Published online Nov 27, 2014. doi: 10.5411/wji.v4.i3.149
Revised: May 17, 2014
Accepted: July 17, 2014
Published online: November 27, 2014
Processing time: 258 Days and 20.1 Hours
Drug induced liver injury (DILI) is a common condition of increasing incidence. Many environmental and genetic factors are involved in its pathogenesis, and immunological mechanisms are also thought to contribute to the development and severity of DILI. This review summarizes current understanding of the immunological pathogenesis of DILI and discusses the perspective for clinical applications.
Core tip: Drug-induced liver injury (DILI) is a common liver disease that occurs frequently after drug ingestion. DILI can be classified as predictable or idiosyncratic. The former is dose dependent, has a short latency period, and results from direct toxicity of the drug or its metabolite(s). Idiosyncratic DILI may be more problematic because it is usually unpredictable and is found more frequently in the clinical setting. This type of DILI is due to an allergic reaction or the toxicity of metabolites generated via individual drug metabolism reactions. Various factors, such as environmental, genetic and immunological reactions, may be associated with adduct formation and the onset of DILI. This review summarizes current knowledge on the immunological aspects of DILI, including its pathogenesis, diagnosis and treatment.
- Citation: Tajiri K, Shimizu Y. Immunological aspects of drug-induced liver injury. World J Immunol 2014; 4(3): 149-157
- URL: https://www.wjgnet.com/2219-2824/full/v4/i3/149.htm
- DOI: https://dx.doi.org/10.5411/wji.v4.i3.149
Drug-induced liver injury (DILI) is a common liver disease that occurs frequently after drug ingestion including herbal medicine. Its incidence is reported to be increasing[1]. Clinical manifestations of DILI vary from transient mild elevation of liver enzymes to fulminant liver failure leading to death. DILI can be divided into three types according to the pattern of liver injury; hepatocellular [alanine aminotransferase (ALT) ≥ 3 upper limit of normal (ULN) and ALT/ alkaline phosphatase (ALP) ratio ≥ 5], cholestatic (ALP ≥ 2 ULN and ALT/ALP ratio ≤ 2), and mixed type (2 < ALT/ALP ratio < 5)[2]. In addition, DILI can be classified as predictable or idiosyncratic[3]. The former is dose dependent, has a short latency period, and results from direct toxicity of the drug or its metabolite(s); e.g., acetaminophen. Idiosyncratic DILI may be more problematic because it is usually unpredictable and is found more frequently in the clinical setting. This type of DILI is due to an allergic reaction or the toxicity of metabolites generated via individual drug metabolism reactions. Various factors, such as environmental, genetic and immunological reactions, may be associated with adduct formation and the onset of DILI. This review will summarize current knowledge on the immunological aspects of DILI, including its pathogenesis, diagnosis and treatment.
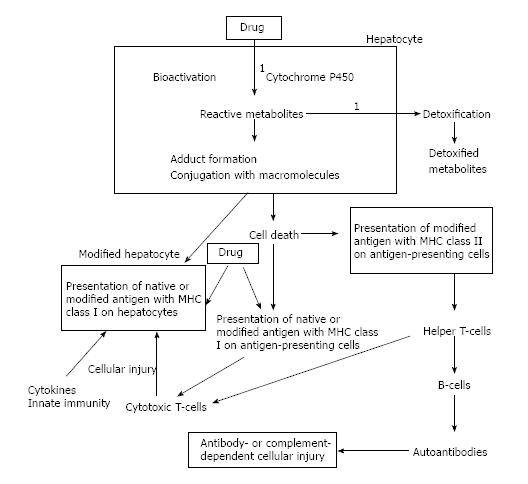
DLII is thought to occur through direct toxic or allergic effects of drugs. However, there is another mechanism, in which DILI is induced by toxic or reactive metabolites produced through abnormal metabolism. Most drugs are fat-soluble, with the liver being the main organ for their biotransformation and elimination. Drug elimination by the liver may be determined by several reactions, such as hepatic blood flow, hepatic metabolism and biliary extraction. Drug metabolism in the liver largely depends on the hydroxylation activity of cytochrome P (CYP) 450 enzymes (Phase 1 reaction); the products are unstable or reactive metabolites; and the process is affected by various factors, such as genetic alterations[4]. The hydroxylated metabolites are then conjugated by gluconization, sulfonization, glutathionization, or acetylation (Phase 2 reaction), followed by their transport to the extracellular space as water-soluble, stable and detoxified products (Phase 3 reaction). These final metabolites are excreted into the urine or bile juice. During this process, most of the targets for immune attack are thought to be unstable and reactive metabolites conjugated with intracellular proteins or macromolecules. These newly formed antigens are recognized by immune cells. Activation of the immune system generates autoantibodies and cell-mediated immune responses, leading to injury to hepatocytes[5] (Figure 1).
