Published online Jan 9, 2023. doi: 10.5492/wjccm.v12.i1.35
Peer-review started: September 24, 2022
First decision: October 21, 2022
Revised: November 3, 2022
Accepted: November 23, 2022
Article in press: November 23, 2022
Published online: January 9, 2023
Processing time: 101 Days and 7.9 Hours
Arginine vasopressin is a neuropeptide produced in the hypothalamus and released by the posterior pituitary gland. In addition to maintaining plasma osmolarity, under hypovolemic or hypotensive conditions, it helps maintain plasma volume through renal water reabsorption and increases systemic vascular tone. Its synthetic analogues are widely used in the intensive care unit as a continuous infusion, in addition to hospital floors as an intravenous or intranasal dose. A limited number of cases of hyponatremia in patients with septic or hemo
A 39-year-old man fell off a forklift and sustained an axial load injury to his cranium. He had no history of previous trauma. Examination was normal except for motor and sensory deficits. The Imagine test showed endplate fracture at C7 and acute traumatic disc at C7 with cortical degeneration. He underwent cervical discectomy and fusion, laminectomy, and posterior instrumented fusion. After intensive care unit admission post-surgery, he developed hyponatremia of 121-124 mEq/L post phenylephrine and vasopressin infusion to maintain blood pressure maintenance. He was evaluated for syndrome of inappropriate secretion of antidiuretic hormone, hypothyroid, adrenal-induced, or diuretic-induced hyponatremia. At the end of extensive evaluation for the underlying cause of hyponatremia, vasopressin was discontinued. He was also put on fluid restriction, given exogenous desmopressin, and a dextrose 5% in water infusion to prevent osmotic demyelination syndrome caused by sodium overcorrection which im
The presentation of vasopressin-induced hyponatremia is uncommon in normotensive patients, and the most difficult aspect of this condition is determining the underlying cause of hypo
Core Tip: While hyponatremia can have many causes, vasopressin-induced hyponatremia in normotensive patients is unusual. Since the coronavirus disease 2019 pandemic, vasopressin use has increased in intensive care units across the country, and vasopressin-induced hyponatremia is likely underrated. We have intervened by discontinuing vasopressin, which led to rapid overcorrection of the sodium, and thus required temporary exogenous desmopressin and a dextrose 5% in water infusion. Through his care, the patient's serum sodium returned to normal and he made a full recovery. This makes our case unique.
- Citation: Lathiya MK, Pepperl E, Schaefer D, Al-Sharif H, Zurob A, Cullinan SM, Charokopos A. Vasopressin-induced hyponatremia in an adult normotensive trauma patient: A case report. World J Crit Care Med 2023; 12(1): 35-40
- URL: https://www.wjgnet.com/2220-3141/full/v12/i1/35.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v12.i1.35
Under normal conditions, the major physiological role of vasopressin (also known as antidiuretic hormone) is the maintenance of serum osmolarity via the regulation of water balance, through the activation of aquaporin channels in the renal collecting duct. Vasopressin is also an important endogenously released stress hormone, particularly during shock; it exerts vasoconstrictive properties via arginine vasopressin receptor 1A receptors. Consequently, this hormone has been used clinically as a vasoconstrictive, norepinephrine-sparing agent in the treatment of septic shock and other forms of hypotension[1]. Furthermore, it is utilized in the treatment central diabetes insipidus, von Willebrand disease, Hemophilia A, and nocturnal enuresis. However, vasopressin therapy carries a number of serious potential side effects, including cardiac and gastrointestinal complications, anaphylaxis, cutaneous gangrene, and venous thrombosis[2]. Vasopressin-induced hyponatremia is a rare adverse effect that has only recently been identified in patients suffering from vasodilatory shock[3]. Hyponatremia is mechanistically justified based on vasopressin’s mechanism of action. In this article, we will discuss a case of clinically significant vasopressin-induced hyponatremia in a normotensive patient in the Mayo Clinic Health System Intensive Care Unit (ICU).
We present a case of successfully treated vasopressin induced hyponatremia in normotensive patient.
A 39-year-old previously healthy male presented as a high acuity trauma after falling off a forklift, sustaining an axial load injury on his cranium. He was evaluated in the emergency department and workup was negative for neurologic findings. His initial vital signs in the emergency department were normal and the patient had a Glasgow Coma Scale of 15. He was protecting his airway and had bilaterally clear lung sounds without chest wall trauma. He was noted to have a scalp laceration with no active bleeding.
He has no known prior history of trauma and illness.
He has no known prior personal and family history.
