Published online Mar 28, 2015. doi: 10.5412/wjsp.v5.i1.111
Peer-review started: September 29, 2014
First decision: December 17, 2014
Revised: January 10, 2015
Accepted: January 30, 2015
Article in press: February 2, 2015
Published online: March 28, 2015
Processing time: 186 Days and 3.1 Hours
Treating pain in patients with terminal cancer is challenging but essential part of their care. Most patients can be managed with pharmacological options but for some these pain control methods are inadequate. Ablative spinal procedures offer an alternative method of pain control for cancer patients with a terminal diagnosis that are failing to have their pain controlled sufficiently by other methods. This paper provides a review of ablative spinal procedures for control of cancer pain. Patient selection, surgical methods, outcomes and complications are discussed in detail for cordotomy, dorsal root entry zone (DREZ) lesioning and midline myelotomy. Cordotomy is primarily done by a percutaneous method and it is best suited for patients with unilateral somatic limb and trunk pain such as due to sarcoma. Possible complications include unilateral weakness possibly respiratory abnormalities. Approximately 90% of patients have significant immediate pain relief following percutaneous cordotomy but increasing portions of patients have pain recurrence as the follow-up period increases beyond one year. The DREZ lesion procedure is best suited to patients with plexus invasion due to malignancy and pain confined to one limb. Possible complications of DREZ procedures include hemiparesis and decreased proprioception. Midline myelotomy is best suited for bilateral abdominal, pelvic or lower extremity pain. Division of the commissure is necessary to address bilateral lower extremity pain. This procedure is relatively rare but published case series demonstrate satisfactory pain control for over half of the patients undergoing the procedure. Possible complications include bilateral lower extremity weakness and diminished proprioception below the lesion level. Unlike cordotomy and DREZ this procedure offers visceral pain control as opposed to only somatic pain control. Ablative spinal procedures offer pain control for terminal cancer patients that are not able to managed medically. This paper provides an in depth review of these procedures with the hope of improving education regarding these underutilized procedures.
Core tip: Pain is a significant symptom that degrades the quality of life for terminally ill cancer patients. For many terminally ill oncology patients medical management is sufficient. However, some patient’s will fail medical management or have unwanted side effects from their medical regimen. Patient’s failing medical management may warrant consideration for interventional procedures such as cordotomy, dorsal root entry zone or midline myelotomy. Of these three procedures only midline myelotomy can address visceral pain, the others are best suited to somatic pain. This review discusses surgical anatomy, patient selection and surgical nuances of these techniques.
- Citation: Lake WB, Konrad PE. Cordotomy procedures for cancer pain: A discussion of surgical procedures and a review of the literature. World J Surg Proced 2015; 5(1): 111-118
- URL: https://www.wjgnet.com/2219-2832/full/v5/i1/111.htm
- DOI: https://dx.doi.org/10.5412/wjsp.v5.i1.111
Pain is a common problem for cancer patients and may significantly degrade their quality of life. Most commonly management for these patients includes medical therapies such as opiates, steroids and NSAIDs. For bony metastases radiation can alleviate pain. However, some cancer patients will have pain that fails medical management. In these patients, particularly those with progressive disease and a terminal prognosis, ablative neurosurgical procedures can be considered. The spinal cord is central to the pain pathway and ablative spinal procedures are a logical choice for cancer patients with severe pain that has failed other therapies[1].
In 1912 Spiller et al[2] first described cordotomy, division of the anterior spinothalmic tracts in the treatment of pain. As initially described cordotomy was an open procedure. Later advancement in fluoroscopy and electrical monitoring made percutaneous cordotomy possible[3]. At some centers cordotomy is now performed in a CT guided fashion[4].
Lesioning of the dorsal root entry zone (DREZ), is another ablative spinal procedure that can be used in cancer patients. This procedure was first described by Sindou et al[5] in the 1970s. Nashold et al[6] went on to modify the procedure and recommend its use deafferentation pain associated with brachial plexus avulsion[5,6]. Now the DREZ procedure can be employed for pain control in cancer patients with inoperable upper thoracic tumors compressing the brachial plexus, such as Pancoast tumors[7].
