Published online Feb 4, 2012. doi: 10.5492/wjccm.v1.i1.4
Revised: October 18, 2011
Accepted: December 21, 2011
Published online: February 4, 2012
Cardiac arrest is one of the leading causes of death and represents maximal stress in humans. After restoration of spontaneous circulation, post-cardiac arrest syndrome is the predominant disorder in survivors. Besides the post-arrest brain injury, the post-resuscitation myocardial stunning, and the systemic ischemia/reperfusion response, this syndrome is characterized by adrenal insufficiency, a disorder that often remains undiagnosed. The pathophysiology of adrenal insufficiency has not been elucidated. We performed a comprehensive search of three medical databases in order to describe the major pathophysiological disturbances which are responsible for the occurrence of the disorder. Based on the available evidence, this article will help physicians to better evaluate and understand the hidden yet deadly post-cardiac arrest adrenal insufficiency.
- Citation: Chalkias A, Xanthos T. Post-cardiac arrest syndrome: Mechanisms and evaluation of adrenal insufficiency. World J Crit Care Med 2012; 1(1): 4-9
- URL: https://www.wjgnet.com/2220-3141/full/v1/i1/4.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v1.i1.4
Sudden cardiac death is one of the leading causes of death in Europe as it affects 350 000-700 000 individuals per year[1]. Although restoration of spontaneous circulation (ROSC) may be achieved, prognostication for cardiac arrest victims remains dismal, as only 17% survive to hospital discharge[2]. Patients with ROSC have not only suffered a situation characterized by maximal stress, but are also going to pass through the “Clashing Rocks” of post-cardiac arrest syndrome. This syndrome combines three major pathophysiological processes, the post-arrest brain injury, the post-cardiac arrest myocardial dysfunction, and the systemic ischemia/reperfusion syndrome[3]. Post-cardiac arrest syndrome has been characterized as a sepsis-like syndrome because it is associated with increased immuno-inflammatory status, hemodynamic instability and multiple organ dysfunction[4]. Recent studies have shown that adrenal insufficiency frequently occurs after ROSC and compromises the outcome of victims[5,6]. Although this matter is not recent, the pathophysiology of post-arrest adrenal insufficiency has not been elucidated.
The PubMed, CINAHL and Scopus databases were comprehensively searched for relevant studies, using keywords: ‘‘cardiac arrest’’, ‘‘post-cardiac arrest syndrome’’, ‘‘adrenal insufficiency’’. All human case reports, animal studies, reviews and randomized controlled studies were included in our search and cross-referencing was performed using the bibliographies from the articles obtained. Selection of studies was based on the population, outcomes, research method, and results of the studies. Pediatric studies were not included.
In order to present significant science, lesser quality studies were excluded from our research to reduce the risk of errors and bias. More specifically, from 62 records identified through database searching and 4 additional records identified through other sources, 5 duplicates were removed. The remaining articles were assessed for eligibility and 4 articles were excluded due to systematic errors. Finally, 57 articles were included in this qualitative synthesis. This article reviews the basic pathophysiological disturbances which are responsible for the emergence of post-cardiac arrest adrenal insufficiency.
The adrenal glands are located at the top of the kidneys in the retroperitoneum. In each gland there are two distinct regions, an inner medulla which is richly innervated by preganglionic sympathetic fibers and is the source of catecholamines, and an outer cortex which secretes several hormones.
The adrenal cortex, the outer portion of the adrenal gland, secretes hormones directly into the bloodstream which have an effect on the body’s metabolism, on chemicals in the blood, and on certain body characteristics. These hormones are glucocorticoids, mineralocorticoids, and androgens. Glucocorticoids have potent anti-inflammatory and immunosuppressive properties. The secretion of these hormones is controlled by a close integration between the nervous and endocrine systems[7]. Cortisol and other glucocorticoids are secreted in response to adrenocorticotropic hormone (ACTH). In healthy subjects, 90% of plasma cortisol is bound to globulin and albumin, and only 10% is in the free or biologically active form[8].
