Published online Sep 9, 2021. doi: 10.5492/wjccm.v10.i5.183
Peer-review started: February 21, 2021
First decision: May 6, 2021
Revised: May 16, 2021
Accepted: July 29, 2021
Article in press: July 29, 2021
Published online: September 9, 2021
Processing time: 199 Days and 11.2 Hours
The novel coronavirus, which was declared a pandemic by the World Health Organization in early 2020 has brought with itself major morbidity and mortality. It has increased hospital occupancy, heralded economic turmoil, and the rapid transmission and community spread have added to the burden of the virus. Most of the patients are admitted to the intensive care unit (ICU) for acute hypoxic respiratory failure often secondary to acute respiratory distress syndrome (ARDS). Based on the limited data available, there have been different opinions about the respiratory mechanics of the ARDS caused by coronavirus disease 2019 (COVID-19). Our article provides an insight into COVID-19 pathophysiology and how it differs from typical ARDS. Based on these differences, our article explains the different approach to ventilation in COVID-19 ARDS compared to typical ARDS. We critically analyze the role of positive end-expiratory pressure (PEEP) and proning in the ICU patients. Through the limited data and clinical experience are available, we believe that early proning in COVID-19 patients improves oxygenation and optimal PEEP should be titrated based on individual lung compliance.
Core Tip: Optimizing and titrating the positive end-expiratory pressure (PEEP) in acute respiratory distress syndrome (ARDS) patients has been studied widely in the critical care world. However, the ARDS caused by coronavirus disease 2019 (COVID-19) possesses a challenge due to relatively preserved compliance in the early phase of this disease and questions the guidelines which have been long established. Proning, though tedious and cumbersome, which has been traditionally proved to improve oxygenation and survival benefits in ARDS patients has been extensively applied in COVID-19 patients. This article critically analyzes the role of PEEP and proning in COVID-19 patients.
- Citation: Gandhi KD, Sharma M, Taweesedt PT, Surani S. Role of proning and positive end-expiratory pressure in COVID-19. World J Crit Care Med 2021; 10(5): 183-193
- URL: https://www.wjgnet.com/2220-3141/full/v10/i5/183.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v10.i5.183
As of February 2021, coronavirus disease 2019 (COVID-19) has accounted for over 3 million deaths worldwide and over 500000 deaths in the United States alone according to the World Health Organization[1]. In a study done in New York City, including 5700 hospitalized COVID-19 patients, 14.2% of patients required intensive care unit (ICU), and 90% of the patient admitted to the ICU were mechanically ventilated[2]. In a small study done with 245 patients, 20% of hospitalized COVID-19 patients were triaged to the ICU secondary to worsening respiratory failure and acute respiratory distress syndrome (ARDS)[3]. Timing of intubation has been a matter of debate for years but given the pandemic, it is more important now than ever to evaluate the risk and benefits associated with early or late intubation. While the early intubation strategy was used in the earlier phases of the pandemic, it was found that early intubation is associated with higher mortality, and the decision to mechanically ventilate the patient should be made cautiously for each patient[4]. Given the high burden of the ICU admission and mechanical ventilation associated with COVID-19 infection, it is imperative to understand the underlying respiratory mechanics related to ARDS and to critically review the application of traditional ventilation management on this novel disease.
ARDS is defined as new or worsening non-cardiogenic respiratory failure with PaO2 to FiO2 ratio less than 300 and presence of bilateral infiltrates on the imaging occurring within 1 wk of original clinical insult as mentioned in Table 1. ARDS severity can be further categorized based on the PaO2/FiO2 ratio (P/F ratio), where severity is significantly associated with mortality as shown in Table 2[5].
| ARDS definition | |
| Onset | Within 1 wk of a known clinical insult or new or worsening respiratory symptoms |
| Chest imaging | Bilateral opacities – not fully explained by effusions, lobar/lung collapse, or nodules on either Chest X-ray or computed X-ray tomography scan |
| Origin of edema | Respiratory failure not fully explained by heart failure or fluid overload; Need objective assessment (e.g., echocardiogram) to exclude hydrostatic edema if no risk factors present |
| Oxygenation | PaO2/FiO2 ratio < 300 with PEEP > 5 cm/H2O |
| PaO2/FiO2 ratio (with PEEP > 5 cm/H2O) | ARDS severity | Mortality (95%CI) |
| 200-300 | Mild | 27% (24-30) |
| 100-200 | Moderate | 32% (29-34) |
| < 100 | Severe | 45% (42-48) |
The basic etiology for ARDS includes non-cardiogenic pulmonary edema, shunt-related hypoxemia, and reduced aeration of lungs thus contributing to decreased lung compliance. Management of ARDS as outlined by ARDSnet protocol includes low tidal volume, optimizing PEEP for plateau pressure less than 30, prone positioning[6].
