Published online Nov 27, 2014. doi: 10.5411/wji.v4.i3.185
Revised: July 10, 2014
Accepted: August 27, 2014
Published online: November 27, 2014
Processing time: 170 Days and 17.1 Hours
Monocytes are effector immune cells but a precise analysis of their role in immune response has been precluded by their heterogeneity. Indeed, human monocytes are composed of at least three different subsets with different phenotypic characteristics and functional properties, the so-called classical, intermediate and non-classical monocytes. A review of the literature shows that these monocyte subsets are differently affected during viral, bacterial, parasitic and fungal infections. The expansion of the CD16+ compartment (intermediate and non-classical monocytes) is typically observed in the majority of infectious diseases and the increased proportion of CD16+ monocytes is likely related to their activation through their direct interaction with the pathogen or the inflammatory context. In contrast, the number of non-classical and intermediate monocytes is decreased in Q fever endocarditis, suggesting that complex mechanisms govern the equilibrium among monocyte subsets. The measurement of monocyte subsets would be useful in better understanding of the role of monocyte activation in the pathophysiology of infectious diseases.
Core tip: In this review of the literature we show that monocyte subsets are differently affected during viral, bacterial, parasitic and fungal infections. We observe that the CD16+ compartment (intermediate and non-classical monocytes) is typically increased in the majority of infectious diseases. The measurement of monocyte subsets would be useful in better understanding of the role of monocyte activation in the pathophysiology of infectious diseases.
- Citation: Ka MB, Olive D, Mege JL. Modulation of monocyte subsets in infectious diseases. World J Immunol 2014; 4(3): 185-193
- URL: https://www.wjgnet.com/2219-2824/full/v4/i3/185.htm
- DOI: https://dx.doi.org/10.5411/wji.v4.i3.185
Human monocytes arise from bone marrow progenitors with myeloid-restricted differentiation potential and then circulate in the blood for a few days before migrating into tissues[1]. Monocytes differentiate into macrophages and dendritic cells (DCs) during inflammation and less efficiently in the steady state[2].
Monocytes play a pivotal role in the immune response as effector cells. These cells are equipped with pattern recognition receptors (PRRs) and phagocytic receptors necessary for the ingestion and elimination of microbes and damaged cells[3,4]. They express adhesion molecules and chemokine receptors, which are required to migrate toward inflamed or infected tissues[5]. Monocytes also initiate the adaptive immune response through their ability to produce cytokines and to differentiate into DCs, the major antigen-presenting cells (APCs)[6]. Finally, monocytes play critical roles in homeostasis and tissue repair[7]. A fundamental property of monocytes consists of their high plasticity[8]. They may adopt a biphasic response to a unique signal, first releasing inflammatory cytokines such as interleukin (IL)-6 and IL-1β[9] and then releasing immunoregulatory cytokines such as IL-10 and transforming growth factor (TGF)-β, thus an avoiding excessive inflammatory response[10]. We recently demonstrated that the gene expression program of human monocytes is determined by the time scale of the stimulation: although macrophage polarization genes are expressed in early stimulated monocytes, this expression is lost when the stimulation is sustained[11].
Another difficulty in analyzing the precise role of monocytes in the immune response is related to their heterogeneity, as they are composed of at least three different subsets with different phenotypic characteristics and functional properties. The aim of this review is to summarize what is known regarding the functions of monocyte subsets and to describe the evolution of these monocyte subsets during infectious diseases.
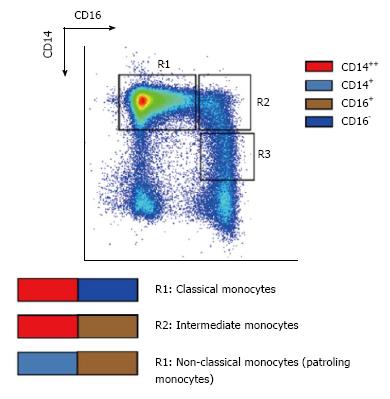
Human monocytes were initially defined as an homogeneous population on the basis of morphology, cytochemistry (monocyte-specific esterase) and flow cytometry measurements, such as light scattering and the expression of CD14, the receptor of bacterial lipopolysaccharides (LPS)[12]. Multi-color flow cytometry using antibodies against CD14 and CD16, the low affinity receptor for IgG, has revealed their heterogeneity, consisting of three subsets[12,13]. The “classical monocytes” that represent approximately 90% of circulating monocytes highly express CD14 but not CD16 (CD14++CD16- cells). Other circulating monocytes express CD16: “non-classical monocytes” representing approximately 5% of circulating monocytes, express low levels of CD14 but highly express CD16 (CD14+CD16+cells) and “intermediate monocytes”, which highly express CD14 with the concomitant expression of CD16 (CD14++CD16+ cells)[14] (Figure 1). However, the notion of intermediate monocytes is still debated. For Ziegler-Heitbrock and Hofer, they are only a transition from[14], conversely, for Hijdra et al[15], they consist of a true population of monocytes, as revealed by the expression of chemokine and Tumor Necrosis Factor (TNF) receptors. Because only the level of CD14 expression allows the distinction between non-classical monocytes and intermediate monocytes and many papers do not explicitly make this distinction, we propose referring to them collectively as CD16+ monocytes[1] and precisely defining the type of monocyte subset when it is documented.