Immunological mechanisms leading to DILI have been widely explored using experimental animal models and predictable drugs such as acetaminophen. In DILI, the balance between pro- and anti-inflammatory responses resulting from the activation of the innate immune system in the liver determines tissue susceptibility and the severity of liver injury[6].
Acetaminophen induced liver injury (AILI) involves glutathione depletion and covalent binding of a metabolite of acetaminophen to mitochondrial protein, both essential mechanisms of hepatocyte death. Acetaminophen hepatotoxicity is dependent upon its metabolic transformation to the reactive metabolite N-acetyl-p-benzo-quinone imine (NAPQI) by CYP450[7]. Following the phase 2 process of conjugation detoxification, NAPQI becomes covalently bound to hepatic mitochondrial protein, leading to cell death. Cell damage induces the release of damage-associated molecular patterns (DAMPS), such as HMGB-1 and heat shock proteins (HSP), which then activate the innate immune system[8].
In animal models of AILI, innate immune cells such as macrophages and NK/NKT cells play a pivotal role in protection against acetaminophen[9-11]. Depletion or inactivation of hepatic macrophages markedly reduced the severity of AILI by inhibiting cytokine production, especially of tumor necrosis factor (TNF)-α, soon after acetaminophen administration[12], but induced more severe liver injury at later times, possibly due to the suppression of delayed production of prostaglandin by hepatic macrophages[9]. These macrophages were shown to be derived from circulating monocytes that infiltrate the liver, but not from resident Kupffer cells[13], with TNF-α regarded as an essential participant in the macrophage dependent etiology of AILI[14-16].
In contrast, the administration of anti-NK1.1 antibody to deplete NK and NKT cells protected mice from AILI, probably due to a reduction in IFN-γ concentrations[10]. Starvation-induced ketone production has been observed in NKT cell-deficient mice, resulting in increases in CYP2E1-mediated reactive metabolites and enhanced susceptibility to AILI[11]. More recently, however, NK and NKT cells were shown to contribute to protection against AILI only in the presence of dimethyl sulfoxide[17], suggesting NK and NKT cells may have little involvement in protecting these mice against AILI. NK/NKT cells are generally regarded as being involved in the immunopathogenesis of AILI, with IFN-γ being a key molecule. Various cytokines and chemokines are produced in response to IFN-γ, with these factors promoting neutrophil infiltration[10,18]. NKT cells have also been reported involved in a mouse model of halothane-induced liver injury by recruiting neutrophils to the liver, with these neutrophils playing a pathogenic role in liver injury[19]. In contrast, eosinophil depletion was found to reduce the severity of halothane-induced liver injury in a mouse model, whereas depletion of neutrophils failed to reduce the severity of liver injury[20]. This study found that eosinophils accumulated exclusively around areas of hepatocellular necrosis, suggesting a pathogenic role of eosinophils in liver injury similar to that observed in many patients with DILI[20]. Recently, regulatory T-cells were also reported to reduce the severity of trifluroacetyl chloride-induced DILI by decreasing the hepatic levels of pro-inflammatory cytokines[21].
Dendritic cells (DCs) have also been reported to play a protective role in AILI. Hepatic DCs have been reported to suppress the severity of AILI, at least in part, by preventing NK cell activation and inducing neutrophil apoptosis[22].
In contrast, interleukin (IL)-10 null mice were found to be more susceptible to AILI, suggesting that a counter-regulatory anti-inflammatory response also modulates liver injury[23]. Furthermore, receptors involved in the innate immune system, such as toll-like receptors (TLR)-4 and 9 have been found to play roles in promoting inflammatory responses and subsequent pathogenesis of AILI[24,25]. Collectively, the innate immune system is closely associated with the onset and severity of AILI, which may explain, at least in part, the pathogenesis of AILI in mice.
Cytokines and immune cells have been reported associated with the pathogenesis of DILI but not of AILI. For example, IL-4 was reported to mediate dicloxacillin-induced liver injury in a mouse model[26], and IL-17 and IL-1β were found to be involved in the onset of diclofenac-induced liver injury in mice[27]. Diclofenac has been reported to inhibit TNF-α induced survival signals through nuclear factor-κB and to sensitize hepatocytes to apoptosis[28]. Thus, the activation of the innate immune system in the liver may play an important role in immune-mediated idiosyncratic DILI[6,29].
Although experimental data support the hypothesis that DILIs are induced through the activation of the innate immune system within the liver, different cell populations may be involved in each DILI. Moreover, these data are derived from mice with a uniform genetic background and environment.