Several authors including Mansuy et al[8], Gildenberg et al[9] and Nauta et al[10] have discussed midline myelotomy with or without commissurotomy[8-10]. Mansuy et al[8] first noted visceral pain relief despite limited division of the anterior commissure of the spinal cord. This led to anatomical studies that demonstrated visceral pain pathways in the deep dorsal columns close to the midline. Subsequent modifications of the procedure resulted in limited myelotomy sparing the anterior commissure and interrupting ascending pathways, deep in the dorsal columns close to the midline, that conduct visceral pain information[11].
This article will review the various ablative spinal procedures for control of cancer pain. Spinal anatomy and physiology as it relates to pain will be reviewed. After this foundation, a discussion of patient and procedure selection will follow. Each ablative procedure type will be discussed along with the relevant literature. At the conclusion the reader should understand ablative spinal procedures and their role in treating cancer pain.
Appropriate diagnosis and management of pain, as with many other neurological problems, depends upon localizing the lesion. Therefore to employ ablative spinal procedures in the management of pain a thorough understanding of the pain generation and conduction is necessary. Numerous sources are available that provide a detailed discussion of spinal cord anatomy[12,13]. The following description the focus will be on the anatomy of the pain pathway and surgically relevant surrounding structures. One method of reviewing the anatomy of the pain pathway is to proceed in an anatomic order. Beginning with the first order neuron, such as the spinal ganglion, and then progress sequentially to the terminal cortical neuron in the pathway. Bearing in mind this variability a discussion of the anatomical principals of pain transmission follows.
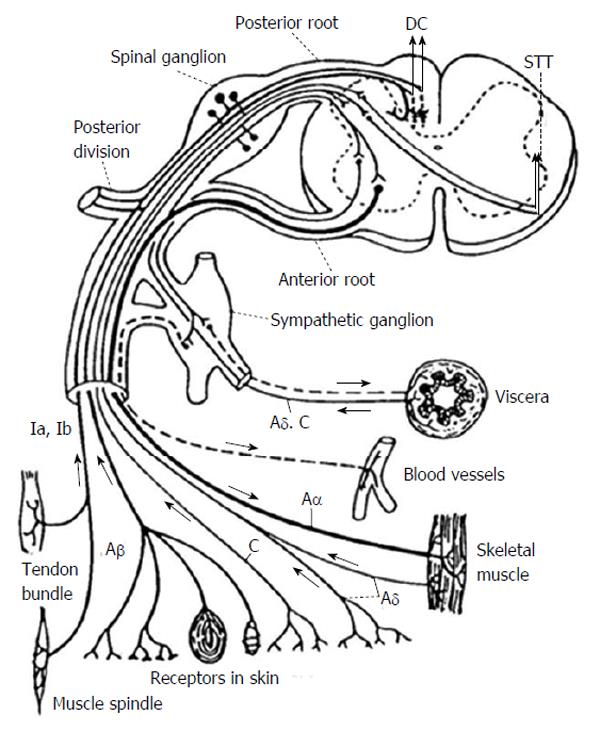
The pain pathway generally starts with effectors, such as bare nerve endings, Paccinian corpuscles, muscle spindles, etc., communicating through various nerve fiber types as sensory information flows towards the dorsal root ganglion. First order neurons for pain perception lie in the dorsal root ganglion. For the pathway conducting pain and temperature these first order neurons synapse with second order neurons in the dorsal horn in Rexed lamina I-V[14]. Most of the first order nociceptive fibers from the dorsal root ganglion synapse in the lamina at the entering level however a portion of the fibers travel rostrally or caudally in Lissauer’s tract. First order neurons responsible for proprioception enter the dorsal root entry zone travelling along the medial aspect of the dorsal root and travel in the posterior columns to synapse in the Nucleus Gracilis or Nucleus Cuneatus for the lower and upper extremities respectively. Figure 1 demonstrates the basic architecture of the first order neurons as they relate to the dorsal root ganglion[15,16].
