Published online Jan 9, 2022. doi: 10.5492/wjccm.v11.i1.48
Peer-review started: June 2, 2021
First decision: July 31, 2021
Revised: August 4, 2021
Accepted: December 23, 2021
Article in press: Deccember 23, 2021
Published online: January 9, 2022
Processing time: 216 Days and 7.4 Hours
Since the beginning of corona virus disease 2019 (COVID-19) pandemic, there has been a widespread use of remdesivir in adults and children. There is little known information about its outcomes in patients with end stage renal disease who are on dialysis.
To assess the clinical outcomes with use of remdesivir in adult patients with end stage kidney failure on hemodialysis.
A retrospective, multicenter study was conducted on patients with end stage renal disease on hemodialysis that were discharged after treatment for COVID-19 between April 1, 2020 and December 31, 2020. Primary endpoints were oxygen requirements, time to mortality and escalation of care needing mechanical ventilation.
A total of 45 patients were included in the study. Twenty patients received remdesivir, and 25 patients did not receive remdesivir. Most patients were caucasian, females with diabetes mellitus and hypertension being the commonest comorbidities. There was a trend towards reduced oxygen requirement (beta = -25.93, X2 (1) = 6.65, P = 0.0099, probability of requiring mechanical ventilation (beta = -28.52, X2 (1) = 22.98, P < 0.0001) and mortality (beta = -5.03, X2 (1) = 7.41, P = 0.0065) in patients that received remdesivir compared to the control group.
Larger studies are justified to study the effects of remdesivir in this high-risk population with end stage kidney disease on dialysis.
Core Tip: Little known information exists regarding the efficacy of remdesivir in corona virus disease 2019 patients with end stage renal disease on dialysis. Use of remdesivir was associated with a trend towards reduced oxygen requirement, reduced probability of progression to mechanical ventilation and better prognosis. Larger studies are justified in this high risk, vulnerable population.
- Citation: Selvaraj V, Lal A, Finn A, Tanzer JR, Baig M, Jindal A, Dapaah-Afriyie K, Bayliss G. Efficacy of remdesivir for hospitalized COVID-19 patients with end stage renal disease. World J Crit Care Med 2022; 11(1): 48-57
- URL: https://www.wjgnet.com/2220-3141/full/v11/i1/48.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v11.i1.48
Corona virus disease 2019 (COVID-19) is a clinical syndrome arising from infection with severe acute respiratory syndrome - coronavirus 2 (SARS-CoV-2) coronavirus that has led to several hospitalizations and intensive care unit admissions. Remdesivir, a viral RNA polymerase inhibitor, has demonstrated in vitro activity against viruses such as Middle East Respiratory Syndrome - CoV (MERS-CoV), Ebola, and SARS-CoV1.
In the Adaptive COVID-19 Treatment Trial-1 (ACTT-1), remdesivir was noted to reduce the median time to recovery when compared to the placebo group (10 vs 15 d)[1]. The Infectious Diseases Society of America (IDSA) recommended the use of remdesivir in hospitalized patients with severe COVID-19 with SpO2 < 94%, including patients on supplemental oxygen or mechanical ventilation[2]. The World Health Organization (WHO) issued a ‘weak or conditional’ recommendation against the use of remdesivir in hospitalized COVID-19 patients[3]. Despite this, the use of remdesivir is widespread in hospitalized COVID-19 patients. Many of the clinical trials on remdesivir excluded COVID-19 patients with severe renal dysfunction (CrCl < 30 mL/min/1.73m2). Little is known about clinical outcomes with use of remdesivir in COVID-19 patients with severe renal dysfunction or end-stage renal disease (ESRD) who are on hemodialysis (HD).
As remdesivir has poor water solubility, Sulfobutylether-β-Cyclodextrin (SBECD) is added to the intravenous preparation as a vehicle. Dialysis and renal replacement therapy readily remove SBECD, and significant accumulation of SBECD only occurs when dialysis is held for prolonged periods in ESRD patients. Voriconazole is another medication that has been safely used in patients with kidney failure using the same carrier (SBECD)[4].