The patient had a loss of sensation below the level of the nipples, loss of motor function of his lower extremities, and no rectal tone. In terms of upper extremity motor function, he had good power and grip strength in right hand but decreased on the left hand. No thoracic or lumbar spine step-off nor back trauma stigmata were noted.
To rule out hyperglycemia-induced hyponatremia, we measured serum glucose and calculated corrected sodium. In order to rule out pseudohyponatremia, we measured serum protein and lipid profiles. We also performed a complete blood count and measured urinary osmolarity, plasma osmolarity, random urine sodium level, serum cortisol level, serum adrenocorticotropic hormone level, and serum thyroid-stimulating hormone level to rule out differential diagnosis of hypoosmotic hyponatremia.
On imaging in the emergency department, a focused assessment with sonography in trauma exam was done and was negative. A computerized tomography (CT) scan of the head was negative for acute intracranial processes, as well as negative CT scans of the thoracic and lumbar spines. CT scan of the cervical spine revealed a superior endplate fracture at C7, and an magnetic resonance imaging revealed what appeared to be an acute traumatic disc at the C7 Level with cortical degeneration. Subsequent CT angiogram of the neck revealed no acute vascular injuries.
The definitive diagnosis in the case described is vasopressin-induced hyponatremia in a normotensive state.
The patient was taken to the odd ratio where he underwent C6-C7anterior cervical discectomy and fusion, C6-C7 Laminectomy, and C4-T2 posterior instrumented fusion, as well as closure of the patient’s scalp laceration.
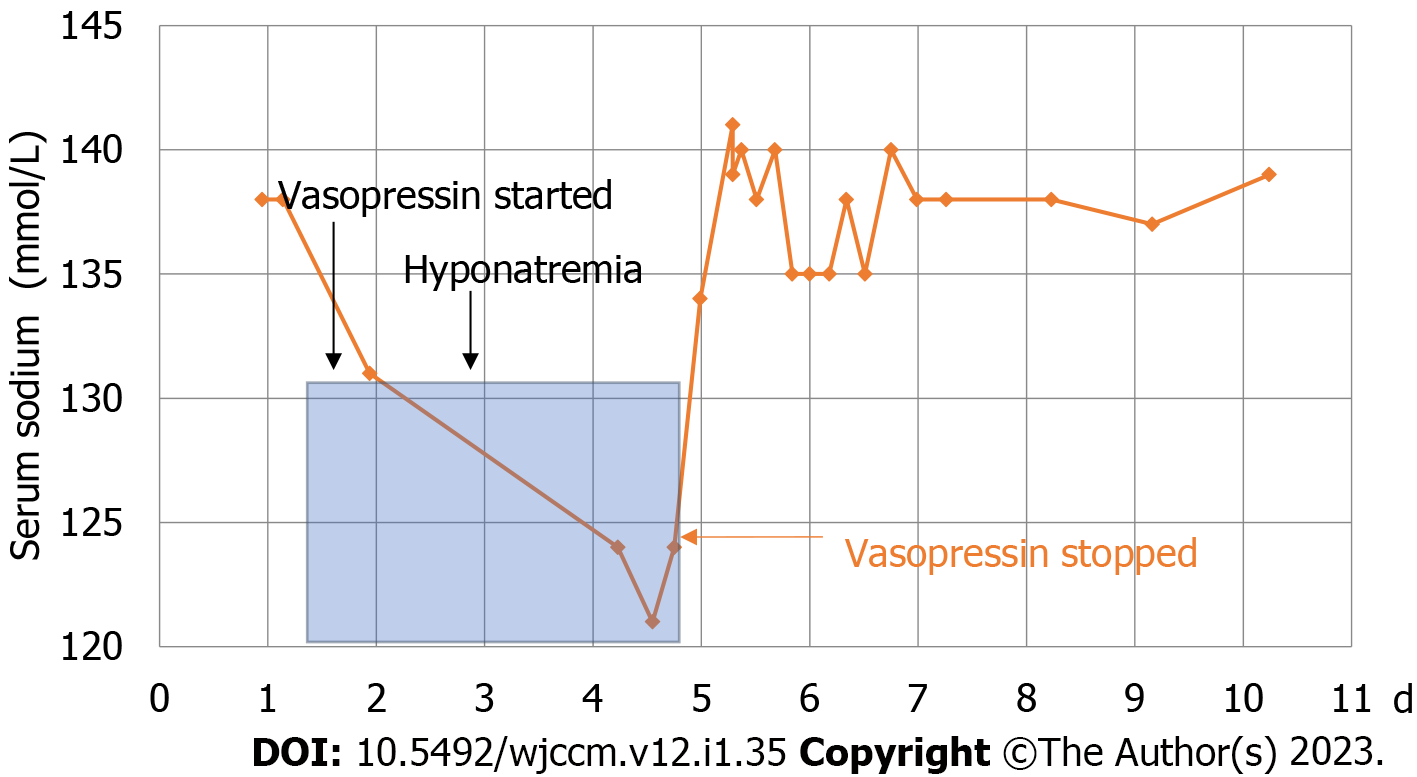
He admitted to the critical care unit on phenylephrine postoperatively for maintenance of a mean arterial pressure (MAP) greater than 85mmHg. On day 2 and day 3 of ICU care, infusion of vasopressin was initiated with the addition of phenylephrine intermittently to maintain MAP goals of 80-85 mmHg to augment cord perfusion for 7 d from the time of his injury. His sodium level on ICU day 3 was 131 mmol/L. On day 4, he had an episode of hypoxemia and cough with a chest x-ray that revealed perihilar infiltrates concerning for possible volume overload. Furosemide was given and arterial blood gas showed minimal hypoxemia with normal pCO2 and was managed on a high-flow nasal cannula. However, we encountered new onset hyponatremia with sodium levels 121-124 mmol/L and noted high urine osmolarity and high urine sodium. He was evaluated for syndrome of inappropriate secretion of antidiuretic hormone (SIADH), hypothyroid, adrenal-induced, or diuretic-induced hyponatremia. He was placed on fluid restriction without much improvement, so subsequently the vasopressin infusion was discontinued. After discontinuation of vasopressin, over ICU days 4 and 5, subsequent sodium levels at 6 h, 12 h, and 18 h were 124, 134, and 141 mmol/L respectively (Figure 