The phenotypic properties of CD16- (classical) and CD16+ (intermediate and non-classical) human monocytes are summarized in Table 1. CD16+ monocytes express lower levels of CD64 than CD16- monocytes but highly express HLA-DR, CD86, and CD49d compared with CD16- monocytes[16,17], demonstrating an activated phenotype. The expression of PRRs and chemokine receptors varies according to the monocyte subset. The intermediate monocytes express higher levels of Toll-like receptor (TLR)-2 and TLR4 than classical and non-classical monocytes[17,18]. The non-classical monocytes do not express CCR2, the membrane receptor of the chemokine CCL2, making them likely unable to migrate in response to CCL2. In contrast, classical and intermediate monocytes express CCR2 and migrate in response to CCL2[19,20]. Intermediate monocytes, but not classical and non-classical monocytes, express CCR5[19-21]. The responses to classical agonists of monocytes vary according to the monocyte subset. For instance Lipopolysaccharide (LPS) stimulation of classical monocytes, but not intermediate monocytes, decreases the membrane expression of CD163; hence, the majority of soluble CD163 found in plasma originates from classical monocytes[22,23].
| Markers | Classical monocytes | Intermediatemonocytes | Nonclassical-monocytes | Ref. |
| CD14 | ++ | ++ | + | [1] |
| CD16 | - | + | + | [1] |
| CD86 | + | ++ | ++ | [17] |
| CD64 | ++ | + | + | [17] |
| HLA-AB | + | ++ | + | [21] |
| HLA-DR | + | ++ | + | [21,25] |
| CCR1 | ++ | + | - | [21] |
| CCR2 | ++ | - | - | [15,20,21] |
| CCR5 | + | ++ | - | [15,20,21] |
| CXCR1 | ++ | - | - | [21] |
| CXCR2 | ++ | - | - | [15,21] |
| CX3CR1 | ++ | + | - | [15,20] |
| CD62L | ++ | - | - | [20,21] |
The functional properties of monocyte subsets are also different (Table 2). The phagocytosis of Staphylococcus aureus and Escherichia coli is lower in non-classical monocytes than in intermediate monocytes and classical monocytes, a property likely related to the expression of CD14[18]. The non-classical monocytes produce less reactive oxygen species (ROS) in response to ligands of TLR4, TLR7 or TLR8 than the classical monocytes[24,25]. In addition non-classical and intermediate monocytes produce lower levels of cytokines, including granulocyte colony-stimulating factor, IL-6, IL-10 and CCL2 in response to LPS stimulation than classical monocytes[20]. The monocyte subsets likely play different roles as APCs. CD16+ monocytes express higher levels of HLA-DR than classical monocytes[12,26], suggesting that they are potent APCs. It has been shown that CD16+ monocytes are more efficient in presenting tetanus toxoid to CD4+ T cells than classical monocytes[27]. Taken together, these results suggest that CD16+ monocytes are activated under homeostatic conditions but they are less responsive to monocyte stimuli than CD16- monocytes.
| Functions | Classicalmonocytes | Intermediatemonocytes | Nonclassical-monocytes | Ref. |
| Phagocytosis | ++ | ++ | + | [25] |
| MHC II processing | + | ++ | + | [25] |
| Antigen presentation | + | ++ | + | [25] |
| CD4+ T cell proliferation | + | ++ | + | [25] |
| Transendothelial migration | - | - | ++ | [15] |
| Patrolling endothelium | - | - | ++ | [24] |
| Virus sensing | - | - | ++ | [24] |
| TNF production | + | - | ++ | [24] |
| IL-1β production | + | ++ | ++ | [24] |
| CCL2 production | ++ | - | - | [24] |
| IL-10 production | ++ | - | - | [24] |
Interestingly, monocytes are known to act as precursors of macrophages or DCs. It has been shown that the ability of monocyte subsets to differentiate into DCs is different according the monocyte subset. Indeed, non-classical monocytes are more prone to becominge DCs with a higher capacity to induce T cell proliferation and IL-4 production by CD4+ T cells[28]. In addition, the functional properties of monocyte-derived macrophages are dependent on the type of monocyte subset. It has been recently shown that the macrophages derived from CD16+ monocytes are more phagocytic than those derived from classical monocytes; they also exhibit a specific gene expression program[29].
The investigation of monocyte functions has benefited from the use of mouse models, though it remains unclear whether monocyte subsets are similar in humans and mice. Murine monocytes can be separated into at least two subpopulations, Gr1+ and Gr1- monocytes. The major subset of murine monocytes is composed of “inflammatory” Gr1+ monocytes that produce high levels of TNF, ROS and nitric oxide (NO) but low levels of IL-10 upon in vivo infection with bacteria such as Listeria monocytogenes or parasites such as Toxoplasma gondii[30]. Gr1+ monocytes also produce type I interferons (IFNs) in response to viral ligands[31]. Murine Gr1+ monocytes resemble human classical monocytes based on surface marker expression, gene expression and a reduced ability to produce inflammatory cytokines[32,33]. In contrast, the minor subset of murine monocytes does not express Gr1. These Gr1- monocytes patrol the blood vasculature, differentiate into macrophages after extravasation into tissues and are likely associated with tissue repair[34,35]. Murine Gr1- monocytes, which resemble human CD16+ monocytes, are described as the main producers of inflammatory cytokines such as TNF and IL-1β in response to LPS[26].
The existence of different subsets of monocytes likely has pathophysiological consequences. An expansion of the CD16+ monocyte subsets inflammatory diseases including hemophagocytic lymphohistiocytosis[36], asthma[37], sarcoidosis[38], peridontitis[39], atopic eczema[40], pancreatitis[41] and alveolar proteinosis[42] has been observed. Despite immunosuppressive therapy, the CD16+ monocyte compartment is also increased in kidney transplant patients, suggesting that this subset may be involved in the persistent, allograft-induced inflammatory reaction[43]. In patients with colorectal cancer, the percentage of intermediate monocytes is mainly increased at the onset of the disease[44], and this subset is also increased in adult survivors of childhood acute lymphoblastic leukemia[13].
