Published online Dec 9, 2023. doi: 10.5492/wjccm.v12.i5.236
Peer-review started: May 30, 2023
First decision: August 2, 2023
Revised: August 20, 2023
Accepted: September 11, 2023
Article in press: September 11, 2023
Published online: December 9, 2023
Processing time: 190 Days and 9.9 Hours
Over the last three years, research has focused on examining cardiac issues arising from coronavirus disease 2019 (COVID-19) infection, including the emergence of new-onset atrial fibrillation (NOAF). Still, no clinical study was conducted on the persistence of this arrhythmia after COVID-19 recovery. Our objective was to co
Core Tip: In this literature review, we have observed resemblances between the fundamental pathophysiology of coronavirus disease 2019 (COVID-19)-related new-onset atrial fibrillation (NOAF) and the mechanisms proposed for the persistence of AF, particularly those involving oxidative stress and reactive oxygen species. The mechanisms responsible for the deve
- Citation: Talaei F, Banga A, Pursell A, Gage A, Pallipamu N, Seri AR, Adhikari R, Kashyap R, Surani S. New-onset atrial fibrillation among COVID-19 patients: A narrative review. World J Crit Care Med 2023; 12(5): 236-247
- URL: https://www.wjgnet.com/2220-3141/full/v12/i5/236.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v12.i5.236
As atrial fibrillation (AF) incidence was approaching an epidemic proportion[1], in January 2020, the world health organization announced the preliminary determination of a novel coronavirus in Wuhan, China. By March 2020, the novel virus was recognized as a global pandemic[2,3]. AF was reported as the most common arrhythmia in a multicenter review of coronavirus disease 2019 (COVID-19) cases in 76 countries, with a prevalence of 19% to 21% of all hospitalized cases[4]. The new onset of AF (NOAF) among COVID-19 patients (referred to as COVID-19-related NOAF) raises con
In individuals whose AF has progressed, it has previously been observed that various factors, such as oxidative stress, atrial dilatation, calcium overload, inflammation, and myofibroblast activation, interact in a way that significantly con
Similar to past pandemics in history, the COVID-19 pandemic presents a chance to broaden our knowledge despite the challenges it poses[3]. Our objective was to explore the underlying mechanisms of NOAF in COVID-19 patients, with a particular emphasis on factors that could sustain the occurrence of this arrhythmia. To peruse this, a comprehensive, structured literature search was conducted through EMBASE and MEDLINE for articles published between December 2019 and May 20, 2023, that reported the pathophysiology of NOAF after COVID-19 and those persisting AF. Also, the latest data on incidence, morbidity-mortality, and management of NOAF in COVID-19 were investigated. The search terms include each of the following terms individually and in combination: “new-onset atrial fibrillation”, “NOAF”, “AF persistence”, “persistent atrial fibrillation”, “SARS-CoV-2”, “COVID-19”, “SARS”, coronavirus as described in the Supplementary Table 1[12]. Two investigators (Talaei F and Banga A) independently screened studies for eligibility. We focused primarily on published research articles, systematic reviews, and observational cohorts. The title, abstract, and keywords were checked for relevance initially. Studies were excluded if not written in English.
NOAF is defined as AF detected after diagnosis of COVID-19 without a prior history[13]. In the course of the disease, AF might indefinitely appear as short (< 7 d) self-limiting episodes (i.e., paroxysmal). However, it is more likely to transform into long-lasting forms of AF[14]. AF is considered persistent when perpetually lasting more than seven days[15,16].
An October 2021 meta-analysis involving over 21000 hospitalized COVID-19 patients revealed that NOAF had a pre
A report from the American Heart Association COVID-19 Cardiovascular Disease Registry revealed that 5.4% of patients hospitalized for COVID-19 infection developed NOAF during their hospital stay. Moreover, NOAF was asso
COVID-19-related NOAF was demonstrated to be an independent prognostic factor for in-hospital embolic events, irrespective of anticoagulant use and prolonged hospital stay. Potential co-factors contributing to the development of NOAF could include older age, arterial hypertension, a history of myocardial infarction, renal dysfunction, and elevated D-dimers[19], which align with previously reported risk factors associated with the emergence of NOAF in critically ill patients[20].
There are ongoing debates regarding underlying mechanisms involved in provoking arrhythmias in COVID-19 infection. While some attribute arrhythmias and hence AF directly to the virus itself[21]; others highlight the connection between inflammatory markers and arrhythmias, considering it as a consequence of a systemic illness not exclusive to COVID-19[11,22]. A third group points towards the long-term changes required to make atrial structural abnormalities and the relatively short incubation period of COVID-19 and concludes that it might be a symptom of prior undetected structural heart diseases[23,24].
There are limited studies concerning the pathophysiology of NOAF in COVID-19; nonetheless, several of them are built upon earlier research conducted on severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) or Middle East respiratory syndrome coronavirus (MERS-CoV). Considering the resemblances in structure and potential pathogenicity among MERS-CoV, SARS-CoV-1, and SARS-CoV-2[25], various mechanisms have been suggested to elucidate the pro
Angiotensin-converting enzyme-2 (ACE2)-related signaling pathways, endothelial dysfunction, spike protein inte
Modulation of myocardial ACE2 expression: ACE2 is found in abundance in the lungs. It is thought to play an essential role in the pathogenesis of SARS-CoV-2-associated severe acute respiratory syndrome by acting as a receptor for this family of viruses. However, this enzyme is not exclusive to the lungs, as it is also highly expressed in the heart and kid
The catalytic action of this enzyme in the heart leads to the degradation of angiotensin-II (Ang II) to cardioprotective Ang1-7. In doing so, ACE2 plays a cardioprotective counterbalance role in the renin-angiotensin-aldosterone system (RAAS)[19].
Binding the viral spike protein to the ACE2 receptor activates catalytic processes that lead to ACE2 shedding, de
Endothelial dysfunction: The cardioprotective role of ACE2 has been discussed, yet it extends beyond that by serving as a regulator of the kallikrein-bradykinin pathway, imparting a significant vasodilator effect. This effect acts as a counterbalance to the vasopressor effect of RAAS[19,31]. Declined vascular levels of ACE2 in COVID-19 patients lead to over
While the impact of AF on the vasculature has been better studied, recent discoveries indicate a bidirectional rela
Spike protein binding to cardiomyocyte: Spike protein of COVID-19 plays an essential role in host cell invasion, inclu
Spike protein also binds to N-acetylneuraminic acid (Neu5Ac), the predominant sialic acid in human cells, including cardiomyocytes. Higher levels of Neu5Ac are associated with left atrial enlargement, and it plays a crucial role in severe coronary artery diseases (CAD) and cardiac fibrosis[38], but how this cardiac fibrosis could lead to AF is under study[23].
