Published online Jan 9, 2023. doi: 10.5492/wjccm.v12.i1.18
Peer-review started: September 13, 2022
First decision: October 21, 2022
Revised: November 15, 2022
Accepted: November 30, 2022
Article in press: November 30, 2022
Published online: January 9, 2023
Processing time: 111 Days and 10.5 Hours
Dexmedetomidine is a centrally acting alpha-2A adrenergic agonist that is com
To systematically review the practice, dosing schema, and outcomes of enteral clonidine use during dexmedetomidine weaning in critically ill adults.
This was a systematic review of enteral clonidine used during dexmedetomidine weaning in critically ill adults (≥ 18 years). Randomized controlled trials, prospective cohorts, and retrospective cohorts evaluating the use of clonidine to wean patients from dexmedetomidine in the critically ill were included. The primary outcomes of interest were dosing and titration schema of enteral clonidine and dexmedetomidine and risk factors for dexmedetomidine withdrawal. Other secondary outcomes included prevalence of adverse events associated with enteral clonidine use, re-initiation of dexmedetomidine, duration of mechanical ventilation, and ICU length of stay.
A total of 3427 studies were screened for inclusion with three meeting inclusion criteria with a total of 88 patients. All three studies were observational, two being prospective and one re
Enteral clonidine is a strategy to wean critically ill patients from dexmedetomidine. There is an association of increased withdrawal symptoms and agitation with the use of a clonidine taper.
Core Tip: In this systematic review of enteral clonidine use during dexmedetomidine weaning in critically ill patients, an association of increased withdrawal symptoms and agitation with the use of a clonidine taper and no difference in intensive care unit length of stay with or without clonidine taper was observed. However, varied techniques and a small total sample size restrict utility of the findings.
- Citation: Rajendraprasad S, Wheeler M, Wieruszewski E, Gottwald J, Wallace LA, Gerberi D, Wieruszewski PM, Smischney NJ. Clonidine use during dexmedetomidine weaning: A systematic review. World J Crit Care Med 2023; 12(1): 18-28
- URL: https://www.wjgnet.com/2220-3141/full/v12/i1/18.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v12.i1.18
In critically ill patients, agitation and delirium lead to poor clinical outcomes, such as prolonged mechanical ventilation, intensive care unit (ICU), and hospital length of stay (LOS)[1,2]. To optimize outcomes related to sedation in ICU patients, the 2018 Society of Critical Care Medicine practice recommendations suggest avoiding benzodiazepines[3]. Dexmedetomidine and propofol are the most commonly used sedatives in this group[3]. They have resulted in a shorter duration of mechanical ventilation as compared to benzodiazepines[3-6].
Dexmedetomidine, an intravenous (IV) alpha-2A adrenergic agonist provides cooperative sedation, sympatholysis, and analgesic-sparing effects without inducing respiratory depression and is frequently used in critically ill patients to treat pain, agitation, and delirium[7,8]. Due to a lack of central depression, dexmedetomidine is an attractive sedative clinically for weaning from mechanical ventilation and awake sedation in non-intubated patients. Dexmedetomidine was licensed by the Food and Drug Administration as a sedative with a 24-h time limit; however, studies have shown that it is safe and effective for up to 5 d with bradycardia and hypotension being the most commonly reported adverse effects[4,9-11]. Other drawbacks have included the cost of drug acquisition and availability only in an IV formulation[12-14]. Sudden cessation of the drug can lead to withdrawal symptoms such as agitation, tachycardia, hypertension, and other hypersympathetic conditions[15]. Clonidine, a structurally comparable alpha-2A that is widely used as an antihypertensive, sedative, and symp
The use of enteral clonidine may be a potential strategy for weaning from dexmedetomidine to prevent withdrawal syndromes[17,18]. However, dexmedetomidine has an eight-fold higher affinity for central alpha-2A receptors than clonidine; as a result, the best dosing and conversion strategies for clonidine in this context are unknown[8]. The purpose of this systematic review was to summarize the available evidence regarding the use of enteral clonidine to prevent withdrawal symptoms during dexmedetomidine weaning.
This was a systematic review designed to assess the use of enteral clonidine to prevent withdrawal syndromes in critically ill adults weaning from dexmedetomidine. The study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses 2020 guidelines[19]. The protocol was a priori registered in the PROSPERO database (No. CRD42022330666).
The systematic search was designed and executed by a skilled medical librarian (DJG). We searched for the concepts of enteral clonidine and dexmedetomidine combined with variant keywords and standardized index terms. The search was performed in April 2022 and included the electronic databases Ovid Evidence-Based Medicine Reviews, Ovid Embase, Ovid Medline, Scopus, and Web of Science Core Collection. The search was limited to the English language and did not include animals or pediatrics. The full search strategy is detailed in the Supplementary Table 1.