Human immunodeficiency virus (HIV) is a lentivirus that efficiently infects CD4+ T cells, leading to their apoptosis and a decreased number of circulating CD4+ T cells. The antiretroviral therapies to date restore the number of circulating CD4+ T cells but are unable to completely eliminate viral infection, as demonstrated by HIV persistence in tissues. Both in vitro and in vivo studies have clearly demonstrated that blood monocytes and tissue macrophages can be infected by HIV[45,46]. During the early phase of HIV infection, the proportion of CD16+ monocytes is increased[47], and this increase in CD16+ monocytes in treatment-naïve HIV-infected patients is correlated with high viral loads and low CD4+ cell counts[48]. Convergent results have been obtained with the infection of non-human primates by simian immunodeficiency virus (SIV), with SIV infecting both CD4+ T cells and monocytes. Following the first description of CD16+ monocytes in cynomolgus monkeys (Macaca fascicularis) nearly two decades ago, an increase in CD16+ monocytes ten days after SIV infection has been observed. Note that increased levels of CD16+ monocytes have also been reported in rhesus monkeys (Macaca mulatta) with lentiviral encephalitis[49]. The treatment of chronically infected macaques with high doses of corticosteroids decreased the proportion of CD16+ monocytes (intermediate monocytes), whereas the other subsets of monocytes were found to be unresponsive to corticosteroids[50]. Highly active antiretroviral therapy (HAART) rescues the amount of intermediate monocytes[51]. The viral efficiency of HAART is also associated with insulin resistance, and it has been reported that the abundance of classical monocytes predicts the risk of insulin resistance and metabolic syndrome during the chronic phase of HIV infection[51].
HIV infection also affects the phenotype of monocyte subsets. The membrane expression of CD163, a receptor involved in the resolution of inflammation and M2 polarization[52] by classical and intermediate monocytes is increased in HIV-1 infection, but HIV-infection does not induce the membrane expression of CD163 in non-classical monocytes[53]. Note that plasma CD163 is not significantly altered by HIV-1 infection, demonstrating that CD163 shedding is not associated with the alteration of the membrane expression of CD163[53]. The exposure of whole blood to HIV enhances the expression of tissue factor (TF) on non-classical monocytes, whereas LPS-activated TLR-4 increases TF expression on all monocyte subsets[47]. The acquisition of such activated phenotypes by non-classical monocytes is reminiscent of the observation in acute coronary syndrome and suggests a potential role of non-classical monocytes in the cardiovascular risk of HIV infection. A recent study reported a decrease in the proportion of non-classical monocytes expressing TF in patients treated with rosuvastatin though anti-retroviral therapy has no effect on monocyte activation[54]. The functional alteration of monocyte subsets is associated with that of the programmed death-1 (PD-1) pathway known to limit the functions of virus-specific T cells during chronic infections such as HIV infection[55]. The expression of PD-1 by monocytes is increased in viremic subjects compared with healthy subjects, but the expression of PD-1 by CD16+ monocytes is twofold higher than that of classical monocytes. The relationship between HIV infection and PD-1 expression likely involves an indirect mechanism in which inflammatory cytokines play a major role[56]. First, the expression of PD-1 by monocyte subsets is not related to viral load in patients with HIV infection. In vitro, viral material such as HIV single-stranded RNA (RNA40) fails to increase PD-1 expression by monocytes. Second, inflammatory cytokines such as TNF, IL-1β and IL-6 increase the expression of PD-1 by monocytes in a dose-dependent manner, and it has been largely demonstrated that the circulating levels of these cytokines are increased in HIV infection[57,58]. Taken together, these results suggest that HIV infection leads to the modulation of monocyte subsets.
Dengue fever, a public health problem in tropical countries, is due to the dengue virus (DENV), a flavivirus that is transmitted to humans via the bite of an Aedes mosquito[59,60]. Monocytes are implicated in protection against DENV infection[61,62]. Indeed, monocytes infected in vitro with DENV produced IFN-α which is protective against viruses[63]. This is confirmed by the increase in DENV titers in mice deficient in IFN receptors[64]. Nevertheless, the role of monocytes is likely more complex. Monocytes are involved in dengue pathogenesis through virus propagation[65], and DENV-specific antibodies promote the infection of monocytes and thus increase the viral burden of individual monocytes[66].
It has been demonstrated that the number of CD16+ monocytes is twofold higher in dengue patients than in healthy controls[67], but the relative role of monocyte subsets in dengue infection remain unclear. In vitro classical monocytes and CD16+ monocytes are susceptible to DENV and produce molecules associated with dengue protection, such as IFN-α, CXCL10 and TNF-related apoptosis-inducing ligand (TRAIL), a cytokine known to induce cell apoptosis[68]. Taken together, these results suggest that classical monocytes and CD16+ monocytes may potentially contribute to anti-dengue responses, however only CD16+ monocytes appear to be affected by DENV infection in vivo.
Hepatitis C is due to an RNA virus (HCV) that affects 160 million individuals worldwide and is responsible for chronic hepatitis and hepatocellular carcinoma[69,70]. It has been recently demonstrated that HCV infects CD16+ monocytes but not classical monocytes in individuals infected with HCV. This specific tropism is related to the expression of CD81, the receptor considered to be necessary for HCV entry into target cells. Hence, CD81 is highly expressed on CD16+ monocytes but not on classical monocytes[71]. These results also suggest that the expression of CD81 by monocyte subsets is associated with the expression of CD16. Furthermore, we can suppose that the monocyte subsets that express CD16 may serve as HCV reservoirs. In hemodialyzed patients with chronic hepatitis, the CD16+ monocyte subset is increased threefold compared with healthy donors[72], suggesting an impact of the viral infection on monocyte distribution. The frequency of CD16+ monocytes is decreased and negatively correlated with viral load in chronic HCV infection. Furthermore the expression of PD-L1 allows the discrimination between chronic HCV infection and spontaneous HCV resolvers[73].