ACTH is secreted under the control of the hypothalamic peptide, corticotrophin-releasing hormone, and binds to receptors in the plasma membrane of cells in the zona fasiculata and reticularis of the adrenal gland[7,9]. Hormone-receptor engagement activates adenyl cyclase, leading to elevated intracellular levels of cyclic adenosine monophosphate which leads ultimately to activation of the enzyme systems involved in biosynthesis of cortisol from cholesterol. Any type of physical or mental stress results in elevation of the cortisol concentration in blood. In contrast, cortisol secretion is suppressed by classical negative feedback loops.
The adrenal medulla, the inner part of the adrenal gland, secretes the catecholamines epinephrine and norepinephrine. Catecholamines are produced mainly by the chromaffin cells of the adrenal medulla from further metabolic modification of dopamine. Catecholamines are released in response to stress and are water-soluble (50% bound to plasma proteins) molecules.
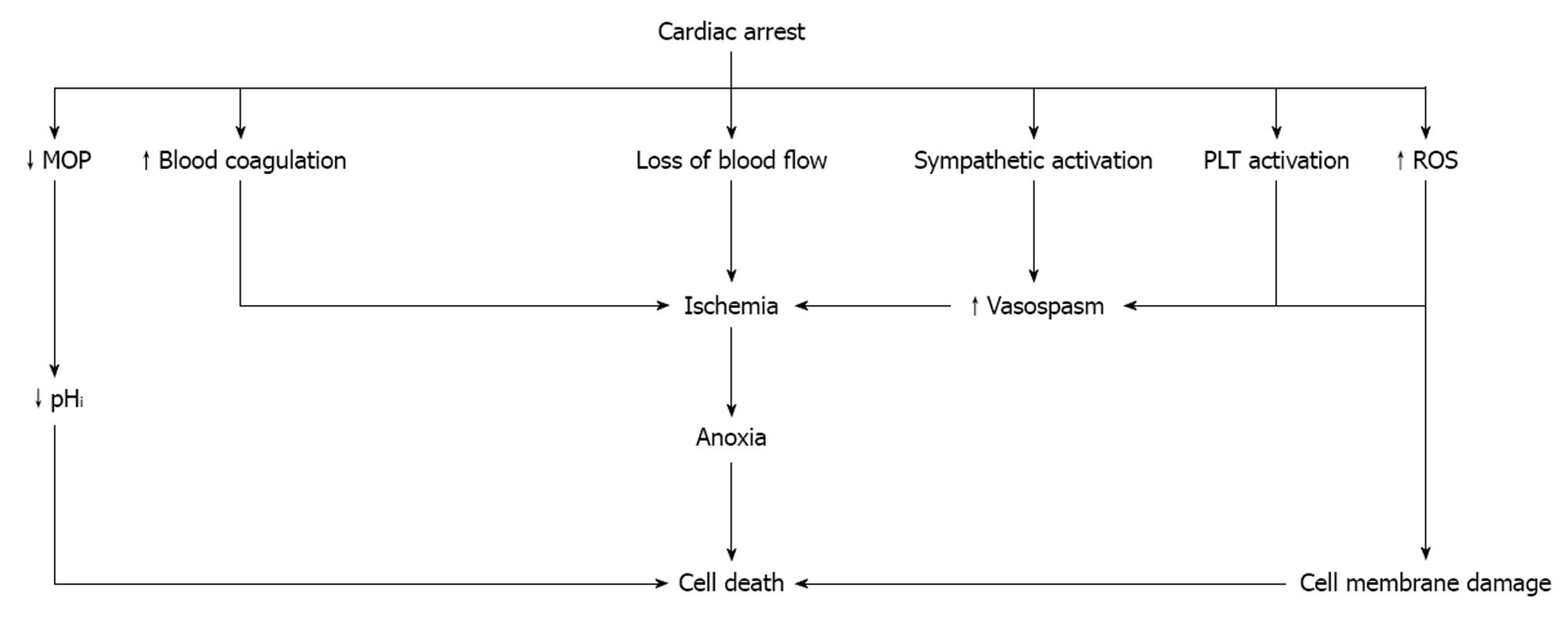
The onset of cardiac arrest causes a unique situation which is characterized by maximal stress. The loss of blood flow results in the withdrawal of hypotension-induced baroreflex and an increase in vascular resistance[9]. Shortly after the arrest, the blood flow to the adrenal glands is gradually reduced and minimizes in a few seconds[10,11]. The acute onset of ischemia activates the sympathetic system and norepinephrine is released by the adrenal glands and the sympathetic nerve terminals[12]. Despite the 10- to 100-fold elevation in endogenous plasma catecholamines, the adrenal blood flow not only remains inadequate, but after a while it worsens due to adrenal microvessel contraction[13]. Adrenomedullin, a vasodilator peptide with a half-life of about 20 min, is partly responsible for this phenomenon as it dampens baroreflex-driven responses and buffers sympathetic actions[14]. The resulting anoxia has a major impact on the function of the adrenal gland which may already be compromised by preexisting conditions affecting the hypothalamic-pituitary-adrenal axis[15].
