INTRODUCTION
Cardiac arrest is a major cause of unexpected death in developed countries, and patients with cardiac arrest generally have a poor prognosis[1,2]. In 1960, artificial ventilation (mouth-to-mouth), chest compression, and electrical defibrillation were integrated into clinical practice. These methods were rediscovered rather than developed from scratch and were used for resuscitation of cardiac arrest patients[3,4]. However, few patients could achieve return of spontaneous circulation (ROSC) with conventional cardiopulmonary resuscitation (CPR). Moreover, despite achieving ROSC, some patients showed re-arrest and many survivors were unable to fully resume their former lifestyles because of severe neurological deficits.
Pretto et al[5] and Safar et al[6] reported the effectiveness of emergency cardiopulmonary bypass for CPR in an animal model, and discussed the possibility of employing cardiopulmonary bypass as a CPR method. However, the system used for extracorporeal assist circulation was cumbersome and not easily available; therefore, it was only used for experimental methods. Recent progress in medical engineering has enabled the development of centrifugal pumps, membrane oxygenators, and thin wall cannulae, which provide easy percutaneous cannulation of the femoral artery and vein. Therefore the cardiopulmonary bypass system has now become small and portable and circulatory support can be easily provided to cardiac arrest or shock patients. In 1983, Phillips et al[7] performed extracorporeal cardiopulmonary resuscitation (ECPR) using a system comprising thin wall cannulae that enable percutaneous cannulation and a centrifugal pump in 5 patients, of whom 3 patients survived. In 1989, the International Resuscitation Research Center began clinical studies and reported favorable results: 40 of 187 patients (21%) survived[8]. Veno-arterial extracorporeal membrane oxygenation (ECMO), also known as percutaneous cardiopulmonary bypass, emergency cardiopulmonary bypass, portable cardiopulmonary bypass, or percutaneous cardiopulmonary support, provide rapid temporal circulatory assistance to patients with shock or cardiac arrest. Martin et al[9] reported that percutaneous cardiopulmonary bypass could be initiated in emergency department. Veno-arterial ECMO is performed in emergency departments and can be used for performing ECPR in patients with out-of-hospital cardiac arrest[9-11]. Recently, Chen et al[12,13] reported that ECPR is superior to conventional CPR in in-hospital cardiac arrest patients. Although there is no sufficient evidence to support the efficacy of ECPR in out-of-hospital cardiac arrest patients, encouraging results have been obtained in small case series[10,11,14]. The recently published CPR guidelines recommend ECPR for patients with limited cardiac arrest that may be caused by accidental hypothermia or drug toxicity[15,16]. Further studies are necessary to assess the efficacy and feasibility of ECPR in out-of-hospital cardiac arrest patients.