Cytomegalovirus (CMV) is a herpes virus of medical importance in immune-compromised individuals. CMV has a tropism for immune and non-immune cells in vivo and in vitro, yet peripheral blood leukocytes are involved in viremia and latency, regardless of the immune status of the patient[74]. Monocytes are likely latent reservoirs and support viral dissemination by benefiting from the maturation of monocytes into permissive macrophages and dendritic cells. CMV encodes inflammatory viral chemokines required for viral dissemination. A recent study proposed that patrolling monocytes acquire the virus from the initial site of infection and deliver to the spleen and salivary glands where CMV can persist. Analysis of the recruitment of patrolling monocytes reveals two phases: the first phase is necessary for the activation of natural killer (NK) response; and the second phase, involving viral chemokine and CX3CR1, the marker of patrolling monocytes, is required for the amplification of monocyte recruitment. Although this study revealed a previously undescribed role for this minority monocyte subset as a latent reservoir, it is not clear whether this finding can be extrapolated to human disease[75].
The study of monocyte subsets in bacterial infections is in its infancy. In patients with severe bacterial sepsis, the number of CD16+ monocytes is dramatically increased[76]. Another report shows that the proportion of intermediate and non-classical monocytes increases during sepsis. CD16+ monocytes show a reduced ability to engulf a bacterium such as E. coli, express low levels of CD86 and HLA-DR, and poorly presents antigen to T cells[77]. The hemolytic uremic syndrome observed in children is due to bacterial toxins. The acute period of this disease is characterized by an increased proportion of CD16+ monocytes that express higher levels of CD16 and lower levels of CD14 compared with those of healthy age-matched children. In addition, HLA-DR expression by classical monocytes is decreased in this patients, and this lower expression of HLA-DR is related to the severity of the disease[78]. In patients with tuberculosis, the percentage and absolute numbers of CD16+ monocytes are increased[79]; nevertheless, some authors did not find changes in the proportion of CD16+ and CD16- monocytes during tuberculosis[80]. When expanded, these monocytes exhibit decreased expression of markers associated with maturation and differentiation and also functional alterations. These alteration include a decrease in phagocytosis potential, a tendency toward cell death and an increased production of TNF after stimulation with live M. tuberculosis[79]. In addition, CD16+ monocytes differentiate into cells that poorly express CD1a and CD209 (DC-SIN) and with a low capacity for presenting mycobacterial antigens. It is likely that this differentiated cell populations contributes to the impairment of DC maturation during tuberculosis[81]. The expansion of these monocytes is amplified in patients with HIV co-infection[82]. Q fever is an acute infectious disease caused by Coxiella burnetii, an obligate intracellular bacterium that targets monocytes and macrophages[83], in patients with valvular damage and in immunocompromised patients, the primo-infection may lead to a chronic disease that essentially manifests as endocarditis[83]. We recently found that the distribution of monocyte subsets is altered in patients with Q fever endocarditis, with a decreased number of CD16+ monocytes (non-classical and intermediate monocytes) (submitted manuscript), which to our knowledge, is the first demonstration that minor monocyte subsets are decreased in an infectious disease.
Only a few papers report the modulation of monocyte subsets in parasitic infections. It has been demonstrated that, the proportion of CD16+ monocytes is increased in pregnant women infected with Plasmodium falciparum, the agent of malaria. These CD16+ monocytes express higher levels of CCR5 than classical monocytes[84]. CD16+ monocytes may play a major in the pathogenesis of maternal malaria because placental plasma concentrations of chemokines such as CCL3 and IL-8 are increased and are associated with placental monocyte infiltration[84,85]. Nevertheless, classical monocytes appear to be critical for the control of Toxoplasma gondii infection in mice[86] and Leishmania brasiliensis in humans via the generation of reactive oxygen species[87].
Aspergillus fumigatus (A. fumigatus) is an environmental fungus that causes life-threatening infections in neutropenic patients. Inhaled A. fumigatus spores (conidia) germinate in the lung and form hyphae that invade blood vessels and disseminate to other tissues[88]. It has been clearly demonstrated that monocyte subsets contribute differently to the defense against A. fumigatus infection. Indeed, classical monocytes are efficient at restricting conidial germination in vitro whereas CD16+ monocytes fail to suppress the germination of conidia. The efficiency of monocyte subsets in controlling A. fumigatus germination is likely dependent on inflammatory cytokines. Although classical monocytes do not secrete TNF following infection, CD16+ monocytes produce high levels of TNF and IL-1β[89]. These results are rather surprising because CD16+ monocytes are thought to be more mature and share features with tissue macrophages and, thus, might be expected to have stronger antimicrobial properties[26]. These data suggest that CD16+ monocytes are the subset that is the most efficient in the control of A. fumigatus infection.
Candida albicans (C. albicans) is responsible of the majority of fungal infections. In 30% of healthy subjects, C. albicans is present as commensal yeast. However when host defense mechanisms are impaired, C. albicans can cause mucocutaneous infections, or disseminate into the bloodstream, thereby infecting multiple organs[90]. Monocytes are associated with systemic candidosis. While the uptake and killing of C. albicans by classical monocytes and CD16+ monocytes are similar, classical monocytes stimulated with heat-killed yeasts produce higher levels of IL-1β and prostaglandin E2 (PGE2) than CD16+ monocytes[91]. It has also been demonstrated that the production of IL-1β by classical monocytes favors the production of IL-17A by CD4+ T lymphocytes and that PGE2 regulates inflammation[92-94]. In addition, the higher production of IL-1β and PGE2 by classical monocytes is associated with increased membrane expression of the mannose receptor (MR)[92,95], suggesting that classical monocytes instead play an immunoregulatory role. These results suggest that only classical monocytes are able to initiate antifungal Th17 responses in human CD4+ T lymphocytes.