Optimizing PEEP by titrating it, increases pressure at the end of expiration and keeps the damaged alveoli open to facilitate ventilation. Low tidal volume decreases transpulmonary pressure and decreases the risk for ventilator-induced lung injury. Some studies have shown driving pressure as a predictor of mortality in ARDS patients[7]. Driving pressure is measured by subtracting the PEEP from the plateau pressure, which can also be expressed as the ratio of tidal volume and respiratory system compliance. Prone positioning enhances oxygen saturation by improving the ventilation-perfusion ratio by redistributing the blood flow to the better-ventilated lung units.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an enveloped, positive-stranded RNA virus. The virus has a great affinity for human angiotensin-converting enzyme (ACE)-2 receptors, which are expressed mainly on Type II pneumocytes but also upper respiratory tract epithelial cells, vascular endothelium, and small intestine enterocytes. Viral infection results in excessive immune response leading to a cytokine storm and thus resulting in systemic inflammatory syndrome and multiorgan failure. It is also believed that viral infection also results in endothelial dysfunction, increased thrombin formation, thus stimulating a hypercoagulable state and thrombosis. This in turn causes thrombosis of the pulmonary vasculature, leading to hypoxic respiratory failure. The exact patho
Histopathological study of lungs affected by SARS-CoV-2 as compared to H1N1 and SARS provides further insight into the pathophysiology underlying this disease. Histopathologically, acute lung injury includes diffuse alveolar damage (DAD), acute fibrinous and organizing pneumonia (AFOP), and organizing pneumonia (OP).
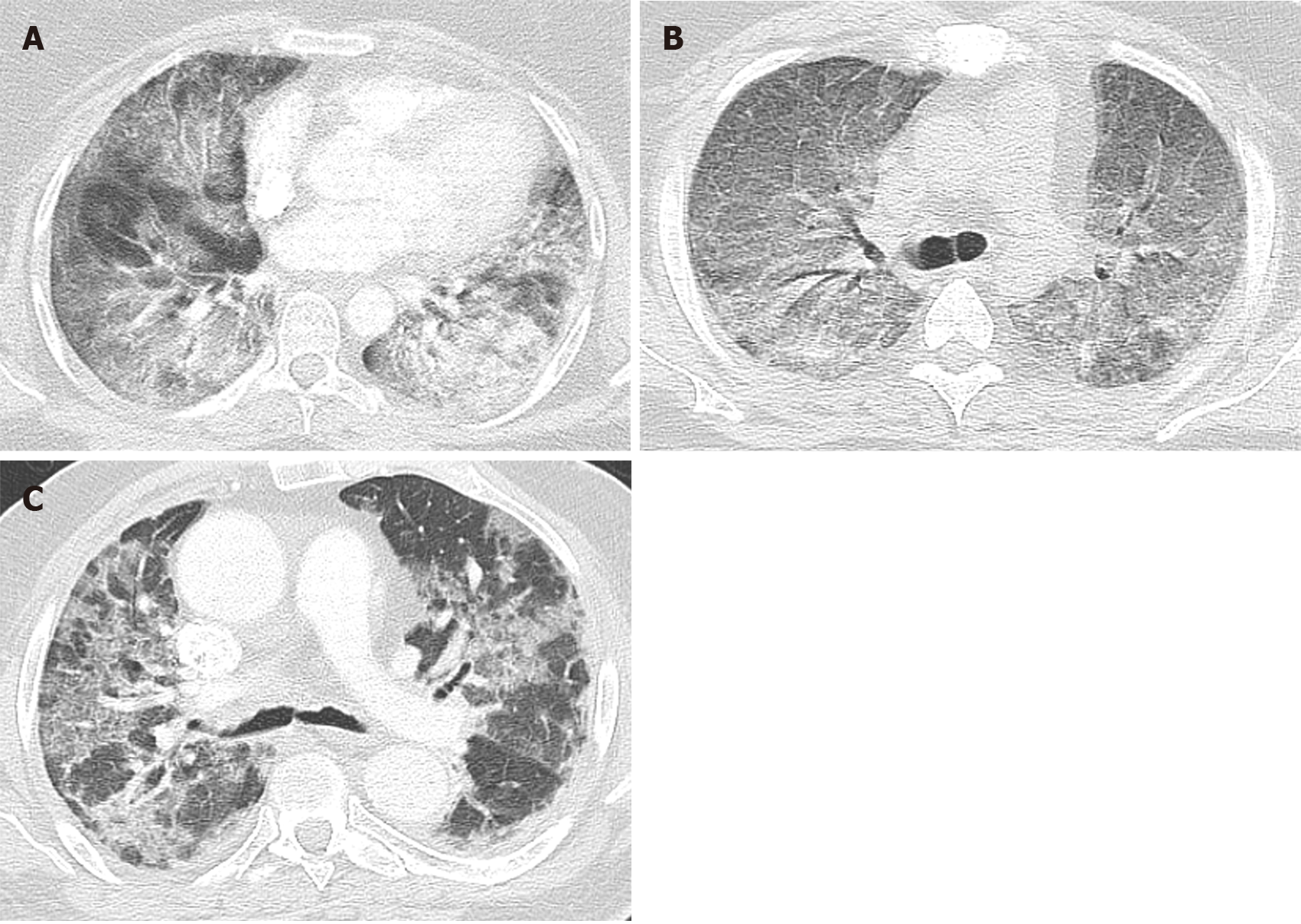
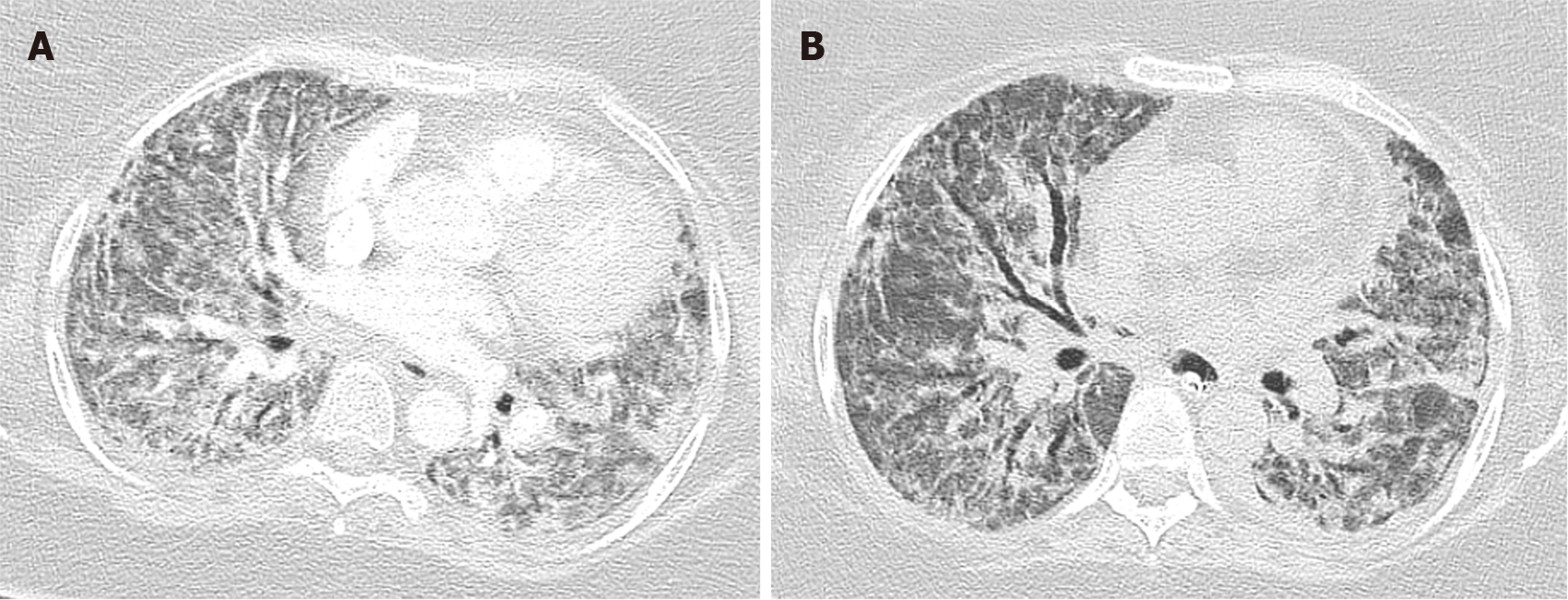
Diffuse alveolar damage is the most common pattern seen in typical ARDS patients, which is the most severe form of acute lung injury. It is caused by alveolar and endothelial cell damage causing fluid and cellular exudation and disruption of the blood-air barrier. DAD is divided into three phases: (1) Acute exudative phase: It occurs within 1 wk of the injury. It is characterized by damage to the alveolar wall causing hyaline membrane formation, edema, and alveolar membrane thickening. Vascular thrombosis