The cellular response to oxygen is coordinated by the hypoxia-inducible factor (HIF) and its regulator, the Von Hippel-Lindau tumor suppressor protein[16]. HIF1 consists of a heterodimer of two proteins, the HIF1-α which accumulates under hypoxic conditions and activates transcription of endothelial nitric oxide synthase (eNOS), and the HIF1-β which is constitutively expressed[17,18]. Hypoxia also induces p53 protein accumulation and initiation of apoptosis, while p53 directly interacts with HIF1-α and limits hypoxia-induced expression of HIF1-α[19]. If the restoration of blood flow is not recovered quickly, the adrenal gland damage will be permanent due to cell death[20,21].
In response to the stress of global ischemia, various inflammatory cytokines are synthesized and released[22], while the complement cascade is activated resulting in chemotaxis and adherence of polymorphonuclear leukocytes (PMNs)[19], increased vascular permeability, activation of blood coagulation, platelet activation, and endothelial and tissue damage[23-26]. At the same time, toxic reactive oxygen species (ROS) and cytokines, two of the factors responsible for post-arrest adrenal insufficiency, are released from the activated PMNs (Table 1). In addition, the activated platelets release vasoactive substances causing vasoconstriction which is further enhanced by expression of cyclooxygenase-2 in response to hypoxia and ischemia, the presence of several cytokines, and by increased oxidative stress[27,28].
| Adrenal gland ischemia and anoxia |
| Increased inflammatory response |
| Oxidative stress |
| Ischemia/reperfusion injury |
| Activation of apoptosis and programmed cell death |
| Malfunction of hypothalamic-pituitary-adrenal axis |
| Down-regulation of adrenal cell membrane receptors |
| Adrenomedullin secretion |
| Abnormalities in nitric oxide production |
| Drugs administered during cardiopulmonary resuscitation |
| Low levels of cortisol binding protein |
| Hypoalbuminemia |
Intracellular acidosis which is established shortly after the development of anoxia causes mitochondrial oxidative phosphorylation to stop resulting in adenosine triphosphate (ATP) depletion and acceleration of anaerobic glycolysis. The concentration of pyruvate increases and hydrogen ions and lactate are produced[29]. The prolongation of ischemia further decreases the intracellular pH, while damage to the cell membrane by ROS leads to a progressive increase in membrane permeability and severe derangements of intracellular electrolytes (Figure 1).
During optimal cardiopulmonary resuscitation (CPR), the cardiac output is between 25% and 40% of pre-arrest values[30]. Although the peak systolic arterial pressure ranges between 60 and 80 mmHg[31,32], the adrenal gland blood flow is minimal as most of the blood pumped out of the heart supplies the brain and coronary arteries. The exact amount of blood supplying the adrenal glands during CPR is unknown. However, based on the available evidence, it should not differ significantly from the amount of blood which flows before the initiation of resuscitation[30]. Regarding this issue, two possibilities exist; either the adrenal glands remain anoxic and/or a small amount of blood is flowing into the glands. In the first case, the resuscitability of the glands is further compromised as cell death continues due to the prolongation of ischemia. In the second case, the small amount of blood flowing into the glands promotes the onset of the ischemia/reperfusion response (I/R) which is characterized by ROS generation and neutrophil activation. Despite the deleterious effects of I/R, the low concentrations of oxygen which are transferred to the adrenal glands enhance the production of small amounts of ATP which contribute to tissue survival.
