Published online Jul 9, 2021. doi: 10.5492/wjccm.v10.i4.66
Peer-review started: February 10, 2021
First decision: March 31, 2021
Revised: April 13, 2021
Accepted: June 2, 2021
Article in press: June 2, 2021
Published online: July 9, 2021
Processing time: 147 Days and 0 Hours
Sepsis can develop during the body’s response to a critical illness leading to multiple organ failure, irreversible shock, and death. Sepsis has been vexing health care providers for centuries due to its insidious onset, generalized metabolic dysfunction, and lack of specific therapy. A common factor underlying sepsis is the characteristic hypermetabolic response as the body ramps up every physiological system in its fight against the underlying critical illness. A hypermetabolic response requires supraphysiological amounts of energy, which is mostly supplied via oxidative phosphorylation generated ATP. A by-product of oxidative phosphorylation is hydrogen peroxide (H2O2), a toxic, membrane-permeable oxidizing agent that is produced in far greater amounts during a hypermetabolic state. Continued production of mitochondrial H2O2 can overwhelm cellular reductive (antioxidant) capacity leading to a build-up within cells and eventual diffusion into the bloodstream. H2O2 is a metabolic poison that can inhibit enzyme systems leading to organ failure, microangiopathic dysfunction, and irreversible septic shock. The toxic effects of H2O2 mirror the clinical and laboratory abnormalities observed in sepsis, and toxic levels of blood H2O2 have been reported in patients with septic shock. This review provides evidence to support a causal role for H2O2 in the pathogenesis of sepsis, and an evidence-based therapeutic intervention to reduce H2O2 levels in the body and restore redox homeostasis, which is necessary for normal organ function and vascular responsiveness.
Core Tip: Sepsis mortality remains unacceptably high because there is no specific treatment to prevent or reverse the multiple organ failure and refractory hypotension that develops in this condition. An evidence-based analysis suggests that impaired systemic redox homeostasis caused by the toxic accumulation of hydrogen peroxide has a causal role in the pathogenesis of this often fatal illness. The data imply that restoration of redox homeostasis by therapeutic reduction of hydrogen peroxide will significantly reduce the morbidity and mortality associated with sepsis. A therapeutic intervention to reduce systemic levels of hydrogen peroxide is presented.
- Citation: Pravda J. Sepsis: Evidence-based pathogenesis and treatment. World J Crit Care Med 2021; 10(4): 66-80
- URL: https://www.wjgnet.com/2220-3141/full/v10/i4/66.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v10.i4.66
Medicine has made fantastic strides over the past century. Our intricate knowledge of disease has been spearheaded by amazing advances in laboratory techniques that allow us to identify and instigate changes at the molecular level. This has led to an explosion of data accompanied by a detailed insight into pathological processes that perpetuate disease states leading to the identification of potential therapeutic targets, which can be exploited for new and more effective therapeutic interventions. However, while laboratory research is an extremely useful tool to obtain a pathophysiological snapshot of disease it cannot, on its own, identify the pathogenesis, and for some diseases, a creative theoretical approach is the only way to get "upstream" where novel insights may shed light on difficult clinical problems.
A prime example is sepsis, a systemic process with a high fatality rate that ultimately leads to microangiopathic dysfunction, refractory hypotension, multiple organ failure, and death. Worldwide, someone dies of sepsis every 3 s with 20% of global deaths being sepsis-related for a total of 11 million deaths annually and growing. Sepsis is thought to be a hyper-immune response to infection[1]. But in over 40% of sepsis cases there is no identifiable infectious agent, and culture positivity is not independently associated with mortality in sepsis[2-6]. These observations suggest that infection can be sufficient but is not absolutely necessary for sepsis to develop. It also suggests an endogenous process that is common to both infectious and non-infectious conditions (i.e., multiple body trauma, pancreatitis, post-surgery, etc.), which is set in motion, ultimately leading to sepsis. Finally, the profound immunosuppression occurring during sepsis[7] suggests a non-immune contemporaneous process as the proximate causal factor in the development of the sepsis syndrome. This raises the consideration that the immune system is failing for the same reason other organs fail.
From a metabolic perspective, there is evidence of impaired mitochondrial oxygen utilization in sepsis despite normal oxygen tension[4,8-10]. This suggests a mitochondrial-derived agent capable of interfering with oxygen utilization by inhibiting substrate oxidation during the tricarboxylic acid (Krebs) cycle or oxidative phosphorylation. The close association of hyperlactatemia with adverse sepsis outcomes despite the absence of tissue hypoxia or impaired tissue oxygenation provides further evidence that implicates impairment of mitochondrial oxidative metabolism as discussed in more detail below[11,12].
The identification of mitochondrial abnormalities in sepsis focuses attention on bioenergetics and suggests that the common link between infectious and non-infectious origins of sepsis is not an immune response but a hypermetabolic state that sends mitochondrial metabolism into “overdrive” causing dysfunction of vital intramitochondrial bioenergetic processes. This reduces the problem of sepsis to the identification of a mitochondrial-generated molecule whose production is scaled up during hypermetabolism and is capable of inhibiting enzymes in the Krebs cycle and/or the electron transport chain (ETC). This is likely to be a small molecule that is normally eliminated within mitochondria since most people do not develop sepsis during a clinical hypermetabolic response.
A prime element that fulfills these theoretical requirements is hydrogen peroxide (H2O2), a small, cell-membrane permeable highly toxic oxidizing agent that is produced within mitochondria as a result of electron transport chain auto-oxidation[13]. H2O2 must be immediately eliminated to prevent cell damage and is removed by the following series of reactions (Figure 1)[14-16].
Studies have shown that blood H2O2 is significantly elevated in human sepsis and septic shock with values reported up to 558 μmol/L, which is over 100 times the normal upper limit of 5 μmol/L and over ten times 50 μmol/L upper limit at which
Other clinical abnormalities observed in sepsis such as hypotension, coagulopathy, encephalopathy, microangiopathic and cardiac dysfunction, erythrocyte rigidity, methemoglobinemia, glutathione depletion, mitochondrial damage, and lymphocyte apoptosis are also documented adverse effects of H2O2, all of which contribute to multiple organ failure and lymphocytopenia observed in sepsis[22-25].
But where does all this H2O2 come from? Although leukocytes such as neutrophils can produce large amounts of H2O2 during the respiratory burst[26], the profound immunosuppression[7,27-30] during advanced stages of sepsis suggests a significant non-immune contribution to the persistently elevated blood H2O2 levels observed in advanced sepsis and septic shock. Significant depletion of tissue glutathione in muscle, lung, and erythrocytes in addition to plasma thiol depletion (albumin cys34) suggests these tissues have become H2O2 generators contributing to elevated blood H2O2 in sepsis patients[22,31,32].
