Published online Mar 9, 2023. doi: 10.5492/wjccm.v12.i2.63
Peer-review started: October 14, 2022
First decision: November 22, 2022
Revised: December 9, 2022
Accepted: February 1, 2023
Article in press: February 1, 2023
Published online: March 9, 2023
Processing time: 143 Days and 18.3 Hours
Several studies of spontaneous intracerebral hemorrhage (SICH) patients have shown apoptotic changes in brain samples after hematoma evacuation. However, there have been no data on the association between blood concentrations of sol
To determine whether there is an association between blood sFas concentrations and SICH patient mortality.
We included patients with severe and supratentorial SIH. Severe was defined as having Glasgow Coma Scale < 9. We determined serum sFas concentrations at the time of severe SICH diagnosis.
We found that non-surviving patients (n = 36) compared to surviving patients (n = 39) had higher ICH score (P = 0.001), higher midline shift (P = 0.004), higher serum sFas concentrations (P < 0.001), and lower rate of early hematoma evacuation (P = 0.04). Multiple logistic regression analysis showed an association between serum sFas concentrations and 30-d mortality (odds ratio = 1.070; 95% confidence interval = 1.014-1.129; P = 0.01) controlling for ICH score, midline shift, and early hematoma evacuation.
The association of blood sFas concentrations and SICH patient mortality is a novel finding in our study.
Core Tip: Several studies of spontaneous intracerebral hemorrhage (SICH) patients have shown apoptotic changes in brain samples after hematoma evacuation. However, there are no data on the association of blood concentrations of soluble fas (sFas) (the main surface death receptor of the extrinsic apoptosis pathway) with SICH patient prognosis. The objective of our study was to determine whether there is an association between blood sFas concentrations and SICH patient mortality. The association of blood sFas concentrations with SICH patient mortality is a novel finding of this study.
- Citation: Lorente L, Martín MM, Pérez-Cejas A, Ramos-Gómez L, Solé-Violan J, Cáceres JJ, Jiménez A, González-Rivero AF. Elevated soluble fas blood concentrations in patients dying from spontaneous intracerebral hemorrhage. World J Crit Care Med 2023; 12(2): 63-70
- URL: https://www.wjgnet.com/2220-3141/full/v12/i2/63.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v12.i2.63
Spontaneous intracerebral hemorrhage (SICH) leads to many disabilities and deaths annually worldwide[1]. Several studies of SICH patients undergoing surgical hematoma evacuation have shown apoptotic changes in brain samples from areas of hematoma compared with areas of the healthy brain[2-8]. Apoptosis can be activated by the release of mitochondrial cytochrome c into the cytoplasm (named the mitochondrial or intrinsic apoptosis pathway) or by the binding of a surface death receptor to its ligand (named extrinsic apoptosis pathway). The main surface death receptor is Fas, and its ligand is the FasL[2-8]. When binding between Fas and FasL occurs, a death signal appears and the the extrinsic pathway is activated. This death signal is responsible for the activation of caspase-8 (initiator caspase in the extrinsic apoptosis pathway), which leads to the activation of caspase-3 (the main effector caspase in extrinsic and intrinsic apoptosis pathways). Finally, caspase-3 is responsible for cell death[2-8]. Lower plasma Fas concentrations have been found in SICH patients than in healthy controls[9]. However, there are no data on the association between blood Fas concentrations and SICH patient prognosis.
Thus, the objective of this study was to determine whether there is an association between blood Fas concentrations and SICH patient mortality.
The following five Spanish Intensive Care Units recruited patients from 2016 to 2017 in this observational and prospective study: H General de La Palma, H Insular de Las Palmas de Gran Canaria, H Universitario de Canarias (San Cristóbal de La Laguna), H Universitario Nuestra Señora de Candelaria (Santa Cruz de Tenerife), and H Universitario Dr. Negrín (Las Palmas de Gran Canaria). The study was performed with approval of the research ethic committee of each hospital, and written informed consent was provided by a family member of each patient.
We recruited 75 patients (29 females and 46 males) with severe and supratentorial SICH. Severe was defined as Glasgow coma scale (GCS) < 9[10]. We excluded patients aged < 18 years, pregnancy, malignant disease, or limited interventions order at hospital admission. In addition, we excluded patients with traumatic hemorrhage, hemorrhagic transformation of brain infarction, infratentorial hemorrhage or primary intraventricular hemorrhage (IVH). We also excluded patients in whom SICH was due to aneurysm, arteriovenous malformation, anticoagulant treatment, or fibrinolytic treatment.