Cytokine storm: The sustained infiltration of neutrophils, macrophages, and CD4+ T-lymphocytes associated with the COVID-19 cytokine storm can promote the transformation of fibroblasts to myofibroblasts, which in the long run could lead to pathological cardiac remodeling and fibrosis[39].
On the other hand, atherosclerotic plaques in the coronary artery are more prone to rupture in the state of inflammation anticipated by cardiac injury and arrhythmias. Production of several cytokines, either by inducing direct myo
Hypoxemia: COVID-19 causes hypoxia by several different mechanisms that can transform into acute respiratory syndrome[42]. Pneumonia in COVID-19 deteriorates gas exchange and complicates cell metabolism. This enhances anaerobic fermentation, resulting in intracellular acidosis and oxygen free radicals destroying the phospholipid layer of the cell membrane. Meanwhile, the hypoxia-induced influx of calcium ions also leads to injury and subsequent apoptosis of injured cardiomyocytes[43]. Also, COVID-19 systemic infection, being a situation of increased cardiometabolic deman
The above should also add hypoxemia-induced dynamic changes in transmural pressure gradients, promoting increased pulmonary pressure, which leads to tricuspid regurgitation and further impairment in the right atrium, followed by possible changes in atrial conduction properties and refractoriness[45].
Gramley et al[46] previously observed a close association between prolonged hypoxic and increased angiogenic markers in the atrium with AF. With the persistence of hypoxia, an endoglin called CD105 would up-regulate, which is a homolog to the type III receptor of transforming growth factor-β, leading to ECM formation. It was hypothesized that cardiac hypoxia could provoke AF through the hypoxia-inducible factor pathway and over-expression of connective tissue growth factor and angiogenic genes like vascular endothelial growth factor[46].
Autonomic nervous system alteration: Severe infections generally activate the sympathetic nervous system (SNS), which also relates to AF[23]. Among cytokines released in COVID-19 infection, IL-6 can hyperactivate SNS, either centrally by a hypothalamus-mediated mechanism or peripherally via the left stellate ganglia[47].
SNS activation likely increases calcium influx into the cardiomyocytes and calcium overload in the sarcoplasmic reticulum, further increasing the frequency of spontaneous diastolic calcium releases, resulting in delayed afterdepolarizations and triggered action potentials, increasing the likelihood of AF induction[48].
On the other hand, hypoxemia might activate the parasympathetic system. Combined sympathetic and vagal acti
Fluid and electrolyte abnormality: Renal dysfunction in COVID-19 can lead to a decrease in serum potassium levels due to increased excretion[50]. Increased ACE/ACE2 ratio imbalance would also affect the RAS and potassium metabolism[51]. Increased ACE2 degradation augments RAS activity, increasing sodium and water reabsorption and collateral increase potassium excretion[52]. Hypokalemia frequently happens in hospitalized patients with COVID-19, with reported rates ranging from 41% to 55% of cases[53]. The occurrence of hypokalemia, which increases resting potential, leads to cell membrane hyperpolarization, thus accelerating atrial conduction and potentially creating a susceptibility to AF. Hypokalemia, by increasing resting potential, leads to cell membrane hyperpolarization, thus accelerating atrial conduction, which could possibly predispose to AF[54]. Hypokalemia frequently happens in hospitalized patients with COVID-19, reported in 41% to 55% of cases[55].
Growing evidence highlights a strong link between CAD and AF, and several observational studies have indicated that CAD and AF aggravate each other. Shared risk factors encompassing hypertension, diabetes mellitus, and obesity subs
Evidence points to an intricate relationship between atrial tissue excitability and neuronal remodeling with ischemia at the microcirculatory level. CAD adversely affects AF by promoting progression via re-entry and increasing the excitability of atrial tissue as a result of ischemia and electrical inhomogeneity. AF, in turn, accelerates atherosclerosis and, together with enhanced thrombogenicity and hypercoagulability contribute to micro and macrothrombi throughout the cardi
Patients with CAD associated with NOAF or persistent AF have significantly higher morbidity and mortality, predisposing to heart failure, life-threatening ventricular arrhythmias, and major adverse cardiovascular events[57]. A recent comprehensive analysis supports heightened AF risk in CAD patients, yet a causal AF-to-CAD link remains unestablished[56]. Management of concurrent CAD and AF centers on anti-thrombotic strategies, balancing stroke prevention and stent thrombosis avoidance while cautiously mitigating bleeding risk. Current guidelines recommend up to one year of combined oral anticoagulant (OAC) and antiplatelet therapy, preferably P2Y12 inhibitors or OAC monotherapy. How
Recognition: NOAF recognition in patients with COVID-19 can be done with electrocardiography, telemetry, or implan
Evaluation: The initial evaluation of COVID-19-related NOAF parallels the standard management for AF. This involves conducting a routine two-dimensional transthoracic echocardiogram to assess for structural irregularities. However, if indications of heart failure, hemodynamic instability, unexplained clinical deterioration, or planned cardioversion are pre
Transesophageal echocardiography should be obviated by the early start of anticoagulation in NOAF to detect left atrium thrombi as a potential source of systemic embolism in AF and can be used to guide the timing of cardioversion or catheter ablation procedures[53].
Treatment goals: Treatment goals are regardless of the type, treatment goals encompass three primary objectives: Managing heart rate during episodes of AF; and achieving the restoration, sustained maintenance of normal sinus rhythm (rhythm control), and mitigating the risk of systemic or cerebral embolism linked to the heightened embolic risk asso
Rate and rhythm control: The contemporary therapy of AF with rate control vs rhythm control strategies is still disputed, there is a scarcity of data regarding the effectiveness of rhythm and rate control approaches for COVID-19-related AF. Current recommendations are based on acute management of AF in COVID-19 disease and long-term data is not avai
Hospitalized patients who have developed COVID-19-related NOAF and are undergoing antiviral treatment while maintaining hemodynamic stability should give precedence to discontinuing their anti-arrhythmic medications. Instead, the preferred approach involves initiating rate control therapy using beta-blockers or non-dihydropyridine calcium channel blockers, along with or without digoxin, unless contraindicated[58]. This approach ensures the safe administration of antiviral medication without the potential risk of QT prolongation[53,58].
Amidst a COVID-19 infection, the potential for QT interval-related risks could be heightened due to the simultaneous utilization of anti-arrhythmic medications with other QT-prolonging medications (such as hydroxychloroquine, azith
Unless dealing with highly symptomatic AF cases, such as individuals with AF-related heart failure or those experiencing medically refractory AF resulting in frequent emergency room visits, all AF ablation procedures ought to be delayed for a minimum of three months after recovering from a COVID-19 infection[53].