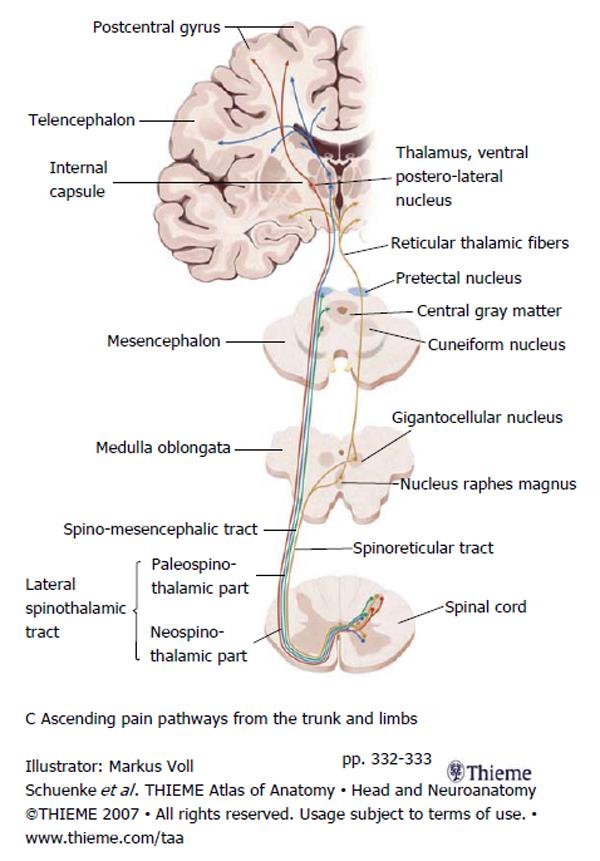
Second order neurons from the Rexed lamina I-V project through the spinal cord carrying nociceptive information. The majority of axons project through the anterior commissure of the spinal cord to the contralateral spinothalamic tract (STT). The STT is located in the anterolateral quadrant of the spinal cord just anterior to the corticospinal tract and lateral to the diaphragmatic reticulospinal tract. The STT ascends and projections are given off, in a caudal to rostral fashion, to the: nucleus raphes magnus, gigantocellular magnus, cuneiform nucleus, periaqueductal gray and the pretectal nucleus. Finally the STT terminates in the ventral postero-lateral nucleus of the thalamus. From there third order neurons project to the sensory cortex where conscious pain is processed. Figure 2 summarizes the path of the second and third order neurons[17-19].
As noted in the introduction observations regarding visceral pain control following midline myelotomy with minimal commissurotomy suggested visceral pain afferent tracts in the midline at the depth of the dorsal columns. Animal studies performed by Willis demonstrated the existence of the deep dorsal column visceral pain pathways[20]. Midline myelotomy became recognized as a method for controlling intractable visceral bilateral abdominal or pelvic pain[10,11].
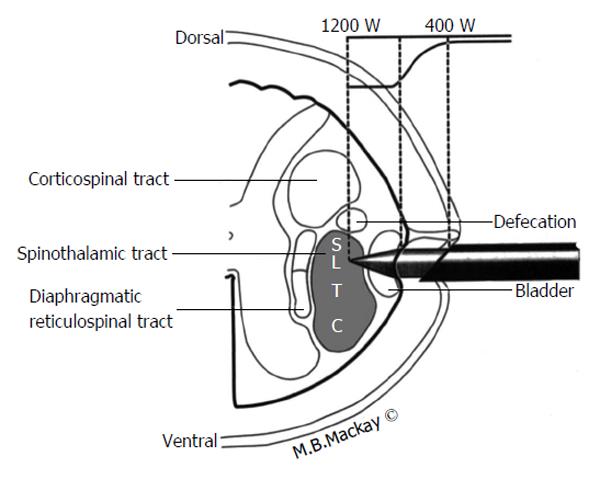
In addition to describing pain pathways it is useful to discuss structures with close anatomical relationship with the areas of the pain pathway. Some of the structures surrounding the pain pathways risk inadvertent injury if spinal anatomy isn’t properly understood. Figure 3 demonstrates many of the important anatomical relationships of the STT in the high cervical region and the underlying somatotopy as well. Initially one should note the location of the STT in the anterolateral quadrant of the cord is just anterior to the corticospinal tract and that the STT lies anterior to the dentate ligament. Lesions deviating too posteriorly may result in hemiparesis or loss of bowel control. Lesions excessively medial can damage respiratory interneurons in the diaphragmatic reticulospinal tract leading to respiratory complications. Finally in the case of the midline myelotomy or commissurotomy it must be remembered that the anterior spinal artery, the primary blood supply for the majority of the spinal cord lies just anterior by millimeters, so care must be taken to preserve this vascular structure[3,21].