In contrast to mice, genetic background and environment are diverse in humans, and most patients who develop this disease are receiving multiple drugs. The conditions under which DILI develops are therefore more complex in humans, making the diagnosis of DILI and the analysis of immunological mechanisms more difficult.
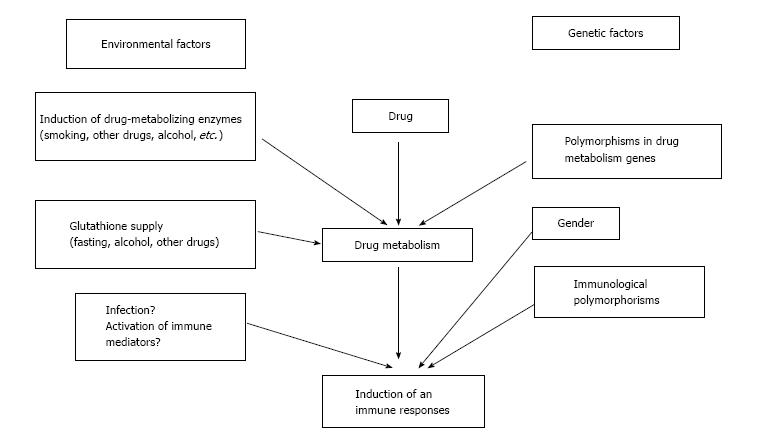
The pathogenesis of DILI in humans may be influenced by a variety of genetic and environmental effects on drug metabolism and immunological responses. These factors may, in turn, be associated with the onset and severity of DILI in humans (Figure 2)[30].
Pro- and anti-inflammatory responses have been observed in patients with DILI. For example, a study of 111 patients with DILI due to acetaminophen overdose included measurements of the plasma concentrations of cytokines such as IL-6, IL-8, IL-10 and monocyte chemoattractant protein (MCP)-1[31]. In that study, the concentrations of IL-6, IL-8, and MCP-1 were elevated in patients with elevated serum ALT[31], with MCP-1 concentration most closely associated with the severity of toxicity. Thus, the hepatotoxicity in patients with acetaminophen overdose is thought to be due to the direct toxicity of the acetaminophen metabolite, whereas the severity of toxicity may be immunologically determined.
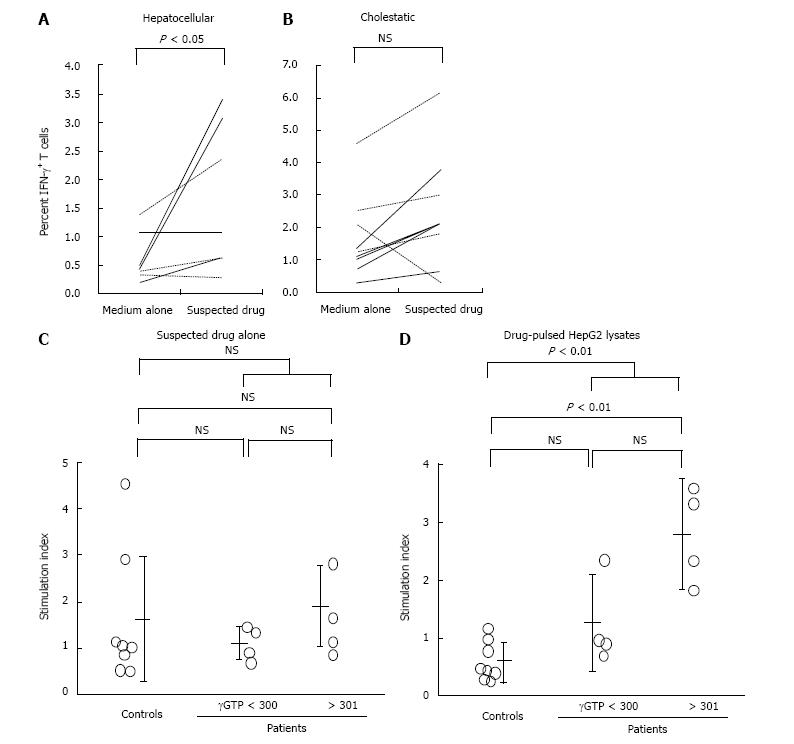
The expression of various cytokines and their association with the severity of liver injury have been reported in patients with acute liver failure[32]. For example, IL-6 and TNF-α have been associated with disease progression, whereas IL-10 was associated with disease protection. A study of 39 patients with acute liver failure due to DILI found that serum IL-17 and IL-21 concentrations were elevated[33] and that autoantibodies were more frequently found in patients with than without DILI, suggesting that autoimmune responses are involved in its pathogenesis[34]. Elevation of the serum cytokines, IL-1β, IL-10, IL-12, IL-13, and TNF-α preceded an increase in liver enzymes in a patient with DILI[35]. Moreover, CD8+ T cells were reported to produce IFN-γ when peripheral mononuclear cells of patients with the hepatocellular type of DILI were stimulated with the causative drug (Figure 3A and B). In contrast, CD14+ monocytes from patients with the cholestatic type of DILI were found to produce TNF-α upon stimulation by lysates of HepG2 cells that were incubated with the causative drug (Figure 3C and D)[36]. Thus, the mechanisms responsible for two types of DILI, hepatocellular and cholestatic, may be different, with the immune system activated by both the drug itself and its reactive metabolites.