Prevention of thromboembolic events: As a general guideline, for patients with a history of prior stroke, transient ischemic attack, or a CHA2DS2-VASc score > 2 who subsequently develop AF, oral anticoagulation is recommended[15]. Given that hospitalized COVID-19 patients are generally over the age of 65 and often have multiple underlying health conditions, a significant proportion of individuals with AF necessitate prolonged anticoagulation therapy[11]. Hemodynamically stable COVID-19 patients presenting with atrial AF during their hospitalization have treatment including unfractionated heparin, low molecular weight heparin, or direct OACs (DOACs). The specific choice among these options is influenced by factors like the suitability of oral administration, renal function, and additional clinical aspects. It’s important to highlight that certain medications for COVID-19 treatment could potentially interact with DOACs. Lop
VKAs are also considered for specific subsets of patients, including individuals with mechanical prosthetic valves or antiphospholipid syndrome. While VKAs typically induce a temporary deficiency of vitamin K, the observed lower levels of vitamin K in patients with COVID-19 compared to healthy individuals suggest a need for additional investigation regarding the utilization of VKAs in COVID-19 patients[61]. The precise mechanisms driving this connection are yet to be fully understood.
The innate tendency of COVID-19 for coagulopathy, characterized by elevated D-dimer and a significant increase in peripheral thromboembolic events observed in NOAF patients, calls for further investigation of the management of COVID-19-related NOAF[10,11]. Since heparins are unlikely to interact with drugs used in COVID-19 treatment, they represent a safe and attractive option for stroke prevention in AF patients who are hospitalized due to COVID-19. Remarkably, beyond their antithrombotic effects, heparins also possess anti-inflammatory properties that could be pertinent in this context[53]. Following recovery from COVID-19, the continuation of long-term anticoagulation should be based on the CHA2DS2-VASc score.
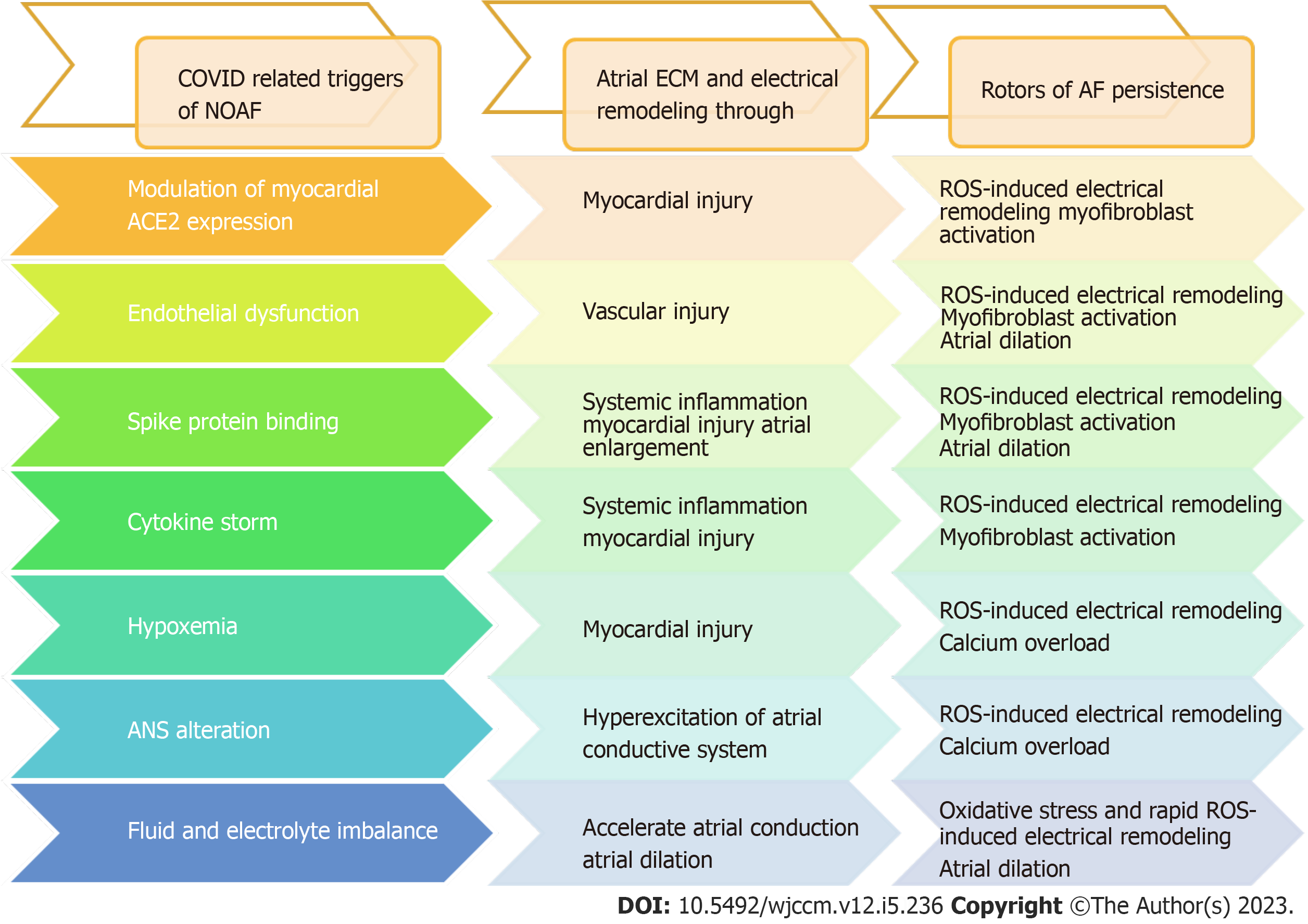
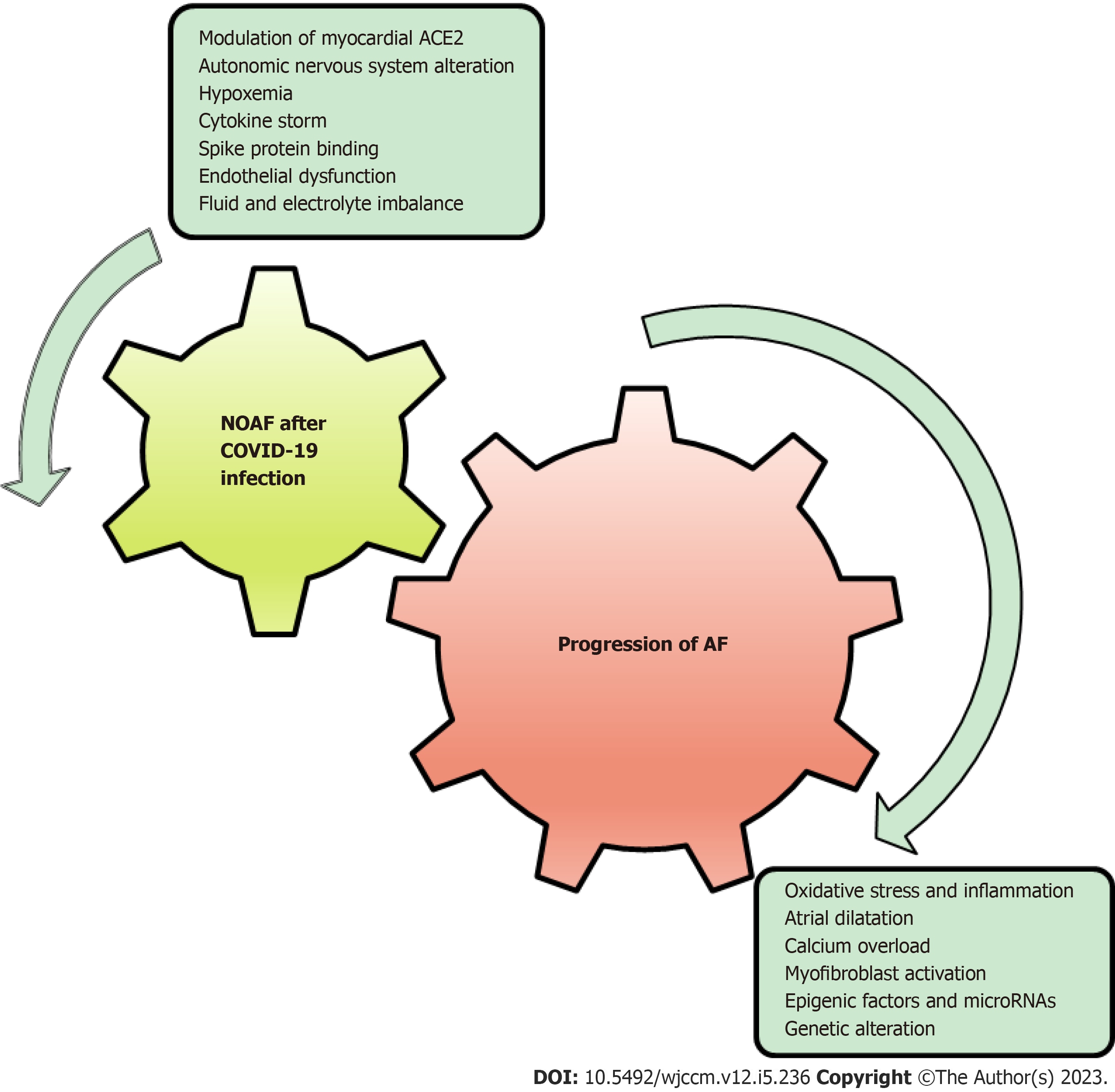
COVID-19-related NOAF is still not well studied. Mechanisms involved in the development of NOAF after COVID-19 infection could potentially lead to atrial remodeling and fibrosis, which can further perpetuate AF, as shown in Figure 1. Clinical studies suggested that the majority of the patients with AF remain paroxysmal, though the electrophysiological substrate underlying AF in those who progress to sustained forms may differ from that of those who remain paroxysmal[62]. However, in this study, a sizable overlap was noted in mechanism inducing COVID-19 associated NOAF and those persisting AF.
The mechanism involved in the progression of AF is a constellation of oxidative stress, inflammation, atrial dilatation, calcium overload, and myofibroblast activation, all of which are likely to be involved in one way or another in AF-induced ECM and electrical remodeling[7,8]. Interestingly, many of these mechanisms seem to be mutual with suggestive models of COVID-19-related NOAF (Figure 2), and looking back to the mutual mechanisms of persistent AF and COVID-19-related NOAF could explain the possible risk of developing persistent AF after NOAF in COVID-19 patients (Table 1 and Figure 2).
| COVID-19-related NOAF | Persistent AF | |
| Etiology and pathophysiology | (1) Diminished availability of ACE-2 receptors contributes to myocardial hypertrophy, vasoconstriction, ROS production, oxidative stress, tissue inflammation, and fibrosis, all of which play a role in the development of AF; (2) Endothelial dysfunction leads to increased vascular permeability and leakage culminating in an overproduction of ROS leading to structural and electrical remodeling predisposing to AF; (3) CD147- and myocyte’s sialic acid-spike protein interaction upregulate the expression of several cytokines and ROS that induce extracellular matrix degradation, cardiac remodeling, and fibrosis; (4) Excessive release of proinflammatory cytokines in cytokine storm leads to ROS production, progressive myocardial cell apoptosis or necrosis, which may lead to conduction disturbances leading to AF; (5) Impaired gas exchanges and intrathoracic pressure swings lead to cardiomyocyte injury and increased frequency of premature atrial beats and induce AF; (6) ANS alteration: SNS-mediated calcium influx increases the frequency of delayed afterdepolarization and triggers AP; PNS