Due to the systemic I/R response, blood coagulation is activated which, together with the effect of catecholamines and the accumulation of activated PMNs and platelets result in microvascular obstruction[33]. These effects are responsible for the low concentration of serum cortisol which is often observed at the end of cardiac arrest[34].
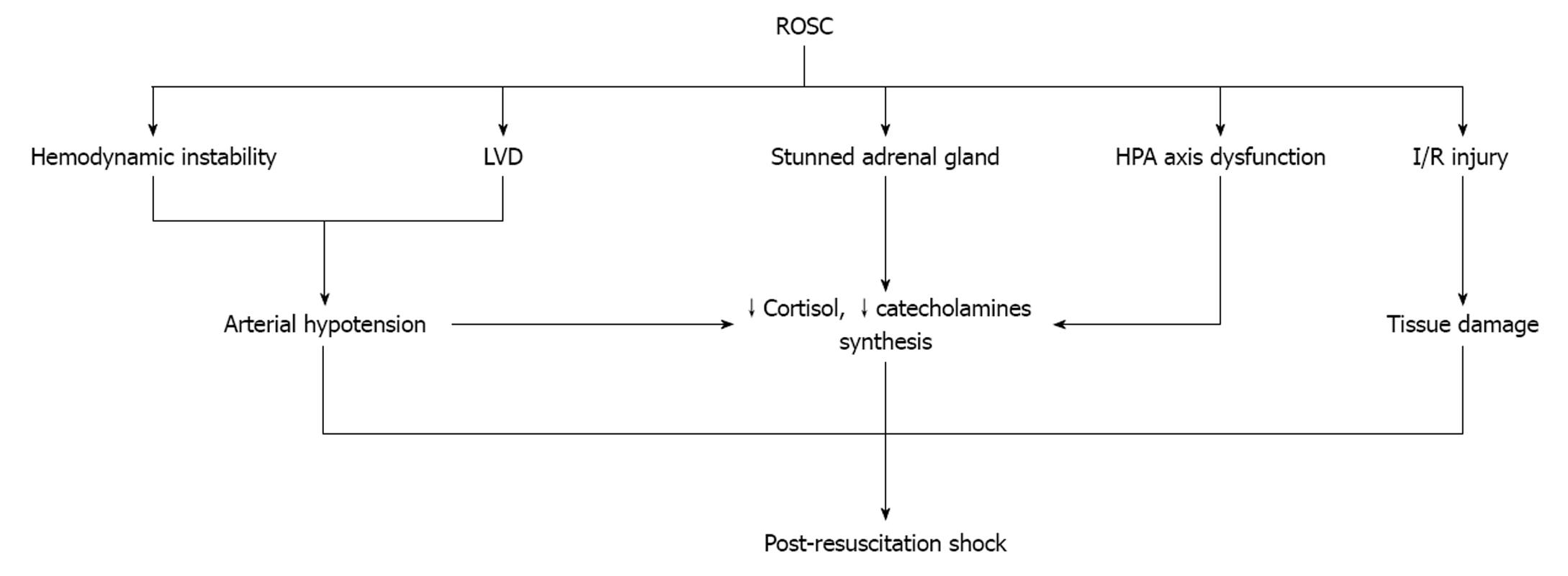
After ROSC, hemodynamic instability and left ventricular dysfunction are the main characteristics of survivors (Figure 2). Although arterial hypotension serves as a stimulus for continued endogenous catecholamine synthesis and release, such a relationship has not been demonstrated[13]. Prengel et al[35] found that the concentrations of plasma catecholamines after ROSC are initially high but gradually decrease during the immediate and early post-resuscitation period[3]. One possible explanation for this phenomenon is that when ROSC is recovered, the high concentrations of endogenous and exogenous catecholamines begin to metabolize. The “stunned” adrenal glands fail to synthesize and release these substances or release small amounts due to several reasons. First, the function of the glands is dependent not only on the number of the cells which survived the period of extreme anoxia, but, also, on the effect of preexisting conditions affecting the hypothalamic-pituitary-adrenal axis[15]. Second, the I/R syndrome inactivates several metabolic enzymes and injures the cells which survived[36]. Third, synthesis and release may decrease after down regulation of the cell’s receptors due to the increased concentrations of plasma catecholamines. Another reason is the increased concentration of adrenomedullin which is secreted in response to increased epinephrine[14]. Finally, post-cardiac arrest brain injury is responsible for the degeneration of selectively vulnerable neuron subpopulations over a period of hours to days[3]. Degeneration of hypothalamus and/or pituitary gland will result in failure of the hypothalamic-pituitary-adrenal axis[37].