The production of mitochondrial H2O2 depends upon the rate of electron transfer through the ETC. The higher the electron transfer rate the greater the production of H2O2. Studies in isolated mitochondria have shown an exponential increase in reactive oxygen species (i.e., H2O2) at strongly polarized levels of mitochondrial membrane potential[33], which can occur in hypermetabolic critically ill patients. Other studies in mice have shown that mitochondrial H2O2 will increase up to 15x the normal rate during state-3 (maximal) respiration[34]. The clinical correlate of state-3 respiration is a hypermetabolic state, which is characterized by tachycardia, tachypnea, leukocytosis, high fever, and significantly enhanced protein biosynthesis. These are the cardinal elements that define the systemic inflammatory response syndrome (SIRS), which accompanies sepsis. This implies that a clinical hypermetabolic response is accom
Due to the limited amount of mitochondrial glutathione available for H2O2 neutralization in addition to high basal levels of mitochondrial H2O2, a sustained hyper
H2O2 is a metabolic poison and the data suggest that sepsis is due to an endogenous H2O2 poisoning secondary to the oxidative damage inflicted by this highly toxic oxidizing agent. Since H2O2 is permeable through cell membranes, elevated blood H2O2 indicates systemic reductive depletion, which perpetuates the production of H2O2[41]. Toxic levels of H2O2 will disrupt cellular function in all body organs, which can lead to multiple organ failure and microvascular dysfunction. Any cell undergoing a hypermetabolic response can deplete its reductive capacity and contribute to total body H2O2 load.
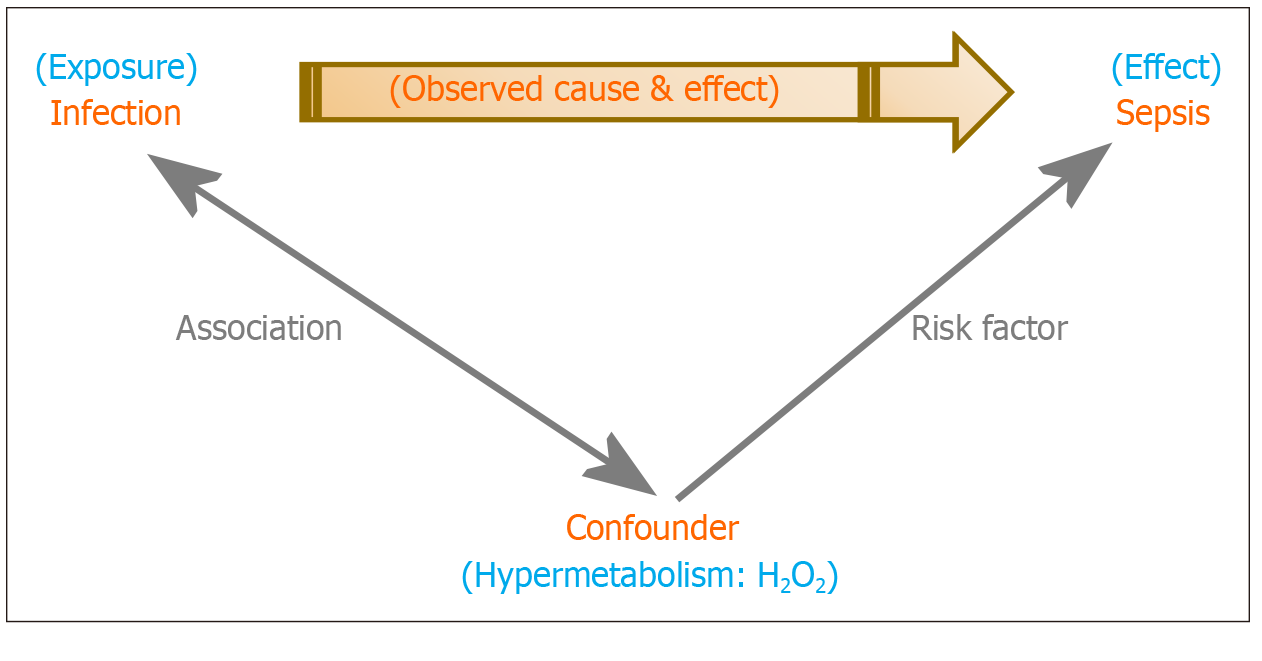
A potential cause and effect relationship between H2O2 and sepsis has likely remained obscure because a hypermetabolic state, which generates H2O2, is a confounding factor in the relationship between infection and sepsis (Figure 2)[42-51].
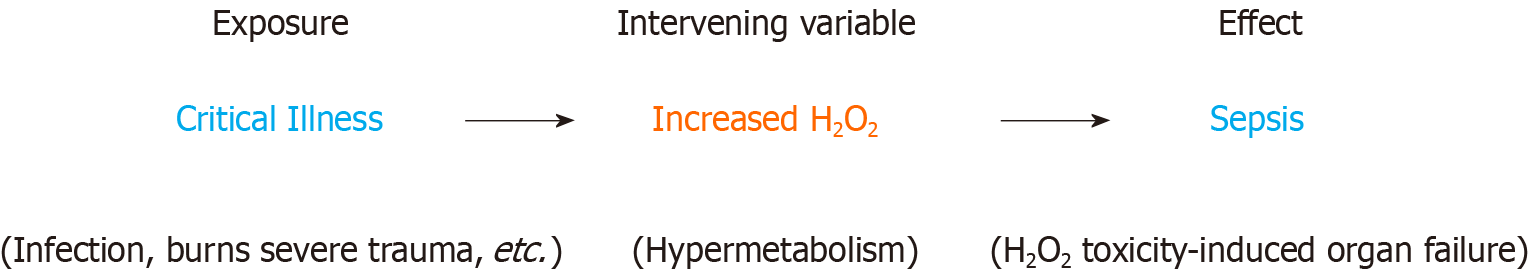
Based on the data, H2O2 is also an intervening variable in the setting of critical illness-associated sepsis (Figure 3)[52-55]. Intervening variables have an important role in therapy as they are mechanistically “closer” to the final effect and can serve as a therapeutic target. The observation that culture-positive sepsis patients on appropriate antibiotics still die suggests an additional factor independent of infection that exerts a significant influence on the clinical outcome of sepsis[5]. In this scenario, the H2O2 induced tissue damage and metabolic dysfunction (the effect) is too severe and can no longer be reversed by treating the infection (the exposure) with antibiotics. As an intervening variable with a postulated causal role in sepsis, H2O2 explains why culture positivity is not independently associated with mortality in sepsis[5] since the data supports H2O2 (and not infection per se) as the proximal causal agent in sepsis.