Eligible studies to be included were those that reported randomized-, crossover-, or parallel-designed clinical trials, prospective and retrospective longitudinal (cohort) studies, and cross-sectional studies (non-longitudinal studies) that reported on the use of enteral clonidine specifically for the purposes of weaning from dexmedetomidine to avoid withdrawal syndromes. Studies were excluded if they reported on pediatric patients (age < 18 years), animal or other non-clinical experiments, case reports, case series, review articles, editorial, and book chapters. Studies using intravenous clonidine or oral alpha-2A agonists other than clonidine were also excluded. No restrictions were placed on date of publication. In addition, a relevant search was performed by Reference Citation Analysis database (https://www.referencecitationanalysis.com/) to supplement and improve the highlights of the latest cutting-edge research results.
Article titles and abstracts were screened by two independent reviewers (SSR, MEW) for inclusion based on the pre-defined inclusion and exclusion criteria. Discrepancies between reviewers were adjudicated by a third independent reviewer (EDW) with consultation of a senior investigator (PMW) if necessary. The full text files of the candidate articles were randomly assigned to the two independent reviewers (SSR, MEW) to screen for final inclusion. Discrepancies between reviewers were adjudicated by a third independent reviewer (EDW) with consultation of a senior investigator (PMW) if necessary. All article screening was performed using Covidence software (Melbourne, Australia).
The data from the final articles meeting inclusion criteria were abstracted from full-text documents by two independent abstractors (JAG, LAW). Disagreements were adjudicated by discussion between the abstractors, and consultation of an adjudicator (EDW) when agreement was unattainable with consultation of a senior investigator (PMW) if necessary. The data abstracted included details regarding the publication information, study design, demographic data, details regarding the dosing schema and protocols, and outcomes information.
The Risk of Bias in Non-randomized Studies of Interventions tool was used to assess for risk of bias[20]. The tool was applied by two independent assessors (JAG, LAW) and disagreements were adjudicated by the senior investigators (PMW, NJS). The risk of bias information was summarized using R version 4.2.1 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria, 2022) and the robvis version 0.3.0 package[21].
The primary outcomes of interest were the dosing and titration schema of enteral clonidine and dexmedetomidine. Secondary outcomes included risk factors for dexmedetomidine withdrawal, incidence of adverse events associated with enteral clonidine use, re-initiation of dexmedetomidine, duration of mechanical ventilation, and ICU LOS. The data were summarized in descriptive format. No inferential analysis was performed.
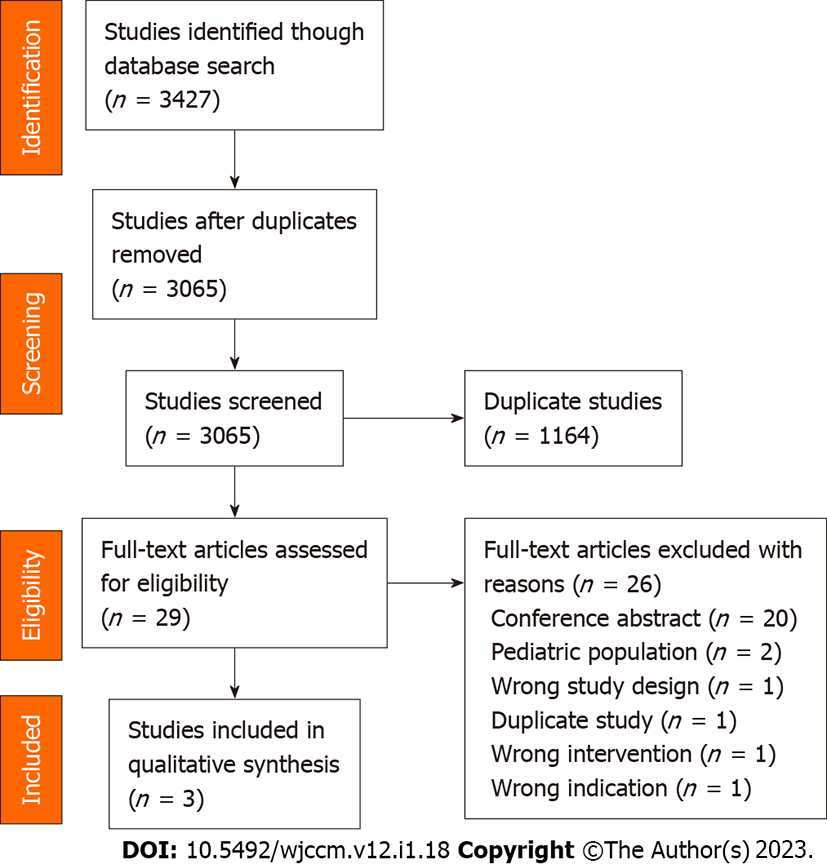
The initial search identified 3427 studies. Following removal of duplicates and excluded records, 29 full-text articles were assessed for eligibility. Three (10.3%) of these met the inclusion criteria and were included in the analysis[22-24]. The results of the systematic search are summarized in Figure 1.