There is a negative correlation between the interval from collapse to the start of CPR and the plasma cortisol level after ROSC[5,38]. Adrenal insufficiency, a consequence of anoxia and high concentrations of epinephrine during cardiac arrest and CPR intervals[5], is correlated with poor outcome[6]. The chemical changes that occur during cardiac arrest predispose to a massive burst of ROS and cytokine production during the first minutes of ROSC which directly inhibit adrenal cortisol synthesis[18,39,40]. Moreover, some drugs administered during CPR inhibit the activity of enzymes involved in cortisol synthesis[41]. The resulting low concentration of cortisol not only adversely affects the post-resuscitation hemodynamic status, but decreases the production of nitric oxide (NO) which possesses anti-inflammatory and anti-ischemic properties[42]. Endothelial NO is a second messenger which is produced by eNOS. NO production is stimulated by a variety of mechanical forces, such as shear stress and cyclic strain, and humoral factors including acetylcholine, vascular endothelial growth factor, and angiotensin-II[43]. Exposure of adrenal endothelial cells to hemodynamic disturbances during cardiac arrest and CPR results in the activation of several signal transduction pathways leading to eNOS activation. eNOS plays a crucial role in the state of blood vessel vasodilatation, modulates platelet aggregation as well as platelet and PMN adhesion to the endothelium, and can interact with various proteins resulting in inhibition of apoptosis[44]. During cardiac arrest and after ROSC, the abnormalities in NO production contribute to the occurrence of adrenal insufficiency and low cortisol creating a harmful vicious cycle[44-49].
The incidence of adrenal insufficiency in the critically ill is 30%-60%, while its most prominent manifestation is hypotension which is refractory to vasopressors[50-52]. Other clinical manifestations of adrenal insufficiency (such as electrolyte abnormalities and hyperpigmentation) are not specific enough to suggest the diagnosis. In mild or chronic cases, the hemodynamic changes are often a reflection of hypovolemia, while in acute adrenal failure the hemodynamic changes are similar to those of hyperdynamic shock.
Adrenal insufficiency should be suspected in any resuscitated patient who develops an unstable or reduced blood pressure of unclear etiology, or has hypotension that is refractory to fluid resuscitation and vasopressors. However, the disorder may not be evident clinically and has to be uncovered by biochemical evidence of abnormal adrenal responsiveness[50]. Unfortunately, there is not enough evidence to support the use of the ACTH test in cardiac arrest patients. This test not only uniquely explores the adrenal reserve, rather than the entire hypothalamic-pituitary-adrenal axis, but, also, it is poorly reproducible. Moreover, the results may not be immediately available and may vary depending on the assay used for analysis. In addition, cortisol transport proteins in blood are diminished in acutely ill patients, while cytokines released during the post-resuscitation period can blunt end-organ responsiveness to cortisol[39]. Consequently, blood cortisol levels may underestimate the severity of abnormal adrenal responsiveness in patients with ROSC.