1). Additionally, on day 5, Nephrology was consulted to manage the over-correction of hyponatremia to reduce the risk of osmotic demyelination syndrome, and he was started on exogenous desmopressin (DDAVP) and dextrose 5% in water (D5W) instead of hypertonic solution, as hypertonic solution is only used in cases of seizure or acute neurological signs and symptoms. Phenylephrine was continued to maintain MAP. On ICU day 6, the patient’s sodium stabilized at 135 mmol/L overnight with DDAVP and free water was discontinued. On day 7, overnight sodium level was noted to stabilize between 135-138 mmol/L. A tertiary survey was performed and no occult injuries were identified. The patient was started on aggressive physical therapy and occupational therapy, and attempts were made to wean off phenylephrine as per Neurosurgery for spinal cord perfusion purposes. He was able to wean off vasopressors but due to orthostatic hypotension symptoms, the patient was started on a low dose of midodrine with subsequent improvement. On day 8, the patient was transferred for inpatient spinal cord rehabilitation, where he continued to demonstrate strength, functional improvement, and coordination of left upper extremity, as well as static and dynamic sitting balance and sensation to light touch and pressure in the lower extremity.
On 3 mo postop follow up, he showed improvement as he was upgraded from American Spinal injury Association Impairment Scale Score (ASIA) B to an ASIA C.
Vasopressin is critical for maintaining normal body osmolality and plasma volume in response to several pathways of stimuli, including osmotic and nonosmotic triggers[4-6]. Its mechanism of action begins with a cyclic adenosine monophosphate signaling cascade in the renal system and culminates with the insertion of aquaporin 2 channels into the apical membrane of the renal collecting duct. The resulting accumulation of these water channels leads to the reabsorption of water into the bloodstream[7-10]. This hormone also serves numerous related functions both when delivered endogenously and exogenously. For instance, in clinical settings, the vasoconstrictive effects of vasopressin correct depleted blood volume in patients facing vasodilatory shock[11]. In the case of this patient, the use of general anesthesia during and following a surgery to address an acute spinal cord injury required external maintenance of MAP for a stated period of seven days. Initially, phenylephrine, an α1-adrenergic receptor, was infused at a maximum dose for these purposes; vasopressin administration began concurrently with phenylephrine to continually reach goal MAPs of > 85 mmHg. Prior to the initiation of vasopressin, basic metabolic panels revealed that sodium levels were within normal limits, defined in this report as 135-145 mmol/L. After vasopressin support, the significant drop in the patient’s sodium level down to 121 mmol/L indicated a state of hyponatremia, and lead to a significant risk for neurologic complications (Figure 1).