activation mediated by intrathoracic pressure swing leads to shortening of right atrial ERP, and APD both induce AF; and (7) Sodium and water resorption increases blood pressure and excretion of potassium increase the resting membrane and enhances depolarization predisposing to AF | Steady generation of ROS triggered by sustained high-electrical activity, followed by intracellular Ca2+ overload together with atrial dilatation, mitochondrial ROS and activation of inflammatory and pro-fibrotic pathways progressively alters gene expression clinically relevant sheep model of persistent AF, leading to myocyte hypertrophy, interstitial fibrosis, and ion channel remodeling, all of which would occur relatively slowly but reach critical levels when AF becomes persistent at a median time of about 2 mo: (1) Oxidative stress by ROS released either by NOX2/4 or mitochondria is the first consequence, the persistence of which leads to shortened APD and RF through reducing rapid L-type Ca2+ current and increasing inward rectifier K+ current promoting the formation and stabilization of rotor that world in a vicious cycle to preserve sustained high electrical activity; and (2) Inflammation leads to profibrotic signaling in response to cardiac injury by promoting fibroblast-to-myofibroblast trans-differentiation leading to either through increased expression of TRP channels or miR-21 resulting in structural remodeling by atrial dilation and fibrosis that maintains AF |
| Risk factors | (1) Older age; (2) A history of myocardial infarction; (3) Renal dysfunction; (4) Raised D-dimer levels; and (5) Hypertension | Risk factors for progression to more persistent forms of AF among patients with paroxysmal AF and varying degrees of CVD per HATCH score is[62]: (1) Heart failure; (2) Older age; (3) Previous transient ischemic attack or stroke; (4) Chronic obstructive pulmonary disease; and (5) Hypertension |
| Outcomes | Among patients hospitalized with COVID-19 infection, 5.4% could develop NOAF. All-cause mortality rates are 45.2% vs 11.9% and MACE is 23.8% vs 6.5% for patients with vs without NOAF[67] | Among patients with persistent AF all-cause mortality rate is 4.41% and MACE is 5.09%[67] |
| Treatment | The initial approach is to enhance the treatment of underlying factors. Hemodynamic instability warrants immediate cardioversion, provided that the risk of embolism is low | Hemodynamic instability warrants immediate cardioversion provided that the risk of embolism is low[15] |
| Rate control therapy is preferred over rhythm control unless hemodynamic instability warrants the addition of rhythm control e.g., with amiodarone | A similar efficacy of rate vs rhythm control in all-cause mortality and MACE had been noted. Thus, current guidelines recommend an individualized decision taking into consideration that a rhythm control is most likely to fail in patients with long-term persistent AF (> 1 yr), in whom atrial substrate alteration is greatest | |
| Anticoagulation: Unfractionated heparin, LMWH is safe to use. Use DOACs with caution as interact with some antiviral medications. VKAs induce a state of vitamin K deficiency that could potentially influence susceptibility to contracting COVID-19 | The choice of anticoagulation should be individualized based on the patient’s comorbidities, like other indications for anticoagulation and renal function |
In the working model of AF perpetuation by Jalife and Kaur[8], oxidative stress and ROS are the cornerstones of main
Nevertheless, Ca2+ overload, together with atrial dilatation, mitochondrial ROS, and activation of inflammatory and pro-fibrotic pathways, progressively alters gene expression. The eventual outcomes of these persistent alterations entail myocyte hypertrophy, interstitial fibrosis, and ion channel remodeling. When these processes collectively escalate to a critical threshold, it could lead to the persistence of AF. In an animal study, after two months of tachypacing, the arrhy
In addition to Ang II, sustained AF is fostered by the release of proinflammatory cytokines and tissue injury mediators such as TNF-α, IL-6, and IL-8. While the initial purpose of this cascade is to facilitate a beneficial “self-destroy and rebuild” process[66], its continuous activation is a widely recognized initiator of fibroblast-to-myofibroblast trans
Conversely, potential risk factors associated with COVID-19-related NOAF, such as advanced age, hypertension, and a previous myocardial infarction, exhibit resemblances to independent factors that are linked with the progression toward persistent AF[62].