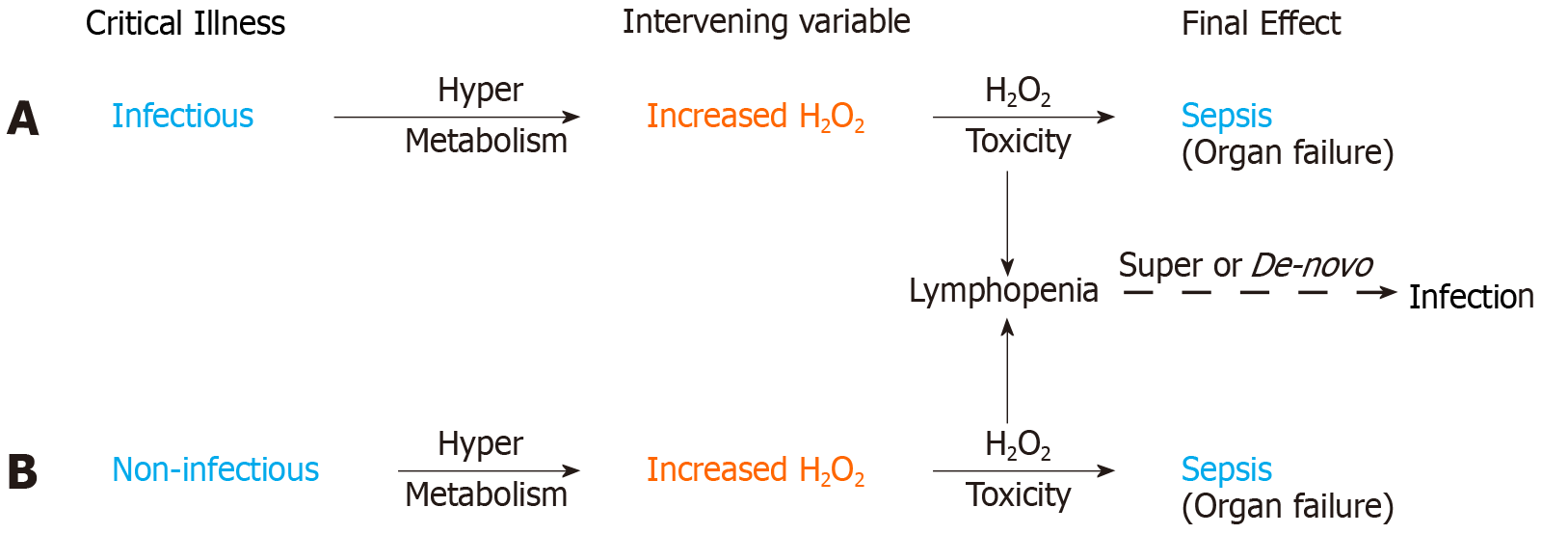
All hypermetabolic states (infectious and non-infectious), have the potential of generating excess H2O2, which can accumulate to toxic levels leading to bioenergetic organ failure and sepsis. The relationship between exposure (infection) and con
Studies have shown that certain antibiotics can cause mitochondrial dysfunction accompanied by a significant production of H2O2[46]. This implies that patients must have sufficient residual reductive capacity to deal with the oxidative stress imposed by antibiotic treatment, underscoring the critical need to begin antibiotics along with reductive therapy as early as possible during the course of infection-associated sepsis. Reductive therapy encompasses any treatment that increases reductive (antioxidant) capacity, i.e., glutathione, protein thiols, etc. The purpose of which (in sepsis) is to augment the patient’s reductive (antioxidant) capacity to neutralize H2O2.
For the patient, the clinical benefits of limiting exposure to H2O2 go beyond discharge from the hospital because H2O2 can damage mitochondrial DNA. Mitochondrial DNA (mtDNA) is highly vulnerable to H2O2 induced oxidative damage due to the proximity of mtDNA to the electron transport chain, both of which reside on the matrix side of the inner mitochondrial membrane. Exposure of mtDNA to H2O2 will inflict base mutations and nucleotide mispairing that upon transcription result in the incorporation of mutated protein subunits into the electron transport chain (ETC). Mutated ETC components interfere with electron transport resulting in augmented electron leakage with increased H2O2 generation[47-52]. This establishes a self-amplifying vicious cycle with ever greater production of H2O2 and mtDNA damage, which can lead to prolonged metabolic and bioenergetic dysfunction in sepsis survivors and contribute to the post-sepsis syndrome.
H2O2 induced impaired redox homeostasis as a primary mechanism of disease is a novel pathogenesis that is supported by experimental evidence and is grounded in fundamental concepts of redox biology, redox biochemistry, and bioenergetics. Similar to electrolyte balance and acid/base buffering systems, redox homeostasis is a vital homeostatic mechanism required for normal cellular function and should be assessed in all critically ill patients.
Since most H2O2 is a product of mitochondrial electron transport chain activity, clinical manifestations of H2O2 begin with its effects on cellular metabolism. Indeed, with almost 40% of all cellular reactions being redox reactions[53], the potential for H2O2 induced oxidative impairment of cellular metabolism and bioenergetics cannot be overstated, especially since blood H2O2 levels reported in sepsis exceed cellular cytotoxic tolerances by several-fold[17]. The mechanisms of H2O2 toxicity mirror the clinical manifestations of sepsis and include:
Elevated blood lactate is common among patients with sepsis and is associated with significantly greater mortality[12]. Toxic levels of H2O2 can inhibit enzymes in the Krebs cycle and electron transport chain leading to hyperlactatemia and bioenergetic failure characteristic of advanced sepsis[54-59]. H2O2 increases cellular lactate by interrupting mitochondrial oxidative energy flux (directional oxidation), which is needed to maintain the proton motive force (electrochemical proton gradient) that fuels pyruvate import into the mitochondrial matrix[60,61]. Studies have shown that H2O2 inhibits a variety of enzymes including enzymes within the Krebs’ cycle such as aconitase, alpha-ketoglutarate dehydrogenase, and Succinate Dehydrogenase[55-57,62].
Once inhibited, the Krebs cycle can no longer supply sufficient reducing equivalents (NADH, FADH2) needed to sustain the mitochondrial proton gradient. Diminished Krebs cycle supplied reducing equivalents can decrease (and eventually collapse) the mitochondrial proton gradient. This will impair the proton motive force needed for pyruvate translocase in the inner mitochondrial membrane to transport pyruvate into mitochondria in symport with a proton[60,61]. The end result is increased cytosolic pyruvate and subsequent conversion to lactate with resulting hyperlactatemia[11]. Thus, in sepsis, hyperlactatemia can be a manifestation of H2O2 toxicity, in which case the reduction of serum lactate alone has no effect on the outcome of sepsis[63,64].
The effect of a dysfunctional Krebs cycle on serum lactate levels can be seen with the inherited deficiency of alpha-ketoglutarate dehydrogenase, which is associated with severe congenital hyperlactatemia[65]. Under these circumstances, increasing inspired oxygen will not lower serum lactate since the problem is with the diminished supply of electrons to the electron transport chain, which collapses the proton gradient dissipating the proton motive force, and not the availability of oxygen.
Studies have shown substantial lactate production from the lungs of patients with septic shock[66]. Hypoperfusion or hypoxia is highly unlikely given that the lungs are continuously bathed in oxygen and receive the entire cardiac output. However, when combined with other studies showing decreased lung glutathione in sepsis, H2O2 toxicity is a strong possibility. Therapeutic removal of H2O2 (discussed below) can contribute to the normalization of bioenergetic function and serum lactate.
It’s worth noting that the mitochondrial proton motive force fuels both ATP synthase and nicotinamide nucleotide transhydrogenase both of which are located in the inner mitochondrial membrane. The former is needed to synthesize ATP while the latter is required to generate mitochondrial NADPH, a critical source of reducing equivalents for the regeneration of mitochondrial glutathione needed to neutralize
A common feature during the progression of sepsis is anemia. Several factors can contribute to the development of sepsis-associated anemia however, sepsis per se is independently associated with the development of anemia, and healthy erythrocytes exposed to plasma from sepsis patients undergo eryptosis[67,68]. H2O2 induced oxidative stress initiates erythrocyte suicidal cell death known as eryptosis leading to cell shrinkage and clearance from the blood[68-71]. Thus, H2O2 initiated eryptosis may contribute to sepsis-related anemia.
Low serum calcium is a common finding in patients with sepsis and critical illness, with reported prevalence rates of up to 80%[72]. Hypocalcemia may be due to one or more of various causes[73]. However, during sepsis, calcium is shifted into red blood cells with significant increases in erythrocyte calcium of more than twice the control value[74]. Given that about 85% of all cells in the body are red blood cells, this shift may significantly contribute to sepsis-associated hypocalcemia[75]. Erythrocytes exposed to oxidative stress (i.e., H2O2) activate calcium-permeable cation channels leading to calcium entry into the cell[71]. Significantly increased lymphocyte calcium has also been reported in sepsis[76]. This suggests that the elevated blood H2O2 reported in sepsis may cause a more generalize intracellular shift of calcium.