While hyponatremia is a relatively common affliction of hospitalized patients, the ramifications of unrecognized or untreated cases can be devastating for patient outcomes. With a decrease in serum sodium, the corresponding increase in intracellular osmolality can result in excess entrance of water into the cells[12,13]. This swelling of cells may engender several effects specifically secondary to brain edema, ranging from those as mild as headaches, lethargy, and nausea to ones as extreme as comas, seizures, and even eventual death[14]. Fortunately, hyponatremia can be carefully corrected via multiple treatments that encourage slow re-establishment of the standard osmotic gradient. Rapid overcorrection can become quickly dangerous to the patient, as brain cell dehydration leads to osmotic demyelination and numerous neurological defects[13,15]. The preferred intervention in correcting hyponatremia depends largely on the cause of this electrolyte imbalance, namely whether it can be described as isotonic, hypertonic, or hypotonic[13].
The investigation of the etiology of hyponatremia in this patient required a systematic approach to eliminating all possible causes of hyponatremia in a stable, normotensive, post-surgical trauma patient. First, it should be noted that blood glucose was within the normal range, noted as 110 mg/dL during the onset of hyponatremia, which concluded that hyperglycemia was not present. Neither hyperlipidemia nor hyperproteinemia were suspected as the serum was not lipemic, the patient did not exhibit jaundice, or have a history of plasma cell dyscrasia. These inquiries, along with a measured serum osmolality of 268 mOsm/kg, outside of defined normal limits of 276-306 mOsm/kg, suggested hypotonic hyponatremia. His euvolemic status and elevated urine sodium level, on the other hand, made hypovolemic hyponatremia less likely, and there was no other obvious cause of SIADH. However, while encephalic trauma can cause transient changes in water-electrolyte balance, this patient's initial serum electrolytes levels, including sodium, potassium, bicarbonate, and chloride, were normal, and his urine output was also normal[16]. His urine output and lack of primary central nervous injury made cerebral salt wasting less likely. We became concerned for hyponatremia secondary to the recent vasopressin infusion[13]. In a combined diagnostic and therapeutic approach, cessation of vasopressin led to prompt improvements in serum sodium. The hyponatremia corrected too rapidly, reaching a high sodium level of 141 mmol/L. After DDAVP and D5W, this overcorrection was reversed and stabilization of sodium levels within the normal range ensued. These observed changes in sodium levels, both upon beginning and stopping vasopressin, heightened suspicions of vasopressin-induced hyponatremia.
Both in the intensive care setting and otherwise, the finding of vasopressin-induced hyponatremia, is still emerging and rare. A few previous studies have detailed cases of hyponatremia secondary to vasopressin administration in the treatment of specific conditions. For example, a 2015 study reviews the cases of two young adult ICU patients treated with vasopressin for septic shock; both patients developed hyponatremia following initiation of the medication, but quickly rebounded from hyponatremia following discontinuation of vasopressin. The aforementioned study also mentions the role of catecholamines, corticosteroids, and endotoxins in counteracting the potentially detrimental effects of vasopressin in shock patients[3]. Another study in 1984 compared the utility of intravenous somatostatin and vasopressin in cirrhotic patients with variceal bleeding; researchers discovered that a complication common only to the vasopressin group was hyponatremia[17]. Similarly, a 1995 case report follows a post-caesarean section patient that received intravenous vasopressin to control esophageal and gastric varices. This serum sodium of this patient dropped significantly following vasopressin infusion but rebounded quickly following discontinuation of the medication[18].
Following his axial loading injury, this trauma patient exhibited periods of hyponatremia while recovering in critical care. While the etiology of this deficiency was initially unknown, the vasopressin infusion’s mechanism of action was thought-provoking. Similar inductions of hyponatremia by vasopressors have been documented in patients suffering from septic shock, as well as in patients undergoing treatment for variceal hemorrhage; however, these consequences have not been examined in a stable, normotensive, adult patient[3,17,18]. Our study diverges from past literature through the patient presentation and the indication for vasopressin treatment and the lack of shock. A stable, normotensive, adult trauma patient received vasopressin post-operation to maintain MAP. The result in this case report illustrates the shared result of hyponatremia remedied through the cessation of vasopressin. As far as we are aware, potential hyponatremia complications include cerebral edema, non-cardiogenic pulmonary edema, permanent neurological damage, and death. Finding the underlying cause of hyponatremia is always necessary in order to prevent life-threatening complications. Although the majority of hyponatremia's underlying causes are treatable and reversible, rare causes such as vasopressin-induced hyponatremia should be considered. Due to the widespread use of this medication in hospitals, both critical care physicians and hospitalists should be aware of this vasopressin side effect. Ultimately, the potential for hyponatremia per vasopressin commands further study across all demographics, indications, and settings. An improved understanding of the risks and mechanisms of vasopressin-induced hyponatremia would better inform clinical decision-making and possible prophylactic salt restriction for these patients.