Circulating monocytes has been classically considered a homogeneous cell population, but in recent years it has become clear that they are composed of different subsets. A review of the literature shows that monocyte subsets are differently affected in infectious diseases caused by varied pathogens including virus, bacteria, parasites and fungi. In the majority of cases, an expansion of the CD16+ compartment is observed, and the increase in CD16+ monocytes is likely related to their activation through their direct interaction with the pathogen or through cytokines. More surprisingly, it has also been found that the relative number of non-classical and intermediate monocytes is decreased in Q fever endocarditis, suggesting that complex mechanisms govern the equilibrium between monocyte subsets. The measurement of monocyte subsets would be useful in better understanding of the role of monocyte activation in the pathophysiology of infectious diseases.
| 1. | Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74-e80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1942] [Cited by in RCA: 1874] [Article Influence: 117.1] [Reference Citation Analysis (1)] |
| 2. | Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2322] [Cited by in RCA: 2297] [Article Influence: 143.6] [Reference Citation Analysis (0)] |
| 3. | Wong-Baeza I, Alcántara-Hernández M, Mancilla-Herrera I, Ramírez-Saldívar I, Arriaga-Pizano L, Ferat-Osorio E, López-Macías C, Isibasi A. The role of lipopeptidophosphoglycan in the immune response to Entamoeba histolytica. J Biomed Biotechnol. 2010;2010:254521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Ishii KJ, Koyama S, Nakagawa A, Coban C, Akira S. Host innate immune receptors and beyond: making sense of microbial infections. Cell Host Microbe. 2008;3:352-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 387] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 5. | Williams DW, Eugenin EA, Calderon TM, Berman JW. Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J Leukoc Biol. 2012;91:401-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 176] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 6. | Rossol M, Heine H, Meusch U, Quandt D, Klein C, Sweet MJ, Hauschildt S. LPS-induced cytokine production in human monocytes and macrophages. Crit Rev Immunol. 2011;31:379-446. [PubMed] |
| 7. | Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1742] [Cited by in RCA: 2363] [Article Influence: 157.5] [Reference Citation Analysis (0)] |
| 8. | Karlmark KR, Tacke F, Dunay IR. Monocytes in health and disease - Minireview. Eur J Microbiol Immunol (Bp). 2012;2:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Zimmermann HW, Trautwein C, Tacke F. Functional role of monocytes and macrophages for the inflammatory response in acute liver injury. Front Physiol. 2012;3:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 206] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 10. | Possamai LA, Antoniades CG, Anstee QM, Quaglia A, Vergani D, Thursz M, Wendon J. Role of monocytes and macrophages in experimental and human acute liver failure. World J Gastroenterol. 2010;16:1811-1819. [PubMed] |
| 11. | Mehraj V, Textoris J, Ben Amara A, Ghigo E, Raoult D, Capo C, Mege JL. Monocyte responses in the context of Q fever: from a static polarized model to a kinetic model of activation. J Infect Dis. 2013;208:942-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527-2534. [PubMed] |
| 13. | Ziegler-Heitbrock L, Hofer TP. Toward a refined definition of monocyte subsets. Front Immunol. 2013;4:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 232] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 14. | Ancuta P, Rao R, Moses A, Mehle A, Shaw SK, Luscinskas FW, Gabuzda D. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J Exp Med. 2003;197:1701-1707. [PubMed] |
| 15. | Hijdra D, Vorselaars AD, Grutters JC, Claessen AM, Rijkers GT. Phenotypic characterization of human intermediate monocytes. Front Immunol. 2013;4:339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Strauss-Ayali D, Conrad SM, Mosser DM. Monocyte subpopulations and their differentiation patterns during infection. J Leukoc Biol. 2007;82:244-252. [PubMed] |
| 17. | Urra X, Villamor N, Amaro S, Gómez-Choco M, Obach V, Oleaga L, Planas AM, Chamorro A. Monocyte subtypes predict clinical course and prognosis in human stroke. J Cereb Blood Flow Metab. 2009;29:994-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 18. | Skrzeczyńska-Moncznik J, Bzowska M, Loseke S, Grage-Griebenow E, Zembala M, Pryjma J. Peripheral blood CD14high CD16+ monocytes are main producers of IL-10. Scand J Immunol. 2008;67:152-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 219] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 19. | Rogacev KS, Seiler S, Zawada AM, Reichart B, Herath E, Roth D, Ulrich C, Fliser D, Heine GH. CD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur Heart J. 2011;32:84-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 262] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 20. | Wong KL, Tai JJ, Wong WC, Han H, Sem X, Yeap WH, Kourilsky P, Wong SC. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118:e16-e31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 852] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 21. | Weber C, Belge KU, von Hundelshausen P, Draude G, Steppich B, Mack M, Frankenberger M, Weber KS, Ziegler-Heitbrock HW. Differential chemokine receptor expression and function in human monocyte subpopulations. J Leukoc Biol. 2000;67:699-704. [PubMed] |