With an understanding of the anatomy and physiology of the pain pathways we are now prepared to discuss patient and procedure selection. Patients selected for ablative spinal procedures must have a medically refractory malignant pain syndrome and a life expectancy of a few years or less. Additionally, the location of the malignant pain must be conduced through the pain pathway in a circuit that allows safe surgical lesioning. Malignant pain is necessitated because pain treated by ablative spinal procedures has a high rate of recurrence in patients with non-malignant pain. The pain previously treated by the lesion may recur or a new central neuropathic pain, typified by dysthesias, may result below the level of the lesion. For a satisfactory result the duration of time before the pain recurrence, or the onset of neuropathic pain, must be longer than the life span of the patient. Therefore, patients with cancer and a remaining lifespan of a few years or less are best suited to ablative spinal procedures[3,22].
Appropriate patient selection also depends on pain location, namely is the pain located in a region such that the corresponding pain pathway is amenable to surgical interruption while maintaining an acceptable side effect profile. Patient and procedure selection are inextricably linked, therefore it is useful organize patient and procedure selection together.
Open, or more commonly in recent years percutaneous, cordotomy is best suited for patients with malignant unilateral limb, pelvis or trunk pain. Cordotomy involves lesioning of the STT, nociception below the level of the lesion is interrupted. The side effects of this procedure include numbness and dysthesias below the level of the lesion. However, this may be an acceptable trade off in cancer patients suffering from severe medically intractable pain. Bilateral cordotomy procedures generally are not an option particularly in the high cervical region[23,24]. This is because respiratory interneurons lie just medial to the STT and if damaged bilaterally may result in respiratory depression. Unilateral limb pain due to brachial plexus avulsion is not a good option for treatment by cordotomy because patients often find the resulting dysthesias unacceptable and the pain can recur or new central neuropathic pain can occur due to the patient’s lifespan. Generally satisfactory pain control begins 3 or 4 levels below the level of the cordotomy lesion due to the presence of Lissauer’s tract conducting pain fibers rostrally before they cross over to join the STT[3,21].
The dorsal root entry zone ablation alternatively known as the DREZ procedure is often considered for patients that suffer from pain due to brachial plexus avulsion. Other patients appropriate for the DREZ procedure include cancer patients suffering from medically intractable pain from tumors invading the brachial plexus, also known as Pancoast tumors, or for patients with spinal cord injury suffering from pain at the level of their injury. Unfortunately, DREZ is not generally helpful for patients with constant burning pain or limb pain associated with shoulder or pelvic pain[23,25,26].
Commissurotomy lesions the anterior commissure of the spinal cord and therefore interrupts nociceptive signals for several levels in the vicinity of the lesion. This procedure is useful in controlling malignant bilateral abdominal, pelvic or lower extremity pain. It represents a reasonable alternative to bilateral cordotomy. Generally the commissure is ablated in the conus to T10 region. Loss of proprioception can occur due to damage of the posterior columns as they are splayed apart during the course of the procedure. Care must also be taken to avoid the anterior spinal artery since it lies only millimeters away from the commissure[27].
Midline myelotomy with or without commissurotomy may also offer control of bilateral abdominal, pelvic or lower extremity pain. Lesioning of the posterior columns, particularly in the deep medial region, was noted to reduce visceral pain in addition to providing the predicted bilateral somatic pain control. These observations coupled with animal data that indicated a visceral pain pathway located deep in the posterior columns at the midline led to greater use of midline myelotomy without commissurotomy. Gildenerg and Hirshberg have even proposed limited midline myelotomy for the treatment of visceral pain[9,10].
At this point it is important to emphasize that in most cases visceral pain also travels through the sympathetic chain and through cranial nerves. This is a key concept. In general with the exception of the above mentioned midline myelotomy, ablative spinal procedures are best suited for controlling somatic cancer related pain, such as that from an extremity sarcoma, because somatic pain fibers travel exclusively through the spinal cord. Ablative spinal procedures in most cases do not effectively interrupt all pathways for visceral pain because this sensory modality is conducted through cranial nerves and the sympathetic chain in addition to the deep midline posterior column tracts. Figure 1 for an illustration of this concept[10,20,23].