We considered that SICH was due to hypertension if the patient was hypertensive and had no other cause of SICH. We considered that SICH was due to amyloid angiopathy if the patient was not hypertensive and any other cause of SICH was recorded. We considered that SICH was due to arteriovenous malformation or aneurysm if some of those findings were shown in computed tomography angiography. We considered that SICH was due to anticoagulant treatment or fibrinolytic treatment if some of those drugs were administered to the patient.
We registered the following data: Age, sex, GCS, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, fibrinogen, international normalized ratio, platelets, activated partial throm
We collected serum samples at the time of severe SICH diagnosis and froze the samples at -80 °C. We determined all soluble fas (sFas) concentrations at the same time with a Human Fas enzyme-linked immunoassay (ELISA) Kit (Elabscience, Houston, TX, United States), which had 19 pg/mL as the detection limit and < 6% as the intra- and inter-assay variation coefficients. This kit uses sandwich ELISA as the method. The micro ELISA plate provided in this kit was pre-coated with an antibody specific to human Fas. The optical density (OD) was measured with spectrophotometry at a wavelength of 450 ± 2 nm. The OD value was proportional to the concentration of human Fas. The concentration of human Fas in samples was calculated by comparing the OD of the samples with the standard curve. Some of those patients were included in our previous publication determining serum sFasL concentrations, and serum sFas concentrations were determined in the current work[14].
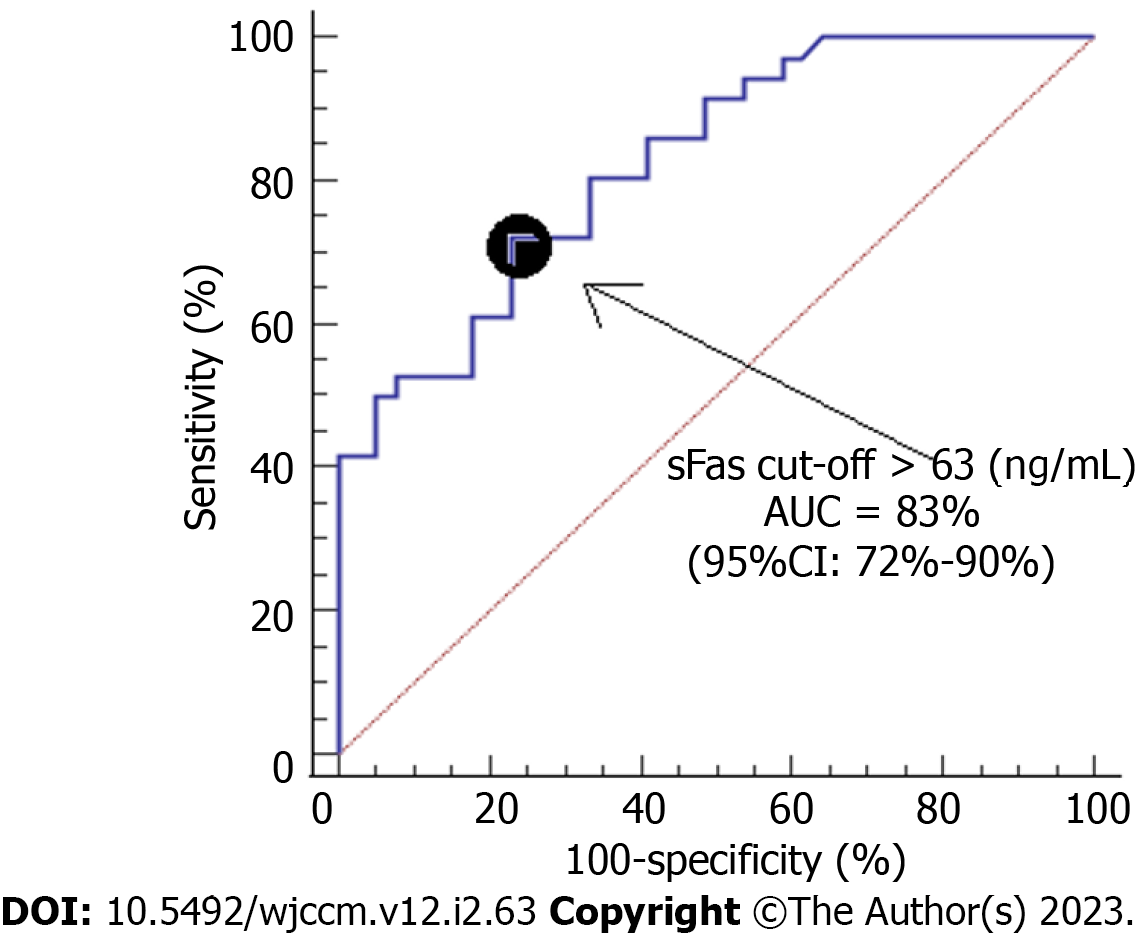
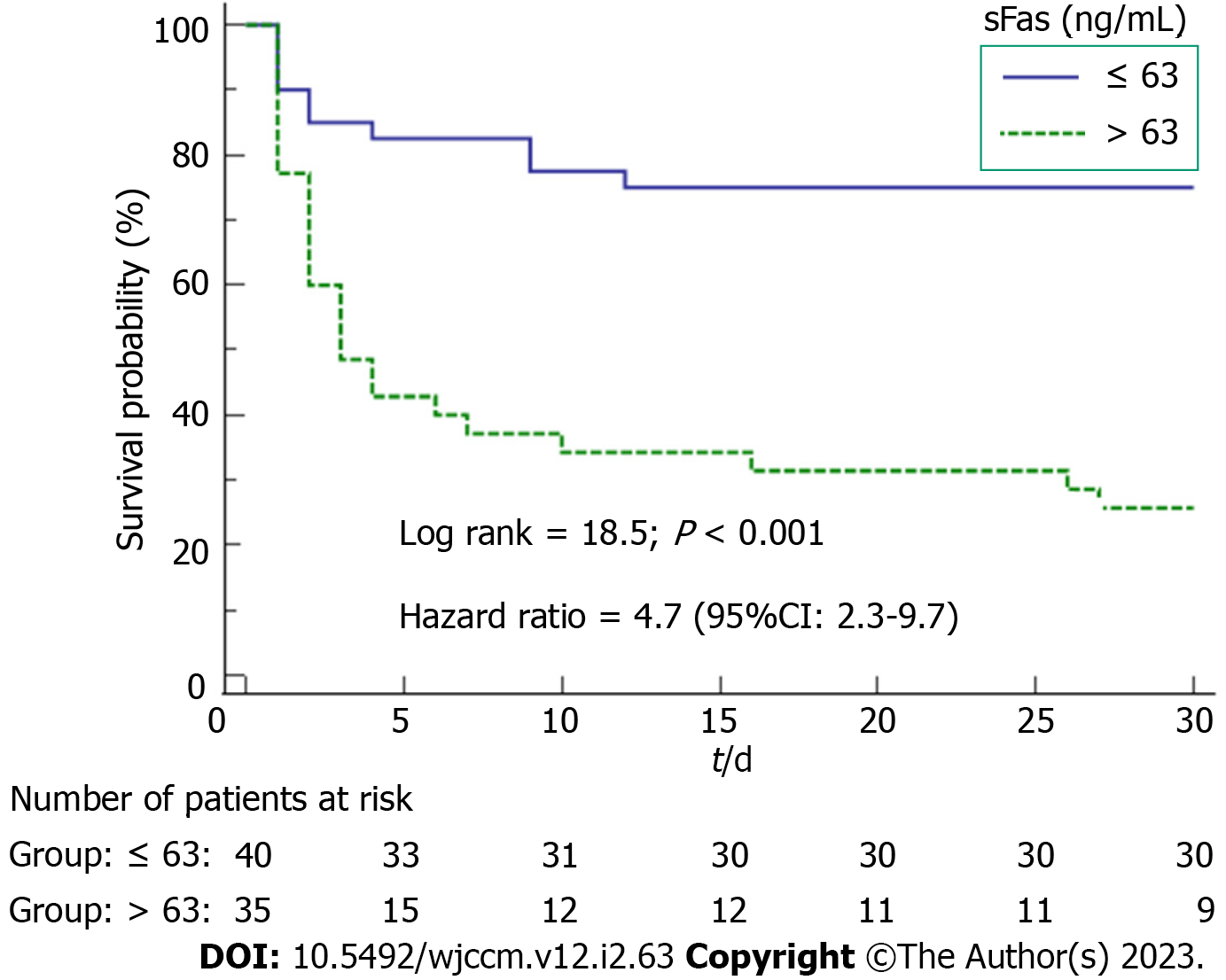
We described continuous variables as medians (interquartile ranges) and categorical variables as frequencies (percentages). We compared continuous variables by the Wilcoxon-Mann-Whitney test and categorical variables by the chi-square test. The estimation of 30-d mortality prediction for serum sFas concentrations was performed using receiver operating characteristic analysis. We constructed Kaplan-Meier curves of 30-d mortality in patients with serum sFas concentrations higher and lower than 63 ng/mL (which was the Youden J index). We analyzed the possible association of serum Fas concentrations and SICH patient mortality controlling for ICH score, midline shift, and early hematoma evacuation. Statistical analyses were performed using LogXact 4.1 (Cytel Co., Cambridge, MA, United States) and SPSS 17.0 (SPSS Inc., Chicago, IL, United States), and P < 0.05 was considered statistically significant.