and microthrombi are also frequently seen in DAD, even in absence of a systemic hypercoagulable state as a result of local inflammation. Angiographic studies done on typical ARDS patients have also shown the presence of thrombosis in its early phase. Chest imaging within 24 h to 48 h may be normal. Computed-tomography (CT) of the chest in acute phase of ARDS after 48 h commonly shows bilateral diffused patchy opacity with ventro-dorsal gradient of density predominant in dependent area (Figure 1A)[9]. Bilateral ground-glass opacity (Figure 1B) and crazy paving pattern can also be found in early phase (Figure 1C); (2) Subacute organizing phase or proliferative phase: It occurs 1 wk after the initial pulmonary injury and is characterized by fibrin organization, fibroblast migration, and collagen secretion. intra-alveolar hyaline membrane gets organized into fibrotic tissue. Reactive atypical changes in type II pneumocytes and squamous metaplasia is also noted. Some DAD resolves after this phase, whereas others progress to the chronic fibrotic phase. Diffuse coarse reticular opacity can be found on chest imaging in this phase (Figure 2A)[9]; and (3) Chronic fibrotic phase: It occurs weeks to months after the initial injury and is characterized by progressive architectural remodeling and interstitial fibrosis. CT chest typically reveals persistent ground-glass densities and coarse reticulations (Figure 2B)[9]. DAD is considered as the pathognomonic histological feature of ARDS. It can be present in isolation or in combination with AFOP and/or OP.
AFOP is characterized by fibrin balls in alveoli with organization caused by fibroblast migration and collagen secretion. It can be seen along with DAD. OP can also be seen either in isolation or with DAD or AFOP. It is characterized by intraluminal tufts of fibroblasts and immature collagen tissue in alveolar ducts and distal airspaces.
A study showed that early SARS-CoV-2 is associated with diffuse alveolar damage characterized by vascular congestion, intra-alveolar edema, patchy inflammatory cellular infiltration but hyaline membrane formation is not prominent. Hyaline thrombi were found in the blood vessels. Whereas late stage of SARS-CoV-2 infection has a combination of diffuse alveolar damage and microvascular damages resulting in fibrinous exudation characteristic of AFOP[10].