Generally the patients being treated with ablative spinal cord procedures should have preoperative spinal imaging such as MRI of the spine in the area of interest or CT myelography in the area of interest. Such imaging can elucidate any relevant anatomical variations such as local metastases syringomyelia, spinal deformity or in the case of brachial plexus avulsions, pseudomeningoceles[14]. Consultation with the patient’s oncologist regarding goals of care, overall health condition and prognosis is also necessary. In the immediate preoperative and postoperative period some practitioners advocate corticosteroids, such as dexamethasone 4-6 mg every 6 h, to reduce spinal cord edema.
The patient is positioned prone. A hemi laminotomy or a full laminectomy is performed to allow access to the spinal cord contralateral to the pain symptoms at a spinal level 3-4 segments above the level of the symptoms. If possible one generally performs a laminotomy or partial laminectomy to reduce the risk of post laminectomy kyphosis. In chosing the level for the lesion it is important to note how the correspondence between spinal level and vertebral level. In the cervical cord the spinal level corresponds closely to the vertebral level. As one transitions more caudally the spinal and vertebral levels become progressively more discordant such that the sacral and coccygeal levels lie at the conus which generally terminates at L1. Once the lamina has be opened at the appropriate level the dura and arachnoid can be opened and tacked up. The dentate ligament is separated from the dura and used to rotate the spinal cord slightly to bring the anterolateral quadrant into better view. A Weck blade placed at a right angle in a Ryder needle driver is then used to divide the anterolateral quadrant of the spinal cord to a depth of 3-4 mm. An angled dissector is then swept through the same cut. The dura is closed in a water tight fashion and the fascia and skin are closed in the usual fashion[2,21,22].
In addition to the usual concerns for infection and CSF leak some complications specific to open cordotomy include: hemiparesis (usually transient occurs in 10%-30%), respiratory depression (particularly in cervical cordotomies), mirror pain (usually in thoracic cordotomies), and urinary disturbances. The complication rate for open cordotomy is on the order of 5%-10%, but for patients in debilitating medically recalcitrant pain this may be an appropriate trade off. In approximately 50% of patients undergoing this procedure the pain relief will be total and immediate. Approximately 25% will have immediate partial pain relief and the remainder will have no improvement in pain. Review of large case series indicates that some patients will have recurrence of pain if they survive to 1 year or longer[21,22,28].
With the advent of improved fluoroscopic equipment and the availability of radiofrequency ablation percutaneous cordotomy became more common than open cordotomy. Unlike open cordotomy, and the other procedures discussed here, percutaneous cordotomy can be performed without general anesthesia and this is a significant advantage in the cancer population since many of these patients have significant comorbidities. The percutaneous cordotomy also allows the practioner to perform a test lesion and this may improve the success of the procedure compared to open cordotomy[3].
The patient is placed supine in a fluoroscopy suite with the head immobilized and light sedation administered. Some local anesthetic is infiltrated to ease the orthogonal passage of the needle into the C1-2 interspace under fluoroscopic guidance (CT guidance has also been described). Injection of water-soluble, contrast such as Omnipaque, is used to verify that the needle is positioned 1-2 mm anterior to the dentate ligament. Figure 3 provides an anatomical schematic of the percutaneous cordotomy procedure. The needle is then advanced into the anterolateral quadrant of the spinal cord. At this point a change in impedence from approximately 300 Ohms to approximately 1000 Ohms will be detected and this verifies entry into the spinal parenchyma. Low frequency (approximately 3 Hz) electrical stimulation with the needle will demonstrate twitching of the ipsilateral neck if the needle position is close to the anterior rootlets. If low frequency electrical stimulation causes limb or trunk contraction this means the needle is too close to the corticospinal tract and should be repositioned. High frequency stimulation (approximately 100 Hz) should produce contralateral sensory phenomenon that corresponds to the area of desired pain control. This verifies an appropriate needle position. If the patient has ipsilateral arm or head paresthesias this means the needle is too posterior. Once appropriate needle position is determined using stimulation and patient exam it is time to create the lesion. This is done using an electrode with 2 mm of exposed tip. Heating the electrode to a temperature of 70 °C-80 °C for one minute creates the lesion. The patient is examined to verify analgesia and to evaluate for reduced pinprick sensation in the area of intended pain control[3,4,23].