Progression from paroxysmal to more sustained forms of AF is associated with increased adverse events, including thromboembolic events, although the long-term outcomes of COVID-19-related NOAF infection are unknown (Figure 1)[67]. Early recognition of COVID-19-related NOAF is essential due to the high mortality risk associated with it. It is unknown if the management of COVID-19-related NOAF should follow the same pattern as routine management of paroxysmal or persistent AF. The disturbed coagulation system resulting from COVID-19 infection appears to elevate the potential for thromboembolic events in individuals with NOAF, although this necessitates additional research and con
While the conclusions drawn from this review are limited due to its non-experimental nature, it is evident that among various factors contributing to the development of COVID-19-related NOAF, some have the potential to perpetuate AF. These factors include modulation of myocardial ACE2 expression, spike protein binding, cytokine storm, endothelial dysfunction, increased permeability, and hypoxemia, which have the potential to induce atrial, ECM, or electrical remodeling, thereby perpetuating AF. To gain a more comprehensive understanding, further fundamental studies are required to explore the interplay between these factors. Additionally, prospective long-term studies are necessary to investigate the outcomes of patients who develop NOAF after experiencing COVID-19 infection in the long run (Figure 1).
Among several mechanisms that contribute to COVID-19-related NOAF, those exerting oxidative stress, such as modu
| 1. | Miyasaka Y, Barnes ME, Bailey KR, Cha SS, Gersh BJ, Seward JB, Tsang TS. Mortality trends in patients diagnosed with first atrial fibrillation: a 21-year community-based study. J Am Coll Cardiol. 2007;49:986-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 332] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 2. | World Health Organization. WHO Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020. [cited 20 March 2023]. Available from: https://www.who.int/director-general/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020. |
| 3. | Sampath S, Khedr A, Qamar S, Tekin A, Singh R, Green R, Kashyap R. Pandemics Throughout the History. Cureus. 2021;13:e18136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Gopinathannair R, Merchant FM, Lakkireddy DR, Etheridge SP, Feigofsky S, Han JK, Kabra R, Natale A, Poe S, Saha SA, Russo AM. COVID-19 and cardiac arrhythmias: a global perspective on arrhythmia characteristics and management strategies. J Interv Card Electrophysiol. 2020;59:329-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 149] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 5. | World Health Organization. WHO Coronavirus (COVID-19) Dashboard. [cited 20 March 2023]. Available from: https://covid19.who.int/?adgroupsurvey={adgroupsurvey}. |
| 6. | Qian J, Kuang L, Chen F, Liu X, Che L. Prognosis and management of new-onset atrial fibrillation in critically ill patients. BMC Cardiovasc Disord. 2021;21:231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Corradi D. Atrial fibrillation from the pathologist's perspective. Cardiovasc Pathol. 2014;23:71-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Jalife J, Kaur K. Atrial remodeling, fibrosis, and atrial fibrillation. Trends Cardiovasc Med. 2015;25:475-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 229] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 9. | Sandhu RK, Conen D, Tedrow UB, Fitzgerald KC, Pradhan AD, Ridker PM, Glynn RJ, Albert CM. Predisposing factors associated with development of persistent compared with paroxysmal atrial fibrillation. J Am Heart Assoc. 2014;3:e000916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Llitjos JF, Leclerc M, Chochois C, Monsallier JM, Ramakers M, Auvray M, Merouani K. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18:1743-1746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 850] [Cited by in RCA: 930] [Article Influence: 155.0] [Reference Citation Analysis (0)] |
| 11. | Pardo Sanz A, Salido Tahoces L, Ortega Pérez R, González Ferrer E, Sánchez Recalde Á, Zamorano Gómez JL. New-onset atrial fibrillation during COVID-19 infection predicts poor prognosis. Cardiol J. 2021;28:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Chaudhari PB, Banga A. Writing strategies for improving the access of medical literature. World J Exp Med. 2023;13:50-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (3)] |
| 13. | Ergün B, Ergan B, Sözmen MK, Küçük M, Yakar MN, Cömert B, Gökmen AN, Yaka E. New-onset atrial fibrillation in critically ill patients with coronavirus disease 2019 (COVID-19). J Arrhythm. 2021;37:1196-1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Kerr CR, Humphries KH, Talajic M, Klein GJ, Connolly SJ, Green M, Boone J, Sheldon R, Dorian P, Newman D. Progression to chronic atrial fibrillation after the initial diagnosis of paroxysmal atrial fibrillation: results from the Canadian Registry of Atrial Fibrillation. Am Heart J. 2005;149:489-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 249] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 15. | January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071-2104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1393] [Cited by in RCA: 1612] [Article Influence: 134.3] [Reference Citation Analysis (0)] |