We found that non-surviving patients (n = 36) with respect to surviving patients (n = 39) had higher age (P = 0.001), APACHE-II score (P < 0.001), ICH score (P = 0.001), ICH volume (P = 0.04), midline shift (P = 0.004), and serum sFas concentrations (P < 0.001). In addition, non-surviving patients with respect to surviving patients had lower GCS (P < 0.001) and lower rate of early hematoma evacuation (P = 0.04) (Table 1).
| Variable | Surviving, n = 39 | Non-surviving, n = 36 | P value |
| Sex, n (%) | 0.35 | ||
| Female | 13 (33.3) | 16 (44.4) | |
| Male | 26 (66.6) | 20 (55.6) | |
| Age in yr, n (median P 25-75) | 57 (51-63) | 68 (57-75) | 0.001 |
| Cause of SIH, n (%) | 0.99 | ||
| Hypertension | 35 (89.7) | 33 (91.7) | |
| Amyloid angiopathy | 4 (10.3) | 3 (8.3) | |
| Volume of SIH in cc, n (median P 25-75) | 41 (23-66) | 72 (29-98) | 0.04 |
| Transtentorial herniation, n (%) | 2 (5.1) | 2 (5.6) | 0.99 |
| Hydrocephalus, n (%) | 17 (43.6) | 23 (63.9) | 0.11 |
| Intraventricular hemorrhage, n (%) | 13 (33.3) | 20 (56.6) | 0.07 |
| Site of SIH, n (%) | 0.91 | ||
| Lobar | 24 (61.5) | 23 (63.9) | |
| Basal ganglia | 7 (17.9) | 7 (19.4) | |
| Thalamus | 8 (20.5) | 6 (16.7) | |
| Midline shift in mm, n (median P 25-75) | 5 (0-8) | 10 (5-15) | 0.004 |
| GCS, n (median P 25-75) | 8 (6-8) | 4 (3-7) | < 0.001 |
| APACHE-II score, n (median P 25-75) | 19 (15-21) | 25 (23-28) | < 0.001 |
| ICH score, n (median P 25-75) | 2 (1-3) | 3 (2-4) | < 0.001 |
| aPTT in s, n (median P 25-75) | 29 (26-30) | 29 (24-33) | 0.28 |
| Platelets as × 103/mm3, n (median P 25-75) | 208 (161-262) | 200 (143-259) | 0.83 |
| Fibrinogen in mg/dL, n (median P 25-75) | 402 (311-626) | 487 (366-542) | 0.42 |
| INR, n (median P 25-75) | 1.07 (0.94-1.21) | 1.09 (0.90-1.21) | 0.76 |
| Lactic acid in mmol/L, n (median P 25-75) | 1.60 (0.90-2.10) | 1.75 (1.20-2.70) | 0.07 |
| Glycemia in g/dL, n (median P 25-75) | 140 (120-194) | 166 (133-211) | 0.06 |
| Sodium in mEq/L, n (median P 25-75) | 140 (137-143) | 139 (136-145) | 0.79 |
| Creatinine in mg/dL, n (median P 25-75) | 0.80 (0.60-0.91) | 0.80 (0.60-1.10) | 0.90 |
| PaO2/FIO2 ratio, n (median P 25-75) | 296 (194-375) | 270 (214-387) | 0.83 |
| Early hematoma evacuation, n (%) | 15 (38.5) | 6 (16.7) | 0.04 |
| sFas in ng/mL, n (median P 25-75) | 22 (17-63) | 141 (49-286) | < 0.001 |
We found that serum sFas concentrations had an area under the curve for mortality prediction of 83% (95% confidence interval [CI] = 72%-90%; P < 0.001) (Figure 1). The mortality prediction for serum sFas concentrations cutoff point of 63 ng/mL had sensitivity of 72% (55%-86%), specificity of 77% (61%-89%), negative likelihood ratio of 0.4 (0.2-0.6), positive likelihood ratio of 3.1 (1.7-5.7), negative predictive value of 75% (63%-84%), and positive predictive value of 74% (61%-84%). We found in the Kaplan-Meier analysis that patients with serum sFas concentrations > 63 ng/mL showed higher death risk (hazard ratio = 4.7; 95%CI = 2.3-97; P < 0.001) (Figure 2). Multiple logistic regression analysis showed an association between serum sFas concentrations and 30-d mortality (odds ratio = 1.070; 95%CI = 1.014-1.129; P = 0.01) controlling for ICH score, midline shift, and early hematoma evacuation (Table 2).