Post-cardiac arrest adrenal insufficiency contributes to poor survival. The etiology of this disorder is multifactorial and its severity varies. Unless diagnosed early, most patients will suffer refractory shock. This article encompasses all the available evidence and presents the pathophysiology and the diagnostic limitations of post-cardiac arrest adrenal insufficiency.
| 1. | Koster RW, Baubin MA, Bossaert LL, Caballero A, Cassan P, Castrén M, Granja C, Handley AJ, Monsieurs KG, Perkins GD. European Resuscitation Council Guidelines for Resuscitation 2010 Section 2. Adult basic life support and use of automated external defibrillators. Resuscitation. 2010;81:1277-1292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 433] [Cited by in RCA: 391] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 2. | Eisenberg MS, Mengert TJ. Cardiac resuscitation. N Engl J Med. 2001;344:1304-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 348] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 3. | Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Böttiger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118:2452-2483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1061] [Cited by in RCA: 1153] [Article Influence: 64.1] [Reference Citation Analysis (0)] |
| 4. | Adrie C, Adib-Conquy M, Laurent I, Monchi M, Vinsonneau C, Fitting C, Fraisse F, Dinh-Xuan AT, Carli P, Spaulding C. Successful cardiopulmonary resuscitation after cardiac arrest as a "sepsis-like" syndrome. Circulation. 2002;106:562-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 757] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 5. | Pene F, Hyvernat H, Mallet V, Cariou A, Carli P, Spaulding C, Dugue MA, Mira JP. Prognostic value of relative adrenal insufficiency after out-of-hospital cardiac arrest. Intensive Care Med. 2005;31:627-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Hékimian G, Baugnon T, Thuong M, Monchi M, Dabbane H, Jaby D, Rhaoui A, Laurent I, Moret G, Fraisse F. Cortisol levels and adrenal reserve after successful cardiac arrest resuscitation. Shock. 2004;22:116-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Loriaux DL, Fleseriu M. Relative adrenal insufficiency. Curr Opin Endocrinol Diabetes Obes. 2009;16:392-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Hamrahian AH, Oseni TS, Arafah BM. Measurements of serum free cortisol in critically ill patients. N Engl J Med. 2004;350:1629-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 452] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 9. | Arafah BM. Hypothalamic pituitary adrenal function during critical illness: limitations of current assessment methods. J Clin Endocrinol Metab. 2006;91:3725-3745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 218] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 10. | Kern KB, Elchisak MA, Sanders AB, Badylak SF, Tacker WA, Ewy GA. Plasma catecholamines and resuscitation from prolonged cardiac arrest. Crit Care Med. 1989;17:786-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Lindner KH, Haak T, Keller A, Bothner U, Lurie KG. Release of endogenous vasopressors during and after cardiopulmonary resuscitation. Heart. 1996;75:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 125] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Veldkamp MW, Verkerk AO, van Ginneken AC, Baartscheer A, Schumacher C, de Jonge N, de Bakker JM, Opthof T. Norepinephrine induces action potential prolongation and early afterdepolarizations in ventricular myocytes isolated from human end-stage failing hearts. Eur Heart J. 2001;22:955-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Niemann JT, Garner D. Post-resuscitation plasma catecholamines after prolonged arrest in a swine model. Resuscitation. 2005;65:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Roessler A, Goswami N, Haditsch B, Hinghofer-Szalkay H. Modulation of plasma adrenomedullin by epinephrine infusion during head up tilt. Eur J Appl Physiol. 2011;111:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Ten S, New M, Maclaren N. Clinical review 130: Addison's disease 2001. J Clin Endocrinol Metab. 2001;86:2909-2922. [PubMed] [DOI] [Full Text] |