ECMO SYSTEMS
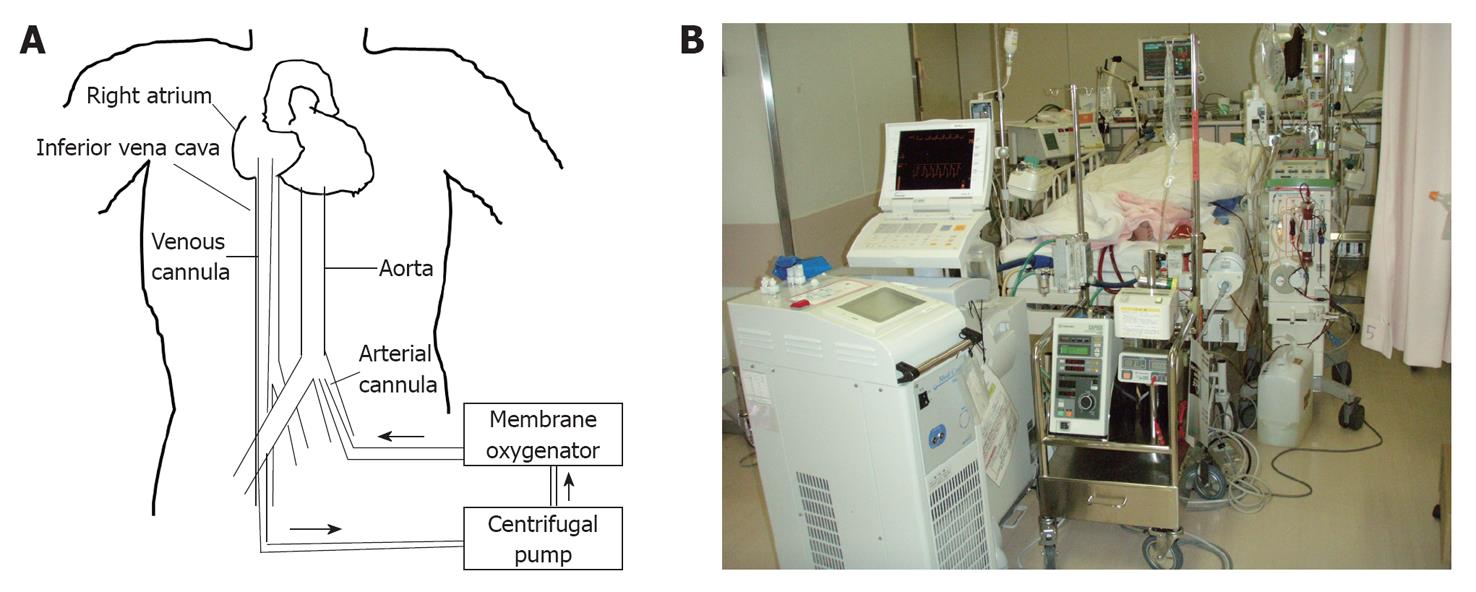
The ECMO system comprises a centrifugal pump, a membrane oxygenator, a heat exchanger, and bypass cannulae (Figure 1A). A membrane oxygenator oxygenates the blood. To rapidly establish circulatory support, the system should be portable. The centrifugal pump for ECMO is small compared with that used in cardiopulmonary bypass for cardiovascular surgery. The veno-arterial ECMO circuit extends from the cannula (inserted from the femoral vein) in the right atrium to the femoral artery and facilitates artificial blood circulation. Arfitifical oxygenation via an oxygenator is necessary, because pulmonary blood circulation is bypassed.
Figure 1 Scheme and picture of the extracorporeal membrane oxygenation system.
A: Scheme; B: Picture.
Both roller and centrifugal pumps are used in cardiopulmonary bypass, while only the centrifugal pump is used in veno-arterial ECMO. The roller pump is simple and blood flow is proportional to the number of rounds. The roller pump creates aspiration pressure strong enough to cause haemolysis or air aspiration if the amount of blood aspirated by the venous cannula is insufficient.
The venous blood aspirated by the venous cannula from the right atrium was oxygenated and returned to the femoral artery in veno-arterial ECMO.
Percutaneous cannulation performed by the widely known Seldinger technique facilitates initiation of veno-arterial ECMO. Arterial cannulae measuring 15-17 French and venous cannulae measuring 17-19 French are usually used. The centrifugal pump pressure is lost largely in these cannulae. If the cannulae are not sufficiently large, haemolysis tends to be severe.
INCLUSION CRITERIA FOR ECPR
Because ECPR is expensive and requires substantial manpower, ECPR cannot be performed for all cardiac arrest patients. Furthermore, the inclusion criteria for ECPR differ in each institution. For example, ECPR has low-grade recommendation in the resuscitation guidelines. The inclusion criteria of ECPR in our hospital are as follows: age of 18-74 years, ventricular fibrillation on electrocardiography during CPR, estimated interval of less than 15 min from the patient’s collapse to initiation of resuscitation, presumed cardiac origin or pulmonary embolism as the cause of the arrest, and ROSC not achievable within 20 min of conventional CPR by medical personnel. Patients were excluded if they had a terminal illness preceding the arrest and acute aortic dissection with pericardial effusion observed on echocardiography[10].