| 16. | Saksena S, Hettrick DA, Koehler JL, Grammatico A, Padeletti L. Progression of paroxysmal atrial fibrillation to persistent atrial fibrillation in patients with bradyarrhythmias. Am Heart J. 2007;154:884-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Li Z, Shao W, Zhang J, Ma J, Huang S, Yu P, Zhu W, Liu X. Prevalence of Atrial Fibrillation and Associated Mortality Among Hospitalized Patients With COVID-19: A Systematic Review and Meta-Analysis. Front Cardiovasc Med. 2021;8:720129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 18. | Rosenblatt AG, Ayers CR, Rao A, Howell SJ, Hendren NS, Zadikany RH, Ebinger JE, Daniels JD, Link MS, de Lemos JA, Das SR. New-Onset Atrial Fibrillation in Patients Hospitalized With COVID-19: Results From the American Heart Association COVID-19 Cardiovascular Registry. Circ Arrhythm Electrophysiol. 2022;15:e010666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 19. | Scialo F, Daniele A, Amato F, Pastore L, Matera MG, Cazzola M, Castaldo G, Bianco A. ACE2: The Major Cell Entry Receptor for SARS-CoV-2. Lung. 2020;198:867-877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 352] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 20. | Seguin P, Signouret T, Laviolle B, Branger B, Mallédant Y. Incidence and risk factors of atrial fibrillation in a surgical intensive care unit. Crit Care Med. 2004;32:722-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 115] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Singh R, Kashyap R, Hutton A, Sharma M, Surani S. A Review of Cardiac Complications in Coronavirus Disease 2019. Cureus. 2020;12:e8034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Musikantow DR, Turagam MK, Sartori S, Chu E, Kawamura I, Shivamurthy P, Bokhari M, Oates C, Zhang C, Pumill C, Malick W, Hashemi H, Ruiz-Maya T, Hadley MB, Gandhi J, Sperling D, Whang W, Koruth JS, Langan MN, Sofi A, Gomes A, Harcum S, Cammack S, Ellsworth B, Dukkipati SR, Bassily-Marcus A, Kohli-Seth R, Goldman ME, Halperin JL, Fuster V, Reddy VY. Atrial Fibrillation in Patients Hospitalized With COVID-19: Incidence, Predictors, Outcomes, and Comparison to Influenza. JACC Clin Electrophysiol. 2021;7:1120-1130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 23. | Gawałko M, Kapłon-Cieślicka A, Hohl M, Dobrev D, Linz D. COVID-19 associated atrial fibrillation: Incidence, putative mechanisms and potential clinical implications. Int J Cardiol Heart Vasc. 2020;30:100631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 24. | Shah A, Kashyap R, Tosh P, Sampathkumar P, O'Horo JC. Guide to Understanding the 2019 Novel Coronavirus. Mayo Clin Proc. 2020;95:646-652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 25. | Lombardi AF, Afsahi AM, Gupta A, Gholamrezanezhad A. Severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), influenza, and COVID-19, beyond the lungs: a review article. Radiol Med. 2021;126:561-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 26. | Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020;5:831-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1095] [Cited by in RCA: 1265] [Article Influence: 210.8] [Reference Citation Analysis (2)] |
| 27. | Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2333] [Cited by in RCA: 2088] [Article Influence: 348.0] [Reference Citation Analysis (0)] |
| 28. | Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116:1097-1100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 674] [Cited by in RCA: 806] [Article Influence: 134.3] [Reference Citation Analysis (0)] |
| 29. | McEwan PE, Gray GA, Sherry L, Webb DJ, Kenyon CJ. Differential effects of angiotensin II on cardiac cell proliferation and intramyocardial perivascular fibrosis in vivo. Circulation. 1998;98:2765-2773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 103] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | South AM, Diz DI, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318:H1084-H1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 527] [Article Influence: 87.8] [Reference Citation Analysis (0)] |
| 31. | van de Veerdonk FL, Netea MG, van Deuren M, van der Meer JW, de Mast Q, Brüggemann RJ, van der Hoeven H. Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome. Elife. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 210] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 32. | Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20:389-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 583] [Cited by in RCA: 809] [Article Influence: 134.8] [Reference Citation Analysis (0)] |
| 33. | Corban MT, Toya T, Ahmad A, Lerman LO, Lee HC, Lerman A. Atrial Fibrillation and Endothelial Dysfunction: A Potential Link? Mayo Clin Proc. 2021;96:1609-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 34. | Guazzi M, Arena R. Endothelial dysfunction and pathophysiological correlates in atrial fibrillation. Heart. 2009;95:102-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Qing E, Hantak M, Perlman S, Gallagher T. Distinct Roles for Sialoside and Protein Receptors in Coronavirus Infection. mBio. 2020;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 36. | Colston JT, Boylston WH, Feldman MD, Jenkinson CP, de la Rosa SD, Barton A, Trevino RJ, Freeman GL, Chandrasekar B. Interleukin-18 knockout mice display maladaptive cardiac hypertrophy in response to pressure overload. Biochem Biophys Res Commun. 2007;354:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Venkatesan B, Valente AJ, Prabhu SD, Shanmugam P, Delafontaine P, Chandrasekar B. EMMPRIN activates multiple transcription factors in cardiomyocytes, and induces interleukin-18 expression via Rac1-dependent PI3K/Akt/IKK/NF-kappaB andMKK7/JNK/AP-1 signaling. J Mol Cell Cardiol. 2010;49:655-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 38. | Hu W, Xie J, Zhu T, Meng G, Wang M, Zhou Z, Guo F, Chen H, Wang Z, Wang S, Liu H, Jiang H. Serum N-Acetylneuraminic Acid Is Associated with Atrial Fibrillation and Left Atrial Enlargement. Cardiol Res Pract. 2020;2020:1358098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Unudurthi SD, Luthra P, Bose RJC, McCarthy JR, Kontaridis MI. Cardiac inflammation in COVID-19: Lessons from heart failure. Life Sci. 2020;260:118482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 40. | Francis Stuart SD, De Jesus NM, Lindsey ML, Ripplinger CM. The crossroads of inflammation, fibrosis, and arrhythmia following myocardial infarction. J Mol Cell Cardiol. 2016;91:114-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 191] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 41. | Madjid M, Vela D, Khalili-Tabrizi H, Casscells SW, Litovsky S. Systemic infections cause exaggerated local inflammation in atherosclerotic coronary arteries: clues to the triggering effect of acute infections on acute coronary syndromes. Tex Heart Inst J. 2007;34:11-18. [PubMed] |