Our hypothesis is that the addition of remdesivir to dexamethasone as part of the treatment regimen in COVID-19 patients with ESRD may have impact on the overall length of stay, need for supplemental oxygen, mortality, and mechanical ventilation. The aim of this study was to evaluate the feasibility and efficacy of using remdesivir in patients with COVID-19 and ESRD on HD.
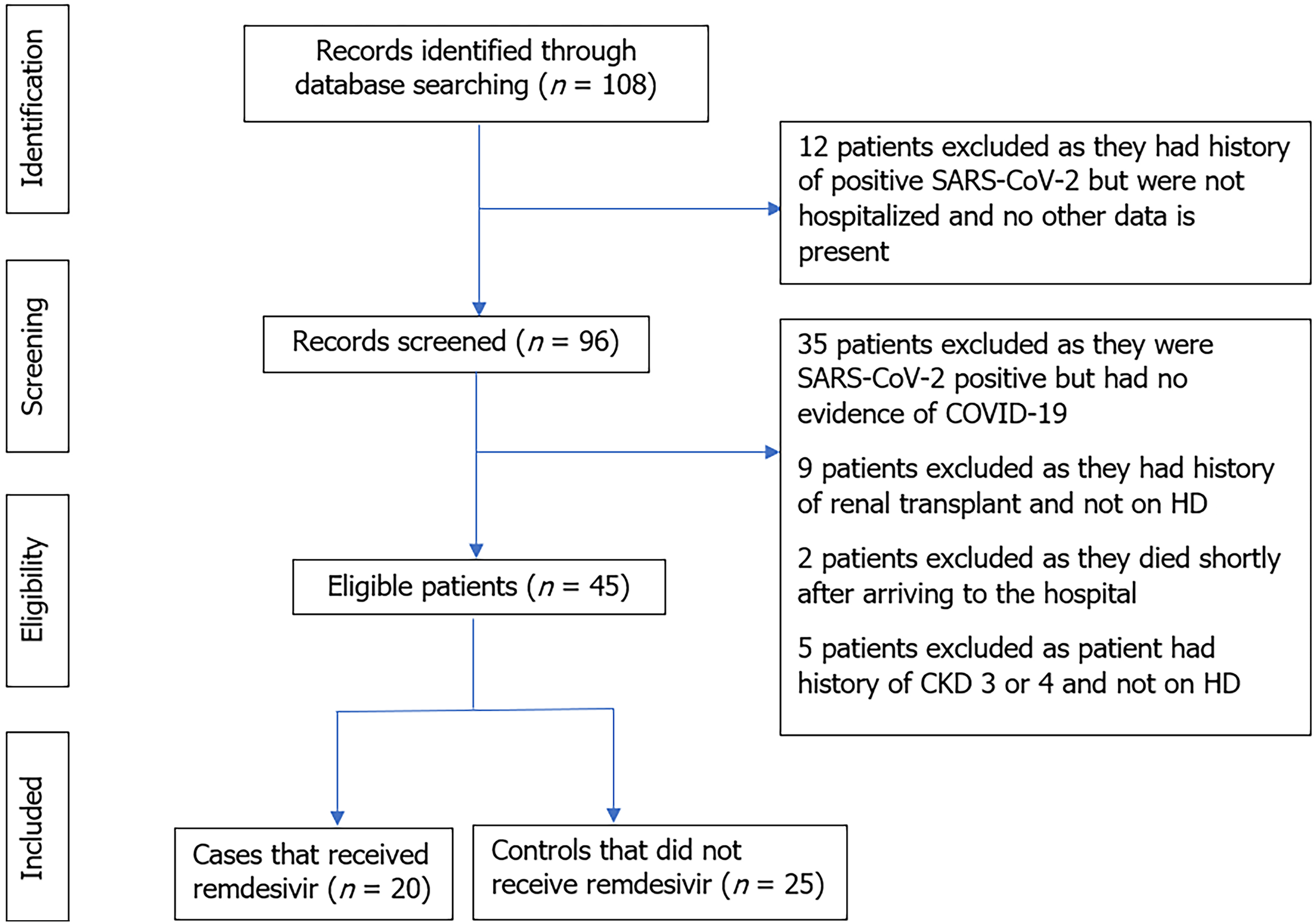
We collected data from two quaternary, acute care hospitals, Rhode Island Hospital (RIH) and The Miriam Hospital (TMH), located in Providence, Rhode Island. All hospitalized patients above the age of 18 years with ESRD on HD from April 1 to December 31, 2020, with a positive polymerase chain reaction (PCR) nasopharyngeal or oropharyngeal SARS-CoV-2 swab were screened for potential study inclusion (Figure 1). ESRD was defined as a GFR of less than 15 mL/min/1.73m2 according to the chronic kidney disease epidemiology collaboration (CKD-EPI) formula. The study was reviewed and approved by the Institutional Review Board of TMH. Data was collected by physicians in the Division of Hospital Medicine at Miriam Hospital (an affiliate of Warren Alpert Medical School of Brown University).
Patients with moderate disease included patients with CRP levels between 50-200 mg/L (normal 0-10 mg/L) and 2-6L/min of oxygen requirement. Patients with severe disease included patients with CRP levels greater than 200 mg/L and oxygen requirements greater than 6 L/min. Prone positioning was instituted in all patients with moderate to severe disease if they could tolerate it.
All patients with ESRD on HD hospitalized with PCR-confirmed COVID-19 in both hospitals were screened for inclusion. To be considered eligible for study inclusion, patients had to meet the following criteria: (1) Hospitalized for at least 48 h, aged ≥18 years; (2) SARS-CoV-2 infection confirmed by RNA PCR test; (3) SpO2 ≤ 94% on room air or requiring supplemental oxygen; and (4) Presence of radiographic evidence of pulmonary infiltrates. These patients were given 200mg of intravenous (iv) remdesivir on day one, followed by 100 mg once daily for 2-10 d or until discharge, death or if there was elevated AST/ALT, with levels greater than ten times the upper limit of normal.