Sepsis-associated hemodynamic instability can progress to septic shock, which carries a high mortality. Oxidative stress due to H2O2 exposure causes extensive cytoskeletal disruption to endothelial cells leading to significant endothelial retraction and microangiopathic dysfunction[22]. The net effect of microvascular H2O2 exposure is microangiopathic dysfunction, impaired vasomotor responsiveness, barrier disruption with edema formation, and irreversible hypotension (septic shock)[22,77]. Studies have reported hypotension in an animal model after intravenous administration of H2O2[25].
Sepsis patients develop profound immunosuppression that begins within days after the onset of sepsis[7,28,30]. Lymphocytes are extremely sensitive to H2O2 induced apoptosis, which occurs at H2O2 concentrations of less than 1 μmol/L[19,20]. Studies report blood H2O2 concentrations in sepsis of up to 558 μmol/L, which is over 500 times the concentration of H2O2 needed to cause lymphocyte apoptosis[17-19]. The ability of high blood H2O2 concentrations to cause generalized lymphocyte apoptosis explains the profound immunosuppression observed in sepsis patients.
Sepsis-associated acute respiratory distress syndrome (ARDS) is a serious compli
Sepsis-associated acute kidney injury (S-AKI) is a life-threatening complication that develops in up to two-thirds of patients with sepsis or septic shock, which in half of the patients develops before seeking medical attention[84]. Once thought to be a consequence of cellular hypoxia leading to acute tubular necrosis, it is now recognized that S-AKI can occur in the setting of normal or increased renal blood flow[84]. Studies suggest a critical role for microcirculatory dysfunction, which is present in every vital organ in animal models and humans with sepsis[84-86]. When combined with studies showing a decreased substrate flux through the Krebs cycle in mice kidneys after the induction of experimental sepsis[87], these effects mirror the known toxic effects of
The renal endothelium is highly vulnerable to oxidative stress with agents such as H2O2, a highly toxic oxidizing agent that can diffuse across cell membranes to impair critical signaling and regulatory function required for microvascular function[90]. Other studies report significant cytotoxicity in human tubular epithelial cells exposed to 100 μmol/L H2O2, while 200 μmol/L exposure caused mitochondrial cytochrome-C translocation to the cytoplasm in addition to significant intracellular increases in H2O2. These concentrations are within the range reported for blood H2O2 in sepsis patients of up to 558 μmol/L[17,91]. H2O2 can inhibit various enzymes involved in oxidative metabolism including Krebs cycle enzymes, ATP synthase, and nucleotide (ADP-ATP) translocase[55-57,92]. The resulting inhibition in mitochondrial oxidative flux may contribute to the increased glycolytic production of lactate by proximal tubule cells observed during sepsis[93]. Increased glycolysis would revert to oxidative phosphorylation when H2O2 induced inhibition of mitochondrial oxidative metabolism is resolved. Lastly, rat renal artery infusion of 70 mmol/L H2O2 (140x that found in human sepsis blood) is reported to cause massive proteinuria without electron microscopic ultrastructural glomerular abnormalities[94]. This is consistent with the minimal postmortem histological findings in human S-AKI[84,86]. This suggests that renal exposure to blood H2O2 levels observed in human sepsis may cause cellular dys
Disseminated intravascular coagulation (DIC) is a life-threatening complication frequently encountered in sepsis that is characterized by the systemic activation of the coagulation system leading to microvascular thrombosis, and potentially life-threatening hemorrhage due to consumption of platelets and coagulation factors[95]. DIC can originate from damage to the microvasculature, which triggers the extrinsic coagulation cascade[96]. H2O2 can cause microvascular injury by peroxidation of endothelial cell membranes, which triggers the expression of tissue factor and subsequent systemic activation of the extrinsic coagulation pathway leading to DIC[97-99]. Intravenous administration of H2O2 is reported to have resulted in fatal sepsis and DIC, underscoring the role of H2O2 induced oxidative stress in both of these conditions[100].
On a more fundamental level, the endothelium is critically involved in preventing inappropriate coagulation by maintaining barrier function and producing several endogenous anticoagulants[101]. The elevated levels of blood H2O2 reported in sepsis can permeate endothelial cells throughout the body causing substantial oxidative stress accompanied by profound disruption in both form and function[77,102]. Studies have reported significant endothelial dysfunction that is associated with mortality and severity of coagulopathy[101]. H2O2 induced endothelial dysfunction can explain why anticoagulants fail to show a survival benefit in sepsis-induced DIC[103] since these agents fail to restore endothelial redox homeostasis.
Sepsis-associated encephalopathy (SAE) is a diffuse cerebral dysfunction ranging from lethargy and lack of concentration to personality changes, delirium, and coma that occurs secondary to sepsis in the absence of direct central nervous system (CNS) infection. SAE affects up to 70% of sepsis patients and is associated with higher mortality and poorer long term outcomes with half of surviving patients suffering from long-term cognitive defects[104,105]. The brain is highly sensitive to H2O2 induced oxidative damage and dysfunction, and studies report dose-dependent cytotoxicity starting at H2O2 exposures of 10 μmol/L[106]. Encephalopathy is reported to occur after the accidental ingestion of H2O2[107]. Encephalopathy was also reported after intravenous administration of H2O2 for alternative medicine therapy[100].
H2O2 is diffusible through cell membranes which facilitates its diffusion into the central nervous system where it can disrupt neuronal and synaptic function. Studies have shown that H2O2 can alter neuron membrane properties and impair synaptic transmission leading to hyperexcitability and epileptiform activity[108,109]. This is notable because epileptic seizures can be a manifestation of SAE. Other studies have demonstrated bioenergetic impairment with decreased ATP biosynthesis and utilization in neurons exposed to H2O2[110,111]. H2O2 has also been reported to alter rat hippocampal synaptic plasticity, which can negatively impact long-term potentiation, learning, and memory[112]. Thus, the presence of elevated levels of blood H2O2 in sepsis can have acute and chronic effects on brain function and cognition.
Sepsis is a life-threatening medical emergency that can precipitously evolve into hemodynamic instability, septic shock, and death. Thus it may not be possible or prudent to wait for a blood H2O2 level if clinical signs of H2O2 toxicity are present. Additionally, it takes some time before free H2O2 can accumulate in the bloodstream given the multiple layers of reductive (antioxidant) defense systems that mito
Within this context, the data support the critical need for reduction of systemic H2O2 in sepsis to prevent bioenergetic organ failure and restore microcirculatory function. Restoration of redox homeostasis by the elimination of excess H2O2 must accompany other therapeutic interventions to optimize clinical responsiveness and outcome. Sodium thiosulfate (STS) is a direct-acting reducing agent that can neutralize H2O2 upon contact.
STS is approved for use in cyanide poisoning with a recommended dose of 12.5 g over slow IV infusion (10 to 20 min) in adults and 250 mg/kg in children[114]. Similar dosing regimens can be considered in sepsis. Repeat dosing can be guided by clinical status, blood reducing capacity (glutathione, plasma thiols), and blood H2O2 levels. The general chemical reaction for the reduction of H2O2 with sodium thiosulfate yields sodium trithionate, sodium sulfate, and water[115].