| 22. | Buechler C, Ritter M, Orsó E, Langmann T, Klucken J, Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol. 2000;67:97-103. [PubMed] |
| 23. | Sulahian TH, Högger P, Wahner AE, Wardwell K, Goulding NJ, Sorg C, Droste A, Stehling M, Wallace PK, Morganelli PM. Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine. 2000;12:1312-1321. [PubMed] |
| 24. | Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375-386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1026] [Cited by in RCA: 972] [Article Influence: 60.8] [Reference Citation Analysis (3)] |
| 25. | Zawada AM, Rogacev KS, Rotter B, Winter P, Marell RR, Fliser D, Heine GH. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood. 2011;118:e50-e61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 440] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 26. | Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, Espevik T, Ziegler-Heitbrock L. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536-3542. [PubMed] |
| 27. | Thomas R, Lipsky PE. Human peripheral blood dendritic cell subsets. Isolation and characterization of precursor and mature antigen-presenting cells. J Immunol. 1994;153:4016-4028. [PubMed] |
| 28. | Randolph GJ, Sanchez-Schmitz G, Liebman RM, Schäkel K. The CD16(+) (FcgammaRIII(+)) subset of human monocytes preferentially becomes migratory dendritic cells in a model tissue setting. J Exp Med. 2002;196:517-527. [PubMed] |
| 29. | Frankenberger M, Hofer TP, Marei A, Dayyani F, Schewe S, Strasser C, Aldraihim A, Stanzel F, Lang R, Hoffmann R. Transcript profiling of CD16-positive monocytes reveals a unique molecular fingerprint. Eur J Immunol. 2012;42:957-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311-317. [PubMed] |
| 31. | Barbalat R, Lau L, Locksley RM, Barton GM. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat Immunol. 2009;10:1200-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 315] [Cited by in RCA: 349] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 32. | Ingersoll MA, Spanbroek R, Lottaz C, Gautier EL, Frankenberger M, Hoffmann R, Lang R, Haniffa M, Collin M, Tacke F. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115:e10-e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 581] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 33. | Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71-82. [PubMed] |
| 34. | Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666-670. [PubMed] |
| 35. | Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037-3047. [PubMed] |
| 36. | Emminger W, Zlabinger GJ, Fritsch G, Urbanek R. CD14(dim)/CD16(bright) monocytes in hemophagocytic lymphohistiocytosis. Eur J Immunol. 2001;31:1716-1719. [PubMed] |
| 37. | Rivier A, Pène J, Rabesandratana H, Chanez P, Bousquet J, Campbell AM. Blood monocytes of untreated asthmatics exhibit some features of tissue macrophages. Clin Exp Immunol. 1995;100:314-318. [PubMed] |
| 38. | Okamoto H, Mizuno K, Horio T. Circulating CD14+ CD16+ monocytes are expanded in sarcoidosis patients. J Dermatol. 2003;30:503-509. [PubMed] |
| 39. | Nagasawa T, Kobayashi H, Aramaki M, Kiji M, Oda S, Izumi Y. Expression of CD14, CD16 and CD45RA on monocytes from periodontitis patients. J Periodontal Res. 2004;39:72-78. [PubMed] |
| 40. | Novak N, Allam P, Geiger E, Bieber T. Characterization of monocyte subtypes in the allergic form of atopic eczema/dermatitis syndrome. Allergy. 2002;57:931-935. [PubMed] |
| 41. | Rahman SH, Salter G, Holmfield JH, Larvin M, McMahon MJ. Soluble CD14 receptor expression and monocyte heterogeneity but not the C-260T CD14 genotype are associated with severe acute pancreatitis. Crit Care Med. 2004;32:2457-2463. [PubMed] |
| 42. | Yoshioka Y, Ohwada A, Harada N, Satoh N, Sakuraba S, Dambara T, Fukuchi Y. Increased circulating CD16+ CD14dim monocytes in a patient with pulmonary alveolar proteinosis. Respirology. 2002;7:273-279. [PubMed] |
| 43. | Scherberich JE, Estner H, Segerer W. Impact of different immunosuppressive regimens on antigen-presenting blood cells in kidney transplant patients. Kidney Blood Press Res. 2004;27:177-180. [PubMed] |
| 44. | Schauer D, Starlinger P, Reiter C, Jahn N, Zajc P, Buchberger E, Bachleitner-Hofmann T, Bergmann M, Stift A, Gruenberger T. Intermediate monocytes but not TIE2-expressing monocytes are a sensitive diagnostic indicator for colorectal cancer. PLoS One. 2012;7:e44450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Ho DD, Rota TR, Hirsch MS. Infection of monocyte/macrophages by human T lymphotropic virus type III. J Clin Invest. 1986;77:1712-1715. [PubMed] |
| 46. | Gartner S, Markovits P, Markovitz DM, Kaplan MH, Gallo RC, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986;233:215-219. [PubMed] |
| 47. | Funderburg NT, Zidar DA, Shive C, Lioi A, Mudd J, Musselwhite LW, Simon DI, Costa MA, Rodriguez B, Sieg SF. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood. 2012;120:4599-4608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 48. | Han J, Wang B, Han N, Zhao Y, Song C, Feng X, Mao Y, Zhang F, Zhao H, Zeng H. CD14(high)CD16(+) rather than CD14(low)CD16(+) monocytes correlate with disease progression in chronic HIV-infected patients. J Acquir Immune Defic Syndr. 2009;52:553-559. [PubMed] |
| 49. | Williams K, Westmoreland S, Greco J, Ratai E, Lentz M, Kim WK, Fuller RA, Kim JP, Autissier P, Sehgal PK. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest. 2005;115:2534-2545. [PubMed] |