For the purposes of this study, we created a control group consisting of hospitalized ESRD patients on HD with PCR-confirmed COVID-19 who did not receive remdesivir (during the same study period). To identify controls, we screened all patients with ESRD on HD who were admitted to both hospitals from April 1 to December 31, 2020 and did not receive remdesivir. After identifying those patients and to minimize selection bias, we used the following inclusion criteria: (1) Hospitalized for at least 48 h, aged ≥18 years; (2) SARS-CoV-2 infection confirmed by PCR test; (3) SpO2 ≤ 94% on room air or requiring supplemental oxygen; and (4) Presence of radiographic evidence of pulmonary infiltrates.
Patients who met the following criteria were excluded: (1) Patients < 18 years of age; (2) Patients with ESRD who received renal transplant and are not on dialysis; and (3) Patients with AST, ALT > 10 times the upper limit of normal. The Nephrology service at Miriam Hospital (an affiliate of Alpert Medical School of Brown University) followed these patients while they were admitted. Patients also received antibiotics if there was a concern for superimposed bacterial infection in addition to the other interventions keeping in line with the institutional standard of care.
Our primary endpoint was comparing the oxygen requirements, time to mortality and escalation of care needing mechanical ventilation in patients that received remdesivir vs control group.
Data were obtained from the Epic Electronic Medical Record system and recorded in a standardized form. Demographic data, laboratory findings, maximum oxygen requirements in Liters Per Minute (LPM), length of stay (LOS), and comorbid conditions were ascertained. Outcome measures were assessed through the date of study completion, hospital discharge or death; whichever came first.