Assessments of genetic factors in patients with DILI found that the low IL-10 producing haplotype was more prevalent, but genetic polymorphisms in IL-10, IL-4 and TNF-α were not related to the risk of developing DILI[37]. In contrast, IL-10 polymorphisms were reported associated with the incidence of docetaxel-induced liver injury[38]. Thus, the balance between pro- and anti-inflammatory cytokines plays a significant role in the pathogenesis of DILI, and the pattern of pro- and anti-inflammatory cytokines may be a candidate biomarker of DILI.
Inhalation of the anesthetic halothane has been explored as an immunoallergic response model. Patients with halothane-induced liver injury were found to produce autoantibodies that recognize autoantigens and neoantigens created by trifluoroacetylation of hepatic proteins. Incubation of halothane-pretreated rabbit hepatocytes with the sera of patients with halothane-induced fulminant hepatitis increased susceptibility to lymphocyte cytotoxicity[39]. Halothane is metabolized by CYP2E1 to form the reactive metabolite acyl halide, which may trigger immune responses. Anti-CYP autoantibodies induced by drugs have also been found in patients with idiosyncratic drug reactions to dihydralazine[40] and tienilic acid[41].
Recently, human monocytic cells have been reported activated by hepatotoxic drugs, such as amiodarone and its metabolite, when co-incubated with CYP3A4 supersomes[42].
Since antigenic peptides are presented on human leukocyte antigen (HLA) molecules, HLA haplotype has been shown associated with the development of various diseases. For example, 57% of Belgian patients with amoxicillin-clavulanate-induced liver injury had the DRB1*1501 allele compared with 11% of the general population[43], with the same allele found in 70% of Scottish patients with amoxicillin-clavulanate-induced liver injury[44]. Furthermore, 53% of patients in the United Kingdom had the HLA-DRB1*15 allele, compared with 30% of the general population[45]. Conversely, 9.8% of patients with DILI had the DRB1*07 allele, compared with 29% of the general population[45]. Study of a Spanish cohort did not show a significant association between DILI and the DRB1*15 allele, but found that the frequency of DQB1*06 was significantly higher in these patients than in the general population[46]. A recent genome-wide association study in a European cohort with amoxicillin-clavulanate-induced liver injury showed that multiple HLA genotypes could affect susceptibility to the onset of the disease[47]. Although several single nucleotide polymorphisms (SNPs) within the HLA were found associated with DILI, the strongest association was observed in SNPs within HLA-DRB1 and an independent association was found with HLA-A*0201. Moreover, HLA-A*0201, DQB1*0602 and amoxicillin-clavulanate-induced liver injury were found to be associated with PTPN22, a gene associated with the development of various autoimmune diseases[47]. Importantly, only SNPs inside the HLA region were significantly associated with amoxicillin-clavulanate-DILI.
Recently, activation of T-cells with a particular HLA genotype has been explored in patients with DILI. Flucloxacillin (Flux)-induced liver injury has been strongly associated with the HLA-B*5701 allele, with approximately 85% of these patients having at least one copy of HLA-B*5701[48]. Flux has been reported to activate naïve CD8+ T-cells when DCs present the drug antigen in patients with HLA-B*5701. Following drug stimulation, T-cells expressing CCR4 and CCR9 were found to secrete IFN-γ, T-helper 2 cytokines, perforin, granzyme B, and FasL[49], with the activation of CD8+ T cells restricted to those with the HLA-B*5701 allele[50]. Hypersensitivity reactions without the formation of conjugates, in which drugs can activate the immune system directly and pharmacologically, have been observed in these types of allergic reaction[51,52], consistent with earlier results[37]. These findings suggest that the HLA genotype and its SNPs contribute significantly to susceptibility to DILI, possibly through abnormal antigen presentation to T cells.
Collectively, various immunological mechanisms, including innate and acquired immunity, are involved in the pathogenesis of DILI. The balance between pro- and anti-inflammatory cytokines may affect the onset and severity of DILI.