Respiratory depression is the most feared complication of percutaneous cordotomy but this is only a concern in bilateral procedures. The complication rate is on the order of 5% and the types of complication described are similar to those noted in open cordotomy: bladder dysfunction, temporary hemiparesis, respiratory abnormality and ataxia. Immediate pain relief following the lesion is reported to be as high as 90%. This improved success rate compared to open cordotomy may be attributable to the fact that physiological testing and patient participation is available for guidance of the lesion since the patient is awake. At 1 year only about half of the patients continue to have pain relief, once again verifying that patients must be suffering from a terminal disease to be an ideal candidate for cordotomy[3,4,23].
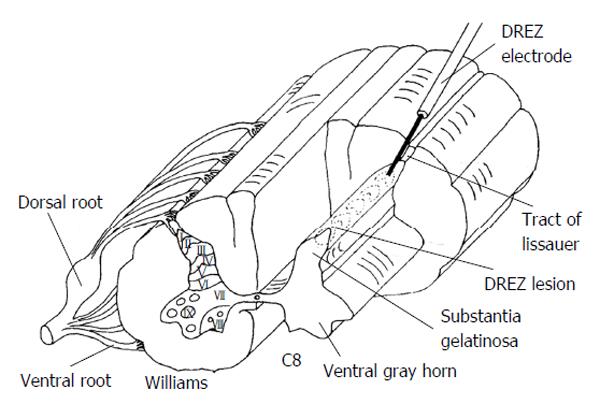
The Dorsal Root Entry Zone or DREZ procedure is useful for malignant pain where the plexus is invaded by cancer and the pain is confined to a limb. This procedure is also useful for treating pain associated with brachial plexus avulsion or for segmental pain at the level of spinal cord injury. Similar to the open cordotomy discussion, the procedure begins with the patient in the prone position. Laminectomy or laminotomy is performed to expose the appropriate levels. In the case of the brachial plexus the laminectomy usually extends from C4-T1 and for conus lesioning the laminectomy is usually from T10 to L2. Some may desire to perform laminoplasty with the hope of reducing post laminectomy kyphosis and to make subsequent operative exposures easier. The dura and arachnoid are opened and tacked up. Next the dorsolateral sulcus of the spinal cord is located; this is where the dorsal roots enter. A Nashold DREZ electrode, with a 2 mm exposed tip is used to created successive lesions by heating the electrode tip to 75 °C for approximately 20 s. The ideal entry point of the electrode is just lateral to the point where roots enter because this also provides for lesioning of Lissauer’s tract. Figure 4 provides a schematic of DREZ lesioning using a radiofrequency probe. An effort should be made to sharply puncture the pia with the electrode to prevent deformation that comes with bluntly pushing the electrode. Somatosensory evoked potential monitoring and motor evoked potential monitoring may provide warning signs if adjacent corticospinal or dorsal column tracts are being damaged. A typical DREZ lesioning case may require approximately 50 radiofrequency lesions and span approximately 4 spinal levels. Lesioning for DREZ should encompass 2 levels above the level of pain to be treated to assure that Lissauer's tract containing non-crossing ipsilateral first order fibers have been interrupted. The surgeon should also keep in mind the shifting orientation of the dorsal horn of the spinal gray matter relative to the sagittal plane. In the cervical spine the dorsal horn of gray matter is 30 degrees off sagittal while in the thoracic spine it is 20 degrees off sagittal. As an alternative to using radiofrequency lesioning for DREZ other have proposed incising the pia and using bipolar coagulation under microscope visualization[23,25,26].
Complications of DREZ include damage to the adjacent corticospinal tract leading to hemiparesis or damage to the dorsal columns causing decreased proprioception and light touch. Hemiparesis seems to be more common in patients undergoing thoracic DREZ. This is thought to be due to the narrower dorsal horn of grey matter in this region. Hemiparesis and loss of proprioception is reported in approximately 10% and 50% respectively. The best results for pain control with DREZ are reported in patients with brachial plexus avulsion pain. Relatively little data is available regarding the efficacy of DREZ procedures in patients with malignant pain due to brachial plexus or lumbar plexus invasion by cancer[23,26,29].