To compare rates of oxygen and ventilator use, generalized linear modeling was used. Estimation was by maximum likelihood using SAS proc genmod software[5]. Mean oxygen use was modeled first as a normal distribution with an identity link, and the progression to mechanical ventilation was modeled as a binomial distribution with a logit link. For the length of stay and patient disposition, survival analysis was used, estimation by SAS proc phreg[6]. Here the length of stay is modeled as a ratio for patients who discharge vs patients who do not survive. The complete outcome data was available for both the cases and controls until death or discharge from the hospital. The risk of patient health deterioration as a function of time is modeled given covariates. Model selection was based on expert medical knowledge as well as the visual examination of residual plots.
Patient experience of COVID-19 pneumonia is highly variable, differences between patients were modeled as conditional on patient health status. Comparisons were made between patients with diabetes because this is a known risk population that would be highly susceptible to disease. Additionally, to identify the specific patients with severe condition, comparisons were also made based on d dimers. Grouping patients by rate of d dimers was selected because there were clear groupings among respondents. The histogram demonstrated a bimodal distribution, with some patients having very few d dimers, and some having many (skew = 2.64, kurtosis = 7.30). To account for this, patients above the mean were classified as “high d dimer” and patients below the mean classified as “low d dimer.” The three-way interaction could then be modeled as a 2 (remdesivir or control) × 2 (diabetic or not diabetic) × 2 (high or low d dimer) ANOVA style design with interactions. While there were data available on corticosteroids, the observational nature of the study raised concerns that this may be a biased estimate because treatments were not given at random. As the research question mainly focused on the clinical outcomes with use of remdesivir, only patients’ health characteristics were used as control variables, rather than introducing the complexity of various drug interactions within a small study sample.
Before analyzing the data, a brief power analysis was done to calibrate the limi
A total of 108 charts were reviewed, of which only 45 met the inclusion criteria. A total of 20 patients received remdesivir while 25 patients were in the control group. Baseline statistics are reported in Table 1. There was no significant difference in length of stay in patients that received remdesivir (M = 13.00 ± 7.35 d) compared to patients that did not receive remdesivir (M = 12.16 ± 8.38 d). Table 2 has the main effect parameter estimates for the primary research questions and covariates, and Table 3 provides the estimated means by risk group for all three endpoints. Oxygen usage was considered first. The main effect of remdesivir was significant and the parameter was negative, indicating that across patients, those who were on remdesivir tended to use less oxygen (beta = -25.93, X2 (1) = 6.65, P = 0.0099). That said, the three-way interaction term was significant (X2 (1) = 6.37, P = 0.0116), indicating that the means varied based on patient risk conditions. Comparing remdesivir and control groups within risk groups, differences were only significant among patients who did not have diabetes (see Table 3).