2Na2S2O3 + 4H2O2 → Na2S3O6 + Na2SO4 + 4H2O
The rationale underlying STS administration in sepsis is to reduce blood H2O2 to normal (less than 30 μmol/L) in order to allow intracellular H2O2 to diffuse down its concentration gradient into the systemic circulation where it can be neutralized by STS. STS is generally well tolerated and is an accepted therapy for cisplatin toxicity and renal failure associated calciphylaxis (25 g three times weekly)[116,117]. High dose STS (up to 16 g per M2 surface area, repeated after 4 h) is reported to be well tolerated in children under 12 years of age[118].
STS is reported to replenish intracellular glutathione, which will aid in the removal of intracellular H2O2 and restoration of redox homeostasis[119,120]. Decreasing serum lactate indicates that H2O2-induced Krebs cycle inhibition and bioenergetic dysfunction are being reversed. Restoration of vascular responsiveness by STS may cause extant vasopressor measures to have an unanticipated amplified effect. Thus, STS administration in critically ill patients should be accompanied by close patient monitoring. Finally, if STS therapy proves to be successful in the treatment of sepsis then treatment with STS should be considered in all critically ill (hypermetabolic) patients in order to restore depleted systemic reducing equivalents before blood H2O2 becomes toxically elevated.
ARDS: Inhaled STS may have a beneficial effect to neutralize H2O2 that has diffused through the alveolar-capillary membrane causing oxidant damage in the alveolar space.
S-AKI: Primary prevention of S-AKI is not possible in all patients because most patients developing S-AKI already have it at presentation. Administration of STS should be considered when patients first seek medical care to initiate primary or secondary prevention.
The evidence supports the use of STS as a specific therapeutic agent for the treatment of sepsis and its associated complications. Given the high mortality, significant societal burden, and absence of a safe and effective treatment for this deadly condition, clinical studies are urgently needed to determine the effectiveness of STS for the treatment of sepsis.
The mortality in sepsis is unacceptably high because there is no specific therapy to treat the sepsis syndrome. H2O2 toxicity mirrors the clinical and laboratory abnormalities observed in sepsis, and toxic levels of blood H2O2 have been reported in this condition. This and other data implicate H2O2 as the causal factor in the pathogenesis of sepsis, which predictably develops accompanied by systemic depletion of reducing equivalents (i.e., glutathione) needed for the reduction (neutralization) of metabolically generated H2O2. Once the body’s reductive (antioxidant) capacity is depleted, H2O2 will continue to be generated and flood the system.
Prolonged supraphysiological production of H2O2 generated by electron transport chain hyperactivity during a hypermetabolic state (such as sepsis) can overwhelm cellular reductive systems leading to H2O2 accumulation within tissues and blood. H2O2 is a highly toxic membrane-permeable metabolic poison that can cause severe bioenergetic dysfunction and cellular damage if allowed to accumulate. Continued exposure can lead to the collapse of systemic redox homeostasis, proton motive force dissipation, organ failure, microvascular dysfunction, and fatal septic shock. Reduction of blood H2O2 is paramount in order to prevent H2O2 toxicity from irreversibly shutting down cellular metabolism.
The data support the use of sodium thiosulfate as a systemic reducing agent with the goal of restoring redox homeostasis by neutralizing excess systemic H2O2. Prophylactic use of sodium thiosulfate in all critically ill (hypermetabolic) patients should be considered before irreversible H2O2 induced bioenergetic failure and microvascular dysfunction develop.
Based on the data, the missing critical intervention to improve patient outcomes and reduce mortality in patients with sepsis and septic shock is the normalization of systemic redox homeostasis. The addition of specialists in redox medicine to the team providing care to critically ill patients can contribute to achieving this heretofore elusive goal.
| 1. | Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, Fleischmann-Struzek C, Machado FR, Reinhart KK, Rowan K, Seymour CW, Watson RS, West TE, Marinho F, Hay SI, Lozano R, Lopez AD, Angus DC, Murray CJL, Naghavi M. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2870] [Cited by in RCA: 4895] [Article Influence: 815.8] [Reference Citation Analysis (7)] |
| 2. | Klein Klouwenberg PM, Cremer OL, van Vught LA, Ong DS, Frencken JF, Schultz MJ, Bonten MJ, van der Poll T. Likelihood of infection in patients with presumed sepsis at the time of intensive care unit admission: a cohort study. Crit Care. 2015;19:319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 201] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 3. | Lin GL, McGinley JP, Drysdale SB, Pollard AJ. Epidemiology and Immune Pathogenesis of Viral Sepsis. Front Immunol. 2018;9:2147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 213] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 4. | Nagar H, Piao S, Kim CS. Role of Mitochondrial Oxidative Stress in Sepsis. Acute Crit Care. 2018;33:65-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 5. | Phua J, Ngerng W, See K, Tay C, Kiong T, Lim H, Chew M, Yip H, Tan A, Khalizah H, Capistrano R, Lee K, Mukhopadhyay A. Characteristics and outcomes of culture-negative vs culture-positive severe sepsis. Crit Care. 2013;17:R202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 261] [Cited by in RCA: 267] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 6. | Molina F, Castaño P, Plaza M, Hincapié C, Maya W, Cataño JC, González J, León A, Jaimes F. Positive Culture and Prognosis in Patients With Sepsis: A Prospective Cohort Study. J Intensive Care Med. 2020;35:755-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Venet F, Rimmelé T, Monneret G. Management of Sepsis-Induced Immunosuppression. Crit Care Clin. 2018;34:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Boekstegers P, Weidenhöfer S, Kapsner T, Werdan K. Skeletal muscle partial pressure of oxygen in patients with sepsis. Crit Care Med. 1994;22:640-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 139] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Fink MP. Cytopathic hypoxia. Mitochondrial dysfunction as mechanism contributing to organ dysfunction in sepsis. Crit Care Clin. 2001;17:219-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 221] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Supinski GS, Schroder EA, Callahan LA. Mitochondria and Critical Illness. Chest. 2020;157:310-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 161] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 11. | Garcia-Alvarez M, Marik P, Bellomo R. Sepsis-associated hyperlactatemia. Crit Care. 2014;18:503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 330] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 12. | Lee SG, Song J, Park DW, Moon S, Cho HJ, Kim JY, Park J, Cha JH. Prognostic value of lactate levels and lactate clearance in sepsis and septic shock with initial hyperlactatemia: A retrospective cohort study according to the Sepsis-3 definitions. Medicine (Baltimore). 2021;100:e24835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 13. | Mailloux RJ. Mitochondrial Antioxidants and the Maintenance of Cellular Hydrogen Peroxide Levels. Oxid Med Cell Longev. 2018;2018:7857251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 14. | Liu SS. Generating, partitioning, targeting and functioning of superoxide in mitochondria. Biosci Rep. 1997;17:259-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 156] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Turrens JF. Superoxide production by the mitochondrial respiratory chain. Biosci Rep. 1997;17:3-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 623] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 16. | Nolfi-Donegan D, Braganza A, Shiva S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020;37:101674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 929] [Article Influence: 154.8] [Reference Citation Analysis (0)] |