Vasopressin-induced hyponatremia is an overall rare occurrence, but this potential consequence should still be suspected under certain conditions to both prevent and mitigate harmful effects. While several previous studies have detailed the development of vasopressin-induced hyponatremia in pediatric, hemorrhage, and shock patients, our case study demonstrates this form of hyponatremia in an adult, normotensive trauma patient recovering in the critical care setting. In brief, our patient’s serum sodium dropped to hyponatremic levels concurrently with the administration of vasopressin and recovered promptly with the cessation of this medication. However, more research is needed to various factors that may be responsible for additive effects, such as additional comorbidities, duration of vasopressin use, dosage, and interaction with other medications.
| 1. | Demiselle J, Fage N, Radermacher P, Asfar P. Vasopressin and its analogues in shock states: a review. Ann Intensive Care. 2020;10:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 2. | Mitra JK, Roy J, Sengupta S. Vasopressin: Its current role in anesthetic practice. Indian J Crit Care Med. 2011;15:71-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Salazar M, Hu BB, Vazquez J, Wintz RL, Varon J. Exogenous Vasopressin-Induced Hyponatremia in Patients With Vasodilatory Shock: Two Case Reports and Literature Review. J Intensive Care Med. 2015;30:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Sklar AH, Schrier RW. Central nervous system mediators of vasopressin release. Physiol Rev. 1983;63:1243-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 120] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Goldsmith SR. Baroreceptor-mediated suppression of osmotically stimulated vasopressin in normal humans. J Appl Physiol (1985). 1988;65:1226-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Sharshar T, Carlier R, Blanchard A, Feydy A, Gray F, Paillard M, Raphael JC, Gajdos P, Annane D. Depletion of neurohypophyseal content of vasopressin in septic shock. Crit Care Med. 2002;30:497-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 126] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Holmes CL, Patel BM, Russell JA, Walley KR. Physiology of vasopressin relevant to management of septic shock. Chest. 2001;120:989-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 378] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 8. | Holmes CL, Landry DW, Granton JT. Science review: Vasopressin and the cardiovascular system part 1--receptor physiology. Crit Care. 2003;7:427-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 235] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 9. | Maybauer MO, Maybauer DM, Enkhbaatar P, Traber DL. Physiology of the vasopressin receptors. Best Pract Res Clin Anaesthesiol. 2008;22:253-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Holt NF, Haspel KL. Vasopressin: a review of therapeutic applications. J Cardiothorac Vasc Anesth. 2010;24:330-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Dünser MW, Mayr AJ, Ulmer H, Knotzer H, Sumann G, Pajk W, Friesenecker B, Hasibeder WR. Arginine vasopressin in advanced vasodilatory shock: a prospective, randomized, controlled study. Circulation. 2003;107:2313-2319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 377] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 12. | Strange K. Regulation of solute and water balance and cell volume in the central nervous system. J Am Soc Nephrol. 1992;3:12-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 141] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Buffington MA, Abreo K. Hyponatremia: A Review. J Intensive Care Med. 2016;31:223-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Seay NW, Lehrich RW, Greenberg A. Diagnosis and Management of Disorders of Body Tonicity-Hyponatremia and Hypernatremia: Core Curriculum 2020. Am J Kidney Dis. 2020;75:272-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 15. | Mount DB. The brain in hyponatremia: both culprit and victim. Semin Nephrol. 2009;29:196-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Corradetti V, Esposito P, Rampino T, Gregorini M, Libetta C, Bosio F, Valsania T, Pattonieri EF, Rocca C, Bianzina S, Dal Canton A. Multiple electrolyte disorders in a neurosurgical patient: solving the rebus. BMC Nephrol. 2013;14:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Kravetz D, Bosch J, Terés J, Bruix J, Rimola A, Rodés J. Comparison of intravenous somatostatin and vasopressin infusions in treatment of acute variceal hemorrhage. Hepatology. 1984;4:442-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 153] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Melo JA, Lee M, Munoz J, Levine SM. Severe hyponatremia and bradycardia associated with intravenous vasopressin therapy for variceal hemorrhage. J Clin Gastroenterol. 1995;20:266-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Esposito P, Italy; Fayed A, Egypt S-Editor: Wang LL L-Editor: A P-Editor: Wang LL