Commissurotomy and midline myelotomy procedures are aimed at treating patients with bilateral abdominal, pelvic or lower extremity pain. Exposure for midline myelotomy/comissurotomy is identical to that described in the open cordotomy or DREZ section. The patient is positioned prone. Midline laminectomy is preformed. The dura and arachnoid are opened and tacked up. Generally levels between T10 and L1 are targeted to provide bilateral abdominal, pelvic or lower extremity pain. The operating microscope is brought into the field after the dura is opened. Dissection is carried out between the dorsal columns in the posterior median sulcas. When the depth is reached an arachnoid knife is used along with fine micro-tipped bipolars to divide the gray matter and the anterior commissure until the pia of the anterior median sulcas is reached. Here care must be taken to avoid injury of the anterior spinal artery. Gildenberg and Hirshberg have also advocated midline myelotomy where a deep medial portion of the dorsal columns is lesioned while sparing the anterior commissure[9]. Nauta further modified this procedure to a confined transverse midline lesion. These procedures seem to serve the function of controlling bilateral visceral pain beginning 2-3 levels below the lesion level[10]. Following the myelotomy the dura and the other tissues are closed in a typical fashion. Complications of the procedure include decreased proprioception and bilateral lower extremity weakness (this occurs in approximately 25%). While the proprioceptive difficulties are well tolerated the lower extremity weakness can represent a significant morbidity. This re-enforces the concept that this procedure is best suited to patients suffering from severe, recalcitrant pelvic or abdominal pain secondary to cancer. To obtain pain relief in the bilateral lower extremities it appears to be necessary to section the commissure while for visceral pain relief midline myelotomy alone seems to be sufficient. This procedure is relatively rare and there are not extensive reports of outcomes. However, it appears that pain relief is excellent in over half of the cases in published series[8,10,11,20,27].
Despite improved pharmacological therapies some terminal cancer patients suffer from severe recalcitrant pain that greatly degrades their quality of life in their final months. With a detailed understanding of the anatomy and physiology of the pain pathway several ablative spinal procedures have been developed that offer a much-needed option for pain relief in this terminally ill population. Cordotomy which can be performed in an open or percutaneous fashion, offers excellent pain relief for patients suffering from unilateral somatic limb or trunk pain secondary to malignancy. The side effect profile for this procedure is favorable but includes transient weakness or occasionally painful dysthesias. The DREZ procedure offers a pain relief for cancer patients suffering from unilateral limb pain, particularly that caused by brachial plexus invasion. Possible complications of DREZ include transient weakness and decreased proprioception. Midline myelotomy is the least commonly performed ablative spinal procedure for pain. This procedure may include lesioning of the deep medial dorsal columns and/or sectioning of the anterior commissure. Midline myelotomy is best suited for patients suffering from a terminal cancer producing bilateral pelvic, abdominal or lower extremity pain. Section of the anterior spinal commissure is necessary if pain control in the bilateral lower extremities is desired. This procedure differs from DREZ and cordotomy because it offers control of visceral as opposed to just somatic pain. The review presented here discusses ablative spinal procedures for cancer pain including patient selection, surgical technique, complications and outcomes. We hope that this will provide needed education on the subject of these underutilized procedures that can provide much needed pain relief to cancer patients in their final days.