| 17. | 19 van Asbeck BS, Braams R, Aarsman JM, Sprong RC, Groenewegen A. Hydrogen Peroxide In Blood Of Patients With Sepsis Syndrome: A Realistic Phenomenon. Crit Care Med. 1995;23: A169. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Forman HJ, Bernardo A, Davies KJ. What is the concentration of hydrogen peroxide in blood and plasma? Arch Biochem Biophys. 2016;603:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 238] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 19. | Halliwell B, Clement MV, Long LH. Hydrogen peroxide in the human body. FEBS Lett. 2000;486:10-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 713] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 20. | Antunes F, Cadenas E. Cellular titration of apoptosis with steady state concentrations of H(2)O(2): submicromolar levels of H(2)O(2) induce apoptosis through Fenton chemistry independent of the cellular thiol state. Free Radic Biol Med. 2001;30:1008-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 182] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Chandel NS. Mitochondria as signaling organelles. BMC Biol. 2014;12:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 301] [Cited by in RCA: 394] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 22. | Pravda J. Metabolic theory of septic shock. World J Crit Care Med. 2014;3:45-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Evans T, Jin H, Elkins N, Shapiro JI. Effect of acidosis on hydrogen peroxide injury to the isolated perfused rat heart. Am J Physiol. 1995;269:H308-H312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Ballinger SW, Van Houten B, Jin GF, Conklin CA, Godley BF. Hydrogen peroxide causes significant mitochondrial DNA damage in human RPE cells. Exp Eye Res. 1999;68:765-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 161] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Shenep JL, Stokes DC, Hughes WT. Lack of antibacterial activity after intravenous hydrogen peroxide infusion in experimental Escherichia coli sepsis. Infect Immun. 1985;48:607-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Weiss SJ. Neutrophil-mediated methemoglobin formation in the erythrocyte. The role of superoxide and hydrogen peroxide. J Biol Chem. 1982;257:2947-2953. [PubMed] |
| 27. | McBride MA, Patil TK, Bohannon JK, Hernandez A, Sherwood ER, Patil NK. Immune Checkpoints: Novel Therapeutic Targets to Attenuate Sepsis-Induced Immunosuppression. Front Immunol. 2020;11:624272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 28. | Ono S, Tsujimoto H, Hiraki S, Aosasa S. Mechanisms of sepsis-induced immunosuppression and immunological modification therapies for sepsis. Ann Gastroenterol Surg. 2018;2:351-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Reddy RC, Chen GH, Tekchandani PK, Standiford TJ. Sepsis-induced immunosuppression: from bad to worse. Immunol Res. 2001;24:273-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD 2nd, Kreisel D, Krupnick AS, Srivastava A, Swanson PE, Green JM, Hotchkiss RS. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594-2605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1072] [Cited by in RCA: 1332] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 31. | Ayar G, Sahin S, Men Atmaca Y, Uysal Yazici M, Neselioglu S, Erel O. Thiol-disulphide homeostasis is an oxidative stress indicator in critically ill children with sepsis. Arch Argent Pediatr. 2019;117:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Turell L, Radi R, Alvarez B. The thiol pool in human plasma: the central contribution of albumin to redox processes. Free Radic Biol Med. 2013;65:244-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 526] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 33. | Gerencser AA, Chinopoulos C, Birket MJ, Jastroch M, Vitelli C, Nicholls DG, Brand MD. Quantitative measurement of mitochondrial membrane potential in cultured cells: calcium-induced de- and hyperpolarization of neuronal mitochondria. J Physiol. 2012;590:2845-2871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 34. | Aon MA, Stanley BA, Sivakumaran V, Kembro JM, O'Rourke B, Paolocci N, Cortassa S. Glutathione/thioredoxin systems modulate mitochondrial H2O2 emission: an experimental-computational study. J Gen Physiol. 2012;139:479-491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 35. | Ribas V, García-Ruiz C, Fernández-Checa JC. Glutathione and mitochondria. Front Pharmacol. 2014;5:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 420] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 36. | Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013;1830:3143-3153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1037] [Cited by in RCA: 1764] [Article Influence: 126.0] [Reference Citation Analysis (0)] |
| 37. | Tang X, Luo YX, Chen HZ, Liu DP. Mitochondria, endothelial cell function, and vascular diseases. Front Physiol. 2014;5:175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 280] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 38. | Stowe DF, Camara AK. Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function. Antioxid Redox Signal. 2009;11:1373-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 358] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 39. | Muyderman H, Nilsson M, Sims NR. Highly selective and prolonged depletion of mitochondrial glutathione in astrocytes markedly increases sensitivity to peroxynitrite. J Neurosci. 2004;24:8019-8028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Marí M, de Gregorio E, de Dios C, Roca-Agujetas V, Cucarull B, Tutusaus A, Morales A, Colell A. Mitochondrial Glutathione: Recent Insights and Role in Disease. Antioxidants (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 41. | Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta. 2006;1757:509-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 750] [Cited by in RCA: 1064] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 42. | Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R, Suman OE, Mlcak RP, Herndon DN. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One. 2011;6:e21245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 367] [Cited by in RCA: 473] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 43. | Efron PA, Mohr AM, Bihorac A, Horiguchi H, Hollen MK, Segal MS, Baker HV, Leeuwenburgh C, Moldawer LL, Moore FA, Brakenridge SC. Persistent inflammation, immunosuppression, and catabolism and the development of chronic critical illness after surgery. Surgery. 2018;164:178-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 44. | Cox MC, Brakenridge SC, Stortz JA, Hawkins RB, Darden DB, Ghita GL, Mohr AM, Moldawer LL, Efron PA, Moore FA. Abdominal sepsis patients have a high incidence of chronic critical illness with dismal long-term outcomes. Am J Surg. 2020;220:1467-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 45. | Stortz JA, Mira JC, Raymond SL, Loftus TJ, Ozrazgat-Baslanti T, Wang Z, Ghita GL, Leeuwenburgh C, Segal MS, Bihorac A, Brumback BA, Mohr AM, Efron PA, Moldawer LL, Moore FA, Brakenridge SC. Benchmarking clinical outcomes and the immunocatabolic phenotype of chronic critical illness after sepsis in surgical intensive care unit patients. J Trauma Acute Care Surg. 2018;84:342-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 46. | Kalghatgi S, Spina CS, Costello JC, Liesa M, Morones-Ramirez JR, Slomovic S, Molina A, Shirihai OS, Collins JJ. Bactericidal antibiotics induce mitochondrial dysfunction and oxidative damage in Mammalian cells. Sci Transl Med. 2013;5:192ra85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 399] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 47. | Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283:1482-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2204] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 48. | Taylor RW, Turnbull DM. Mitochondrial DNA mutations in human disease. Nat Rev Genet. 2005;6:389-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1215] [Cited by in RCA: 1358] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 49. | Ricci C, Pastukh V, Leonard J, Turrens J, Wilson G, Schaffer D, Schaffer SW. Mitochondrial DNA damage triggers mitochondrial-superoxide generation and apoptosis. Am J Physiol Cell Physiol. 2008;294:C413-C422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 50. | Canter JA, Eshaghian A, Fessel J, Summar ML, Roberts LJ, Morrow JD, Sligh JE, Haines JL. Degree of heteroplasmy reflects oxidant damage in a large family with the mitochondrial DNA A8344G mutation. Free Radic Biol Med. 2005;38:678-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 51. | Rose G, Passarino G, Scornaienchi V, Romeo G, Dato S, Bellizzi D, Mari V, Feraco E, Maletta R, Bruni A, Franceschi C, De Benedictis G. The mitochondrial DNA control region shows genetically correlated levels of heteroplasmy in leukocytes of centenarians and their offspring. BMC Genomics. 2007;8:293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 52. | Lee HC, Wei YH. Oxidative stress, mitochondrial DNA mutation, and apoptosis in aging. Exp Biol Med (Maywood). 2007;232:592-606. [PubMed] |