| Remdesivir (n = 20) | Control (n = 25) | |
| Mean age (yr) | 64.20 (± 15.16) | 68.32 (± 12.67) |
| Age groups in years (n, %) | ||
| 18-40 | 2 (10) | 1 (4) |
| 41-64 | 5 (25) | 7 (28) |
| Above 65 | 13 (65) | 17 (68) |
| Females (n, %) | 11 (55) | 12 (48) |
| Race or ethnic group (n, %) | ||
| White or Caucasian | 9 (45) | 12 (48) |
| Hispanic | 5 (25) | 9 (36) |
| Black or African American | 2 (10) | 2 (8) |
| Other | 4 (20) | 2 (8) |
| Tobacco use (n, %) | 11 (55) | 14 (56) |
| Diabetes mellitus (n, %) | 13 (65) | 20 (80) |
| Hypertension (n, %) | 19 (95) | 24 (96) |
| Coronary artery disease/peripheral vascular disease (n, %) | 8 (40) | 9 (36) |
| Congestive heart failure (n, %) | 10 (50) | 12 (48) |
| History of lung disease- no. (%) | 6 (30) | 9 (36) |
| Obesity (BMI>30 kg/m2) (n, %) | 8 (40) | 12 (48) |
| Arrhythmia (n, %) | 6 (30) | 9 (36) |
| Length of stay - d (± SD) | 13.00 (± 7.35) | 12.16 (± 8.38) |
| Treatment (n, %) | ||
| Corticosteroids | 20 (100) | 17 (68) |
| Antibiotics | 13 (65) | 13 (52) |
| Therapeutic anticoagulation | 9 (45) | 11 (44) |
| Outcome: Max O2 | Outcome: Ventilation | Outcome: Time to Mortality | |||||||
| Variable | PE | X2 (1) | p | PE | X2 (1) | P value | PE | X2 (1) | P value |
| Age | 0.32 | 3.25 | 0.0712 | 0.04 | 0.56 | 0.4562 | 0.05 | 1.75 | 0.1860 |
| Tobacco use | 8.59 | 3.82 | 0.0507 | 1.29 | 0.91 | 0.3399 | -0.89 | 0.91 | 0.3398 |
| Female Sex | -9.49 | 5.43 | 0.0198 | -2.94 | 3.80 | 0.0511 | 0.05 | < 0.01 | 0.9529 |
| Black, Hispanic, and Other races | 7.02 | 2.69 | 0.1011 | 2.14 | 1.96 | 0.1614 | 1.18 | 1.91 | 0.1672 |
| Obesity | 5.35 | 1.36 | 0.2444 | 1.46 | 0.74 | 0.3904 | 0.32 | 0.16 | 0.6932 |
| Diabetes | -20.59 | 5.21 | 0.0224 | -4.06 | 3.61 | 0.0575 | -4.17 | 9.25 | 0.0024 |
| High d dimers | -21.50 | 2.22 | 0.1358 | -0.01 | < 0.01 | 0.9971 | -5.86 | 7.41 | 0.0065 |
| Remdesivir | -25.93 | 6.65 | 0.0099 | -28.52 | 22.98 | < 0.0001 | -5.03 | 7.42 | 0.0065 |
| D dimers | Diabetes | Condition | Mean | Z | P value | Cohen's d |
| Outcome: Max O2 | ||||||
| High | Yes | Remdesivir | 28.80 | -0.75 | 0.2260 | 0.43 |
| Control | 36.81 | |||||
| No | Remdesivir | 46.23 | 2.38 | 0.0087 | 1.76 | |
| Control | 13.22 | |||||
| Low | Yes | Remdesivir | 13.99 | -0.33 | 0.3712 | 0.09 |
| Control | 15.72 | |||||
| No | Remdesivir | 8.79 | -2.06 | 0.0199 | 1.38 | |
| Control | 34.72 | |||||
| Outcome: Probability of being on a ventilator | ||||||
| D dimers | Diabetes | Condition | % on ventilator | Z | p | Risk ratio |
| High | Yes | Remdesivir | 6.16 | -1.21 | 0.1125 | 0.11 |
| Control | 55.34 | |||||
| No | Remdesivir | 67.92 | -0.07 | 0.4708 | 0.90 | |
| Control | 75.47 | |||||
| Low | Yes | Remdesivir | 8.22 | 0.27 | 0.3955 | 1.62 |
| Control | 5.07 | |||||
| No | Remdesivir | 0.00 | -4.45 | < 0.0001 | 0.00 | |
| Control | 75.66 | |||||
| Outcome: Time to mortality | ||||||
| D dimers | Diabetes | Condition | Hazard ratio | Z | p | Risk ratio |
| High | Yes | Remdesivir | -3.13 | 0.11 | 0.4570 | 5.78 |
| Control | -4.92 | |||||
| No | Remdesivir | -5.98 | -0.02 | 0.4930 | 0.89 | |
| Control | -5.86 | |||||
| Low | Yes | Remdesivir | -4.84 | -0.12 | 0.4512 | 0.52 |
| Control | -4.17 | |||||
| No | Remdesivir | -5.03 | -2.72 | 0.0032 | 0.01 | |
| Control | 0.00 | |||||
Examining the covariates, the only significant finding at alpha = 0.05 was for sex, such that women tended to have lower oxygen need on average (beta = -9.49, X2 (1) = 4.43, P = 0.0198). In addition, there was a trend for older patients and patients who used tobacco toward higher oxygen use (age: beta = 0.32, X2 (1) = 3.25, P = 0.0712; tobacco use: beta = 8.49, X2 (1) = 3.82, P = 0.0507). We anticipate that with larger sample size these results would reach the threshold of statistical significance.