| 1. | Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of eighteen major cancers in 1985. Int J Cancer. 1993;54:594-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1065] [Cited by in RCA: 1042] [Article Influence: 31.6] [Reference Citation Analysis (1)] |
| 2. | Spiller W, Martin E. The treatment of persistent pain of organic origin in the lower part of the body by division of the anterolateral column of the spinal cord. JAMA. 1912;58:1489-1490. [RCA] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 209] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Tasker RR. Percutaneous cordotomy. Textbook of Stereotactic and Functional Neurosurgery. 2nd ed. Berlin: Springer 2009; 2137-2148. [DOI] [Full Text] |
| 4. | Kanpolat Y, Ugur HC, Ayten M, Elhan AH. Computed tomography-guided percutaneous cordotomy for intractable pain in malignancy. Neurosurgery. 2009;64:ons187-ons93; discussion ons187-ons93;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Sindou MP, Blondet E, Emery E, Mertens P. Microsurgical lesioning in the dorsal root entry zone for pain due to brachial plexus avulsion: a prospective series of 55 patients. J Neurosurg. 2005;102:1018-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Nashold B, Pearlstein R. The DREZ Operation. Park Ridge, IL: The American Association of Neurological Surgeons 1996; . |
| 7. | Konrad P, Caputi F, El-Naggar AO. Radiofrequency dorsal root entry zone lesions for Pain. Textbood of Stereotactic and Functional Neurosurgery. 2nd ed. Berlin: Springer 2009; 2251-2268. [DOI] [Full Text] |
| 8. | Mansuy L, Lecuire J, Acassat L. Technique de la myélotomie commissurale postérieure. Journal de Chirurgie. 1944;60:206-213. |
| 9. | Gildenberg PL, Hirshberg RM. Limited myelotomy for the treatment of intractable cancer pain. J Neurol Neurosurg Psychiatry. 1984;47:94-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 49] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Nauta HJ, Hewitt E, Westlund KN, Willis WD. Surgical interruption of a midline dorsal column visceral pain pathway. Case report and review of the literature. J Neurosurg. 1997;86:538-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 81] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Gildenberg PL. Myelotomy through the years. Stereotact Funct Neurosurg. 2001;77:169-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Almeida TF, Roizenblatt S, Tufik S. Afferent pain pathways: a neuroanatomical review. Brain Res. 2004;1000:40-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 232] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 13. | Romanelli P, Esposito V. The functional anatomy of neuropathic pain. Neurosurg Clin N Am. 2004;15:257-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Mertens P, Guenot M, Hermier M, Jouvet A, Tournut P, Froment JL, Sindou M, Carret JP. Radiologic anatomy of the spinal dorsal horn at the cervical level (anatomic-MRI correlations). Surg Radiol Anat. 2000;22:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Willis WD. Dorsal horn neurophysiology of pain. Ann N Y Acad Sci. 1988;531:76-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Rosenow JM, Henderson JM. Anatomy and physiology of chronic pain. Neurosurg Clin N Am. 2003;14:445-462, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Xiang JP, Liu XL, Xu YB, Wang JY, Hu J. Microsurgical anatomy of dorsal root entry zone of brachial plexus. Microsurgery. 2008;28:17-20. [PubMed] |
| 18. | Romanelli P, Esposito V, Adler J. Ablative procedures for chronic pain. Neurosurg Clin N Am. 2004;15:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Karatas A, Caglar S, Savas A, Elhan A, Erdogan A. Microsurgical anatomy of the dorsal cervical rootlets and dorsal root entry zones. Acta Neurochir (Wien). 2005;147:195-199; discussion 199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Willis WD, Westlund KN. The role of the dorsal column pathway in visceral nociception. Curr Pain Headache Rep. 2001;5:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Jones B, Finlay I, Ray A, Simpson B. Is there still a role for open cordotomy in cancer pain management? J Pain Symptom Manage. 2003;25:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Tomycz L, Forbes J, Ladner T, Kahn E, Maris A, Neimat J, Konrad P. Open thoracic cordotomy as a treatment option for severe, debilitating pain. J Neurol Surg A Cent Eur Neurosurg. 2014;75:126-132. [PubMed] |
| 23. | Burchiel K. Surgical Management of Pain. New York: Thieme 2002; . |
| 24. | Nannapaneni R, Behari S, Todd NV, Mendelow AD. Retracing “Ondine’s curse”. Neurosurgery. 2005;57:354-363; discussion 354-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Samii M, Moringlane JR. Thermocoagulation of the dorsal root entry zone for the treatment of intractable pain. Neurosurgery. 1984;15:953-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Nashold BS, Friedman A, Bullitt E. The status of dorsal root entry zone lesions in 1987. Clin Neurosurg. 1989;35:422-428. [PubMed] |
| 27. | Viswanathan A, Burton AW, Rekito A, McCutcheon IE. Commissural myelotomy in the treatment of intractable visceral pain: technique and outcomes. Stereotact Funct Neurosurg. 2010;88:374-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Atkin N, Jackson KA, Danks RA. Bilateral open thoracic cordotomy for refractory cancer pain: a neglected technique? J Pain Symptom Manage. 2010;39:924-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Rath SA, Seitz K, Soliman N, Kahamba JF, Antoniadis G, Richter HP. DREZ coagulations for deafferentation pain related to spinal and peripheral nerve lesions: indication and results of 79 consecutive procedures. Stereotact Funct Neurosurg. 1997;68:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
P- Reviewer: Amr YM, Cheung CW S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/