| 53. | Jinich A, Flamholz A, Ren H, Kim SJ, Sanchez-Lengeling B, Cotton CAR, Noor E, Aspuru-Guzik A, Bar-Even A. Quantum chemistry reveals thermodynamic principles of redox biochemistry. PLoS Comput Biol. 2018;14:e1006471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 54. | Viola HM, Hool LC. Qo site of mitochondrial complex III is the source of increased superoxide after transient exposure to hydrogen peroxide. J Mol Cell Cardiol. 2010;49:875-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 55. | Tretter L, Adam-Vizi V. Inhibition of Krebs cycle enzymes by hydrogen peroxide: A key role of [alpha]-ketoglutarate dehydrogenase in limiting NADH production under oxidative stress. J Neurosci. 2000;20:8972-8979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 322] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 56. | Tretter L, Adam-Vizi V. Alpha-ketoglutarate dehydrogenase: a target and generator of oxidative stress. Philos Trans R Soc Lond B Biol Sci. 2005;360:2335-2345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 334] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 57. | Nulton-Persson AC, Szweda LI. Modulation of mitochondrial function by hydrogen peroxide. J Biol Chem. 2001;276:23357-23361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 307] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 58. | Levy RJ. Mitochondrial dysfunction, bioenergetic impairment, and metabolic down-regulation in sepsis. Shock. 2007;28:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 59. | Japiassú AM, Santiago AP, d'Avila JC, Garcia-Souza LF, Galina A, Castro Faria-Neto HC, Bozza FA, Oliveira MF. Bioenergetic failure of human peripheral blood monocytes in patients with septic shock is mediated by reduced F1Fo adenosine-5'-triphosphate synthase activity. Crit Care Med. 2011;39:1056-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 60. | Bender T, Martinou JC. The mitochondrial pyruvate carrier in health and disease: To carry or not to carry? Biochim Biophys Acta. 2016;1863:2436-2442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 61. | Zangari J, Petrelli F, Maillot B, Martinou JC. The Multifaceted Pyruvate Metabolism: Role of the Mitochondrial Pyruvate Carrier. Biomolecules. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 62. | Knaus UG. Oxidants in Physiological Processes. Handb Exp Pharmacol. 2021;264:27-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 63. | Stacpoole PW, Harman EM, Curry SH, Baumgartner TG, Misbin RI. Treatment of lactic acidosis with dichloroacetate. N Engl J Med. 1983;309:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 175] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 64. | Stacpoole PW, Wright EC, Baumgartner TG, Bersin RM, Buchalter S, Curry SH, Duncan CA, Harman EM, Henderson GN, Jenkinson S. A controlled clinical trial of dichloroacetate for treatment of lactic acidosis in adults. The Dichloroacetate-Lactic Acidosis Study Group. N Engl J Med. 1992;327:1564-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 199] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 65. | Bonnefont JP, Chretien D, Rustin P, Robinson B, Vassault A, Aupetit J, Charpentier C, Rabier D, Saudubray JM, Munnich A. Alpha-ketoglutarate dehydrogenase deficiency presenting as congenital lactic acidosis. J Pediatr. 1992;121:255-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 57] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 66. | Opdam H, Bellomo R. Oxygen consumption and lactate release by the lung after cardiopulmonary bypass and during septic shock. Crit Care Resusc. 2000;2:181-187. [PubMed] |
| 67. | Jansma G, de Lange F, Kingma WP, Vellinga NA, Koopmans M, Kuiper MA, Boerma EC. 'Sepsis-related anemia' is absent at hospital presentation; a retrospective cohort analysis. BMC Anesthesiol. 2015;15:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 68. | Kempe DS, Akel A, Lang PA, Hermle T, Biswas R, Muresanu J, Friedrich B, Dreischer P, Wolz C, Schumacher U, Peschel A, Götz F, Döring G, Wieder T, Gulbins E, Lang F. Suicidal erythrocyte death in sepsis. J Mol Med (Berl). 2007;85:273-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 190] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 69. | Sun Y, Liu G, Jiang Y, Wang H, Xiao H, Guan G. Erythropoietin Protects Erythrocytes Against Oxidative Stress-Induced Eryptosis In Vitro. Clin Lab. 2018;64:365-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 70. | Repsold L, Joubert AM. Eryptosis: An Erythrocyte's Suicidal Type of Cell Death. Biomed Res Int. 2018;2018:9405617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 146] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 71. | Bissinger R, Bhuyan AAM, Qadri SM, Lang F. Oxidative stress, eryptosis and anemia: a pivotal mechanistic nexus in systemic diseases. FEBS J. 2019;286:826-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 72. | Steele T, Kolamunnage-Dona R, Downey C, Toh CH, Welters I. Assessment and clinical course of hypocalcemia in critical illness. Crit Care. 2013;17:R106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 73. | Tinawi M. Disorders of Calcium Metabolism: Hypocalcemia and Hypercalcemia. Cureus. 2021;13:e12420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 74. | Todd JC 3rd, Mollitt DL. Effect of sepsis on erythrocyte intracellular calcium homeostasis. Crit Care Med. 1995;23:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 75. | Rogers S, Doctor A. Red Blood Cell Dysfunction in Critical Illness. Crit Care Clin. 2020;36:267-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 76. | Zaloga GP, Washburn D, Black KW, Prielipp R. Human sepsis increases lymphocyte intracellular calcium. Crit Care Med. 1993;21:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 77. | Joffre J, Hellman J. Oxidative Stress and Endothelial Dysfunction in Sepsis and Acute Inflammation. Antioxid Redox Signal. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 212] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 78. | Cardinal-Fernández P, Lorente JA, Ballén-Barragán A, Matute-Bello G. Acute Respiratory Distress Syndrome and Diffuse Alveolar Damage. New Insights on a Complex Relationship. Ann Am Thorac Soc. 2017;14:844-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 79. | Sharp C, Millar AB, Medford AR. Advances in understanding of the pathogenesis of acute respiratory distress syndrome. Respiration. 2015;89:420-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 80. | Habib MP, Clements NC. Effects of low-dose hydrogen peroxide in the isolated perfused rat lung. Exp Lung Res. 1995;21:95-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 81. | Seeger W, Hansen T, Rössig R, Schmehl T, Schütte H, Krämer HJ, Walmrath D, Weissmann N, Grimminger F, Suttorp N. Hydrogen peroxide-induced increase in lung endothelial and epithelial permeability--effect of adenylate cyclase stimulation and phosphodiesterase inhibition. Microvasc Res. 1995;50:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 82. | Zhou X, Qian Y, Yuan D, Feng Q, He P. H2 O2 -induced microvessel barrier dysfunction: the interplay between reactive oxygen species, nitric oxide, and peroxynitrite. Physiol Rep. 2019;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 83. | He P, Talukder MAH, Gao F. Oxidative Stress and Microvessel Barrier Dysfunction. Front Physiol. 2020;11:472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 84. | Manrique-Caballero CL, Del Rio-Pertuz G, Gomez H. Sepsis-Associated Acute Kidney Injury. Crit Care Clin. 2021;37:279-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 187] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 85. | Post EH, Kellum JA, Bellomo R, Vincent JL. Renal perfusion in sepsis: from macro- to microcirculation. Kidney Int. 2017;91:45-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 143] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 86. | Peerapornratana S, Manrique-Caballero CL, Gómez H, Kellum JA. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019;96:1083-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 1024] [Article Influence: 146.3] [Reference Citation Analysis (0)] |