Because there are no standard criteria for the diagnosis of DILI, various clinical scales have been developed. The Naranjo Adverse Drug Reactions Probability Scale (NADRPS) was proposed for the assessment of adverse drug reactions in 1981[53]. The NADRPS has been widely used to diagnose DILI due to its simplicity and wide applicability, despite it not being developed specifically for the diagnosis of DILI. The Roussel Uclaf Causality Assessment Method (RUCAM) diagnostic scale, first proposed in 1993[2], has been used to classify the pattern of liver injury into hepatocellular, cholestatic, or mixed type. The RUCAM is based on seven criteria, including temporal relationship, clinical course (response after withdrawal of drug), risk factors, concomitant drugs, exclusion of other non-drug etiologies, likelihood of a reaction based on package labeling, and re-challenge. This method has been widely used as a standardized scale with high reliability, reproducibility and specificity.
The more recently described Maria and Victorino clinical diagnostic scale (CDS) simplified the RUCAM scale by using only five criteria[54]. This scale emphasizes immunoallergic reactions, such as extrahepatic manifestations[55]. Lymphocyte proliferation in response to drugs was observed in over 50% of patients with DILI[54]. In Japan, the diagnostic scale similar to the RUCAM scale includes drug-lymphocyte stimulation tests (DLST) in the diagnosis[56]. Thus, although the diagnosis of DILI is mainly dependent on the course of the disease and the causative drug, some immunological methods have been utilized in its diagnosis because immunological mechanisms are involved in its pathogenesis.
Although these methods of causality assessment for DILI are useful in most of the clinical setting, there may be some pitfalls in the diagnosis of DILI induced by herbal medicine[57,58]. Hepatotoxicity due to herbs has not been fully recognized and the risk may be underestimated. There is a variety of the quality of herbal products, which may makes the evaluation of the causality for DILI by herbs complicated[58]. Furthermore, various extracts from herbs have been reported to have immunomodulatory effects via cytokine production, toll-like receptor binding, and induction of signal transduction via T-cell receptor or dendritic cell maturation[59-62], all of which could be associated with the immunological basis for DILI caused by herbs.
One of the most popular additional diagnostic tests for DILI is the DLST[63]. In this test, lymphocytes collected from the heparinized peripheral blood of patients are incubated with dilutions of the suspected drug, with lymphocyte proliferation evaluated by 3H-thymidine uptake. DLST is widely used in Japan and is incorporated in Japanese diagnostic criteria for DILI (DDW-J scale). However, the sensitivity of this test is below 50% and the lymphocyte response to the suspected drug may not necessarily be related to the liver injury. Another test using the peripheral blood of patients is the leukocyte migration test (LMT), which has been reported more useful than DLST[64]. This test measures the chemotaxis of granulocytes in response to chemotactic factors produced by mononuclear cells after incubation with the suspected drug. Furthermore, a cytokine production test showed high diagnostic sensitivity[36]. In this analysis, a mixture of a HepG2 cell extract and culture medium, which retain metabolic enzyme activities such as CYP450 are incubated with dilutions of the suspected drug, followed by incubation with peripheral blood lymphocytes isolated from patients suspected of having DILI. Intracytoplasmic cytokines, such as IFN-γ, TNF-α and IL-2, of the lymphocytes are finally evaluated by flow cytometry. Although these tests are useful for the diagnosis or identification of a single causative drug, they are not simple to perform and may not be suitable for routine examinations.
Thus, immunological methods detecting lymphocyte reactivity against a causative drug or its derivatives may be helpful for the diagnosis of DILI. At this time, however, this method is not applicable to all types of DILI.
The main treatment strategy for DILI is cessation of the causative drug. Early recognition of the adverse effects of a drug is most important in managing DILI and in preventing severe liver injury. Although few specific treatments for DILI have proven beneficial, there are two exceptions; N-acetylcysteine for acetaminophen toxicity and L-carnitine for valproic acid overdose[65,66].
Patients should be assessed carefully by serial biochemical tests. Severe hepatocellular injury may develop into acute liver failure, and the only effective therapy for the latter may be liver transplantation. Patients with severe liver injury, particularly those with jaundice, should be managed carefully and considered for referral to a liver transplant specialist. Some treatments that can modulate immune functions have been tried in clinical settings, although there is no consensus on their use.
Corticosteroids are of unproven benefit for DILI, but may be used to treat patients with hypersensitivity reactions[67]. The combination of a corticosteroid and ursodeoxycholic acid has been reported safe for patients with DILI, leading to a more rapid reduction in bilirubin and transaminases[68].