A study was conducted to find the difference in lung histopathology in patients affected by SARS, 2009-H1N1 Influenza and SARS-CoV-2. It revealed that the early phase of ARDS affecting the lungs including DAD, AFOP, organizing fibrosis, end-stage fibrosis, and superimposed pneumonia are equally distributed amongst the three causative factors. However, microthrombi and pulmonary thrombosis are more commonly seen in lungs affected by SARS and SARS-CoV-2 viruses as shown in Table 3[11].
| Virus | Number of patients | Diffuse alveolar damage, n (%) | AFOP, n (%) | Organizing fibrosis, n (%) | End-stage fibrosis, n (%) | Superimposed pneumonia, n (%) | Microthrombi, n (%) | Pulmonary thrombosis, n (%) |
| 2009 H1N1 | 287 | 90 | 0.30 | 40 | 3 | 30 | 24 | 6 |
| SARS | 64 | 98 | 9 | 47 | 6 | 31 | 58 | 28 |
| SARS-CoV-2 | 171 | 88 | 4 | 52 | 1 | 32 | 57 | 15 |
Though COVID-19 meets the ARDS criteria based on the Berlin definition, it differs in the way that COVID ARDS has severe hypoxemia with near-normal respiratory system compliance. Gattinoni et al[12] postulated the different phenotypes of COVID pneumonia requiring different approaches to the management.
COVID ARDS can be divided into early phase L type pneumonia and late phase H type pneumonia: (1) L type is characterized by low-weight lungs with low elastance and preserved compliance. These lungs have low recruitability as the amount of non-aerated lung is less. These patients are characterized to be less dyspneic with near-normal compliance. Gattinoni postulated the hypothesis of pulmonary vasoplegia causing hypoxemia. However, various other theories are postulated including damage to the ACE-2 receptors and upregulation of ACE-1 receptors resulting in uneven pulmonary vasoconstriction and hypoxemia; and (2) H type is characterized by high weight lungs with high elastance and decreased compliance. These lungs have increased recruitability due to extensively collapsed lungs. These patients fit into the characteristic feature of ARDS. Hypoxemia is caused by systemic inflammatory syndrome causing alveolar damage.
These phenotypes are a topic of debate as many scholars postulate that these phenotypes are a mere progression of ARDS in which L type is consistent with mild ARDS and H type is consistent with severe ARDS. Gattinoni described these phenotypes based on the study of 16 patients with COVID-19 showing significantly normal compliance and increased shunt fraction compared to typical ARDS patients. However, there have been multiple follow-up studies showing the presence of similar mechanics in the typical ARDS patients with near-normal respiratory system compliance in mild ARDS[13]. The study done in New York amongst 257 patients showed that the baseline respiratory mechanics was comparable to the typical ARDS patients. Per the study, 25% of the patients enrolled did have compliance greater than 38 mL/cm H2O, however, such heterogeneity is also seen in typical ARDS patients[13]. Lower compliance in COVID ARDS has also been seen in smaller studies from Seattle and Boston with median compliances of 29 and 35 respectively[14,15]. Another study showed the heterogeneity amongst compliance and dissociation between respiratory compliance system and hypoxemia in non-COVID ARDS patients. Amongst 1117 ARDS patients, one out of eight patients had preserved compliance whereas three out of four patients had poor respiratory compliance. The study showed that of the patients with preserved compliance, 43% had moderate to severe ARDS with P/F ratio < 150. It also showed an increase in mortality associated with patients with lower respiratory compliance[16]. Thus, the different phenotypes proposed by Gattinoni et al[12] requires further investigation to know whether it is characteristic of typical ARDS or is mainly applicable to COVID ARDS.
While as per Gattinoni et al[12], silent hypoxemia is caused by near-normal respiratory compliance, Tobin et al[17] believe that silent hypoxemia is secondary to underlying following physiological mechanisms.
Per Tobin et al[17], dyspnea is caused by stimulation of respiratory centers which are oversensitive to PaCO2 whereas a decrease in PaO2 from 90 mmHg to 60 mmHg results in no stimulation, and also a drop in PaO2 less than 60 mmHg results in dyspnea in only half of the subjects. Thus, response to hypoxia is influenced by PaCO2. Studies have shown blunted response to hypoxia in elderly and diabetic patients.
The shift of oxygen dissociation curve brought in by increased temperature seen in COVID-19 patients results in a decreased level of saturation even at higher PaO2. Given the carotid bodies are sensitive to PaO2 and not oxygen saturation, the chemoreceptors are not activated, resulting in silent hypoxia. Oxygen saturation measured by pulse oximetry is less reliable once saturation drops below 80%, and the true saturation measured by arterial-blood gas could be 10% higher than that measured by pulse oximetry.
Thus, given the differing thoughts for the underlying physiology, the management approach of the two experts differs widely as shown in Table 4[18,19]. While Gattinoni et al[12] believes in early intubation and mechanical ventilation to prevent patient-self-induced lung injury, Tobin et al[17] believe intubation is a rescue maneuver reserved for hypoxic patients in severe respiratory distress.
| Gattinoni et al[12] | Tobin et al[17] |
| Silent hypoxemia is caused by vasoplegia which increases the respiratory drive and increases the tidal volume, causing negative intrathoracic pressure. Dyspnea is not endorsed in the setting of near-normal respiratory compliance | Silent hypoxemia is caused by underlying physiologic mechanism such as fever causing right shift of oxygen dissociation curve, unreliability of pulse oximeter at SaO2 < 80% and decreased chemoreceptor response to PaO2 < 60 mmHg with normocapnia |
| Increased tidal volume causing progressive increase in negative intrathoracic pressure results in P-SILI | P-SILI needs further research and increase in tidal volume is not associated with requiring intubation, whereas, underlying critical condition leads to intubation |
| Esophageal manometric measurement of work of breathing is crucial to determine the inspiratory efforts of the patient. Esophageal pressure > 15 is associated with increased risk of lung injury and patient should be intubated as early as possible | No data available to support the arbitrary measurement of esophageal pressure as an indication of intubation. Also, insertion of esophageal balloon in dyspneic COVID-19 patients increases the risk for intubation |
| Early intubation is advised along with esophageal manometric measurement of work of breathing | Less liberal use of intubation and mechanical ventilation. Should be used when hypoxia is accompanied with increased work of breathing and severe respiratory distress |
| Spontaneous breathing trials should be implemented only at the end of the weaning process as strong spontaneous efforts raise oxygen demand, edema and P-SILI | Weaning and spontaneous breathing trial should be initiated as early as 24 h after initial intubation |
PEEP applies pressure to the lung during exhalation, thereby, decreasing atelectasis and improving ventilation-perfusion (VQ) mismatch. In general, patients are typically maintained at the PEEP of 5 because it is thought to mimic physiological conditions. PEEP is titrated based on driving pressure and the PEEP-FiO2 table provided by ARDSnetwork guidelines[20]. If a patient requires higher FiO2, increasing the PEEP further improves the oxygen saturation and thereby, allows to lower the FiO2 to safer levels (< 0.60). PEEP can also be titrated by measuring transpulmonary pressure with the help of esophageal manometry or by studying the pressure-flow curve on the ventilator[21].