Next the progression to mechanical ventilation was considered. As before, remdesivir use was associated with much better outcome (beta = -28.52, X2 (1) = 22.98, P < 0.0001). The three-way interaction term was not significant, reducing the model fit overall, however the interactions between remdesivir and each of diabetes and high d dimer status was significant (P < 0.0001), indicating dependencies between patient characteristics and health outcomes. Examining the conditional probabilities of mechanical ventilation need, remdesivir was found to be helpful for patients who were not diabetic and had low d dimer values (P < 0.0001). No covariates showed statistically significant association with the risk of needing a ventilator; female sex reached very close to statistical significance (X2 (1) = 3.80, P = 0.0511), indicating less risk of ventilator use on average (beta = 2.94).
Finally, the time to mortality was examined, providing similar results to the previous analyses. The main effect of remdesivir was significant (X2 (1) = 7.41, P = 0.0065) indicating on average patients on remdesivir had a better prognosis (beta = -5.03). The three-way interaction was not significant (X2 (1) = 0.63, P = 0.4262), however all two-way interactions were significant or close to significant (remdesivir-high d dimers: X2 (1) = 3.56, P = 0.0591; remdesivir-diabetes: X2 (1) = 4.59, P = 0.0322; high d dimers-diabetes: X2 (1) = 4.58, P = 0.0324) indicating dependent risks given patient characteristics. Again, it was specifically patients who did not have diabetes and had low d dimers for whom remdesivir demonstrated to significantly reduced risk (P = 0.0032, risk ratio < 0.01). No covariates demonstrated significant association with COVID-19 pneumonia prognosis.
Our study demonstrated a trend towards lesser oxygen requirement in the group of ESRD patients on HD who received remdesivir for the treatment of COVID-19 pneumonia. There was also a trend towards lower progression to mechanical ventilation in patients with COVID-19 that received remdesivir as compared to the control group. There was a trend towards better prognosis in terms of mortality in patients that received remdesivir compared to patients in the control group. However, due to the smaller number this trend did not reach statistical significance. None of the patients’ treatment was interrupted due to hepatotoxicity. To our knowledge, only case series have been previously published on the safety of remdesivir in COVID-19 patients with ESRD.
Remdesivir is a monophosphoramidate prodrug of a nucleoside analogue and an inhibitor of the viral RNA-dependent RNA polymerase (RDRP). Intracellularly, the prodrug is rapidly converted into GS-704277 and subsequently into a monophosphate form that is finally converted into the active triphosphate form. Dephosphorylation of the monophosphate form produces the nucleoside core (GS-441524), which becomes the predominant circulating plasma metabolite. The triphosphate form acts as an analog of adenosine triphosphate (ATP) and competes for incorporation by RDRP, causing premature chain termination and inhibition of viral replication. Originally developed as an investigational drug for Ebola virus, remdesivir has potent in vitro inhibitory activity against SARS-CoV1, MERS coronavirus, and SARS-CoV2. Remdesivir is usually intravenously administered at a dose of 200 mg once followed by 100 mg daily for a total of 5-10 d in adults and children ≥ 40 kg. The plasma t1/2 of parent remdesivir is 1-2 hours, however the t1/2 of GS-441524 is approximately 20-25 hours[9,10]..