Two of the included studies were prospective with one double cohort observational study and the other an observational pilot study[22,23]. The third was a retrospective observational study[24]. A total of 88 participants were included across the 3 studies. All studies were performed in the United States, and publication dates spanned from 2015 to 2020. Characteristics of all the studies included are detailed in Table 1. Males outnumbered females in all studies, with the most common initial diagnosis on admission being respiratory or heart disease, followed by sepsis, gastrointestinal disorders, trauma, neurological issues, and substance abuse. Indications of dexmedetomidine and enteral clonidine taper use were agitation, delirium, substance abuse, post procedural and intolerance of other sedatives.
| Ref. | Type of study | Study dates | Total patients studied | ICU | Admitting diagnosis | Mechanical ventilation | Age (yr) | Sex | Weight (kg) | BMI (kg/m2) | APACHE score |
| Terry et al[24], 2015 | Single-center, retrospective, observational study | February 1, 2013-February 28, 2014 | 26 | Cardiac surgery 21 (80.7%), thoracic 3 (11.5%), neurology 1 (3.8%), surgical 1 (3.8%) | Respiratory 4 (15.4%), cardiac 20 (76.9%), trauma 1 (3.8%), substance abuse 1 (3.8%) | 4 (14.8%) | 54.4 ± 16.91 | Male: 17 (63%), female: 9 (37%) | NR | 32 ± 3.11 | 18 (14-22)2 |
| Gagnon et al[23], 2015 | Single-center prospective observational pilot study | January, 2014-March, 2014 | 20 | Mixed medical, surgical, neuro ICU | Respiratory 12 (60%), neurologic 1 (5%), trauma 2 (10%), substance abuse 2 (10%), other 3 (15%) | 13 (65%) | 62 (54-73)2 | Male: 13 (65%), female: 7 (35%) | NR | 29.9 (26.5-33.1)2 | 62 (54-80)2 |
| Bhatt et al[22], 2020 | Single-center, prospective, double cohort observational study | November, 2017-December, 2018 | 42 | Medical-surgical 10 (67%) vs 13 (48%), cardiothoracic 3 (20%) vs 8 (30%), neurosurgical 2 (13%) vs 6 (22%) | Respiratory 16 (38.1%), cardiac 12 (28.6%), gastroenterological 5 (11.9%), neurologic 2 (4.8%), trauma 1 (2.4%), sepsis/shock 6 (14.3%) | NR | Clonidine taper: 58 (43-662 vs no taper: 54 (45-66)2 | Male: 27 (64%), female: 15 (36%) | Clonidine taper: 86.9 (67.3-94.1)2 vs no taper 91.6 (78.9-101.1)2 (P = 0.19) | NR | NR |
The decision to initiate enteral clonidine to wean dexmedetomidine was per clinician discretion in all included studies (Table 2). Bhatt et al[22] required at least 72 h of dexmedetomidine prior to enteral clonidine initiation for study inclusion with a median of 167 h [interquartile range (IQR) 115-217.1]; over the entire dexmedetomidine course patients received a mean dose of 0.9 mcg/kg/h and standard deviation of 0.3. Patients in the Gagnon et al[23] study had shorter median dexmedetomidine duration prior to enteral clonidine initiation of 33 h (IQR 21-47.5) at a median rate of 1 mcg/kg/h (IQR 0.7-1.2). Dexmedetomidine duration prior to enteral clonidine was shortest in the Terry et al[24] study with a median of 24 h (IQR 14.5-39) for patients who had dexmedetomidine discontinued within 8 h of clonidine initiation with a median dose at time of clonidine initiation of 0 mcg/kg/h (IQR 0-0.25). The group requiring more than 8 h of enteral clonidine to wean dexmedetomidine received a median of 13 h (IQR 4-32) of dexmedetomidine at a rate of 0.7 mcg/kg/h (IQR 0.45-0.7). Enteral clonidine was initiated in a similar protocolized fashion by Gagnon et al[23] and Bhatt et al[22], starting with 0.3 mg every 6 h. Patients with a dexmedetomidine rate < 0.7 mcg/kg/h, weight < 100 kg, or age > 65 years were initiated on 0.2 mg at the same interval. The doses were reduced by 0.1 mg for bradycardia and hypotension and increased by 0.1 mg for agitation. Dexmedetomidine dose was weaned by 25% every 6 h if no agitation requiring rescue medications had occurred. Terry et al[24] initiated enteral clonidine at 0.1 mg with non-protocolized uptitration every 6-8 h until the Richmond Agitation-Sedation Scale (RASS) goal was met, or hemodynamics prohibited further uptitration. Dexmedetomidine was weaned as soon as patients responded to clonidine as assessed by RASS, Confusion Assessment Method for the Intensive Care Unit (CAM-ICU), and hemodynamics without a defined protocol.