Optimal PEEP is PEEP that maximizes potential benefit (better oxygenation and less atelectrauma) and minimizes potential harm (hemodynamic compromise, volutrauma, and increased dead space). Excessive PEEP can decrease venous return and thus, reducing cardiac output and resulting in hemodynamic compromise. It can also increase volutrauma if excessive PEEP is applied and theoretically can cause VQ mismatch by creating physiologic dead space by improving ventilation and decreased perfusion. Thus, optimal PEEP is essential in managing ventilation in patients with acute respiratory distress syndrome[22].
Higher PEEP does not significantly improve the oxygen in all hypoxic patients. Presumably, PEEP helps only if there are atelectatic lung units that can be recruited. Studies in typical ARDS have also shown that increasing the PEEP in “non-recruitable” lungs results in a further decrease in P/F ratio whereas, in patients with “recruitable” lungs results in improving oxygenation.
Multiple small studies are available that discuss the effects of higher vs lower PEEP on oxygenation and compliance in COVID patients. A study of 14 mechanically ventilated patients showed that a decrease in PEEP resulted in an increase in lung compliance and a decrease in dead space ventilation in 13 out of 14 patients whereas in 1 patient it showed an increase in respiratory compliance with an increase in PEEP[23]. Another study done in Greece including 17 mechanically ventilated patients within 2-3 d of intubation, showed a decrease in PEEP by 25%-30% increasing the respiratory compliance and a decrease in hypercapnia with no change in P/F ratio[24]. A study matched 30 patients of COVID ARDS with typical ARDS patients and showed the difference in respiratory mechanics at PEEP of 5 and 15. There was a significant increase in the P/F ratio with an increase in PEEP in both COVID ARDS and typical ARDS with no significant change in compliance at either of the PEEP level. In COVID-19 patients, lung recruitment was independent of the oxygenation and respiratory mechanic changes due to PEEP[25]. Some studies used recruitment to inflation ratio (R/I) which is defined as the ratio between the compliance of recruited lung to that of the respiratory system, as a measure of recruitability. R/I ratio of > 0.5 suggested more potential for lung recruitment with respect to lung inflation. In a small study involving 12 mechanically ventilated patients, lower PEEP was used in poorly recruitable lungs whereas higher PEEP was applied to patients with highly recruitable lungs, however, the difference in respiratory mechanics with different values of PEEP was not studied further[26]. Beloncle et al[27] in a study of 25 patients divided into highly and poorly recruitable lungs based on R/I ratio showed there was no difference in respiratory compliance at PEEP of 5 cm and 15 cm/H2O in both the group of patients, whereas the recruited lung volume was significantly higher at PEEP of 15 compared to a PEEP of 5 in patients with highly recruitable lungs compared to those with poor recruitability. The study also revealed that the P/F ratio was significantly higher at PEEP of 15 cm/H2O in patients with higher recruitability as compared to a PEEP of 5, however, no difference in the P/F ratio with a change in PEEP was noticed in the lower recruitability group. In a small study with 19 typical ARDS patients (non-COVID), 9 patients were recruitable where oxygenation improved with high PEEP, whereas the other 10 patients did not show significant improvement in oxygen saturation with high PEEP[28]. Similar findings with the heterogeneity in the respiratory system compliance have been found in the COVID ARDS, though the presence of higher compliance is seen more in COVID ARDS which might be consistent with mild ARDS.
Thus, we believe that COVID ARDS though has higher compliance, PEEP should be optimized and individualized for each patient based on titration according to FiO2 or esophageal manometry.
Mechanisms by which proning improves oxygenation are still debated. In ARDS patients, dorsal lung units are involved more with relative sparing of ventral lung units. However, due to gravitational force, perfusion is better in the dorsal lung units compared to the ventral units. Proning helps redistribution of the blood flow, thus causing the well-aerated ventral units to have more perfusion[29]. Similarly, proning also improves ventilation in the dorsal lung units, thus improving ventilation-perfusion match. Proning also encourages the drainage of secretion from the lungs. Though proning improves oxygenation, its effect tends to decrease over time and not all patients respond to proning. Traditionally, in ARDS, proning has been shown to improve oxygenation in multiple studies, however, only the PROSEVA trial has shown survival benefits[30]. PROSEVA study included ARDS patients with a P/F ratio < 150, who were prone for >16 h/d for an average of 4 d. Study showed 16% mortality with prone positioning compared to 33% mortality in supine positioning (P value < 0.001).
