations from the European Conference on Infections in Leukemia-6, as well as the ESCMID/ECMM guidelines, advocate the use of a lipid formulation of amphotericin B as first-line therapy for mucormycosis[44,45]. The currently suggested dose for liposomal amphotericin B is 5 mg/kg/day and as high as 10 mg/kg/day for infection of the central nervous system. However, the optimal doses for antifungal agents are still an issue of controversy. In case of renal failure, dose of amphotericin B may be reduced or alternate antifungals such as posaconazole and isavuconazole may be used. Also, in cases of severe disease, rapid progression, or poor general condition, they may be given in addition to amphotericin B[46]. Hyperbaric oxygen may have a role as an adjunct to standard therapy because higher oxygen pressure improves the ability of neutrophils to kill the organism[47] and has been shown to inhibit the germination of fungal spores in vitro[48], although there is a lack of prospective clinical trials to definitely establish its role in the treatment of mucormycosis.
Mucormycosis is usually rapidly progressive, and antifungal therapy alone is often inadequate to control the infection. Surgical debridement has an important role because, various species of mucor may or may not be susceptible to available antifungal agents and some species may even be resistant to amphotericin B. Moreover, the hallmark angioinvasion, thrombosis, and tissue necrosis in mucormycosis result in poor penetration of these agents. Thus, even in vitro susceptibility of the pathogen is not a guarantee of its in vivo efficacy. The killing the pathogen is not sufficient and urgent surgical debridement is thereby necessary to remove the infected and necrotic tissue and optimize cure rates[49].
Currently, mortality rates for mucormycosis vary from 40% to 80% based primarily on predisposing factors and site of involvement. This can rise to 96% for those with disseminated disease[9,50]. Much of the variability in outcome is due to the various forms of the disease. With respect to site of involvement, mortality is shown to be highest among patients with disseminated disease (68%) and lowest in those with cutaneous disease (31%)[16,23].
Independent risk factors associated with significantly increased mortality include disseminated disease, extensive burns, hematological malignancies, associated renal failure, delayed initiation of therapy and neonatal age group[9,16,34]. Conversely, lower mortality is seen in patients with immunocompetent status; without comorbidities; or with localized infection of the sinuses or skin and soft tissues, where early tissue-based diagnosis may be obtained and cure may be possible with early complete surgical debridement[34].
The case reported by us had delay in referral and administration of proper wound care. There could also have been contamination of dressings during 2 mo before reporting to the burn center. Although rapid diagnosis and surgical debridement were done when the patient finally reported to our center, the infection was already at the invasive stage. This further led to hematogenous dissemination with major vessel thrombosis, and pulmonary involvement.
Incidence of mucormycosis complicating burn wounds ranges from 0.1% to 0.6%, which may rise to 10%–15% during localized outbreaks in treatment units. The most common clinical form of mucormycosis in burn patients is cutaneous with higher propensity for dissemination than cutaneous involvement from other causes. Arterial invasion invariably occurs with embolization, thrombosis and infarction. Vascular invasion by the hyphae leads to progressive tissue necrosis. Despite improved understanding of the disease and the availability of more therapeutic options, survival rates in mucormycosis remain poor[9,16,26].
Maximizing survival rates requires rapid diagnostic and therapeutic intervention[34]. Patients with suspected mucormycosis should be referred immediately to a facility with the highest care level. The capability of diagnosing mucormycosis depends on the availability of mycological and histological investigation facilities and trained personnel. When diagnosed, early localized cutaneous mucormycosis treated with aggressive surgical debridement and adjunctive antifungal therapy. Care providers should be especially vigilant for wound infections in patients who demonstrate progressive necrosis outside of the area of initial burn wound. Wound surveillance seems to be the gold standard to avoid the devastating outcome of this rare, life-threatening infection. Treating surgeons must keep a high index of suspicion and send multiple wound biopsies when faced with a nonhealing burn raw area, especially in cases presenting late or with pre-existing immunocompromised state.