ECPR PROCEDURES
The ECPR procedure may be different in each institution because of the use of different software and hardware. The following is the ECPR procedure in Hiroshima City Asa Hospital. The cardiologists and medical engineers there have been trained to set up the ECMO systems within 10 min in all cases. In cases of out-of-hospital cardiac arrest, the physician who receives the telephone call from the out-of-hospital emergency medical personnel evaluates the indication for ECPR and its appropriateness for the patient. The cardiology team prepares for advanced cardiac life support, alerts the catheter laboratory, and prepares the ECMO system before patient arrival. If ECPR is considered appropriate for an out-of-hospital cardiac arrest patient who had not achieved ROSC on arrival to the hospital, advanced cardiac life support is continued according to the guidelines. If ROSC could not be achieved after a second dose of epinephrine, the patient is administered continuous chest compressions and transferred to the catheter laboratory. The femoral vein and artery are percutaneously cannulated to achieve extracorporeal circulation, and circulatory support is initiated in the catheter laboratory. A similar system of transfer to the catheter laboratory is used to establish ECMO for in-hospital cardiac arrest patients if advanced cardiac life support fails. After following these protocols, emergency coronary angiography, percutaneous coronary intervention (PCI), emergency pulmonary angiography, IABP, pulmonary angiography and/or placement of a pulmonary artery catheter were performed if necessary, and patients are transferred from the catheter laboratory to the coronary care unit for further intensive care (Figure 1B). Computed tomography is performed during patient transfer from the catheter laboratory to the coronary care unit. In haemodynamically stable comatose patients treated with ECMO, IABP and/or drugs, mild hypothermia is induced by rapid injection of cold saline, surface cooling, using a heat exchanger attached to the ECMO circuit, and/or direct blood cooling by a coil attached to a circuit for continuous haemodiafiltration[10,17,18]. The ECMO circuit is usually primed with cold saline and the heat exchanger can rapidly induced the target temperature of 32-34 °C in cardiac arrest patients.
SUBSEQUENT THERAPEUTIC INTERVENTIONS
Chest compression can be ceased after veno-arterial ECMO and pump flow are deemed appropriate. Veno-arterial ECMO allows minimum brain and coronary flow. However, it only provides circulatory support and cannot treat the cause of cardiac arrest: the different causes of cardiac arrest, such as acute coronary syndrome, pulmonary embolism, accidental hypothermia, drug intoxication, and electrolytes disorder, require separate attention. As described above, primary PCI for acute myocardial infarction improves the clinical outcomes. Hence, emergency coronary angiography should be performed in cardiac arrest patients without any obvious external cause of cardiac arrest[19].
Therapeutic hypothermia can improve the clinical outcome in out-of-hospital cardiac arrest patients with ventricular fibrillation, and a similar efficacy is anticipated in in-hospital cardiac arrest patients and cardiac arrest patients whose initial recorded rhythm was non-shockable. Before the initiation of ECMO, rapid injection of cold saline may be feasible and effective[20,21]. The ECMO circuit should therefore be primed with cold saline. After the initiation of ECMO, a heat exchanger can rapidly induce mild hypothermia.
WEANING FROM ECMO
If cardiac function shows improvement, pump flow should be decreased to reduce the left ventricular afterload and risk of haemolysis. If pump flow is decreased to 1.5 L/min and left ventricular ejection time is more than 200 msec, weaning from ECMO should be considered. After additional heparin is administered, the circuit should be clamped for 10 min, and vital signs such as heart rate, blood pressure, pulmonary artery pressure, oxygen saturation, and presence of lethal arrhythmia should be observed. If the above parameters appear abnormal, the circuit should be declamped and circulatory support should be immediately restarted. If the above parameters are within the tolerance levels and there is no lethal arrhythmia, the patient can be weaned from ECMO. The cannulae can be removed surgically or by manual compression. Although surgical removal is safe and reliable, we stop bleeding by manual compression without a low incidence of hematoma because the cannulae became thinner compared to previously used cannulae. If the patient develop refractory shock again after the surgical removal of cannulae, ECMO should be restarted. Re-cannulation may present some difficulty for patients with severe peripheral artery disease.
FUTURE DIRECTIONS
The feasibility and efficacy of ECPR for in-hospital cardiac arrest patients have been reported[13]. However, the feasibility, safety, efficacy, and cost-effectiveness of ECPR for out-of-hospital cardiac arrest patients remain unclear. Further studies are necessary to assess these factors. The rate of favorable recovery remains low in refractory cardiac arrest patients despite being treated with ECPR. Therefore, other novel ideas, methods, or procedures are necessary to enable cardiac arrest patients to resume their former lifestyles.
Peer reviewer: Dr. Yan-Ren Lin, MD, Department of Emergency Medicine, Changhua Christian Hospital, 135 Nanshsiao Street, Changhua 500, Taiwan, China
S- Editor Gou SX L- Editor A E- Editor Zheng XM