In hypoxic respiratory failure caused by COVID-19, proning has been extensively applied in both non-intubated awake patients and intubated patients[31]. Though many studies are available, the sample size of each study is very limited[32]. Multiple studies showed that early proning in non-intubated awake patients improves oxygenation and results in the prevention of intubation. A study revealed that early awake proning combined with high flow nasal cannula in 10 COVID-19 patients in China resulted in the prevention of intubation[33], though the study is limited by the sample size. At baseline, these patients' PF ratio varied from 89 to 200, thus, having a varied spectrum of diseased patients, and patients were prone for 16 h/d or less as tolerated. After prone positioning, median PaCO2 increased slightly whereas P/F ratio was significantly elevated[32]. Another study showed that early proning in non-intubated patients improves oxygen saturation and decreases respiratory rate. This study also showed a 90-d mortality benefit in prone patients compared to patients who were not prone amongst 60 patients with severe hypoxia secondary to COVID infection[34]. Various other studies including non-intubated, awake patients showed improvement in oxygenation and improved respiratory comfort. Caputo et al[35] revealed that self proning improved oxygen saturation from 84% to 94% in all 50 ED patients included and avoided intubation in 76% of the patients. The remaining 24% of patients showed no significant improvement in oxygenation and required intubation within 24 h of admission. Elharrar et al[36], included 24 awake, non-intubated patients, of which only 63% tolerated proning for > 3 h and of which improvement of oxygenation was seen in 25% of the patients, but oxygenation returned to baseline on supination. In Italy, Sartini et al[37] showed that in 15 non-intubated, awake patients on non-invasive ventilation, early proning showed significant improvement in oxygenation during pronation whereas 80% had sustained improvement even after pronation, whereas 6% worsened after pronation. All the patients had a significant decrease in respiratory rate both during and after pronation. Coppo et al[38] revealed that of 56 included patients, 47 patients could tolerate proning, of which all the patients had significant improvement in oxygenation immediately after proning whereas improved oxygenation was maintained in only 50% of patients after resupination. A few of the relevant studies are shown in Table 5.
Thus, all the studies did show the improvement in oxygenation, however, are limited by the sample size and not all studies showed whether the improvement in oxygenation was sustained. Evidence for the effect on long-term outcomes and endpoints, such as mortality and rate of intubation is lacking. The conclusion is made mainly from case series and case reports, rather than clinical trials. Thus, the low quality of evidence available in support of awake proning needs to be critically analyzed and further researched.
Amongst the ventilated patients with typical and COVID-19 ARDS, proning has been shown to improve oxygenation. Of the 42 intubated patients of COVID-19 ARDS, proning showed initial improvement in oxygenation and P/F ratio. Mortality amongst these patients was 21.4% similar to the PROSEVA study[39]. In another study, among 31 patients who underwent prone ventilation, the P/F ratio increased from a median of 150 mmHg in the supine position to 232 mmHg in the prone position and compliance increased from 33 cm/H2O to 36 cm/H2O. The P/F ratio and compliance were maintained 72 h after initial prone ventilation[15]. In the earlier studies done in China, early prone ventilation amongst 29 patients was significantly associated with improved prognosis and improved oxygenation after 7 d of proning[40].
Proning is not without its complication. Venous stasis can lead to facial and ocular edema, whereas arm extension can lead to brachial plexus neuropathy[41]. Pressure ulcers and pressure necrosis are also common in prone positioning. Thus, additional support should be applied at pressure points such as shoulder, face, and anterior pelvis and frequent repositioning are necessary. Mechanical complications such as device displacement, including dislodging of the endotracheal tube and central lines are also commonly seen in the prone position. In some patients, hemodynamic compromise or oxygen desaturation may also occur. A Specialized prone team consisting of 3-5 members should be employed in each hospital and special attention should be paid to the endotracheal tube and central lines.
Though proning has been shown to improve oxygenation in each study, the technical difficulties associated with it are cumbersome. In the event of a cardiac arrest in a prone patient, even with the help of the expert team, it takes at least 5 min to resupinate the patient and with the risk of displacement of the endotracheal tube. Disconnection of the central lines and injury to staff and/or patients can occur. Prone cardiopulmonary resuscitation (CPR) has been used previously in neurosurgical patients where turning the patients would result in neural damage. During prone CPR, chest compressions are applied over the scapula or thoracic spine with or without counter-pressure on the sternum. Defibrillation can also be done by placing the defibrillator pads on specific locations among the prone patients[42]. Newer methods to do prone CPR, echocardiogram, central line placement have been adopted to accommodate proning as a therapeutic intervention. In our clinical experience, even bronchoscopy can be done in the prone positioning.
Proning is contraindicated in patients with a spinal fracture, whereas it is relatively contraindicated in patients with long bone fractures, increased intracranial pressure, and an open abdomen. Massive obesity should not be considered as a contraindication[43].
This review is limited by the small number of studies available to provide adequate evidence. Sample size of all these studies is also very small, limiting our conclusion. Thus, we encourage large randomized study to help provide more concrete information on approaching the ventilation for COVID-19 patients.