| Ref. | Formal protocol | Dexmedetomidine indication | Threshold for clonidine use | Initial clonidine dose | Dexmedetomidine wean | Clonidine taper |
| Terry et al[24], 2015 | No | Primarily for sedation after cardiac surgery | No standard | No standard. 0.1 mg three times daily commonly used | No standard | No standard |
| Gagnon et al[23], 2015 | Yes | Agitation: 12 (60%); Alcohol withdrawal: 3 (15%); Delirium: 2 (10%); Intolerance to other sedatives: 3 (15%) | Hemodynamically stable patients; Favorable response to DEX for 12-24 h | 0.2-0.5 mg every 6 h; Start at 0.2 mg with DEX doses of < 0.7 µg/kg/h, weight < 100 kg or age > 65 yr; Start with 0.5 mg every 6 h for all other patients | Decrease DEX dose by 25% of baseline within 6 h of clonidine administration (as long as no rescue meds were needed for agitation) | Extend the dosing interval to every 8, 12 and 24 h every 1-2 d as tolerated until discontinuation |
| Bhatt et al[22], 2020 | Yes | No clear selection criteria; patients with substance withdrawal were excluded | Variable; Clonidine taper and DEX wean started together | 0.3 or 0.2 mg every 6 h; Start at 0.2 mg with DEX < 0.7 µg/kg/h, weight < 100 kg, age > 65 yr old; Start with 0.3 mg every 6 h for all other patients | Decrease DEX dose by 25% of baseline from 0 h to 6 h, and continue dose reduction by 25% every 6 h while on clonidine | Extend the dosing interval to every 8, 12 and 24 h every 1-2 d as tolerated until discontinuation |
Patients spent a median of 19 h (IQR 9.5-23) on dexmedetomidine after enteral clonidine initiation in the Bhatt et al[22] study. Dexmedetomidine was utilized for a median of 23 h (IQR 2-53) for patients in the Gagnon et al[23] study after enteral clonidine was started. Terry et al[24] separately evaluated patients able to wean off dexmedetomidine within 8 h of enteral clonidine initiation from those requiring more than 8 h. Of 26 patients included, 17 (65%) were weaned off dexmedetomidine within 8 h with a median transition time of 1 h (IQR 0.5-4.25). Patients requiring more than 8 h to wean off dexmedetomidine after clonidine initiation had a median transition time of 28 h (IQR 20-56.5).
After dexmedetomidine discontinuation, Bhatt et al[22] tapered enteral clonidine by increasing the interval every 24 h from 6 h to 8h, then 12h, then 24 h, followed by clonidine discontinuation without any individual dose reduction. Gagnon et al[23] also increased the dosing interval in the same manner every 24-48 h without dose reduction. For signs of clonidine withdrawal, the previously tolerated dose was reinitiated for several days and then an attempted taper resumed on the same protocol. Terry et al[24] did not describe any subsequent enteral clonidine taper.
Dexmedetomidine re-initiation: No patients had dexmedetomidine restarted for documented enteral clonidine failure, albeit transition failure was identified as inability to wean dexmedetomidine after 8 h (Table 3)[24]. Failed transition had a median transition time of 28 h (IQR = 20-56.5). Patients who failed transition had alcohol withdrawal, septic shock, endocarditis, lung transplant and aortic valve replacement[24]. None of the patients were restarted on dexmedetomidine in the observation pilot trial[23]. Bhatt et al[22] showed 93% of patients were able to stop dexmedetomidine within 24 h of enteral clonidine initiation. No explicit details on re-initiation were provided.
| Outcomes data | Terry etal[24], 2015 | Gagnon etal[23], 2015 | Bhatt etal[22], 2020 |
| Breakthrough withdrawal | NR | 11 | Taper 11 (73%); No taper 16 (59%)2 |
| Discharged on clonidine | Out of ICU: 14 (54%); Out of hospital: 6 (23%) | 5 (25%) | NR |
| Use of other agents | |||
| Propofol | NR | No individual data | Taper: 5 (33%); No taper: 8 (30%) |
| Ketamine | NR | NR | Taper: 1 (6.7%); No taper: 6 (22.2%) |
| Benzodiazepines | Clonidine: 2 (22%); No clonidine: 5 (29%) | No individual data | Taper: 3 (20%); No taper: 3 (11%) |
| Antipsychotics | Clonidine: 4 (44%); No clonidine: 3 (18%) | DEX maintenance dose | Taper: 9 (60%); No taper: 10 (37%) (P = 0.2) |
| Opioids | Clonidine: 7 (78%); No clonidine: 13 (76%) | No individual data | No individual data |
| Hemodynamic changes | |||
| Tachycardia | NR | NR | Taper: 12 (80%); No taper: 20 (74%) |
| Hypertension | NR | DEX maintenance dose: 0; Transition: 0; Clonidine maintenance: 0; Clonidine taper final day: 0; Post clonidine: 1 (6%) | Taper: 6 (40%); No taper: 8 (30%) |
| Bradycardia | NR | DEX maintenance dose: 0; Transition: 0; Clonidine maintenance: 1 (5%); Clonidine taper final day: 1 (6%); Post clonidine: 0 | 0 |
| Hypotension | Clonidine: 4 (44%); No clonidine: 6 (35%) | DEX maintenance dose: 8 (40%); Transition: 7 (35%); Clonidine maintenance: 4 (20%); Clonidine taper final day: 2 (12%); Post clonidine: 2 (25%) | 0 |
| Sedation assessment score | RASS; Clonidine: 0 (-2 to 2); No clonidine: 0 (0-2) | SAS Score outside the goal of 3-4; DEX maintenance: 10 (50%); Transition: 10 (50%); Clonidine maintenance: 9 (45%); Clonidine taper final day 13 (76%); Post clonidine: 2 (25%) | NR |
| CAM ICU | Clonidine: 4 (44%); No clonidine: 3 (18%), P = 0.036 | DEX maintenance: 10 (50%); Transition: 11 (55%); Clonidine maintenance: 9 (45%); Clonidine taper final day: 13 (76%); Post clonidine: 3 (38%) | Taper: 11 (73%); No taper: 17 (63%) |
| Duration of mechanical ventilation (d), median (IQR) | NR | 3.5 (0, 10.5) | NR |
| Hospital length of stay (d), median (IQR) | 8 (4, 10.5) | 16.5 (10.5, 29.5) | NR |
| ICU length of stay (d), median (IQR) | 12.5 (7, 28) | 9.5 (5, 16.5) | Taper: 22.7; No taper: 17 |
| Mortality | 0 | 2 (10%) | NR |
Duration of mechanical ventilation: Seventeen (37%) patients were mechanically ventilated of the 2 studies that reported this data[23,24]. Gagnon et al[23] reported duration of mechanical ventilation of 3.5 d (IQR 0-10.5) and mechanical ventilation free days of 24.5 (IQR 15.3, 28)[23]. One study, despite having the bulk of its patients admitted with respiratory diagnosis to the ICU, did not provide data regarding the need for supplemental oxygenation or ventilation[22].