| 42. | Nitsure M, Sarangi B, Shankar GH, Reddy VS, Walimbe A, Sharma V, Prayag S. Mechanisms of Hypoxia in COVID-19 Patients: A Pathophysiologic Reflection. Indian J Crit Care Med. 2020;24:967-970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 43. | Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, Bi Z, Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1374] [Cited by in RCA: 1248] [Article Influence: 208.0] [Reference Citation Analysis (0)] |
| 44. | Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr. 2020;14:247-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 591] [Cited by in RCA: 572] [Article Influence: 95.3] [Reference Citation Analysis (0)] |
| 45. | Linz D, Hohl M, Nickel A, Mahfoud F, Wagner M, Ewen S, Schotten U, Maack C, Wirth K, Böhm M. Effect of renal denervation on neurohumoral activation triggering atrial fibrillation in obstructive sleep apnea. Hypertension. 2013;62:767-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 46. | Gramley F, Lorenzen J, Jedamzik B, Gatter K, Koellensperger E, Munzel T, Pezzella F. Atrial fibrillation is associated with cardiac hypoxia. Cardiovasc Pathol. 2010;19:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 47. | Lazzerini PE, Boutjdir M, Capecchi PL. COVID-19, Arrhythmic Risk, and Inflammation: Mind the Gap! Circulation. 2020;142:7-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 189] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 48. | Denham NC, Pearman CM, Caldwell JL, Madders GWP, Eisner DA, Trafford AW, Dibb KM. Calcium in the Pathophysiology of Atrial Fibrillation and Heart Failure. Front Physiol. 2018;9:1380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 49. | Linz D, Elliott AD, Hohl M, Malik V, Schotten U, Dobrev D, Nattel S, Böhm M, Floras J, Lau DH, Sanders P. Role of autonomic nervous system in atrial fibrillation. Int J Cardiol. 2019;287:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 50. | Malladi S, Hamid K, Pendyala NC, Veerapaneni V, Deliwala S, Dubre D, Elian SA, Singh A. Management of stable coronary artery disease and atrial fibrillation with anti-thrombotic therapy: A systematic review and meta-analysis. Medicine (Baltimore). 2021;100:e27498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Chen Q, Xu L, Dai Y, Ling Y, Mao J, Qian J, Zhu W, Di W, Ge J. Cardiovascular manifestations in severe and critical patients with COVID-19. Clin Cardiol. 2020;43:796-802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 52. | Fanaroff AC, Li S, Marquis-Gravel G, Giri J, Lopes RD, Piccini JP, Wang TY. Atrial Fibrillation and Coronary Artery Disease: A Long-Term Perspective on the Need for Combined Antithrombotic Therapy. Circ Cardiovasc Interv. 2021;14:e011232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 53. | Nguyen TN, Qureshi MM, Klein P, Yamagami H, Mikulik R, Czlonkowska A, Abdalkader M, Sedova P, Sathya A, Lo HC, Mansour OY, Vanguru HR, Lesaine E, Tsivgoulis G, Loochtan AI, Demeestere J, Uchino K, Inoa V, Goyal N, Charidimou A, Siegler JE, Yaghi S, Aguiar de Sousa D, Mohammaden MH, Haussen DC, Kristoffersen ES, Lereis VP, Scollo SD, Campbell BCV, Ma A, Thomas JO, Parsons MW, Singhal S, Slater LA, Tomazini Martins R, Enzinger C, Gattringer T, Rahman A, Bonnet T, Ligot N, De Raedt S, Lemmens R, Vanacker P, Vandervorst F, Conforto AB, Hidalgo RCT, de Oliveira Neves L, Martins RT, Mora Cuervo DL, Rebello LC, Santiago IB, Lameirinhas da Silva I, Sakelarova T, Kalpachki R, Alexiev F, Catanese L, Cora EA, Goyal M, Hill MD, Kelly ME, Khosravani H, Lavoie P, Peeling L, Pikula A, Rivera R, Chen HS, Chen Y, Huo X, Miao Z, Yang S, Bedekovic MR, Bralic M, Budincevic H, Corredor-Quintero AB, Lara-Sarabia OE, Cabal M, Tenora D, Fibrich P, Herzig R, Hlaváčová H, Hrabanovska E, Hlinovsky D, Jurak L, Kadlcikova J, Karpowicz I, Klecka L, Kovar M, Lauer D, Neumann J, Palouskova H, Reiser M, Rekova P, Rohan V, Skoda O, Škorňa M, Sobotková L, Sramek M, Zakova L, Christensen H, Drenck N, Iversen HK, Truelsen TC, Wienecke T, Sobh K, Ylikotila P, Alpay K, Strbian D, Bernady P, Casenave P, Dan M, Faucheux JM, Gentric JC, Magro E, Sabben C, Reiner P, Rouanet F, Bohmann FO, Boskamp S, Mbroh J, Nagel S, Nolte CH, Ringleb PA, Rosenkranz M, Poli S, Thomalla G, Karapanayiotides T, Koutroulou I, Kargiotis O, Palaiodimou L, Barrientos Guerra JD, Huded V, Menon B, Nagendra S, Prajapati C, Sylaja PN, Krishna Pramana NA, Sani AF, Ghoreishi A, Farhoudi M, Hokmabadi ES, Raya TA, Kalmanovich SA, Ronen L, Sabetay SI, Acampa M, Adami A, Castellan L, Longoni M, Ornello R, Renieri L, Bigliani CR, Romoli M, Sacco S, Salmaggi A, Sangalli D, Zini A, Doijiri R, Fukuda H, Fujinaka T, Fujita K, Imamura H, Sakai N, Kanamaru T, Kimura N, Kono R, Miyake K, Sakaguchi M, Sakai K, Sonoda K, Todo K, Miyashita F, Tokuda N, Matsumaru Y, Matsumoto S, Ohara N, Shindo S, Takenobu Y, Yoshimoto T, Toyoda K, Uwatoko T, Yagita Y, Yamada T, Yamamoto N, Yamamoto R, Yazawa Y, Sugiura Y, Waweru PK, Baek JH, Lee SB, Seo KD, Sohn SI, Arsovska AA, Chan YC, Wan Zaidi WA, Jaafar AS, Gongora-Rivera F, Martinez-Marino M, Infante-Valenzuela A, Groppa S, Leahu P, Coutinho JM, Rinkel LA, Dippel DWJ, van Dam-Nolen DHK, Ranta A, Wu TY, Adebayo TT, Bello AH, Nwazor EO, Sunmonu TA, Wahab KW, Ronning OM, Sandset EC, Al Hashmi AM, Ahmad S, Rashid U, Rodriguez-Kadota L, Vences MÁ, Yalung PM, Hao Dy JS, Pineda-Franks MC, Co CO, Brola W, Debiec A, Dorobek M, Karlinski MA, Labuz-Roszak BM, Lasek-Bal A, Sienkiewicz-Jarosz H, Staszewski J, Sobolewski P, Wiacek M, Zielinska-Turek J, Araujo AP, Rocha M, Castro P, Cruz VT, Ferreira PV, Ferreira P, Nunes AP, Fonseca L, Marto JP, Pinho E Melo T, Rodrigues M, Silva ML, Dimitriade A, Falup-Pecurariu C, Hamid MA, Venketasubramanian N, Krastev G, Mako M, Ayo-Martin O, Hernández-Fernández F, Blasco J, Rodríguez-Vázquez A, Cruz-Culebras A, Moniche F, Montaner J, Perez-Sanchez S, García Sánchez MJ, Guillán Rodríguez M, Jood K, Nordanstig A, Mazya