| 87. | Waltz P, Carchman E, Gomez H, Zuckerbraun B. Sepsis results in an altered renal metabolic and osmolyte profile. J Surg Res. 2016;202:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 88. | Patil NK, Parajuli N, MacMillan-Crow LA, Mayeux PR. Inactivation of renal mitochondrial respiratory complexes and manganese superoxide dismutase during sepsis: mitochondria-targeted antioxidant mitigates injury. Am J Physiol Renal Physiol. 2014;306:F734-F743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 89. | McCarthy C, Kenny LC. Therapeutically targeting mitochondrial redox signalling alleviates endothelial dysfunction in preeclampsia. Sci Rep. 2016;6:32683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 90. | Ratliff BB, Abdulmahdi W, Pawar R, Wolin MS. Oxidant Mechanisms in Renal Injury and Disease. Antioxid Redox Signal. 2016;25:119-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 528] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 91. | Cao Y, Xu J, Cui D, Liu L, Zhang S, Shen B, Wu Y, Zhang Q. Protective effect of carnosine on hydrogen peroxide-induced oxidative stress in human kidney tubular epithelial cells. Biochem Biophys Res Commun. 2021;534:576-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 92. | Tatsumi T, Kako KJ. Effects of hydrogen peroxide on mitochondrial enzyme function studied in situ in rat heart myocytes. Basic Res Cardiol. 1993;88:199-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 93. | Sun J, Zhang J, Tian J, Virzì GM, Digvijay K, Cueto L, Yin Y, Rosner MH, Ronco C. Mitochondria in Sepsis-Induced AKI. J Am Soc Nephrol. 2019;30:1151-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 193] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 94. | Yoshioka T, Ichikawa I, Fogo A. Reactive oxygen metabolites cause massive, reversible proteinuria and glomerular sieving defect without apparent ultrastructural abnormality. J Am Soc Nephrol. 1991;2:902-912. [PubMed] |
| 95. | Adelborg K, Larsen JB, Hvas AM. Disseminated intravascular coagulation: epidemiology, biomarkers, and management. Br J Haematol. 2021;192:803-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 96. | Iba T, Umemura Y, Watanabe E, Wada T, Hayashida K, Kushimoto S; Japanese Surviving Sepsis Campaign Guideline Working Group for disseminated intravascular coagulation. Diagnosis of sepsis-induced disseminated intravascular coagulation and coagulopathy. Acute Med Surg. 2019;6:223-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 97. | Ambrosio G, Tritto I, Golino P. Reactive oxygen metabolites and arterial thrombosis. Cardiovasc Res. 1997;34:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 98. | Penn MS, Patel CV, Cui MZ, DiCorleto PE, Chisolm GM. LDL increases inactive tissue factor on vascular smooth muscle cell surfaces: hydrogen peroxide activates latent cell surface tissue factor. Circulation. 1999;99:1753-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 99. | van Vught LA, Uhel F, Ding C, Van't Veer C, Scicluna BP, Peters-Sengers H, Klein Klouwenberg PMC, Nürnberg P, Cremer OL, Schultz MJ, van der Poll T; MARS consortium. Consumptive coagulopathy is associated with a disturbed host response in patients with sepsis. J Thromb Haemost. 2021;19:1049-1063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 100. | Wetter DA, Davis MD. Ulceration of the arm attributed to a spider bite and treated with intravenous hydrogen peroxide: a cautionary tale. Arch Dermatol. 2006;142:1658-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 101. | Walborn A, Rondina M, Mosier M, Fareed J, Hoppensteadt D. Endothelial Dysfunction Is Associated with Mortality and Severity of Coagulopathy in Patients with Sepsis and Disseminated Intravascular Coagulation. Clin Appl Thromb Hemost. 2019;25:1076029619852163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 102. | Dolmatova EV, Wang K, Mandavilli R, Griendling KK. The effects of sepsis on endothelium and clinical implications. Cardiovasc Res. 2021;117:60-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 195] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 103. | Inata Y. Should we treat sepsis-induced DIC with anticoagulants? J Intensive Care. 2020;8:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 104. | 108 Mazeraud A, Righy C, Bouchereau E, et al Septic-associated encephalopathy: a comprehensive review. Neurotherapeutics 2020;17:392-403. [RCA] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 219] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 105. | Chung HY, Wickel J, Brunkhorst FM, Geis C. Sepsis-Associated Encephalopathy: From Delirium to Dementia? J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 106. | Desagher S, Glowinski J, Premont J. Astrocytes protect neurons from hydrogen peroxide toxicity. J Neurosci. 1996;16:2553-2562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 418] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 107. | Cannon G, Caravati EM, Filloux FM. Hydrogen peroxide neurotoxicity in childhood: case report with unique magnetic resonance imaging features. J Child Neurol. 2003;18:805-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 108. | Ohashi M, Hirano T, Watanabe K, Katsumi K, Ohashi N, Baba H, Endo N, Kohno T. Hydrogen peroxide modulates synaptic transmission in ventral horn neurons of the rat spinal cord. J Physiol. 2016;594:115-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 109. | Frantseva MV, Perez Velazquez JL, Carlen PL. Changes in membrane and synaptic properties of thalamocortical circuitry caused by hydrogen peroxide. J Neurophysiol. 1998;80:1317-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 110. | Teepker M, Anthes N, Fischer S, Krieg JC, Vedder H. Effects of oxidative challenge and calcium on ATP-levels in neuronal cells. Neurotoxicology. 2007;28:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 111. | Wang Y, Floor E. Hydrogen peroxide inhibits the vacuolar H+-ATPase in brain synaptic vesicles at micromolar concentrations. J Neurochem. 1998;70:646-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 112. | Kamsler A, Segal M. Hydrogen peroxide modulation of synaptic plasticity. J Neurosci. 2003;23:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 149] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 113. | Santhanam S, Venkatraman A, Ramakrishna BS. Impairment of mitochondrial acetoacetyl CoA thiolase activity in the colonic mucosa of patients with ulcerative colitis. Gut. 2007;56:1543-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 114. | 118 US Department of Health and Human Services. Chemical Hazards emergency medical management. [cited 3 February 2021] Available from: https://chemm.nlm.nih.gov/countermeasure_sodium-thiosulfate.htm#indication. |
| 115. | Pravda J. Hydrogen peroxide and disease: towards a unified system of pathogenesis and therapeutics. Mol Med. 2020;26:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 116. | Tsang RY, Al-Fayea T, Au HJ. Cisplatin overdose: toxicities and management. Drug Saf. 2009;32:1109-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 185] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 117. | Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 290] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 118. | Neuwelt EA, Gilmer-Knight K, Lacy C, Nicholson HS, Kraemer DF, Doolittle ND, Hornig GW, Muldoon LL. Toxicity profile of delayed high dose sodium thiosulfate in children treated with carboplatin in conjunction with blood-brain-barrier disruption. Pediatr Blood Cancer. 2006;47:174-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 119. | Enongene EN, Sun PN, Mehta CS. Sodium thiosulfate protects against acrylonitrile-induced elevation of glial fibrillary acidic protein levels by replenishing glutathione. Environ Toxicol Pharmacol. 2000;8:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 120. | Hayden MR, Tyagi SC, Kolb L, Sowers JR, Khanna R. Vascular ossification-calcification in metabolic syndrome, type 2 diabetes mellitus, chronic kidney disease, and calciphylaxis-calcific uremic arteriolopathy: the emerging role of sodium thiosulfate. Cardiovasc Diabetol. 2005;4:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 148] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited Manuscript
Specialty type: Critical care medicine
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shou ST S-Editor: Ma YJ L-Editor: A P-Editor: Wang LYT