ICU LOS: All three studies reported ICU LOS; two of the studies appear to have similar ICU LOS in patients that had weaning protocol with no comparison[23,24]. Only one study specifically evaluated and found no statistically significant difference in ICU LOS between enteral clonidine taper vs no taper (22.7 d vs 17 d; P = 0.3) and time to discharge after dexmedetomidine wean in either group (7.2 d vs 7 d; P = 0.69)[22].
Adverse events: Terry et al[24] did not specify symptoms associated with enteral clonidine withdrawal. Gagnon et al[23] discovered that only one patient met withdrawal criteria (blood pressure > 180/120 mmHg) after stopping enteral clonidine despite a 6-d taper; this patient was also tapering off methadone and clonazepam. Bhatt et al[22] provided significant withdrawal data as defined by ³2 of: heart rate > 90, CAM positive, RASS > 1, systolic blood pressure > 140, or Withdrawal Assessment Tool Version 1 (WAT-1) > 2. Patients who experienced at least two withdrawal symptoms from dexm
Socioeconomic factors: Gagnon et al[23] reported an estimated $15360-$52140 cost reduction with enteral clonidine usage based on drug acquisition cost alone assuming a minimum of 24 h of enteral clonidine in place of dexmedetomidine per patient and a maximum of substituting the entire enteral clonidine course with continuous dexmedetomidine[23]. Bhatt et al[22] reported an average cost savings of $1553 per patient, also based solely on drug acquisition costs. Gagnon et al[23] reported 25% (5/20) of the patients were discharged on enteral clonidine with 20% (4/20) receiving instructions to taper off the medication. Terry et al[24] discovered 54% (14/26) of patients were continued on enteral clonidine at ICU transfer with 23% (6/26) of patients being discharged home on clonidine unintentionally.
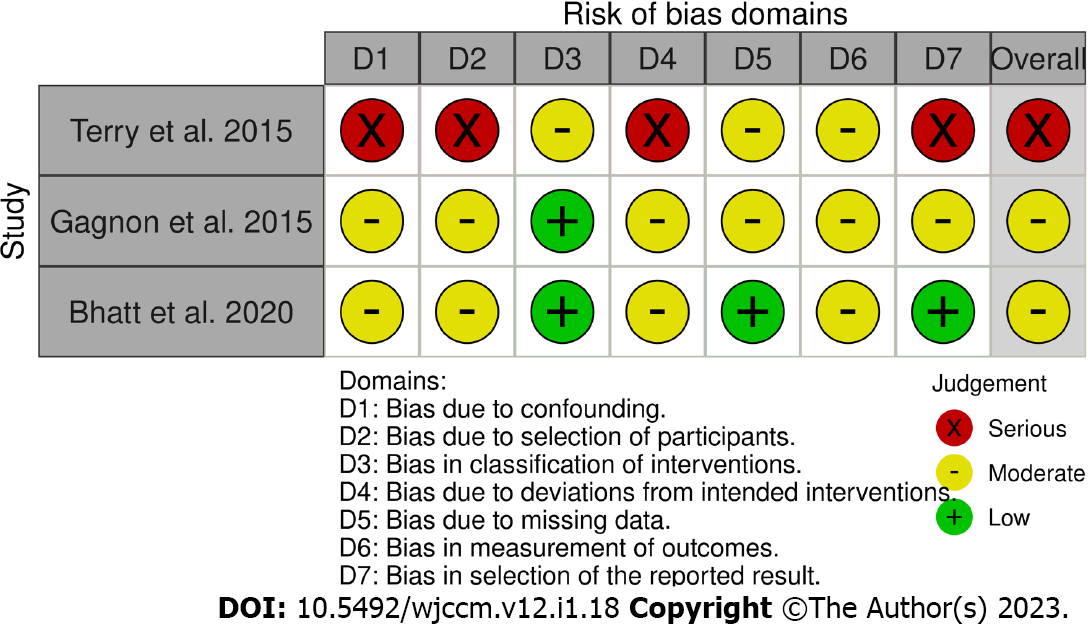
Risk of bias: In the risk of bias assessment, two studies[22,23] were deemed to be moderate risk and one study[24] was deemed to be serious risk (Figure 2). The primary reasons for a serious risk of bias were confounding, participant selection, and deviations from the intended interventions given the observational nature of the design[24]. Additionally, confounding, participant selection, and outcomes measurements were common reasons for a moderate risk of bias in the other studies[22,23].
This systematic review of the literature summarized the use of enteral clonidine for weaning of parental dexmedetomidine in the critically ill, dosing and titration schema of enteral clonidine and dexmedetomidine, prevalence of adverse events associated with clonidine use, re-initiation of dexmedetomidine, duration of mechanical ventilation and ICU LOS. Meta-analysis was not feasible due to differences in methodology, patients, and procedures that led to variation in the reported results between studies.
Weaning off dexmedetomidine with enteral clonidine has gained much attention for the potential benefits of reduced ICU LOS and costs. Clonidine has shown promise in minimizing the withdrawal symptoms associated with cessation of prolonged dexmedetomidine[25]. Clonidine, like dexm
The results of this systematic review leave many unanswered questions regarding the optimal utility of enteral clonidine in the setting of dexmedetomidine weaning. It is difficult to draw comparisons among the available data from the three studies due to the heterogeneity of the groups studied. There seems to be a common dosing scheme for enteral clonidine in the setting of weaning from dexmedetomidine based on Gagnon et al[23] and their institutional experience with the medication. However, the process of determining who received enteral clonidine in the reviewed studies was largely left to clinician discretion, limiting the ability to draw conclusions about the impact of clonidine. For example, although Bhatt et al[22] demonstrated a higher incidence of agitation and rescue antipsychotic dosing in the patient group receiving enteral clonidine, potential confounders include unknown patient factors that led to higher dexmedetomidine dosing and the clinician’s need to provide clonidine as a treatment rather than to evaluate its comparative effect vs. dexmedetomidine taper alone.
Dexmedetomidine is typically restricted to use in areas with critical care personnel and monitoring available such as the ICU and the perioperative care area. While it is valuable to have a study design with an inclusive patient population, the inclusion of both medical and postoperative patients in the studies reviewed pose challenges to the generalizability of the findings. For example, the sedation needs for a cardiac surgery patient in a rapid recovery protocol and the rapidity of sedation and mechanical ventilation liberation is often quite different than the medical patient requiring both treatment and stability after an acute cardiorespiratory insult requiring escalation to critical care needs. Terry et al[24] was highly skewed toward a post cardiac surgery population, whereas Gagnon et al[23] and Bhatt et al[22] included more mixed medical-surgical patients. The numerically lower total duration of dexmedetomidine in Terry et al[24] may have allowed for a lower general dose of enteral clonidine (i.e. 0.1 mg per dose) compared to the standard 0.2-0.3 mg clonidine doses used in the other two studies. However, given the lack of detail regarding exact dosing plan and the liberty clinicians were allotted regarding dosing selection, it is difficult to draw specific conclusions beyond the generalities offered in the study methods.
Adverse events from dexmedetomidine withdrawal included anxiety, agitation, decreased sleep, loose stools, emesis, tremors, and increased secretions[26]. Similarly, well-described phenomenon attributed to cessation of adrenal catecholamine secretion blockade and a subsequent surge in their circulating levels is associated with clonidine withdrawal resulting in rise in blood pressure, agitation, insomnia, and palpitations[27]. Risk factors for withdrawal are not known and were not identified in the studies reviewed. Further understanding of the risk factors for withdrawal and targeting appropriate patients for weaning could help minimize harm and improve quality of care. Patients on the enteral clonidine taper appeared to have more withdrawal symptoms than patients on dexmedetomidine taper. Re-initiation of dexmedetomidine was not explicitly addressed in any of the studies for withdrawal and should be an area of further investigation.
Although cost-effectiveness data is limited, the anticipated cost savings from drug acquisition ranged from $819 to $2338 per patient in two of the studies that reported data[22,23]. This price solely includes the drug acquisition cost and excludes the additional costs associated with dexmedetomidine, such as a dedicated ICU service line, monitoring, and titration. As a result, the shorter time on dexmedetomidine infusion following clonidine commencement may be greatly understated by these values.
Notably, in the two studies that reported information on enteral clonidine continuation at discharge from the hospital, approximately 25% of patients were still taking the medication[23,24]. Terry et al[24] also reported over half of patients were still taking enteral clonidine upon transfer from the intensive care unit. Several medications started in the ICU to expedite discharge, including antipsychotics and midodrine, are frequently prolonged without proper indication during transfer and upon discharge[28,29]. An order set and medication reconciliation during transitions of care may be helpful techniques for preventing the unintentional continuation of clonidine.
Strengths of this systematic review include the identification of a feasible enteral clonidine dosing strategy protocolized by Gagnon et al[23] that has been applied to other institutions as evidenced by Bhatt et al[22] and the elucidation of areas that could be optimized when utilizing enteral clonidine for dexmedetomidine weaning such as appropriate discontinuation prior to hospital discharge and the potential association of increased hypersympathetic withdrawal symptoms with its use. This systematic review has several limitations. All three studies have insufficient sample sizes, preventing the detection of withdrawal symptoms. Only one study had a matched control group, despite selection bias based on withdrawal risk assessment, which was not reported in any of the studies. Indications for weaning protocol varied according to the patient group and ICU site. There was heterogeneity of the research and data regarding the start date of clonidine weaning. The broad use of clinician discretion in the determination of enteral clonidine use and dosing limits the ability to systematically evaluate the available literature. Lastly, due to the heterogeneity in the reporting of the outcomes, quantitative meta-analysis was not possible.
Enteral clonidine has been utilized as a strategy to wean patients from parenteral dexmedetomidine due to similar mechanisms of action and potential for reduced costs and shorter ICU requirements. However, guidance on an appropriate taper strategy and resultant outcomes is limited. This systematic review investigated the literature related to weaning dexmedetomidine with and without an enteral clonidine taper. While there are some patterns in dosing schedules among the studies included, there is no consensus regarding an ideal taper strategy and the decision to utilize an enteral clonidine taper is left to clinical judgment. There may be an association of increased withdrawal symptoms and agitation with the use of an enteral clonidine taper, however we did not observe any appreciable difference in ICU LOS with or without a clonidine taper. Further research into risk factors for withdrawal, dose, and duration of dexmedetomidine use followed with appropriate clonidine dose and taper is needed.
Clonidine, an enterally available alpha-2A adrenergic agonist, may be a suitable agent to taper off parenteral dexmedetomidine (centrally acting alpha-2A adrenergic agonist) and reduce withdrawal syndromes. This could lead to reduced intensive care unit (ICU) length of stay (LOS), among other outcomes. However, limited data exist on this topic.
To determine if oral clonidine is useful to wean off parenteral dexmedetomine and reduce ICU LOS.
To systematically review the practice, dosing schema, and outcomes of enteral clonidine use during dexmedetomidine weaning in critically ill adults.
This was a systematic review of randomized controlled trials, prospective and retrospective cohorts, on the use of enteral clonidine during dexmedetomidine weaning in critically ill adults (≥ 18 years). The primary outcomes of interest were dosing and titration schema of enteral clonidine and dexm
Three observational studies were included (two prospective and one retrospective). Weaning time ranged from 13 to 167 h on average. The adverse events associated with enteral clonidine use were higher than patients on dexmedetomidine taper alone with increased agitation. The re-initiation of dexmedetomidine was not documented in any study. Only 17 (37%) patients were mechanically ventilated with median duration of 3.5 d for 13 patients in one of the 2 studies. ICU lengths of stay were similar.
Enteral clonidine is a strategy to wean critically ill patients from parenteral dexmedetomidine. However, there is an association of increased withdrawal symptoms and agitation with the use of a clonidine taper.
It is unclear if oral clonidine is useful in weaning from dexmedetomidine. More data are needed in terms of both dosing schedule and outcomes.
| 1. | Woods JC, Mion LC, Connor JT, Viray F, Jahan L, Huber C, McHugh R, Gonzales JP, Stoller JK, Arroliga AC. Severe agitation among ventilated medical intensive care unit patients: frequency, characteristics and outcomes. Intensive Care Med. 2004;30:1066-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Jaber S, Chanques G, Altairac C, Sebbane M, Vergne C, Perrigault PF, Eledjam JJ. A prospective study of agitation in a medical-surgical ICU: incidence, risk factors, and outcomes. Chest. 2005;128:2749-2757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJC, Pandharipande PP, Watson PL, Weinhouse GL, Nunnally ME, Rochwerg B, Balas MC, van den Boogaard M, Bosma KJ, Brummel NE, Chanques G, Denehy L, Drouot X, Fraser GL, Harris JE, Joffe AM, Kho ME, Kress JP, Lanphere JA, McKinley S, Neufeld KJ, Pisani MA, Payen JF, Pun BT, Puntillo KA, Riker RR, Robinson BRH, Shehabi Y, Szumita PM, Winkelman C, Centofanti JE, Price C, Nikayin S, Misak CJ, Flood PD, Kiedrowski K, Alhazzani W. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med. 2018;46:e825-e873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1292] [Cited by in RCA: 2419] [Article Influence: 345.6] [Reference Citation Analysis (0)] |
| 4. | Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, Shintani AK, Thompson JL, Jackson JC, Deppen SA, Stiles RA, Dittus RS, Bernard GR, Ely EW. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644-2653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1014] [Cited by in RCA: 1001] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 5. | Fraser GL, Devlin JW, Worby CP, Alhazzani W, Barr J, Dasta JF, Kress JP, Davidson JE, Spencer FA. Benzodiazepine versus nonbenzodiazepine-based sedation for mechanically ventilated, critically ill adults: a systematic review and meta-analysis of randomized trials. Crit Care Med. 2013;41:S30-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 203] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 6. | Parida S, Theerth KA. Dexmedetomidine: A drug for all seasons? Indian J Anaesth. 2021;65:789-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 7. | Keating GM. Dexmedetomidine: A Review of Its Use for Sedation in the Intensive Care Setting. Drugs. 2015;75:1119-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 279] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 8. | Gertler R, Brown HC, Mitchell DH, Silvius EN. Dexmedetomidine: a novel sedative-analgesic agent. Proc (Bayl Univ Med Cent). 2001;14:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 427] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 9. | Abuhasna S, Al Jundi A, Abdelatty W, Urrahman M. Evaluation of long-term infusion of dexmedetomidine in critically ill patients: A retrospective analysis. Int J Crit Illn Inj Sci. 2012;2:70-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Ozaki M, Takeda J, Tanaka K, Shiokawa Y, Nishi S, Matsuda K, Doi M, Kakihana Y, Fujino Y, Takinami M, Kawai M. Safety and efficacy of dexmedetomidine for long-term sedation in critically ill patients. J Anesth. 2014;28:38-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, Whitten P, Margolis BD, Byrne DW, Ely EW, Rocha MG; SEDCOM (Safety and Efficacy of Dexmedetomidine Compared With Midazolam) Study Group. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1142] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 12. | Bioc JJ, Magee C, Cucchi J, Fraser GL, Dasta JF, Edwards RA, Devlin JW. Cost effectiveness of a benzodiazepine vs a nonbenzodiazepine-based sedation regimen for mechanically ventilated, critically ill adults. J Crit Care. 2014;29:753-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Dasta JF, Kane-Gill SL, Pencina M, Shehabi Y, Bokesch PM, Wisemandle W, Riker RR. A cost-minimization analysis of dexmedetomidine compared with midazolam for long-term sedation in the intensive care unit. Crit Care Med. 2010;38:497-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Kaur M, Singh PM. Current role of dexmedetomidine in clinical anesthesia and intensive care. Anesth Essays Res. 2011;5:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 165] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 15. | Bouajram RH, Bhatt K, Croci R, Baumgartner L, Puntillo K, Ramsay J, Thompson A. Incidence of Dexmedetomidine Withdrawal in Adult Critically Ill Patients: A Pilot Study. Crit Care Explor. 2019;1:e0035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Glaess SS, Attridge RL, Christina Gutierrez G. Clonidine as a strategy for discontinuing dexmedetomidine sedation in critically ill patients: A narrative review. Am J Health Syst Pharm. 2020;77:515-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Arenas-López S, Riphagen S, Tibby SM, Durward A, Tomlin S, Davies G, Murdoch IA. Use of oral clonidine for sedation in ventilated paediatric intensive care patients. Intensive Care Med. 2004;30:1625-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Cunningham FE, Baughman VL, Peters J, Laurito CE. Comparative pharmacokinetics of oral versus sublingual clonidine. J Clin Anesth. 1994;6:430-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 50750] [Article Influence: 10150.0] [Reference Citation Analysis (2)] |
| 20. | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7683] [Cited by in RCA: 12443] [Article Influence: 1244.3] [Reference Citation Analysis (2)] |
| 21. | McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 3228] [Article Influence: 538.0] [Reference Citation Analysis (0)] |
| 22. | Bhatt K, Thompson Quan A, Baumgartner L, Jia S, Croci R, Puntillo K, Ramsay J, Bouajram RH. Effects of a Clonidine Taper on Dexmedetomidine Use and Withdrawal in Adult Critically Ill Patients-A Pilot Study. Crit Care Explor. 2020;2:e0245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 23. | Gagnon DJ, Riker RR, Glisic EK, Kelner A, Perrey HM, Fraser GL. Transition from dexmedetomidine to enteral clonidine for ICU sedation: an observational pilot study. Pharmacotherapy. 2015;35:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Terry K, Blum R, Szumita P. Evaluating the transition from dexmedetomidine to clonidine for agitation management in the intensive care unit. SAGE Open Med. 2015;3:2050312115621767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Albertson TE, Chenoweth J, Ford J, Owen K, Sutter ME. Is it prime time for alpha2-adrenocepter agonists in the treatment of withdrawal syndromes? J Med Toxicol. 2014;10:369-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Haenecour AS, Seto W, Urbain CM, Stephens D, Laussen PC, Balit CR. Prolonged Dexmedetomidine Infusion and Drug Withdrawal In Critically Ill Children. J Pediatr Pharmacol Ther. 2017;22:453-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Hunyor SN, Hansson L, Harrison TS, Hoobler SW. Effects of clonidine withdrawal: possible mechanisms and suggestions for management. Br Med J. 1973;2:209-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 99] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Rizvi MS, Nei AM, Gajic O, Mara KC, Barreto EF. Continuation of Newly Initiated Midodrine Therapy After Intensive Care and Hospital Discharge: A Single-Center Retrospective Study. Crit Care Med. 2019;47:e648-e653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Gilbert B, Morales JR, Searcy RJ, Johnson DW, Ferreira JA. Evaluation of Neuroleptic Utilization in the Intensive Care Unit During Transitions of Care. J Intensive Care Med. 2017;32:158-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arslan M, Turkey; Karim HMR, India S-Editor: Zhang H L-Editor: A P-Editor: Zhang H