| 16. | Jiang BH, Zheng JZ, Leung SW, Roe R, Semenza GL. Transactivation and inhibitory domains of hypoxia-inducible factor 1alpha. Modulation of transcriptional activity by oxygen tension. J Biol Chem. 1997;272:19253-19260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 473] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 17. | O'Rourke JF, Tian YM, Ratcliffe PJ, Pugh CW. Oxygen-regulated and transactivating domains in endothelial PAS protein 1: comparison with hypoxia-inducible factor-1alpha. J Biol Chem. 1999;274:2060-2071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 184] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Damert A, Ikeda E, Risau W. Activator-protein-1 binding potentiates the hypoxia-induciblefactor-1-mediated hypoxia-induced transcriptional activation of vascular-endothelial growth factor expression in C6 glioma cells. Biochem J. 1997;327:419-423. [PubMed] |
| 19. | Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med. 2001;7:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 706] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 20. | Papathanassoglou ED, Moynihan JA, Ackerman MH. Does programmed cell death (apoptosis) play a role in the development of multiple organ dysfunction in critically ill patients? a review and a theoretical framework. Crit Care Med. 2000;28:537-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 115] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Didenko VV, Wang X, Yang L, Hornsby PJ. Expression of p21(WAF1/CIP1/SDI1) and p53 in apoptotic cells in the adrenal cortex and induction by ischemia/reperfusion injury. J Clin Invest. 1996;97:1723-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | El-Menyar AA. The resuscitation outcome: revisit the story of the stony heart. Chest. 2005;128:2835-2846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Chenoweth DE, Cooper SW, Hugli TE, Stewart RW, Blackstone EH, Kirklin JW. Complement activation during cardiopulmonary bypass: evidence for generation of C3a and C5a anaphylatoxins. N Engl J Med. 1981;304:497-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 774] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 24. | Marks RM, Todd RF, Ward PA. Rapid induction of neutrophil-endothelial adhesion by endothelial complement fixation. Nature. 1989;339:314-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 115] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Stahl GL, Reenstra WR, Frendl G. Complement-mediated loss of endothelium-dependent relaxation of porcine coronary arteries. Role of the terminal membrane attack complex. Circ Res. 1995;76:575-583. [PubMed] |
| 26. | Adler S, Baker PJ, Johnson RJ, Ochi RF, Pritzl P, Couser WG. Complement membrane attack complex stimulates production of reactive oxygen metabolites by cultured rat mesangial cells. J Clin Invest. 1986;77:762-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Golino P, Piscione F, Benedict CR, Anderson HV, Cappelli-Bigazzi M, Indolfi C, Condorelli M, Chiariello M, Willerson JT. Local effect of serotonin released during coronary angioplasty. N Engl J Med. 1994;330:523-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 113] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Adderley SR, Fitzgerald DJ. Oxidative damage of cardiomyocytes is limited by extracellular regulated kinases 1/2-mediated induction of cyclooxygenase-2. J Biol Chem. 1999;274:5038-5046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 287] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 29. | Ellis D, Noireaud J. Intracellular pH in sheep Purkinje fibres and ferret papillary muscles during hypoxia and recovery. J Physiol. 1987;383:125-141. [PubMed] |
| 30. | Rubertsson S, Grenvik A, Wiklund L. Blood flow and perfusion pressure during open-chest versus closed-chest cardiopulmonary resuscitation in pigs. Crit Care Med. 1995;23:715-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Chandra NC, Tsitlik JE, Halperin HR, Guerci AD, Weisfeldt ML. Observations of hemodynamics during human cardiopulmonary resuscitation. Crit Care Med. 1990;18:929-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Swenson RD, Weaver WD, Niskanen RA, Martin J, Dahlberg S. Hemodynamics in humans during conventional and experimental methods of cardiopulmonary resuscitation. Circulation. 1988;78:630-639. [PubMed] |
| 33. | Park JL, Lucchesi BR. Mechanisms of myocardial reperfusion injury. Ann Thorac Surg. 1999;68:1905-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 226] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 34. | Schultz CH, Rivers EP, Feldkamp CS, Goad EG, Smithline HA, Martin GB, Fath JJ, Wortsman J, Nowak RM. A characterization of hypothalamic-pituitary-adrenal axis function during and after human cardiac arrest. Crit Care Med. 1993;21:1339-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 93] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Prengel AW, Lindner KH, Ensinger H, Grünert A. Plasma catecholamine concentrations after successful resuscitation in patients. Crit Care Med. 1992;20:609-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Sharma AB, Sun J, Howard LL, Williams AG, Mallet RT. Oxidative stress reversibly inactivates myocardial enzymes during cardiac arrest. Am J Physiol Heart Circ Physiol. 