| 50. | Moniuszko M, Liyanage NP, Doster MN, Parks RW, Grubczak K, Lipinska D, McKinnon K, Brown C, Hirsch V, Vaccari M. Glucocorticoid Treatment at Moderate Doses of SIVmac251-Infected Rhesus Macaques Decreases the Frequency of Circulating CD14(+)CD16(++) Monocytes But Does Not Alter the Tissue Virus Reservoir. AIDS Res Hum Retroviruses. 2014;Mar 3; Epub ahead of print. [PubMed] |
| 51. | Shikuma CM, Chow DC, Gangcuangco LM, Zhang G, Keating SM, Norris PJ, Seto TB, Parikh N, Kallianpur KJ, Nakamoto BK. Monocytes expand with immune dysregulation and is associated with insulin resistance in older individuals with chronic HIV. PLoS One. 2014;9:e90330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Schaer DJ, Alayash AI, Buehler PW. Gating the radical hemoglobin to macrophages: the anti-inflammatory role of CD163, a scavenger receptor. Antioxid Redox Signal. 2007;9:991-999. [PubMed] |
| 53. | Tippett E, Cheng WJ, Westhorpe C, Cameron PU, Brew BJ, Lewin SR, Jaworowski A, Crowe SM. Differential expression of CD163 on monocyte subsets in healthy and HIV-1 infected individuals. PLoS One. 2011;6:e19968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 54. | Funderburg NT, Jiang Y, Debanne SM, Storer N, Labbato D, Clagett B, Robinson J, Lederman MM, McComsey GA. Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy. Clin Infect Dis. 2014;58:588-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 55. | Palmer BE, Neff CP, Lecureux J, Ehler A, Dsouza M, Remling-Mulder L, Korman AJ, Fontenot AP, Akkina R. In vivo blockade of the PD-1 receptor suppresses HIV-1 viral loads and improves CD4+ T cell levels in humanized mice. J Immunol. 2013;190:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 56. | Said EA, Dupuy FP, Trautmann L, Zhang Y, Shi Y, El-Far M, Hill BJ, Noto A, Ancuta P, Peretz Y. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med. 2010;16:452-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 383] [Cited by in RCA: 368] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 57. | Breen EC, Rezai AR, Nakajima K, Beall GN, Mitsuyasu RT, Hirano T, Kishimoto T, Martinez-Maza O. Infection with HIV is associated with elevated IL-6 levels and production. J Immunol. 1990;144:480-484. [PubMed] |
| 58. | Than S, Hu R, Oyaizu N, Romano J, Wang X, Sheikh S, Pahwa S. Cytokine pattern in relation to disease progression in human immunodeficiency virus-infected children. J Infect Dis. 1997;175:47-56. [PubMed] |
| 59. | Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10:S98-109. [PubMed] |
| 61. | Durbin AP, Vargas MJ, Wanionek K, Hammond SN, Gordon A, Rocha C, Balmaseda A, Harris E. Phenotyping of peripheral blood mononuclear cells during acute dengue illness demonstrates infection and increased activation of monocytes in severe cases compared to classic dengue fever. Virology. 2008;376:429-435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 62. | Jessie K, Fong MY, Devi S, Lam SK, Wong KT. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J Infect Dis. 2004;189:1411-1418. [PubMed] |
| 63. | Diamond MS, Roberts TG, Edgil D, Lu B, Ernst J, Harris E. Modulation of Dengue virus infection in human cells by alpha, beta, and gamma interferons. J Virol. 2000;74:4957-4966. [PubMed] |
| 64. | Fink K, Ng C, Nkenfou C, Vasudevan SG, van Rooijen N, Schul W. Depletion of macrophages in mice results in higher dengue virus titers and highlights the role of macrophages for virus control. Eur J Immunol. 2009;39:2809-2821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 65. | Chen LC, Shyu HW, Lin HM, Lei HY, Lin YS, Liu HS, Yeh TM. Dengue virus induces thrombomodulin expression in human endothelial cells and monocytes in vitro. J Infect. 2009;58:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 66. | Bosch I, Xhaja K, Estevez L, Raines G, Melichar H, Warke RV, Fournier MV, Ennis FA, Rothman AL. Increased production of interleukin-8 in primary human monocytes and in human epithelial and endothelial cell lines after dengue virus challenge. J Virol. 2002;76:5588-5597. [PubMed] |
| 67. | Azeredo EL, Neves-Souza PC, Alvarenga AR, Reis SR, Torrentes-Carvalho A, Zagne SM, Nogueira RM, Oliveira-Pinto LM, Kubelka CF. Differential regulation of toll-like receptor-2, toll-like receptor-4, CD16 and human leucocyte antigen-DR on peripheral blood monocytes during mild and severe dengue fever. Immunology. 2010;130:202-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 68. | Wong KL, Chen W, Balakrishnan T, Toh YX, Fink K, Wong SC. Susceptibility and response of human blood monocyte subsets to primary dengue virus infection. PLoS One. 2012;7:e36435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 69. | Ashfaq UA, Javed T, Rehman S, Nawaz Z, Riazuddin S. An overview of HCV molecular biology, replication and immune responses. Virol J. 2011;8:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 70. | Calland N, Dubuisson J, Rouillé Y, Séron K. Hepatitis C virus and natural compounds: a new antiviral approach? Viruses. 2012;4:2197-2217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 71. | Coquillard G, Patterson BK. Determination of hepatitis C virus-infected, monocyte lineage reservoirs in individuals with or without HIV coinfection. J Infect Dis. 2009;200:947-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 72. | Nockher WA, Scherberich JE. Expanded CD14+ CD16+ monocyte subpopulation in patients with acute and chronic infections undergoing hemodialysis. Infect Immun. 1998;66:2782-2790. [PubMed] |