| 1. | Burdge JJ, Rea F, Ayers L. Noncandidal, fungal infections of the burn wound. J Burn Care Rehabil. 1988;9:599-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006;19:403-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1053] [Cited by in RCA: 1290] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 3. | Still JM Jr, Law EJ, Belcher KE, Spencer SA. A comparison of susceptibility to five antifungal agents of yeast cultures from burn patients. Burns. 1995;21:167-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Wright JB, Lam K, Hansen D, Burrell RE. Efficacy of topical silver against fungal burn wound pathogens. Am J Infect Control. 1999;27:344-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 215] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 5. | Beck-Sagué C, Jarvis WR. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980-1990. National Nosocomial Infections Surveillance System. J Infect Dis. 1993;167:1247-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 718] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 6. | Slavin M, van Hal S, Sorrell TC, Lee A, Marriott DJ, Daveson K, Kennedy K, Hajkowicz K, Halliday C, Athan E, Bak N, Cheong E, Heath CH, Orla Morrissey C, Kidd S, Beresford R, Blyth C, Korman TM, Owen Robinson J, Meyer W, Chen SC; Australia and New Zealand Mycoses Interest Group. Invasive infections due to filamentous fungi other than Aspergillus: epidemiology and determinants of mortality. Clin Microbiol Infect. 2015;21:490.e1-490.10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 7. | Park BJ, Pappas PG, Wannemuehler KA, Alexander BD, Anaissie EJ, Andes DR, Baddley JW, Brown JM, Brumble LM, Freifeld AG, Hadley S, Herwaldt L, Ito JI, Kauffman CA, Lyon GM, Marr KA, Morrison VA, Papanicolaou G, Patterson TF, Perl TM, Schuster MG, Walker R, Wingard JR, Walsh TJ, Kontoyiannis DP. Invasive non-Aspergillus mold infections in transplant recipients, United States, 2001-2006. Emerg Infect Dis. 2011;17:1855-1864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 252] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 8. | Chakrabarti A, Chatterjee SS, Das A, Panda N, Shivaprakash MR, Kaur A, Varma SC, Singhi S, Bhansali A, Sakhuja V. Invasive zygomycosis in India: experience in a tertiary care hospital. Postgrad Med J. 2009;85:573-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 154] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 9. | Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Kontoyiannis DP, Walsh TJ. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1793] [Cited by in RCA: 2037] [Article Influence: 97.0] [Reference Citation Analysis (1)] |
| 10. | Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13:236-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 702] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 11. | Chakrabarti A, Singh R. Mucormycosis in India: unique features. Mycoses. 2014;57 Suppl 3:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 12. | Paltauf A. Mycosis mucorina. Virchows Arch Path Anat. 1885;102:543-553. |
| 13. | Gleissner B, Schilling A, Anagnostopolous I, Siehl I, Thiel E. Improved outcome of zygomycosis in patients with hematological diseases? Leuk Lymphoma. 2004;45:1351-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 146] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Morrison A, Polisena J, Husereau D, Moulton K, Clark M, Fiander M, Mierzwinski-Urban M, Clifford T, Hutton B, Rabb D. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care. 2012;28:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 907] [Cited by in RCA: 856] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 15. | Pagano L, Offidani M, Fianchi L, Nosari A, Candoni A, Picardi M, Corvatta L, D'Antonio D, Girmenia C, Martino P, Del Favero A; GIMEMA (Gruppo Italiano Malattie EMatologiche dell'Adulto) Infection Program. Mucormycosis in hematologic patients. Haematologica. 2004;89:207-214. [PubMed] |