Various immunological mechanisms are involved in the pathogenesis of the unpredictable type of DILI. To date, however, analyses of these mechanisms have been unsatisfactory, and additional studies are required to better understand the pathogenesis of DILI and its diagnosis, to predict the extent of injury in each subject and to manage these patients.
| 1. | Verma S, Kaplowitz N. Diagnosis, management and prevention of drug-induced liver injury. Gut. 2009;58:1555-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 2. | Danan G, Benichou C. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323-1330. [PubMed] |
| 3. | Kaplowitz N. Drug-induced liver injury. Clin Infect Dis. 2004;38 Suppl 2:S44-S48. [PubMed] |
| 4. | Andrade RJ, Robles M, Ulzurrun E, Lucena MI. Drug-induced liver injury: insights from genetic studies. Pharmacogenomics. 2009;10:1467-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Liu ZX, Kaplowitz N. Immune-mediated drug-induced liver disease. Clin Liver Dis. 2002;6:755-774. [PubMed] |
| 6. | Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov. 2005;4:489-499. [PubMed] |
| 7. | Raucy JL, Lasker JM, Lieber CS, Black M. Acetaminophen activation by human liver cytochromes P450IIE1 and P450IA2. Arch Biochem Biophys. 1989;271:270-283. [PubMed] |
| 8. | Laverty HG, Antoine DJ, Benson C, Chaponda M, Williams D, Kevin Park B. The potential of cytokines as safety biomarkers for drug-induced liver injury. Eur J Clin Pharmacol. 2010;66:961-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Ju C, Reilly TP, Bourdi M, Radonovich MF, Brady JN, George JW, Pohl LR. Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice. Chem Res Toxicol. 2002;15:1504-1513. [PubMed] |
| 10. | Liu ZX, Govindarajan S, Kaplowitz N. Innate immune system plays a critical role in determining the progression and severity of acetaminophen hepatotoxicity. Gastroenterology. 2004;127:1760-1774. [PubMed] |
| 11. | Martin-Murphy BV, Kominsky DJ, Orlicky DJ, Donohue TM, Ju C. Increased susceptibility of natural killer T-cell-deficient mice to acetaminophen-induced liver injury. Hepatology. 2013;57:1575-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Goldin RD, Ratnayaka ID, Breach CS, Brown IN, Wickramasinghe SN. Role of macrophages in acetaminophen (paracetamol)-induced hepatotoxicity. J Pathol. 1996;179:432-435. [PubMed] |
| 13. | Holt MP, Cheng L, Ju C. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J Leukoc Biol. 2008;84:1410-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 343] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 14. | Gardner CR, Laskin JD, Dambach DM, Sacco M, Durham SK, Bruno MK, Cohen SD, Gordon MK, Gerecke DR, Zhou P. Reduced hepatotoxicity of acetaminophen in mice lacking inducible nitric oxide synthase: potential role of tumor necrosis factor-alpha and interleukin-10. Toxicol Appl Pharmacol. 2002;184:27-36. [PubMed] |
| 15. | Boess F, Bopst M, Althaus R, Polsky S, Cohen SD, Eugster HP, Boelsterli UA. Acetaminophen hepatotoxicity in tumor necrosis factor/lymphotoxin-alpha gene knockout mice. Hepatology. 1998;27:1021-1029. [PubMed] |
| 16. | Ishida Y, Kondo T, Tsuneyama K, Lu P, Takayasu T, Mukaida N. The pathogenic roles of tumor necrosis factor receptor p55 in acetaminophen-induced liver injury in mice. J Leukoc Biol. 2004;75:59-67. [PubMed] |
| 17. | Masson MJ, Carpenter LD, Graf ML, Pohl LR. Pathogenic role of natural killer T and natural killer cells in acetaminophen-induced liver injury in mice is dependent on the presence of dimethyl sulfoxide. Hepatology. 2008;48:889-897. [PubMed] |
| 18. | Ishida Y, Kondo T, Ohshima T, Fujiwara H, Iwakura Y, Mukaida N. A pivotal involvement of IFN-gamma in the pathogenesis of acetaminophen-induced acute liver injury. FASEB J. 2002;16:1227-1236. [PubMed] |
| 19. | Cheng L, You Q, Yin H, Holt MP, Ju C. Involvement of natural killer T cells in halothane-induced liver injury in mice. Biochem Pharmacol. 2010;80:255-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Proctor WR, Chakraborty M, Chea LS, Morrison JC, Berkson JD, Semple K, Bourdi M, Pohl LR. Eosinophils mediate the pathogenesis of halothane-induced liver injury in mice. Hepatology. 2013;57:2026-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Cho J, Kim L, Li Z, Rose NR, Talor MV, Njoku DB. Sex bias in experimental immune-mediated, drug-induced liver injury in BALB/c mice: suggested roles for Tregs, estrogen, and IL-6. PLoS One. 2013;8:e61186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Connolly MK, Ayo D, Malhotra A, Hackman M, Bedrosian AS, Ibrahim J, Cieza-Rubio NE, Nguyen AH, Henning JR, Dorvil-Castro M. Dendritic cell depletion exacerbates acetaminophen hepatotoxicity. Hepatology. 2011;54:959-968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Bourdi M, Masubuchi Y, Reilly TP, Amouzadeh HR, Martin JL, George JW, Shah AG, Pohl LR. Protection against acetaminophen-induced liver injury and lethality by interleukin 10: role of inducible nitric oxide synthase. Hepatology. 2002;35:289-298. [PubMed] |