MV, Moreira TTP, Bernava G, Beyeler M, Bolognese M, Carrera E, Dobrocky T, Karwacki GM, Keller E, Hsieh CY, Boonyakarnkul S, Churojana A, Aykac O, Ozdemir AÃ, Bajrami A, Senadim S, Hussain SI, John S, Banerjee S, Kwan J, Krishnan K, Lenthall R, Matthews A, Wong K, Zhang L, Altschul D, Asif KS, Bahiru Z, Below K, Biller J, Ruland S, Chaudry SA, Chen M, Chebl A, Cibulka J, Cistrunk L, Clark J, Colasurdo M, Czap A, de Havenon A, D'Amato S, Dharmadhikari S, Grimmett KB, Dmytriw AA, Etherton MR, Ezepue C, Farooqui M, Feske SK, Fink L, Gasimova U, Guzik AK, Hakemi M, Hovingh M, Khan M, Jillela D, Kan PT, Khatri R, Khawaja AM, Khoury NN, Kiley NL, Kim BS, Kolikonda MK, Kuhn AL, Lara S, Linares G, Linfante I, Lukovits TG, Lycan S, Male SS, Maali L, Mancin J, Masoud H, Mohamed GA, Monteiro A, Nahab F, Nalleballe K, Ortega-Gutierrez S, Puri AS, Radaideh Y, Rahangdale RH, Rai A, Ramakrishnan P, Reddy AB, Rojas-Soto DM, Romero JR, Rost NS, Rothstein A, Omran SS, Sheth SA, Siddiqui AH, Starosciak AK, Tarlov NE, Taylor RA, Wang MJ, Wolfe J, Wong KH, Le HV, Nguyen QV, Pham TN, Nguyen TT, Phan HT, Ton MD, Fischer U, Michel P, Strambo D, Martins SO, Zaidat OO, Nogueira RG; and the SVIN COVID-19 Global Stroke Registry. Global Impact of the COVID-19 Pandemic on Stroke Volumes and Cerebrovascular Events: A 1-Year Follow-up. Neurology. 2023;100:e408-e421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 54. | Kardalas E, Paschou SA, Anagnostis P, Muscogiuri G, Siasos G, Vryonidou A. Hypokalemia: a clinical update. Endocr Connect. 2018;7:R135-R146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 188] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 55. | Alfano G, Ferrari A, Fontana F, Perrone R, Mori G, Ascione E, Magistroni R, Venturi G, Pederzoli S, Margiotta G, Romeo M, Piccinini F, Franceschi G, Volpi S, Faltoni M, Ciusa G, Bacca E, Tutone M, Raimondi A, Menozzi M, Franceschini E, Cuomo G, Orlando G, Santoro A, Di Gaetano M, Puzzolante C, Carli F, Bedini A, Milic J, Meschiari M, Mussini C, Cappelli G, Guaraldi G; Modena Covid-19 Working Group (MoCo19). Hypokalemia in Patients with COVID-19. Clin Exp Nephrol. 2021;25:401-409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 56. | Yan T, Zhu S, Xie C, Zhu M, Weng F, Wang C, Guo C. Coronary Artery Disease and Atrial Fibrillation: A Bidirectional Mendelian Randomization Study. J Cardiovasc Dev Dis. 2022;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 57. | Batta A, Hatwal J, Batta A, Verma S, Sharma YP. Atrial fibrillation and coronary artery disease: An integrative review focusing on therapeutic implications of this relationship. World J Cardiol. 2023;15:229-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 74] [Article Influence: 24.7] [Reference Citation Analysis (10)] |
| 58. | Rattanawong P, Shen W, El Masry H, Sorajja D, Srivathsan K, Valverde A, Scott LR. Guidance on Short-Term Management of Atrial Fibrillation in Coronavirus Disease 2019. J Am Heart Assoc. 2020;9:e017529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 59. | Mujović N, Dobrev D, Marinković M, Russo V, Potpara TS. The role of amiodarone in contemporary management of complex cardiac arrhythmias. Pharmacol Res. 2020;151:104521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 60. | Kang Y, Chen T, Mui D, Ferrari V, Jagasia D, Scherrer-Crosbie M, Chen Y, Han Y. Cardiovascular manifestations and treatment considerations in COVID-19. Heart. 2020;106:1132-1141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 266] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 61. | Dofferhoff ASM, Piscaer I, Schurgers LJ, Visser MPJ, van den Ouweland JMW, de Jong PA, Gosens R, Hackeng TM, van Daal H, Lux P, Maassen C, Karssemeijer EGA, Vermeer C, Wouters EFM, Kistemaker LEM, Walk J, Janssen R. Reduced Vitamin K Status as a Potentially Modifiable Risk Factor of Severe Coronavirus Disease 2019. Clin Infect Dis. 2021;73:e4039-e4046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 62. | de Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen RJ, van den Heijkant AC, Allessie MA, Crijns HJ. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 540] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 63. | Dobrev D, Nattel S. New insights into the molecular basis of atrial fibrillation: mechanistic and therapeutic implications. Cardiovasc Res. 2011;89:689-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 64. | Mattson MP, Chan SL. Calcium orchestrates apoptosis. Nat Cell Biol. 2003;5:1041-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 337] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 65. | Martins RP, Kaur K, Hwang E, Ramirez RJ, Willis BC, Filgueiras-Rama D, Ennis SR, Takemoto Y, Ponce-Balbuena D, Zarzoso M, O'Connell RP, Musa H, Guerrero-Serna G, Avula UM, Swartz MF, Bhushal S, Deo M, Pandit SV, Berenfeld O, Jalife J. Dominant frequency increase rate predicts transition from paroxysmal to long-term persistent atrial fibrillation. Circulation. 2014;129:1472-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 66. | Xu X, Lai Y, Hua ZC. Apoptosis and apoptotic body: disease message and therapeutic target potentials. Biosci Rep. 2019;39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 664] [Article Influence: 94.9] [Reference Citation Analysis (0)] |
| 67. | Link MS, Giugliano RP, Ruff CT, Scirica BM, Huikuri H, Oto A, Crompton AE, Murphy SA, Lanz H, Mercuri MF, Antman EM, Braunwald E; ENGAGE AF-TIMI 48 Investigators. Stroke and Mortality Risk in Patients With Various Patterns of Atrial Fibrillation: Results From the ENGAGE AF-TIMI 48 Trial (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction 48). Circ Arrhythm Electrophysiol. 2017;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 158] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: Society of Critical Care Medicine; American College of CHEST Physician.
Specialty type: Critical care medicine
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Batta A, India; Mahmoud MZ, Saudi Arabia; Yu L, Singapore S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