| Variable | Odds ratio | 95%CI | P value |
| Serum sFas in ng/mL | 1.070 | 1.014-1.129 | 0.01 |
| ICH score as points | 47.71 | 2.24-1012.34 | 0.01 |
| Midline shift in mm | 1.758 | 1.133-2.727 | 0.01 |
| Early hematoma evacuation as yes vs no | 0.002 | 0.001-0.210 | 0.01 |
Several studies of SICH patients have shown apoptotic changes in brain samples after hematoma evacuation[2-8]. However, there are no data on the association of blood concentrations of sFas with SICH patient prognosis. Our study reports the novel findings of the existence of higher serum sFas concentrations in non-survivor than survivor SICH patients and the existence of an association between serum sFas concentrations and 30-d mortality controlling SICH severity and early hematoma evacuation.
Fas is the main surface death receptor of the apoptosis extrinsic pathway. After binding to its specific receptor (FasL), a death signal appears that is responsible for the activation of caspase-8 activation[2-8]. Afterwards, when this initiator caspase of the apoptosis extrinsic pathway (caspase-8) is activated, the activation of executor caspase (caspase-3) occurs. Finally, activation of this executor caspase is responsible for apoptotic cellular death[2-8]. Thus, it is possible that the findings of our study showing higher serum sFas concentrations in non-survivor with respect to survivor patients may reflect a lower apoptosis degree due to lower activation of the apoptosis extrinsic pathway in survivor patients. However, a limitation of our study was the fact that apoptotic brain damage was not assessed. In addition, the absence of serum sFas concentrations during patient evolution and in healthy subjects were other limitations. A promising finding is that the administration of Fas/FasL system inhibitors is associated with a reduction of neuronal cell death in rat models of brain ischemia[15-17]. Therefore, we believe that the findings from our study showing higher serum sFas concentrations in non-survivor with respect to survivor SICH patients and those findings from brain ischemia animal models showing the reduction of neuronal cell death using Fas/FasL system inhibitors could motivate research on the Fas/FasL system and its modulation in SICH patients.
The association of blood sFas concentrations and SICH patient mortality is a novel finding in our study.
Several studies of spontaneous intracerebral hemorrhage (SICH) patients have shown apoptotic changes in brain samples after hematoma evacuation.
There are no data on the association of blood concentrations of soluble fas (sFas) (the main surface death receptor of extrinsic apoptosis pathway) with SICH patient prognosis.
To determine whether there is an association between blood sFas concentrations and SICH patient mortality.
We included patients with severe and supratentorial SICH. Severe was defined as having Glasgow coma scale < 9. We determined serum sFas concentrations at the time of severe SICH diagnosis.
We found that non-surviving patients (n = 36) compared to surviving patients (n = 39) had higher ICH score (P = 0.001), higher midline shift (P = 0.004), higher serum sFas concentrations (P < 0.001), and lower rate of early hematoma evacuation (P = 0.04). Multiple logistic regression analysis showed an association between serum sFas concentrations and 30-d mortality (odds ratio = 1.070; 95% confidence interval = 1.014-1.129; P = 0.01) controlling for ICH score, midline shift, and early hematoma evacuation.
The association of blood sFas concentrations and SICH patient mortality is a novel finding in our study.