The intravenous preparation of remdesivir also contains a solubilizing agent, SBECD. Every 100 mg of remdesivir contains 3-6 g of SBECD (maximum recom
The results from our feasibility study are hypothesis generating. We see interesting trends towards lower oxygen requirements, and reduced progression to mechanical ventilation in the ESRD patients that received remdesivir as a part of the treatment for COVID-19. If remdesivir is an efficacious treatment as hypothesized, it would be expected that patients receiving this treatment would have better outcomes. This was observed in the data, at least for patients who were lower risk (i.e., not diabetic, low d dimer rates). This provides early support for remdesivir, though larger studies could show the effect of remdesivir on these patient centric outcomes.
Our study has many limitations. Firstly, only 68% of the patients in the control group received dexamethasone. However, all the patients in the remdesivir group received dexamethasone. This is mainly because some patients in the control group presented before July 2020 when dexamethasone use was not considered standard of care. In place of dexamethasone, alternative treatments such as hydroxychloroquine and convalescent plasma were used. Steroids were only used in these patients if they were in septic shock requiring vasopressors. Secondly, the sample size was relatively small. The study may not have been adequately powered to detect a significant difference. However, being a feasibility study, we did not expect the results to be statistically significant. Lastly, being a retrospective study, the study design has inherent biases such as selection and confounding biases.
The use of remdesivir in COVID-19 patients with ESRD showed a trend towards lesser oxygen requirements, lower progression to mechanical ventilation and survived longer. Our feasibility study is hypothesis generating and these patterns need further exploration with larger studies. Further research is also needed to study the clinical effects of remdesivir in COVID-19 patients with CKD stage 4 or 5 that are not on hemodialysis.
Little known information exists regarding the efficacy of remdesivir in COVID-19 patients with end stage renal disease on dialysis.
With increasing use of remdesivir in COVID-19 patients we need more information about specific group of patients who could potentially benefit from the use of this medication and its safety profile.
To assess the clinical outcomes with use of remdesivir in adult patients with end stage kidney failure on hemodialysis.
A multicenter, retrospective study was conducted on COVID-19 patients with end stage renal disease on hemodialysis that were discharged from the hospital between April 1st and December 31st, 2020. The primary outcomes were oxygen requirements, time to mortality and escalation of care needing mechanical ventilation.
A total of 45 patients were included in the study. Twenty patients received remdesivir, while 25 patients did not receive remdesivir. Most of the patients were females, Caucasians, and had diabetes mellitus and hypertension as the commonest comorbidities. There was a trend towards reduced oxygen requirement (beta = -25.93, X2 (1) = 6.65, P = 0.0099, probability of requiring mechanical ventilation (beta = -28.52, X2 (1) = 22.98, P < 0.0001) and mortality (beta = -5.03, X2 (1) = 7.41, P = 0.0065) in patients that received remdesivir compared to the control group.
Larger studies are justified to study the effects of remdesivir in this high-risk population with end stage kidney disease on dialysis.
We believe that larger studies (both observational and randomized clinical trials) are warranted to further confirm the findings of this study.