| 73. | Zheng J, Liang H, Xu C, Xu Q, Zhang T, Shen T, Lu F. An unbalanced PD-L1/CD86 ratio in CD14(++)CD16(+) monocytes is correlated with HCV viremia during chronic HCV infection. Cell Mol Immunol. 2014;11:294-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 74. | Reeves M, Sinclair J. Aspects of human cytomegalovirus latency and reactivation. Curr Top Microbiol Immunol. 2008;325:297-313. [PubMed] |
| 75. | Daley-Bauer LP, Roback LJ, Wynn GM, Mocarski ES. Cytomegalovirus hijacks CX3CR1(hi) patrolling monocytes as immune-privileged vehicles for dissemination in mice. Cell Host Microbe. 2014;15:351-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 76. | Fingerle G, Pforte A, Passlick B, Blumenstein M, Ströbel M, Ziegler-Heitbrock HW. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood. 1993;82:3170-3176. [PubMed] |
| 77. | Skrzeczyñska J, Kobylarz K, Hartwich Z, Zembala M, Pryjma J. CD14+CD16+ monocytes in the course of sepsis in neonates and small children: monitoring and functional studies. Scand J Immunol. 2002;55:629-638. [PubMed] |
| 78. | Fernández GC, Ramos MV, Gómez SA, Dran GI, Exeni R, Alduncín M, Grimoldi I, Vallejo G, Elías-Costa C, Isturiz MA. Differential expression of function-related antigens on blood monocytes in children with hemolytic uremic syndrome. J Leukoc Biol. 2005;78:853-861. [PubMed] |
| 79. | Castaño D, García LF, Rojas M. Increased frequency and cell death of CD16+ monocytes with Mycobacterium tuberculosis infection. Tuberculosis (Edinb). 2011;91:348-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 80. | Barcelos W, Martins-Filho OA, Guimarães TM, Oliveira MH, Spíndola-de-Miranda S, Carvalho BN, Toledo Vde P. Peripheral blood mononuclear cells immunophenotyping in pulmonary tuberculosis patients before and after treatment. Microbiol Immunol. 2006;50:597-605. [PubMed] |
| 81. | Balboa L, Romero MM, Laborde E, Sabio Y García CA, Basile JI, Schierloh P, Yokobori N, Musella RM, Castagnino J, de la Barrera S. Impaired dendritic cell differentiation of CD16-positive monocytes in tuberculosis: role of p38 MAPK. Eur J Immunol. 2013;43:335-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 82. | Vanham G, Edmonds K, Qing L, Hom D, Toossi Z, Jones B, Daley CL, Huebner B, Kestens L, Gigase P. Generalized immune activation in pulmonary tuberculosis: co-activation with HIV infection. Clin Exp Immunol. 1996;103:30-34. [PubMed] |
| 83. | Raoult D, Marrie T, Mege J. Natural history and pathophysiology of Q fever. Lancet Infect Dis. 2005;5:219-226. [PubMed] |
| 84. | Jaworowski A, Kamwendo DD, Ellery P, Sonza S, Mwapasa V, Tadesse E, Molyneux ME, Rogerson SJ, Meshnick SR, Crowe SM. CD16+ monocyte subset preferentially harbors HIV-1 and is expanded in pregnant Malawian women with Plasmodium falciparum malaria and HIV-1 infection. J Infect Dis. 2007;196:38-42. [PubMed] |
| 85. | Abrams ET, Brown H, Chensue SW, Turner GD, Tadesse E, Lema VM, Molyneux ME, Rochford R, Meshnick SR, Rogerson SJ. Host response to malaria during pregnancy: placental monocyte recruitment is associated with elevated beta chemokine expression. J Immunol. 2003;170:2759-2764. [PubMed] |
| 86. | Dunay IR, Fuchs A, Sibley LD. Inflammatory monocytes but not neutrophils are necessary to control infection with Toxoplasma gondii in mice. Infect Immun. 2010;78:1564-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 155] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 87. | Novais FO, Nguyen BT, Beiting DP, Carvalho LP, Glennie ND, Passos S, Carvalho EM, Scott P. Human classical monocytes control the intracellular stage of Leishmania braziliensis by reactive oxygen species. J Infect Dis. 2014;209:1288-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 88. | Brown JM. Fungal infections in bone marrow transplant patients. Curr Opin Infect Dis. 2004;17:347-352. [PubMed] |
| 89. | Serbina NV, Cherny M, Shi C, Bleau SA, Collins NH, Young JW, Pamer EG. Distinct responses of human monocyte subsets to Aspergillus fumigatus conidia. J Immunol. 2009;183:2678-2687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 90. | Perlroth J, Choi B, Spellberg B. Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Med Mycol. 2007;45:321-346. [PubMed] |
| 91. | Smeekens SP, van de Veerdonk FL, Joosten LA, Jacobs L, Jansen T, Williams DL, van der Meer JW, Kullberg BJ, Netea MG. The classical CD14⁺⁺ CD16⁻ monocytes, but not the patrolling CD14⁺ CD16⁺ monocytes, promote Th17 responses to Candida albicans. Eur J Immunol. 2011;41:2915-2924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 92. | Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576-587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1001] [Cited by in RCA: 1004] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 93. | Boniface K, Bak-Jensen KS, Li Y, Blumenschein WM, McGeachy MJ, McClanahan TK, McKenzie BS, Kastelein RA, Cua DJ, de Waal Malefyt R. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J Exp Med. 2009;206:535-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 351] [Cited by in RCA: 392] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 94. | Napolitani G, Acosta-Rodriguez EV, Lanzavecchia A, Sallusto F. Prostaglandin E2 enhances Th17 responses via modulation of IL-17 and IFN-gamma production by memory CD4+ T cells. Eur J Immunol. 2009;39:1301-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 95. | Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1353] [Cited by in RCA: 1361] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
P- Reviewer: Vogt G, Zhang LL S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