| 16. | Jeong W, Keighley C, Wolfe R, Lee WL, Slavin MA, Kong DCM, Chen SC. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019;25:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 605] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 17. | Alsuwaida K. Primary cutaneous mucormycosis complicating the use of adhesive tape to secure the endotracheal tube. Can J Anaesth. 2002;49:880-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Petrikkos G, Skiada A, Sambatakou H, Toskas A, Vaiopoulos G, Giannopoulou M, Katsilambros N. Mucormycosis: ten-year experience at a tertiary-care center in Greece. Eur J Clin Microbiol Infect Dis. 2003;22:753-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Antonetti J, Killyon GW, Chang P, McCauley RL. Microvascular transfer of burned tissue for mandibular reconstruction. J Burn Care Res. 2009;30:536-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Thielen BK, Barnes AMT, Sabin AP, Huebner B, Nelson S, Wesenberg E, Hansen GT. Widespread Lichtheimia Infection in a Patient with Extensive Burns: Opportunities for Novel Antifungal Agents. Mycopathologia. 2019;184:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Adam RD, Hunter G, DiTomasso J, Comerci G Jr. Mucormycosis: emerging prominence of cutaneous infections. Clin Infect Dis. 1994;19:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 138] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Kerr OA, Bong C, Wallis C, Tidman MJ. Primary cutaneous mucormycosis masquerading as pyoderma gangrenosum. Br J Dermatol. 2004;150:1212-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Quinio D, Karam A, Leroy JP, Moal MC, Bourbigot B, Masure O, Sassolas B, Le Flohic AM. Zygomycosis caused by Cunninghamella bertholletiae in a kidney transplant recipient. Med Mycol. 2004;42:177-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Boelaert JR, de Locht M, Van Cutsem J, Kerrels V, Cantinieaux B, Verdonck A, Van Landuyt HW, Schneider YJ. Mucormycosis during deferoxamine therapy is a siderophore-mediated infection. In vitro and in vivo animal studies. J Clin Invest. 1993;91:1979-1986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 221] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 25. | de Locht M, Boelaert JR, Schneider YJ. Iron uptake from ferrioxamine and from ferrirhizoferrin by germinating spores of Rhizopus microsporus. Biochem Pharmacol. 1994;47:1843-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 83] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18:556-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 980] [Cited by in RCA: 928] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 27. | Roilides E, Zaoutis TE, Walsh TJ. Invasive zygomycosis in neonates and children. Clin Microbiol Infect. 2009;15 Suppl 5:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Zaoutis TE, Roilides E, Chiou CC, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Prasad PA, Chu JH, Walsh TJ. Zygomycosis in children: a systematic review and analysis of reported cases. Pediatr Infect Dis J. 2007;26:723-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54 Suppl 1:S23-S34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 896] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 30. | Skiada A, Pagano L, Groll A, Zimmerli S, Dupont B, Lagrou K, Lass-Florl C, Bouza E, Klimko N, Gaustad P, Richardson M, Hamal P, Akova M, Meis JF, Rodriguez-Tudela JL, Roilides E, Mitrousia-Ziouva A, Petrikkos G; European Confederation of Medical Mycology Working Group on Zygomycosis. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect. 2011;17:1859-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 527] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 31. | Boyd AS, Wiser B, Sams HH, King LE. Gangrenous cutaneous mucormycosis in a child with a solid organ transplant: a case report and review of the literature. Pediatr Dermatol. 2003;20:411-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Kordy FN, Al-Mohsen IZ, Hashem F, Almodovar E, Al Hajjar S, Walsh TJ. Successful treatment of a child with posttraumatic necrotizing fasciitis caused by Apophysomyces elegans: case report and review of literature. Pediatr Infect Dis J. 2004;23:877-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 33. | Patiño JF, Castro D. Necrotizing lesions of soft tissues: a review. World J Surg. 1991;15:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 45] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Devauchelle P, Jeanne M, Fréalle E. Mucormycosis in Burn Patients. J Fungi (Basel). 2019;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Espinel-Ingroff A, Chakrabarti A, Chowdhary A, Cordoba S, Dannaoui E, Dufresne P, Fothergill A, Ghannoum M, Gonzalez GM, Guarro J, Kidd S, Lass-Flörl C, Meis JF, Pelaez T, Tortorano AM, Turnidge J. Multicenter evaluation of MIC distributions for epidemiologic cutoff value definition to detect amphotericin B, posaconazole, and itraconazole resistance among the most clinically relevant species of Mucorales. Antimicrob Agents Chemother. 2015;59:1745-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 36. | Guinea J, Escribano P, Vena A, Muñoz P, Martínez-Jiménez MDC, Padilla B, Bouza E. Increasing incidence of mucormycosis in a large Spanish hospital from 2007 to 2015: Epidemiology and microbiological characterization of the isolates. PLoS One. 2017;12:e0179136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 37. | Walsh TJ, Gamaletsou MN, McGinnis MR, Hayden RT, Kontoyiannis DP. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis). Clin Infect Dis. 2012;54 Suppl 1:S55-S60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 254] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 38. | Millon L, Herbrecht R, Grenouillet F, Morio F, Alanio A, Letscher-Bru V, Cassaing S, Chouaki T, Kauffmann-Lacroix C, Poirier P, Toubas D, Augereau O, Rocchi S, Garcia-Hermoso D, Bretagne S; French Mycosis Study Group. Early diagnosis and monitoring of mucormycosis by detection of circulating DNA in serum: retrospective analysis of 44 cases collected through the French Surveillance Network of Invasive Fungal Infections (RESSIF). Clin Microbiol Infect. 2016;22:810.e1-810.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 187] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 39. | Kontoyianis DP, Vartivarian S, Anaissie EJ, Samonis G, Bodey GP, Rinaldi M. Infections due to Cunninghamella bertholletiae in patients with cancer: report of three cases and review. Clin Infect Dis. 1994;18:925-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Mori T, Egashira M, Kawamata N, Oshimi K, Nakamura K, Oguri T, Aida H, Hiruma A, Ichinohe M. [Zygomycosis: two case reports and review of reported cases in the literature in Japan]. Nihon Ishinkin Gakkai Zasshi. 2003;44:163-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Tietz HJ, Brehmer D, Jänisch W, Martin H. [Incidence of endomycoses in the autopsy material of the Berlin Charité Hospital]. Mycoses. 1998;41 Suppl 2:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Nithyanandam S, Jacob MS, Battu RR, Thomas RK, Correa MA, D'Souza O. Rhino-orbito-cerebral mucormycosis. A retrospective analysis of clinical features and treatment outcomes. Indian J Ophthalmol. 2003;51:231-236. [PubMed] |
| 43. | Khor BS, Lee MH, Leu HS, Liu JW. Rhinocerebral mucormycosis in Taiwan. J Microbiol Immunol Infect. 2003;36:266-269. [PubMed] |
| 44. | Tissot F, Agrawal S, Pagano L, Petrikkos G, Groll AH, Skiada A, Lass-Flörl C, Calandra T, Viscoli C, Herbrecht R. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica. 2017;102:433-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 454] [Article Influence: 45.4] [Reference Citation Analysis (1)] |
| 45. | Cornely OA, Arikan-Akdagli S, Dannaoui E, Groll AH, Lagrou K, Chakrabarti A, Lanternier F, Pagano L, Skiada A, Akova M, Arendrup MC, Boekhout T, Chowdhary A, Cuenca-Estrella M, Freiberger T, Guinea J, Guarro J, de Hoog S, Hope W, Johnson E, Kathuria S, Lackner M, Lass-Flörl C, Lortholary O, Meis JF, Meletiadis J, Muñoz P, Richardson M, Roilides E, Tortorano AM, Ullmann AJ, van Diepeningen A, Verweij P, Petrikkos G; European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group; European Confederation of Medical Mycology. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin Microbiol Infect. 2014;20 Suppl 3:5-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 488] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 46. | Santos J, Espigado P, Romero C, Andreu J, Rivero A, Pineda JA. Isolated renal mucormycosis in two AIDS patients. Eur J Clin Microbiol Infect Dis. 1994;13:430-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Couch L, Theilen F, Mader JT. Rhinocerebral mucormycosis with cerebral extension successfully treated with adjunctive hyperbaric oxygen therapy. Arch Otolaryngol Head Neck Surg. 1988;114:791-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 71] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Robb SM. Reactions of fungi to exposure to 10 atmospheres pressure of oxygen. J Gen Microbiol. 1966;45:17-29. [RCA] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 49. | Ibrahim AS, Spellberg B, Avanessian V, Fu Y, Edwards JE Jr. Rhizopus oryzae adheres to, is phagocytosed by, and damages endothelial cells in vitro. Infect Immun. 2005;73:778-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 50. | Kennedy KJ, Daveson K, Slavin MA, van Hal SJ, Sorrell TC, Lee A, Marriott DJ, Chapman B, Halliday CL, Hajkowicz K, Athan E, Bak N, Cheong E, Heath CH, Morrissey CO, Kidd S, Beresford R, Blyth C, Korman TM, Robinson JO, Meyer W, Chen SC; Australia and New Zealand Mycoses Interest Group of the Australasian Society for Infectious Diseases. Mucormycosis in Australia: contemporary epidemiology and outcomes. Clin Microbiol Infect. 2016;22:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elsiesy H, Saudi Arabia; Pan L, China S-Editor: Fan JR L-Editor: Kerr C P-Editor: Cai YX