For patients suffering from COVID-19, early proning is an inexpensive therapeutic intervention to improve oxygenation. In patients with ARDS secondary to COVID-19, PEEP should be titrated individually based on the compliance of the respiratory system and proning should still be encouraged given drastic improvement in oxygenation. Further randomized clinical trials are suggested among the COVID patients to address these important clinical issues.
| 1. | World Health Organization. WHO COVID-19 Dashboard. 2021 [cited 21 January 2021]. Available from: https://covid19.who.int/table. |
| 2. | Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; Consortium and the NC-19 R. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052-2059. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 6573] [Article Influence: 1095.5] [Reference Citation Analysis (0)] |
| 3. | Hashmi MD, Alnababteh M, Vedantam K, Alunikummannil J, Oweis ES, Shorr AF. Assessing the need for transfer to the intensive care unit for Coronavirus-19 disease: Epidemiology and risk factors. Respir Med. 2020;174:106203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Wunsch H. Mechanical Ventilation in COVID-19: Interpreting the Current Epidemiology. Am J Respir Crit Care Med. 2020;202:1-4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 157] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 5. | Force TADT; V Marco Ranieri; Gordon D Rubenfeld, B Taylor Thompson, Niall D Ferguson, Ellen Caldwell, Eddy Fan, Luigi Camporota, Arthur S Slutsky. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA. 2012;307:2526-2533. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1812] [Cited by in RCA: 4416] [Article Influence: 315.4] [Reference Citation Analysis (0)] |
| 6. | Acute Respiratory Distress Syndrome Network. Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8487] [Cited by in RCA: 8456] [Article Influence: 325.2] [Reference Citation Analysis (3)] |
| 7. | Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, Richard JC, Carvalho CR, Brower RG. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1397] [Cited by in RCA: 1702] [Article Influence: 154.7] [Reference Citation Analysis (3)] |
| 8. | Behera S, Jha S, Singh N, Khilnani G, Mahajan A, Kumar S, Kumar A, Sant S. COVID-19: What we all intensivists should know. Saudi Crit Care J. 2020;4:45-57. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Zompatori M, Ciccarese F, Fasano L. Overview of current lung imaging in acute respiratory distress syndrome. Eur Respir Rev. 2014;23:519-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | von der Thüsen J, van der Eerden M. Histopathology and genetic susceptibility in COVID-19 pneumonia. Eur J Clin Invest. 2020;50:e13259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Hariri LP, North CM, Shih AR, Israel RA, Maley JH, Villalba JA, Vinarsky V, Rubin J, Okin DA, Sclafani A, Alladina JW, Griffith JW, Gillette MA, Raz Y, Richards CJ, Wong AK, Ly A, Hung YP, Chivukula RR, Petri CR, Calhoun TF, Brenner LN, Hibbert KA, Medoff BD, Hardin CC, Stone JR, Mino-Kenudson M. Lung Histopathology in Coronavirus Disease 2019 as Compared with Severe Acute Respiratory Sydrome and H1N1 Influenza: A Systematic Review. Chest. 2021;159:73-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 135] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 12. | Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, Camporota L. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099-1102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1016] [Cited by in RCA: 1211] [Article Influence: 201.8] [Reference Citation Analysis (0)] |
| 13. | Schenck EJ, Hoffman K, Goyal P, Choi J, Torres L, Rajwani K, Tam CW, Ivascu N, Martinez FJ, Berlin DA. Respiratory Mechanics and Gas Exchange in COVID-19-associated Respiratory Failure. Ann Am Thorac Soc. 2020;17:1158-1161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 14. | Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, Greninger AL, Pipavath S, Wurfel MM, Evans L, Kritek PA, West TE, Luks A, Gerbino A, Dale CR, Goldman JD, O'Mahony S, Mikacenic C. Covid-19 in Critically Ill Patients in the Seattle Region - Case Series. N Engl J Med. 2020;382:2012-2022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1819] [Cited by in RCA: 1871] [Article Influence: 311.8] [Reference Citation Analysis (0)] |
| 15. | Ziehr DR, Alladina J, Petri CR, Maley JH, Moskowitz A, Medoff BD, Hibbert KA, Thompson BT, Hardin CC. Respiratory Pathophysiology of Mechanically Ventilated Patients with COVID-19: A Cohort Study. Am J Respir Crit Care Med. 2020;201:1560-1564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 329] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 16. | Panwar R, Madotto F, Laffey JG, van Haren FMP. Compliance Phenotypes in Early Acute Respiratory Distress Syndrome before the COVID-19 Pandemic. Am J Respir Crit Care Med. 2020;202:1244-1252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 17. | Tobin MJ, Laghi F, Jubran A. Why COVID-19 Silent Hypoxemia Is Baffling to Physicians. Am J Respir Crit Care Med. 2020;202:356-360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 435] [Cited by in RCA: 381] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 18. | Tobin MJ, Laghi F, Jubran A. Caution about early intubation and mechanical ventilation in COVID-19. Ann Intensive Care. 2020;10:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 187] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 19. | Marini JJ, Gattinoni L. Management of COVID-19 Respiratory Distress. JAMA. 2020;323:2329-2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 706] [Cited by in RCA: 737] [Article Influence: 122.8] [Reference Citation Analysis (0)] |