| 24. | Yohe HC, O’Hara KA, Hunt JA, Kitzmiller TJ, Wood SG, Bement JL, Bement WJ, Szakacs JG, Wrighton SA, Jacobs JM. Involvement of Toll-like receptor 4 in acetaminophen hepatotoxicity. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1269-G1279. [PubMed] |
| 25. | Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, Flavell RA, Mehal WZ. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 321] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 26. | Higuchi S, Kobayashi M, Yoshikawa Y, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. IL-4 mediates dicloxacillin-induced liver injury in mice. Toxicol Lett. 2011;200:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Yano A, Higuchi S, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. Involvement of immune-related factors in diclofenac-induced acute liver injury in mice. Toxicology. 2012;293:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Fredriksson L, Herpers B, Benedetti G, Matadin Q, Puigvert JC, de Bont H, Dragovic S, Vermeulen NP, Commandeur JN, Danen E. Diclofenac inhibits tumor necrosis factor-α-induced nuclear factor-κB activation causing synergistic hepatocyte apoptosis. Hepatology. 2011;53:2027-2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 29. | Aithal GP, Ramsay L, Daly AK, Sonchit N, Leathart JB, Alexander G, Kenna JG, Caldwell J, Day CP. Hepatic adducts, circulating antibodies, and cytokine polymorphisms in patients with diclofenac hepatotoxicity. Hepatology. 2004;39:1430-1440. [PubMed] |
| 30. | Obermayer-Straub P, Manns MP. Immunological mechanisms in liver injury. In Drug-induced liver disease, 1st ed. New York: Marcel Dekker 2003; 125-149. |
| 31. | James LP, Simpson PM, Farrar HC, Kearns GL, Wasserman GS, Blumer JL, Reed MD, Sullivan JE, Hinson JA. Cytokines and toxicity in acetaminophen overdose. J Clin Pharmacol. 2005;45:1165-1171. [PubMed] |
| 32. | Berry PA, Antoniades CG, Hussain MJ, McPhail MJ, Bernal W, Vergani D, Wendon JA. Admission levels and early changes in serum interleukin-10 are predictive of poor outcome in acute liver failure and decompensated cirrhosis. Liver Int. 2010;30:733-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Li J, Zhu X, Liu F, Cai P, Sanders C, Lee WM, Uetrecht J. Cytokine and autoantibody patterns in acute liver failure. J Immunotoxicol. 2010;7:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Adams DH, Ju C, Ramaiah SK, Uetrecht J, Jaeschke H. Mechanisms of immune-mediated liver injury. Toxicol Sci. 2010;115:307-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 226] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 35. | Kakisaka K, Takikawa Y. Elevation of serum cytokines preceding elevation of liver enzymes in a case of drug-induced liver injury. Hepatol Res. 2013;44:E284-E289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Murata H, Shimizu Y, Okada K, Higuchi K, Watanabe A. Detection and analysis of intracytoplasmic cytokines in peripheral blood mononuclear cells in patients with drug-induced liver injury. J Hepatol. 2003;38:573-582. [PubMed] |
| 37. | Pachkoria K, Lucena MI, Crespo E, Ruiz-Cabello F, Lopez-Ortega S, Fernandez MA, Romero-Gomez M, Madrazo A, Durán JA, de Dios AM. Analysis of IL-10, IL-4 and TNF-alpha polymorphisms in drug-induced liver injury (DILI) and its outcome. J Hepatol. 2008;49:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Liang X, Zhang J, Zhu Y, Lu Y, Zhou X, Wang Z, Yu J, Yan Y, Di L, Che L. Specific genetic polymorphisms of IL10-592 AA and IL10-819 TT genotypes lead to the key role for inducing docetaxel-induced liver injury in breast cancer patients. Clin Transl Oncol. 2013;15:331-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Vergani D, Mieli-Vergani G, Alberti A, Neuberger J, Eddleston AL, Davis M, Williams R. Antibodies to the surface of halothane-altered rabbit hepatocytes in patients with severe halothane-associated hepatitis. N Engl J Med. 1980;303:66-71. [PubMed] |