2007;292:H198-H206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Miller JB, Donnino MW, Rogan M, Goyal N. Relative adrenal insufficiency in post-cardiac arrest shock is under-recognized. Resuscitation. 2008;76:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Lindner KH, Strohmenger HU, Ensinger H, Hetzel WD, Ahnefeld FW, Georgieff M. Stress hormone response during and after cardiopulmonary resuscitation. Anesthesiology. 1992;77:662-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 159] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 39. | Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med. 2003;348:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 734] [Cited by in RCA: 643] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 40. | Catalano RD, Parameswaran V, Ramachandran J, Trunkey DD. Mechanisms of adrenocortical depression during Escherichia coli shock. Arch Surg. 1984;119:145-150. [PubMed] |
| 41. | Wagner RL, White PF, Kan PB, Rosenthal MH, Feldman D. Inhibition of adrenal steroidogenesis by the anesthetic etomidate. N Engl J Med. 1984;310:1415-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 314] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 42. | Hafezi-Moghadam A, Simoncini T, Yang Z, Limbourg FP, Plumier JC, Rebsamen MC, Hsieh CM, Chui DS, Thomas KL, Prorock AJ. Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat Med. 2002;8:473-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 426] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 43. | Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1-12. [PubMed] |
| 44. | Ho FM, Lin WW, Chen BC, Chao CM, Yang CR, Lin LY, Lai CC, Liu SH, Liau CS. High glucose-induced apoptosis in human vascular endothelial cells is mediated through NF-kappaB and c-Jun NH2-terminal kinase pathway and prevented by PI3K/Akt/eNOS pathway. Cell Signal. 2006;18:391-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 200] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 45. | Boo YC, Jo H. Flow-dependent regulation of endothelial nitric oxide synthase: role of protein kinases. Am J Physiol Cell Physiol. 2003;285:C499-C508. [PubMed] |
| 46. | Kim JJ, Lim YS, Shin JH, Yang HJ, Kim JK, Hyun SY, Rhoo I, Hwang SY, Lee G. Relative adrenal insufficiency after cardiac arrest: impact on postresuscitation disease outcome. Am J Emerg Med. 2006;24:684-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | de Jong MF, Beishuizen A, de Jong MJ, Girbes AR, Groeneveld AB. The pituitary-adrenal axis is activated more in non-survivors than in survivors of cardiac arrest, irrespective of therapeutic hypothermia. Resuscitation. 2008;78:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 48. | Beishuizen A, Thijs LG, Vermes I. Patterns of corticosteroid-binding globulin and the free cortisol index during septic shock and multitrauma. Intensive Care Med. 2001;27:1584-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 168] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 49. | Goodman S, Sprung CL, Ziegler D, Weiss YG. Cortisol changes among patients with septic shock and the relationship to ICU and hospital stay. Intensive Care Med. 2005;31:1362-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Marik PE, Zaloga GP. Adrenal insufficiency in the critically ill: a new look at an old problem. Chest. 2002;122:1784-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 261] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 51. | Annane D, Sébille V, Troché G, Raphaël JC, Gajdos P, Bellissant E. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA. 2000;283:1038-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 726] [Cited by in RCA: 665] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 52. | Siraux V, De Backer D, Yalavatti G, Mélot C, Gervy C, Mockel J, Vincent JL. Relative adrenal insufficiency in patients with septic shock: comparison of low-dose and conventional corticotropin tests. Crit Care Med. 2005;33:2479-2486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
Peer reviewers: Medha Mohta, MBBS, MD, MAMSD, Department of Anaesthesiology and Critical Care, University College of Medical Sciences and GTB Hospital, Delhi 110095, India; Juan Antonio Llompart-Pou, MD, PhD, Intensive Care Unit, Hospital Universitari Son Espases, Carretera Valldemossa, 79, Palma de Mallorca 07010, Spain
S- Editor Wang JL L- Editor Webster JR E- Editor Zheng XM