| 20. | Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT; National Heart; Lung, and Blood Institute ARDS Clinical Trials Network. Higher vs lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1592] [Cited by in RCA: 1608] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 21. | Sahetya SK, Goligher EC, Brower RG. Fifty Years of Research in ARDS. Setting Positive End-Expiratory Pressure in Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2017;195:1429-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 22. | Levy MM. PEEP in ARDS--how much is enough? N Engl J Med. 2004;351:389-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Roesthuis L, van den Berg M, van der Hoeven H. Advanced respiratory monitoring in COVID-19 patients: use less PEEP! Crit Care. 2020;24:230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Tsolaki V, Siempos I, Magira E, Kokkoris S, Zakynthinos GE, Zakynthinos S. PEEP levels in COVID-19 pneumonia. Crit Care. 2020;24:303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 25. | Grieco DL, Bongiovanni F, Chen L, Menga LS, Cutuli SL, Pintaudi G, Carelli S, Michi T, Torrini F, Lombardi G, Anzellotti GM, De Pascale G, Urbani A, Bocci MG, Tanzarella ES, Bello G, Dell'Anna AM, Maggiore SM, Brochard L, Antonelli M. Respiratory physiology of COVID-19-induced respiratory failure compared to ARDS of other etiologies. Crit Care. 2020;24:529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 128] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 26. | Pan C, Chen L, Lu C, Zhang W, Xia JA, Sklar MC, Du B, Brochard L, Qiu H. Lung Recruitability in COVID-19-associated Acute Respiratory Distress Syndrome: A Single-Center Observational Study. Am J Respir Crit Care Med. 2020;201:1294-1297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 230] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 27. | Beloncle FM, Pavlovsky B, Desprez C, Fage N, Olivier PY, Asfar P, Richard JC, Mercat A. Recruitability and effect of PEEP in SARS-Cov-2-associated acute respiratory distress syndrome. Ann Intensive Care. 2020;10:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 28. | Grasso S, Fanelli V, Cafarelli A, Anaclerio R, Amabile M, Ancona G, Fiore T. Effects of high vs low positive end-expiratory pressures in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171:1002-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Guérin C. Prone positioning acute respiratory distress syndrome patients. Ann Transl Med. 2017;5:289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, Clavel M, Chatellier D, Jaber S, Rosselli S, Mancebo J, Sirodot M, Hilbert G, Bengler C, Richecoeur J, Gainnier M, Bayle F, Bourdin G, Leray V, Girard R, Baboi L, Ayzac L; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159-2168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2391] [Cited by in RCA: 2619] [Article Influence: 201.5] [Reference Citation Analysis (0)] |
| 31. | Douglas WW, Rehder K, Beynen FM, Sessler AD, Marsh HM. Improved oxygenation in patients with acute respiratory failure: the prone position. Am Rev Respir Dis. 1977;115:559-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 55] [Reference Citation Analysis (0)] |
| 32. | Raoof S, Nava S, Carpati C, Hill NS. High-Flow, Noninvasive Ventilation and Awake (Nonintubation) Proning in Patients with Coronavirus Disease 2019 With Respiratory Failure. Chest. 2020;158:1992-2002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 33. | Xu Q, Wang T, Qin X, Jie Y, Zha L, Lu W. Early awake prone position combined with high-flow nasal oxygen therapy in severe COVID-19: a case series. Crit Care. 2020;24:250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 34. | Zang X, Wang Q, Zhou H, Liu S, Xue X; COVID-19 Early Prone Position Study Group. Efficacy of early prone position for COVID-19 patients with severe hypoxia: a single-center prospective cohort study. Intensive Care Med. 2020;46:1927-1929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 35. | Caputo ND, Strayer RJ, Levitan R. Early Self-Proning in Awake, Non-intubated Patients in the Emergency Department: A Single ED's Experience During the COVID-19 Pandemic. Acad Emerg Med. 2020;27:375-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 246] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 36. | Elharrar X, Trigui Y, Dols AM, Touchon F, Martinez S, Prud'homme E, Papazian L. Use of Prone Positioning in Nonintubated Patients With COVID-19 and Hypoxemic Acute Respiratory Failure. JAMA. 2020;323:2336-2338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 264] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 37. | Sartini C, Tresoldi M, Scarpellini P, Tettamanti A, Carcò F, Landoni G, Zangrillo A. Respiratory Parameters in Patients With COVID-19 After Using Noninvasive Ventilation in the Prone Position Outside the Intensive Care Unit. JAMA. 2020;323:2338-2340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 229] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 38. | Coppo A, Bellani G, Winterton D, Di Pierro M, Soria A, Faverio P, Cairo M, Mori S, Messinesi G, Contro E, Bonfanti P, Benini A, Valsecchi MG, Antolini L, Foti G. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med. 2020;8:765-774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 357] [Cited by in RCA: 347] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 39. | Weiss TT, Cerda F, Scott JB, Kaur R, Sungurlu S, Mirza SH, Alolaiwat AA, Augustynovich AE, Li J. Prone positioning for patients intubated for severe acute respiratory distress syndrome (ARDS) secondary to COVID-19: a retrospective observational cohort study. Br J Anaesth. 2021;126:48-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 40. | Huang M, Yang Y, Shang F, Zheng Y, Zhao W, Luo L, Han X, Lin A, Zhao H, Gu Q, Shi Y, Li J, Xu X, Liu K, Deng Y, Cao Q, Wang W. Clinical characteristics and predictors of disease progression in severe patients with COVID-19 infection in Jiangsu Province. Am J Med Sci. 2020;120-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Diprose WK, Bainbridge L, Frith RW, Anderson NE. Bilateral upper limb neuropathies after prone ventilation for COVID-19 pneumonia. Neurol Clin Pract. 2021;11:e211-e213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Mędrzycka-Dąbrowska W, Lewandowska K, Ślęzak D, Dąbrowski S. Prone ventilation of critically ill adults with COVID-19: how to perform CPR in cardiac arrest? Crit Care. 2020;24:258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Messerole E, Peine P, Wittkopp S, Marini JJ, Albert RK. The pragmatics of prone positioning. Am J Respir Crit Care Med. 2002;165:1359-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Critical care medicine
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kajumba MM S-Editor: Wu YXJ L-Editor: A P-Editor: Li X