The beneficial results of blockade of the Fas system in animal models could motivate its investigation in these patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Exbrayat JM, France; Lei Y, China S-Editor: Wang LL L-Editor: Filipodia P-Editor: Wang LL
| 1. | Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, Fung GL, Goldstein JN, Macdonald RL, Mitchell PH, Scott PA, Selim MH, Woo D; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015;46:2032-2060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1852] [Cited by in RCA: 2197] [Article Influence: 199.7] [Reference Citation Analysis (0)] |
| 2. | Zhang XQ, Zhang ZM, Yin XL, Zhang K, Cai H, Ling F. Exploring the optimal operation time for patients with hypertensive intracerebral hemorrhage: tracking the expression and progress of cell apoptosis of prehematomal brain tissues. Chin Med J (Engl). 2010;123:1246-1250. [PubMed] |
| 3. | Wu CH, Ding XY, Wang HY, Ye XB, Huang SY, Huang AM, Li HZ, Wu SY, Yu J, Yan XH. Neural apoptosis and apoptosis-related genes in intracerebral hemorrhage patients. Zhonghua Yixue Zazhi. 2006;86:3073-3076. [PubMed] |
| 4. | Wang YX, Yan A, Ma ZH, Wang Z, Zhang B, Ping JL, Zhu JS, Zhou Y, Dai L. Nuclear factor-κB and apoptosis in patients with intracerebral hemorrhage. J Clin Neurosci. 2011;18:1392-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Bao G, Han Y, Wang M, Xu G. Relationship between cellular apoptosis and the expression of p75 neurotrophin receptor and tyrosine kinase A receptor in tissue surrounding haematoma in intracerebral haemorrhage. J Int Med Res. 2011;39:150-160. [PubMed] |
| 6. | Guo FQ, Li XJ, Chen LY, Yang H, Dai HY, Wei YS, Huang YL, Yang YS, Sun HB, Xu YC, Yang ZL. [Study of relationship between inflammatory response and apoptosis in perihematoma region in patients with intracerebral hemorrhage]. Zhongguo Weizhongbing Jijiuyixue. 2006;18:290-293. [PubMed] |
| 7. | Zhu S, Tang Z, Guo S, Peng L, Fang S, Zhang S. Experimental study on the expression of HIF-1alpha and its relationship to apoptosis in tissues around cerebral bleeding loci. J Huazhong Univ Sci Technolog Med Sci. 2004;24:373-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Qureshi AI, Suri MF, Ostrow PT, Kim SH, Ali Z, Shatla AA, Guterman LR, Hopkins LN. Apoptosis as a form of cell death in intracerebral hemorrhage. Neurosurgery. 2003;52:1041-1047. [PubMed] |
| 9. | Delgado P, Cuadrado E, Rosell A, Alvarez-Sabín J, Ortega-Aznar A, Hernández-Guillamón M, Penalba A, Molina CA, Montaner J. Fas system activation in perihematomal areas after spontaneous intracerebral hemorrhage. Stroke. 2008;39:1730-1734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8080] [Cited by in RCA: 8303] [Article Influence: 159.7] [Reference Citation Analysis (0)] |
| 11. | Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818-829. [PubMed] |
| 12. | Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, Khoury J. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1398] [Cited by in RCA: 1684] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 13. | Hemphill JC 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1304] [Cited by in RCA: 1533] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 14. | Lorente L, Martín MM, Pérez-Cejas A, González-Rivero AF, Ramos-Gómez L, Solé-Violán J, Cáceres JJ, Cabrera J, Uribe L, Ferrer-Moure C, Jiménez A. Mortality prediction of patients with spontaneous intracerebral hemorrhage by serum soluble Fas ligand concentrations. Expert Rev Mol Diagn. 2022;22:233-238. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Yin XH, Yan JZ, Yang G, Chen L, Xu XF, Hong XP, Wu SL, Hou XY, Zhang G. PDZ1 inhibitor peptide protects neurons against ischemia via inhibiting GluK2-PSD-95-module-mediated Fas signaling pathway. Brain Res. 2016;1637:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Yin XH, Han YL, Zhuang Y, Yan JZ, Li C. Geldanamycin inhibits Fas signaling pathway and protects neurons against ischemia. Neurosci Res. 2017;124:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Ullah I, Chung K, Oh J, Beloor J, Bae S, Lee SC, Lee M, Kumar P, Lee SK. Intranasal delivery of a Fas-blocking peptide attenuates Fas-mediated apoptosis in brain ischemia. Sci Rep. 2018;8:15041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |