Published online Mar 9, 2022. doi: 10.5492/wjccm.v11.i2.92
Peer-review started: November 18, 2021
First decision: December 27, 2021
Revised: January 3, 2022
Accepted: January 20, 2022
Article in press: January 20, 2022
Published online: March 9, 2022
Processing time: 104 Days and 11 Hours
Since December 2019, an outbreak of pneumonia caused by severe acute respiratory syndrome - coronavirus-2 (SARS-CoV-2) has led to a life-threatening ongoing pandemic worldwide. A retrospective study by Chow et al showed aspirin use was associated with decreased intensive care unit (ICU) admissions in hospitalized coronavirus disease 2019 (COVID-19) patients. Recently, the RECOVERY TRIAL showed no associated reductions in the 28-d mortality or the progression to mechanical ventilation of such patients. With these conflicting findings, our study was aimed at evaluating the impact of daily aspirin intake on the outcome of COVID-19 patients.
To study was aimed at evaluating the impact of daily aspirin intake on the outcome of COVID-19 patients.
This retrospective cohort study was conducted on 125 COVID-19 positive patients. Subgroup analysis to evaluate the association of demographics and comorbidities was undertaken. The impact of chronic aspirin use was assessed on the survival outcomes, need for mechanical ventilation, and progression to ICU. Variables were evaluated using the chi-square test and multinomial logistic regression analysis.
125 patients were studied, 30.40% were on daily aspirin, and 69.60% were not. Cross-tabulation of the clinical parameters showed that hypertension (P = 0.004), hyperlipidemia (0.016), and diabetes mellitus (P = 0.022) were significantly associated with aspirin intake. Regression analysis for progression to the ICU, need for mechanical ventilation and survival outcomes against daily aspirin intake showed no statistical significance.
Our study suggests that daily aspirin intake has no protective impact on COVID-19 illness-associated survival outcomes, mechanical ventilation, or progression to ICU level of care.
Core Tip: Our study suggests that aspirin has no beneficial effects with regards to progression to intensive care unit (ICU) from the medical floors in coronavirus disease 2019 (COVID-19) positive patients. This study was conducted on the patients presenting during the early phase of the pandemic when there was little evidence on the most beneficial modality of treatment. Over the last 2 years we have learned about the pro-thrombotic nature of COVID-19. Since aspirin is a widely dispensed medication in our adult population, we questioned if its chronic use could have a preventive effect on ICU progression of patients admitted to the medical floors. However, our data analysis suggests that there was no such protective effect.
- Citation: Gogtay M, Singh Y, Bullappa A, Scott J. Retrospective analysis of aspirin's role in the severity of COVID-19 pneumonia. World J Crit Care Med 2022; 11(2): 92-101
- URL: https://www.wjgnet.com/2220-3141/full/v11/i2/92.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v11.i2.92
Since December 2019, an outbreak of pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused a life-threatening ongoing pandemic worldwide[1]. Several nonsteroidal anti-inflammatory drugs (NSAIDs) have been used in patients with SARS-CoV-2 infection, but many remain controversial effects on the disease[2]. Aspirin (acetylsalicylic acid), a popular medicine, exhibits a variety of effects, including alleviating anti-inflammatory response, reducing fever and pain, and blocking viral propagation of RNA viruses (e.g., influenza virus and hepatic C virus)[3]. Moreover, coagulopathy plays a central role in the patho-mechanism of coronavirus disease 2019 (COVID-19), which leads to end-organ complications and death[4-6]. COVID-19 has been linked with increased thromboembolic complications such as venous thro
This single-center retrospective cohort study was conducted on patients that tested COVID-19 positive and were admitted between March and April 2020. IRB approval was obtained before initiating the study. Patient data including demographic information, history of comorbidities like hypertension, hyperlipidemia and diabetes mellitus, medication use like aspirin, P2Y12 inhibitor, warfarin and NOACs, clinical characteristics, and clinical outcomes were retrieved from the hospital database based on the following inclusion and exclusion criteria.
COVID-19 positive in-patients. Adults aged 18 years and older.
Patients with incomplete medical records. Pregnant women and patients aged 17 years and younger.
All the collected data were stored securely in a password-protected computer, and any paper records were securely stored. Only the approved study team had access to data.
Based on intensive retrospective chart review and recording the baseline characteristics of the patients, they were divided into two cohorts. The first cohort consisted of patients taking daily aspirin of at least 81 mg, and those who were not taking daily aspirin were placed in the second cohort. The patients were on chronic daily aspirin prior to contracting COVID-19 and hospitalization. Aspirin intake was recorded as per their pre-admission medication history. For both the cohorts, we calculated various outcomes, which included the percentage of patients progressing to the ICU, percentage of patients requiring oxygen supplementation, and percentage of patients requiring mechanical ventilation. We also calculated survival outcomes for the two groups. Additionally, subgroup analysis was undertaken by comparing various age groups and gender. All the statistical analysis was performed using SPSS (IBM SPSS Statistics for Windows, Version 21.0; IBM Corp, Armonk, NY, United States). Categorical variables were analyzed using the chi-square test; P < 0.05 was considered statistically significant. A multinomial logistic regression analysis was done to study the relationship between various outcomes (ICU admission, intubation rate, and survival rate) and multiple independent variables like the use of aspirin, warfarin, NOACs, P2Y12 inhibitors, and comorbidities like hypertension and diabetes mellitus.
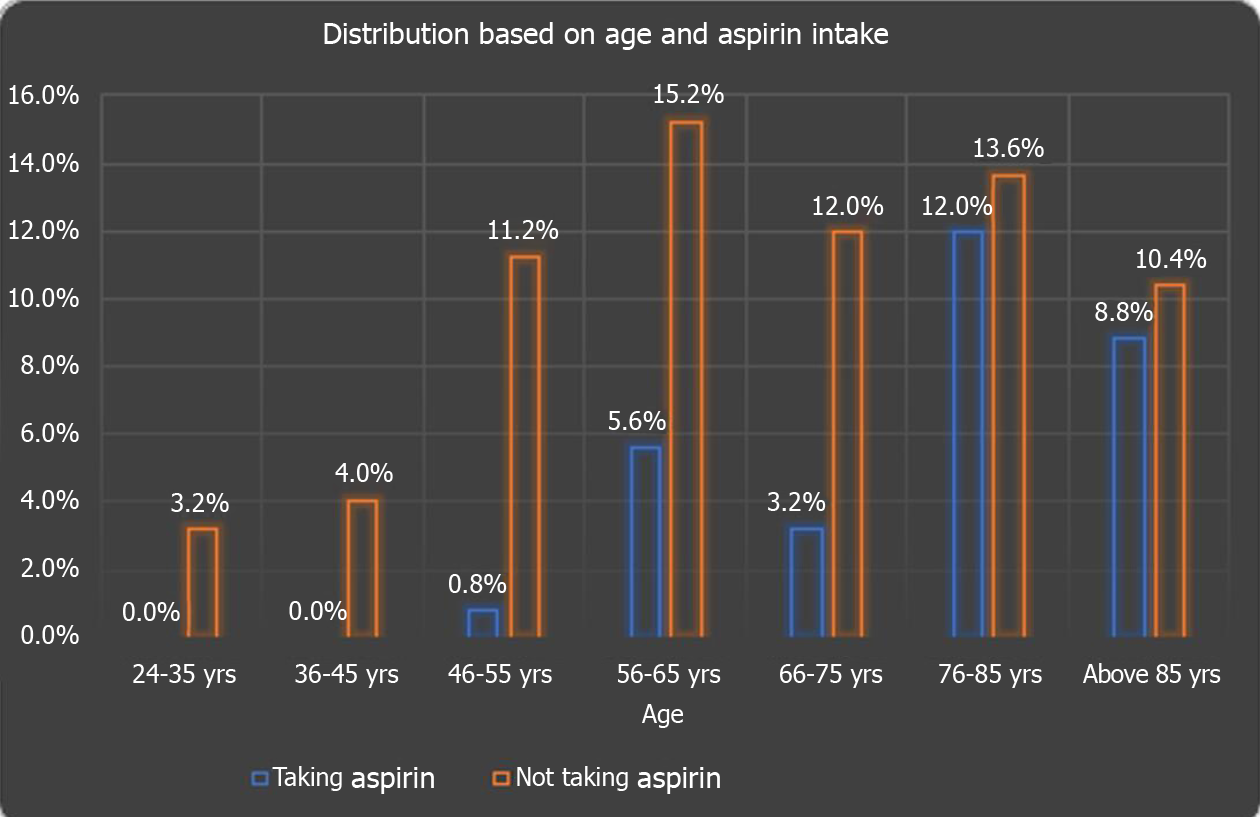
One hundred and twenty-five patients met our inclusion criteria and were stratified for further analysis. Out of them, 38 (30.40%) patients were on daily aspirin, and 87 (69.60%) were not. The majority of the 125 study subjects, i.e, 25.6% of the study subjects, belonged to the age group of 76-85 years, followed by 20.8% in the 56-65 age group. 19.2%, 15.2%, 12%, 4%, and 3.2% of study subjects belonged to above 85, 66-75, 46-55, 36-45, and 24-35 years of age respectively. The chi-square test showed a significant (P = 0.016) difference in age groups of study subjects taking daily aspirin as shown in Figure 1.
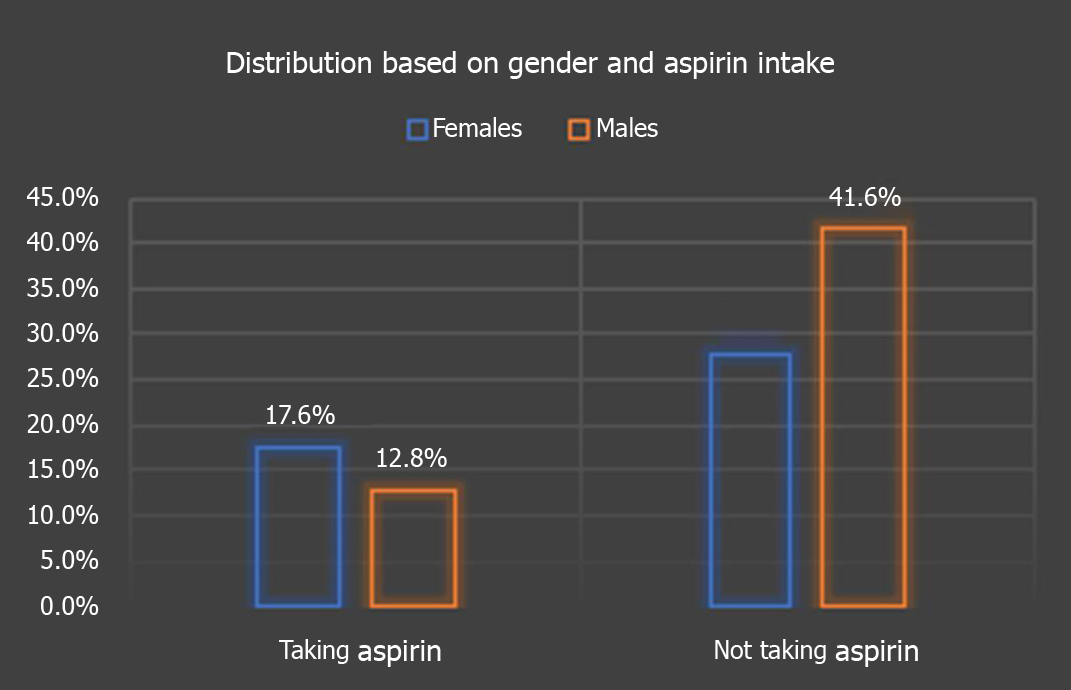
Amongst the 125 patients, we found that 41.6% were males not taking daily aspirin, 28% were females not taking aspirin, 17.6% were women taking daily aspirin, 12.8% were males on daily aspirin (P = 0.068), as depicted in Figure 2.
For those on daily aspirin, 32 (84.21%), 30 (78.94%), and 18 (47.36%) subjects had significant comorbidities like hypertension, hyperlipidemia and diabetes mellitus, respectively. Cross-tabulation of the clinical parameters of study subjects showed that hypertension (P = 0.004), hyperlipidemia (P = 0.016), diabetes mellitus (P = 0.022), were significantly associated with aspirin intake (Table 1).
| Patient characteristics | Aspirin | Total (n = 125) | χ2 value | P value | ||
| Taking (n = 38) | Not taking aspirin (n = 87) | |||||
| Warfarin | Yes | 4 | 5 | 9 | 0.90 | 0.34 |
| Percentage (%) | 3.2 | 4.0 | 7.2 | |||
| No | 34 | 82 | 116 | |||
| Percentage (%) | 27.2 | 65.6 | 92.8 | |||
| Direct oral anticoagulants (NOAC) | Yes | 6 | 9 | 15 | 0.74 | 0.38 |
| Percentage (%) | 4.8 | 7.2 | 12.0 | |||
| No | 32 | 78 | 110 | |||
| Percentage (%) | 25.6 | 62.4 | 88.0 | |||
| P2Y12 inhibitors | Yes | 1 | 5 | 6 | 0.56 | 0.45 |
| Percentage (%) | 0.8 | 4.0 | 4.8 | |||
| No | 37 | 82 | 119 | |||
| Percentage (%) | 29.6 | 65.6 | 95.2 | |||
| Hypertension | Present | 32 | 50 | 82 | 8.38 | 0.004a |
| Percentage (%) | 84.2 | 57.4 | 65.6 | |||
| Absent | 6 | 37 | 43 | |||
| Percentage (%) | 15.78 | 42.5 | 34.4 | |||
| Hyperlipidemia | Present | 30 | 49 | 79 | 5.82 | 0.016a |
| Percentage (%) | 78.9 | 56.32 | 63.2 | |||
| Absent | 8 | 38 | 46 | |||
| Percentage (%) | 21 | 43.6 | 36.8 | |||
| Diabetes Mellitus | Present | 18 | 23 | 41 | 5.25 | 0.022a |
| Percentage (%) | 47.36 | 26.4 | 32.8 | |||
| Absent | 20 | 64 | 84 | |||
| Percentage (%) | 52.6 | 73.5 | 67.2 | |||
| Immunosuppression | Yes | 3 | 4 | 7 | 0.54 | 0.46 |
| Percentage (%) | 7.8 | 4.5 | 5.6 | |||
| No | 35 | 83 | 118 | |||
| Percentage (%) | 92.1 | 95.4 | 94.4 | |||
| ICU admission | Admitted to ICU | 9 | 38 | 47 | 4.50 | 0.034a |
| Percentage (%) | 23.6 | 43.67 | 37.6 | |||
| Remained on medical floors | 29 | 49 | 78 | |||
| Percentage (%) | 90.6 | 56.3 | 62.4 | |||
| Intubation | Yes | 5 | 21 | 26 | 1.93 | 0.16 |
| Percentage (%) | 13.1 | 24.1 | 20.8 | |||
| No | 33 | 66 | 99 | |||
| Percentage (%) | 86.8 | 75.8 | 79.2 | |||
| Outcome (survival) | Survived | 26 | 66 | 92 | 0.75 | 0.38 |
| Percentage (%) | 68.4 | 75.8 | 73.6 | |||
| Died | 12 | 21 | 33 | |||
| Percentage (%) | 31.5 | 24.1 | 26.4 | |||
| PE/DVT | Present | 2 | 1 | 3 | 1.91 | 0.16 |
| Percentage (%) | 5.2 | 1.1 | 2.4 | |||
| Absent | 36 | 86 | 122 | |||
| Percentage (%) | 94.7 | 98.8 | 97.6 | |||
| Oxygen use | Present | 36 | 73 | 109 | 2.77 | 0.096 |
| Percentage (%) | 94.7 | 83.9 | 87.2 | |||
| Absent | 2 | 14 | 16 | |||
| Percentage (%) | 5.2 | 16 | 12.8 | |||
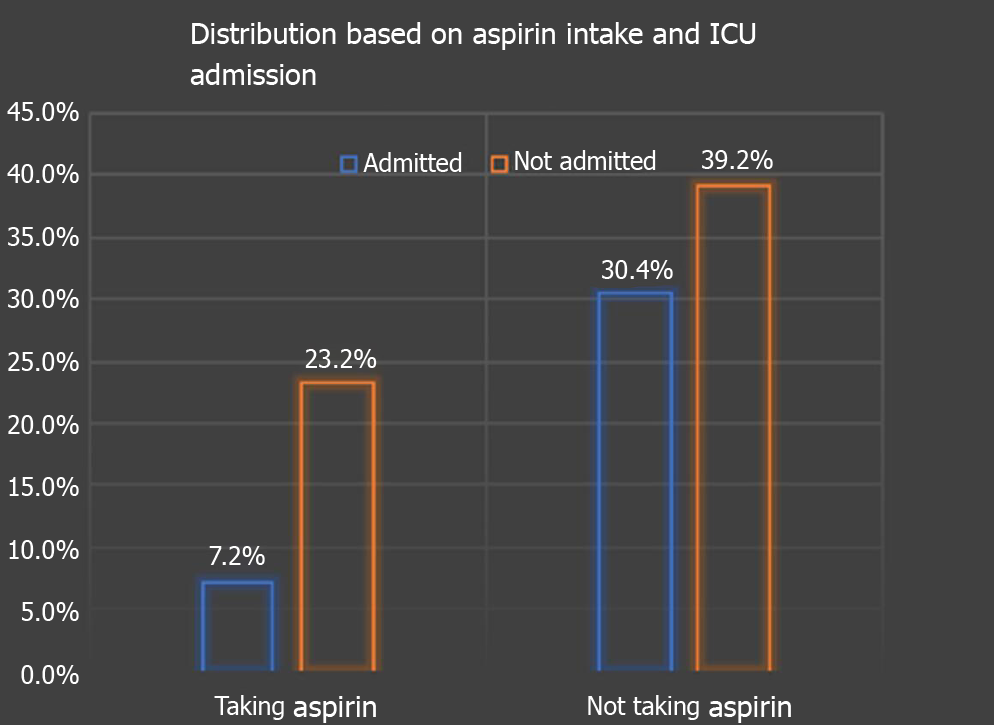
In terms of outcomes, 9 (23.68%) patients were on aspirin vs 38 (43.6%) not on aspirin progressed to requiring ICU level of care (P = 0.034) as depicted in Figure 3. 5 (13.15%) on aspirin required mechanical ventilation contrary to 21 (24.13%) not on aspirin (P = 0.16). 36 (94.73%) of aspirin users required supplemental oxygen vs 73 (83.9%) not on aspirin (P = 0.096). 26 (68.5%) on aspirin survived vs 66 (75.8%), not on aspirin (P = 0.38) as depicted in Table 1.
A multinomial logistic regression analysis was further used to predict the categorical placement of each independent variable (aspirin, warfarin, NOACs, P2Y12 inhibitors, hypertension and diabetes mellitus) against the dependent variables: (1) Progression to ICU (Table 2); (2) Need for mechanical ventilation (Table 3); and (3) Survival outcomes (Table 4).
| Characteristics | Regression coefficients | Standard error | χ2 (wald) | P value | Odds ratio | 95%CI |
| Intercept | -0.45044 | 0.332171 | 1.838826 | 0.175089 | 0.637351 | |
| Aspirin | -1.00047 | 0.46281 | 4.67307 | 0.030639 | 0.367707 | 0.365575-2.269164 |
| Warfarin | 0.382791 | 0.733339 | 0.272467 | 0.601681 | 1.466372 | 0.179321-3.701697 |
| NOAC’s | -0.15984 | 0.616872 | 0.067143 | 0.795543 | 0.852277 | 0.22984-2.520831 |
| P2Y12 inhibitors | 1.098044 | 0.908435 | 1.461005 | 0.22677 | 2.998296 | 0.142169-5.14458 |
| HTN | 0.213851 | 0.424561 | 0.253712 | 0.614473 | 1.238438 | 0.259028-1.790559 |
| DM | 0.018183 | 0.432623 | 0.001767 | 0.966474 | 1.01835 | 0.187667-1.05208 |
| Characteristics | Regression coefficients | Standard error | χ2 (wald) | P value | Odds ratio | 95%CI |
| Intercept | -1.22056 | 0.389142 | 9.83799 | 0.001709 | 0.295063 | |
| Aspirin | -0.83593 | 0.566163 | 2.179995 | 0.139815 | 0.433472 | 0.142903-1.31486 |
| Warfarin | 0.1583 | 0.859459 | 0.033924 | 0.853868 | 1.171517 | 0.217358-6.314246 |
| NOACs | -0.54597 | 0.812938 | 0.451048 | 0.501838 | 0.57928 | 0.117737-2.850114 |
| P2Y12 inhibitors | -0.42413 | 1.139528 | 0.138534 | 0.709742 | 0.654336 | 0.070118-6.106168 |
| HTN | 0.22629 | 0.500756 | 0.20421 | 0.651344 | 1.253939 | 0.469929-3.345963 |
| DM | 0.020291 | 0.510762 | 0.001578 | 0.968312 | 1.020498 | 0.375017-2.776985 |
| Characteristics | Regression coefficients | Standard error | χ2 (wald) | P value | Odds ratio | 95%CI |
| Intercept | 1.689138 | 0.422469 | 15.98599 | 6.38E-05 | 5.41481 | |
| Aspirin | -0.07596 | 0.456833 | 0.027651 | 0.867932 | 0.926849 | 0.378575-2.269164 |
| Warfarin | -0.20489 | 0.772302 | 0.070384 | 0.790778 | 0.814735 | 0.179321-3.701697 |
| NOACs | -0.27293 | 0.610988 | 0.199538 | 0.655094 | 0.761148 | 0.229824-2.520831 |
| P2Y12 inhibitors | -0.1564 | 0.915497 | 0.029184 | 0.864355 | 0.855219 | 0.142169-5.14458 |
| HTN | -0.38415 | 0.49321 | 0.606636 | 0.436057 | 0.681032 | 01.790559 |
| DM | -0.81116 | 0.439766 | 3.402248 | 0.065108 | 0.444344 | 1.05208 |
The analysis showed that aspirin users had an odds ratio of 0.367 (P = 0.03, CI: 0.378-2.26), predicting the odds of a patient taking aspirin progressing to the ICU is 0.3677 higher than those not being on aspirin if all the other predictor variables were held constant as represented in Table 2, though not significant.
The odds ratio of warfarin was 1.466 (P = 0.60, CI: 0.179-3.701) higher risk of ICU transfer than those not on warfarin. NOACs users had an odds ratio of 0.8522 (P = 0.79, CI: 0.229-2.520) and P2Y12 inhibitors were 2.998 (P = 0.22, CI: 0.141-5.144). Similarly, comorbidities (hypertension and diabetes mellitus) showed no significant impact on ICU admissions.
Other dependent variables like the need for mechanical ventilation and survival outcomes of the patients were also analyzed using the same independent variables with no significant association as in Table 3 and Table 4, respectively.
In a multi-center cohort study on COVID-19 patients by Chow et al[14], aspirin use was independently associated with a lower risk of mechanical ventilation, ICU admission, and in-hospital mortality. Given aspirin's wide inexpensive use, it could be the answer we are looking for especially in low-income countries where expensive immunomodulators aren't readily available[14]. But a recent randomized controlled, open-label trial - RECOVERY, compared multiple treatments, including 150 mg aspirin once daily. They found that in hospitalized COVID-19 patients, aspirin was not associated with reductions in 28-d mortality or the risk of progressing to invasive mechanical ventilation or death but was associated with a slight increase in the rate of being discharged alive within 28 d[15]. Given the conflicting nature of recent studies, we sought to evaluate the effect of daily aspirin intake on clinical outcomes in hospitalized patients with COVID-19 and its impact on the rate of COVID-19 positive patient’s progression to ICU.
Our study analyzed 125 patients, of which 38 patients were on daily aspirin use, with a minimum dose of 81 mg. The study showed a significant association in variables such as age groups, hypertension, hyperlipidemia, and diabetes mellitus. This insinuated that our aspirin patients were older, and most of them had significant comorbidities, putting them at risk of severe COVID-19 illness.
At first glance, aspirin showed a possible protective role in progression to ICU on chi-square analysis. It failed to reach significance in multinomial logistic regression analysis. Furthermore, in terms of mortality, patients on aspirin had a higher mortality rate of 32% as compared to only 25% for non-aspirin users. This could be explained by the fact that patients on aspirin were older and had more comorbidities.
Hence, we conclude that aspirin shows no protective role for COVID-19 patients in terms of progression to ICU, survival outcome, and use of mechanical ventilation. Our findings concurred with the results of the RECOVERY trial[15].
Furthermore, bleeding risk is a potential adverse event while on aspirin. In the RECOVERY TRIAL, the incidence of major bleeding events was higher in the aspirin group (1.6% vs 1.0%; absolute difference 0.6%, SE: 0.2%). There were 18 reports of serious adverse events believed related to aspirin, all due to hemorrhagic in nature[15]. Even though we did not assess bleeding risk, this is a serious adverse event to bear in mind.
The advantage of our study is that it was conducted on the cohort of patients that presented at our hospital during the initial phase of the COVID-19 pandemic back in March of 2020. At that time, the use of corticosteroids and remdesivir were not established as the standard of care, and hence our study is not confounded by the effects of these medications.
The limitations of our study include a modest sample size and a retrospective - observational analysis, which limits generalizability and adjustment for confounding variables. We did not collect data on other concomitant medications - like statins or ACEI/ARBs, as most patients on aspirin are usually on the above, due to guideline-directed medical therapy for cardiovascular diseases, which could confound results. Some of our patients had their daily aspirin use discontinued after admission due to inability to tolerate enteral feeds, new bleeding complications, or being started on other anticoagulants owing to COVID-19 complications.
Our study suggests that aspirin does not have beneficial effects regarding progression to ICU from the medical floors in COVID-19 positive patients. Furthermore, it showed no statistically significant impact in reducing rates of mechanical ventilation, oxygen requirement, or decreasing mortality in patients.
In a retrospective study by Chow et al, it was found that aspirin use may be associated with improved outcomes, reduced rates of mechanical ventilation, and decreased intensive care unit (ICU) admissions in hospitalized coronavirus disease 2019 (COVID-19) patients. Given the encouraging findings, the world’s largest randomized controlled open-label trial was performed using approximately 15000 patients in the UK (RECOVERY TRIAL). The patients in the study were allocated to receive aspirin after diagnosis of COVID-19 during in-hospital admission, and the results showed no associated reductions in the 28-d mortality or the progression to mechanical ventilation of such patients. With the above conflicting findings, the present study was designed to evaluate the impact of daily aspirin intake prior to hospitalization on the rate of COVID-19 positive patients’ progression to the ICU.
With the never ending COVID-19 pandemic, it is imperative we find ways to keep patients out of the ICU. We have learnt that COVID-19 illness has major thrombotic and inflammatory effects. Aspirin would seem like an ideal choice to curb these effects. With this in mind, we conducted our study. But surprisingly we found that aspirin has no beneficial effects when it comes to preventing severe COVID-19 illness like ICU admissions. We postulate that patients taking aspirin were also older and had significant comorbidities, putting them at high risk for severe COVID-19. Furthermore, this study was carried out back when the most effective treatment modalities like steroids and remdesivir were not used. Hence, we conclude that aspirin's antiviral, anti-inflammatory and anti-thrombotic properties may not be strong enough to combat the COVID-19 illness.
Present study was designed to evaluate the impact of daily aspirin intake prior to hospitalization on the rate of COVID-19 positive patients’ progression to the ICU.
The idea of using the below methods were modeled after the study by Chow et al and the recovery trial on Aspirin in patients admitted to the hospital with COVID-19. Research methods adopted were the following: (1) Categorical variables, such as demographic information, comorbidities, receipt of investigational therapeutics, type of oxygen support, mechanical ventilation need, and outcomes, were reported as the number and percentage of patients and were compared between groups using the χ2 test. P values < 0.05 were considered statistically significant; and (2) Multinomial logistic regression analysis to control for interplay of confounding from other anti-coagulation agents.
Our study analyzed 125 patients, of which 38 patients were on daily aspirin use, with a minimum dose of 81 mg. The study showed a significant association of aspirin with variables such as age groups, hypertension, hyperlipidemia, and diabetes mellitus. This insinuated that our aspirin patients were older, and most of them had significant comorbidities, putting them at risk of severe COVID-19 illness. At first glance, aspirin showed a possible protective role in progression to ICU on chi-square analysis. It failed to reach significance in multinomial logistic regression analysis. Furthermore, in terms of mortality, patients on aspirin had a higher mortality rate of 32% as compared to only 25% for non-aspirin users. This could be explained by the fact that patients on aspirin were older and had more comorbidities.
We conclude that aspirin shows no protective role for COVID-19 patients in terms of progression to ICU, survival outcome, and use of mechanical ventilation. Our findings concurred with the results of the RECOVERY trial. The advantage of our study is that it was conducted on the cohort of patients that presented at our hospital during the initial phase of the COVID-19 pandemic back in March of 2020. At that time, the use of corticosteroids and remdesivir were not established as the standard of care, and hence our study is not confounded by the effects of these medications.
Given the conflicting results of recent studies on aspirin and COVID-19 illness, it would seem beneficial for future studies to study the effect of chronic daily aspirin use on COVID-19 outcomes. Since our N-126, larger studies with N-1000s may be able to show definitive significance between aspirin and COVID-19. In theory, aspirin is an over the counter, cheap medication with a wide range of properties to battle the ill effects of the virus.
| 1. | Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020;92:568-576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 972] [Cited by in RCA: 853] [Article Influence: 142.2] [Reference Citation Analysis (0)] |
| 2. | Russell B, Moss C, Rigg A, Van Hemelrijck M. COVID-19 and treatment with NSAIDs and corticosteroids: should we be limiting their use in the clinical setting? Ecancermedicalscience. 2020;14:1023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 196] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 3. | Ornelas A, Zacharias-Millward N, Menter DG, Davis JS, Lichtenberger L, Hawke D, Hawk E, Vilar E, Bhattacharya P, Millward S. Beyond COX-1: the effects of aspirin on platelet biology and potential mechanisms of chemoprevention. Cancer Metastasis Rev. 2017;36:289-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 159] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 4. | Huang I, Pranata R, Lim MA, Oehadian A, Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14:1753466620937175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 386] [Cited by in RCA: 337] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 5. | Lim MA, Pranata R, Huang I, Yonas E, Soeroto AY, Supriyadi R. Multiorgan Failure With Emphasis on Acute Kidney Injury and Severity of COVID-19: Systematic Review and Meta-Analysis. Can J Kidney Health Dis. 2020;7:2054358120938573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 6. | Pranata R, Lim MA, Yonas E, Huang I, Nasution SA, Setiati S, Alwi I, Kuswardhani RAT. Thrombocytopenia as a prognostic marker in COVID-19 patients: diagnostic test accuracy meta-analysis. Epidemiol Infect. 2021;149:e40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Barnes GD, Burnett A, Allen A, Blumenstein M, Clark NP, Cuker A, Dager WE, Deitelzweig SB, Ellsworth S, Garcia D, Kaatz S, Minichiello T. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50:72-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 325] [Article Influence: 54.2] [Reference Citation Analysis (1)] |
| 8. | Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 653] [Cited by in RCA: 914] [Article Influence: 152.3] [Reference Citation Analysis (0)] |
| 9. | Porfidia A, Pola R. Venous thromboembolism in COVID-19 patients. J Thromb Haemost. 2020;18:1516-1517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 10. | Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann Intern Med. 2020;173:268-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1577] [Cited by in RCA: 1772] [Article Influence: 295.3] [Reference Citation Analysis (0)] |
| 11. | Abdelwahab HW, Shaltout SW, Sayed Ahmed HA, Fouad AM, Merrell E, Riley JB, Salama R, Abdelrahman AG, Darling E, Fadel G, Elfar MSA, Sabry K, Shah J, Amin H, Nieman GF, Mishriky A, Aiash H. Acetylsalicylic Acid Compared with Enoxaparin for the Prevention of Thrombosis and Mechanical Ventilation in COVID-19 Patients: A Retrospective Cohort Study. Clin Drug Investig. 2021;41:723-732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Bianconi V, Violi F, Fallarino F, Pignatelli P, Sahebkar A, Pirro M. Is Acetylsalicylic Acid a Safe and Potentially Useful Choice for Adult Patients with COVID-19 ? Drugs. 2020;80:1383-1396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 13. | Mohamed-Hussein AAR, Aly KME, Ibrahim MAA. Should aspirin be used for prophylaxis of COVID-19-induced coagulopathy? Med Hypotheses. 2020;144:109975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Chow JH, Khanna AK, Kethireddy S, Yamane D, Levine A, Jackson AM, McCurdy MT, Tabatabai A, Kumar G, Park P, Benjenk I, Menaker J, Ahmed N, Glidewell E, Presutto E, Cain S, Haridasa N, Field W, Fowler JG, Trinh D, Johnson KN, Kaur A, Lee A, Sebastian K, Ulrich A, Peña S, Carpenter R, Sudhakar S, Uppal P, Fedeles BT, Sachs A, Dahbour L, Teeter W, Tanaka K, Galvagno SM, Herr DL, Scalea TM, Mazzeffi MA. Aspirin Use Is Associated With Decreased Mechanical Ventilation, Intensive Care Unit Admission, and In-Hospital Mortality in Hospitalized Patients With Coronavirus Disease 2019. Anesth Analg. 2021;132:930-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 221] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 15. | Group RC; Horby PW, Pessoa-Amorim G, Staplin N, Emberson JR, Campbell M, Spata E, Peto L, Brunskill NJ, Tiberi S, Chew V, Brown T, Tahir H, Ebert B, Chadwick D, Whitehouse T, Sarkar R, Graham C, Baillie JK, Basnyat B, Buch MH, Chappell LC, Day J, Faust SN, Hamers RL, Jaki T, Juszczak E, Jeffery K, Lim WS, Montgomery A, Mumford A, Rowan K, Thwaites G, Mafham M, Haynes R, Landray MJ. Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. 2021. [DOI] [Full Text] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Iglesias J, Watanabe A S-Editor: Liu JH L-Editor: A P-Editor: Liu JH