| 1. | Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC; ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;383:1813-1826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5829] [Cited by in RCA: 5220] [Article Influence: 870.0] [Reference Citation Analysis (0)] |
| 2. | Bhimraj A, Morgan RL, Shumaker AH, Lavergne V, Baden L, Cheng VC, Edwards KM, Gandhi R, Muller WJ, O'Horo JC, Shoham S, Murad MH, Mustafa RA, Sultan S, Falck-Ytter Y. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Clin Infect Dis. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 424] [Cited by in RCA: 594] [Article Influence: 99.0] [Reference Citation Analysis (0)] |
| 3. | Rochwerg B, Agarwal A, Siemieniuk RA, Agoritsas T, Lamontagne F, Askie L, Lytvyn L, Leo YS, Macdonald H, Zeng L, Amin W, Burhan E, Bausch FJ, Calfee CS, Cecconi M, Chanda D, Du B, Geduld H, Gee P, Harley N, Hashimi M, Hunt B, Kabra SK, Kanda S, Kawano-Dourado L, Kim YJ, Kissoon N, Kwizera A, Mahaka I, Manai H, Mino G, Nsutebu E, Preller J, Pshenichnaya N, Qadir N, Sabzwari S, Sarin R, Shankar-Hari M, Sharland M, Shen Y, Ranganathan SS, Souza JP, Stegemann M, De Sutter A, Ugarte S, Venkatapuram S, Dat VQ, Vuyiseka D, Wijewickrama A, Maguire B, Zeraatkar D, Bartoszko JJ, Ge L, Brignardello-Petersen R, Owen A, Guyatt G, Diaz J, Jacobs M, Vandvik PO. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 578] [Article Influence: 96.3] [Reference Citation Analysis (0)] |
| 4. | Kiser TH, Fish DN, Aquilante CL, Rower JE, Wempe MF, MacLaren R, Teitelbaum I. Evaluation of sulfobutylether-β-cyclodextrin (SBECD) accumulation and voriconazole pharmacokinetics in critically ill patients undergoing continuous renal replacement therapy. Crit Care. 2015;19:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Hilbe JM. Generalized linear models. The American Statistician. 1994;48:255-265. |
| 6. | Perperoglou A, le Cessie S, van Houwelingen HC. A fast routine for fitting Cox models with time varying effects of the covariates. Comput Methods Programs Biomed. 2006;81:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Schoenfeld DA. Sample-size formula for the proportional-hazards regression model. Biometrics. 1983;39:499-503. [PubMed] |
| 8. | Cohen J. A power primer. Psychol Bull. 1992;112:155-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 2235] [Article Influence: 131.5] [Reference Citation Analysis (0)] |
| 9. | Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, Montgomery SA, Hogg A, Babusis D, Clarke MO, Spahn JE, Bauer L, Sellers S, Porter D, Feng JY, Cihlar T, Jordan R, Denison MR, Baric RS. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1216] [Cited by in RCA: 1161] [Article Influence: 193.5] [Reference Citation Analysis (0)] |
| 10. | Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4289] [Cited by in RCA: 4613] [Article Influence: 768.8] [Reference Citation Analysis (0)] |
| 11. | Adamsick ML, Gandhi RG, Bidell MR, Elshaboury RH, Bhattacharyya RP, Kim AY, Nigwekar S, Rhee EP, Sise ME. Remdesivir in Patients with Acute or Chronic Kidney Disease and COVID-19. J Am Soc Nephrol. 2020;31:1384-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 12. | Sörgel F, Malin JJ, Hagmann H, Kinzig M, Bilal M, Eichenauer DA, Scherf-Clavel O, Simonis A, El Tabei L, Fuhr U, Rybniker J. Pharmacokinetics of remdesivir in a COVID-19 patient with end-stage renal disease on intermittent haemodialysis. J Antimicrob Chemother. 2021;76:825-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Luke DR, Tomaszewski K, Damle B, Schlamm HT. Review of the basic and clinical pharmacology of sulfobutylether-beta-cyclodextrin (SBECD). J Pharm Sci. 2010;99:3291-3301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 168] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 14. | Davis MR, Pham CU, Cies JJ. Remdesivir and GS-441524 plasma concentrations in patients with end-stage renal disease on haemodialysis. J Antimicrob Chemother. 2021;76:822-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Noncommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Society of Critical Care Medicine, American College of Physicians, American College of Chest Physicians.
Specialty type: Critical care medicine
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Omar BJ S-Editor: Wang LL L-Editor: A P-Editor: Wang LL