| 40. | Bourdi M, Tinel M, Beaune PH, Pessayre D. Interactions of dihydralazine with cytochromes P4501A: a possible explanation for the appearance of anti-cytochrome P4501A2 autoantibodies. Mol Pharmacol. 1994;45:1287-1295. [PubMed] |
| 41. | Lecoeur S, André C, Beaune PH. Tienilic acid-induced autoimmune hepatitis: anti-liver and-kidney microsomal type 2 autoantibodies recognize a three-site conformational epitope on cytochrome P4502C9. Mol Pharmacol. 1996;50:326-333. [PubMed] |
| 42. | Endo S, Toyoda Y, Fukami T, Nakajima M, Yokoi T. Stimulation of human monocytic THP-1 cells by metabolic activation of hepatotoxic drugs. Drug Metab Pharmacokinet. 2012;27:621-630. [PubMed] |
| 43. | Hautekeete ML, Horsmans Y, Van Waeyenberge C, Demanet C, Henrion J, Verbist L, Brenard R, Sempoux C, Michielsen PP, Yap PS. HLA association of amoxicillin-clavulanate--induced hepatitis. Gastroenterology. 1999;117:1181-1186. [PubMed] |
| 44. | O'Donohue J, Oien KA, Donaldson P, Underhill J, Clare M, MacSween RN, Mills PR. Co-amoxiclav jaundice: clinical and histological features and HLA class II association. Gut. 2000;47:717-720. [PubMed] |
| 45. | Donaldson PT, Daly AK, Henderson J, Graham J, Pirmohamed M, Bernal W, Day CP, Aithal GP. Human leucocyte antigen class II genotype in susceptibility and resistance to co-amoxiclav-induced liver injury. J Hepatol. 2010;53:1049-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 46. | Andrade RJ, Lucena MI, Alonso A, García-Cortes M, García-Ruiz E, Benitez R, Fernández MC, Pelaez G, Romero M, Corpas R. HLA class II genotype influences the type of liver injury in drug-induced idiosyncratic liver disease. Hepatology. 2004;39:1603-1612. [PubMed] |
| 47. | Lucena MI, Molokhia M, Shen Y, Urban TJ, Aithal GP, Andrade RJ, Day CP, Ruiz-Cabello F, Donaldson PT, Stephens C. Susceptibility to amoxicillin-clavulanate-induced liver injury is influenced by multiple HLA class I and II alleles. Gastroenterology. 2011;141:338-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 408] [Cited by in RCA: 360] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 48. | Daly AK, Donaldson PT, Bhatnagar P, Shen Y, Pe’er I, Floratos A, Daly MJ, Goldstein DB, John S, Nelson MR. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet. 2009;41:816-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 799] [Cited by in RCA: 746] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 49. | Monshi MM, Faulkner L, Gibson A, Jenkins RE, Farrell J, Earnshaw CJ, Alfirevic A, Cederbrant K, Daly AK, French N. Human leukocyte antigen (HLA)-B*57: 01-restricted activation of drug-specific T cells provides the immunological basis for flucloxacillin-induced liver injury. Hepatology. 2013;57:727-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 50. | Wuillemin N, Adam J, Fontana S, Krähenbühl S, Pichler WJ, Yerly D. HLA haplotype determines hapten or p-i T cell reactivity to flucloxacillin. J Immunol. 2013;190:4956-4964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 51. | Pichler WJ. The p-i Concept: Pharmacological Interaction of Drugs With Immune Receptors. World Allergy Organ J. 2008;1:96-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 52. | Pichler WJ, Naisbitt DJ, Park BK. Immune pathomechanism of drug hypersensitivity reactions. J Allergy Clin Immunol. 2011;127:S74-S81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 53. | Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245. [PubMed] |
| 54. | Maria VA, Victorino RM. Development and validation of a clinical scale for the diagnosis of drug-induced hepatitis. Hepatology. 1997;26:664-669. [PubMed] |
| 55. | Kaplowitz N. Causality assessment versus guilt-by-association in drug hepatotoxicity. Hepatology. 2001;33:308-310. [PubMed] |
| 56. | Watanabe M, Shibuya A. Validity study of a new diagnostic scale for drug-induced liver injury in Japan-comparison with two previous scales. Hepatol Res. 2004;30:148-154. [PubMed] |
| 57. | Teschke R, Frenzel C, Schulze J, Eickhoff A. Herbal hepatotoxicity: challenges and pitfalls of causality assessment methods. World J Gastroenterol. 2013;19:2864-2882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 58. | Teschke R, Wolff A, Frenzel C, Schwarzenboeck A, Schulze J, Eickhoff A. Drug and herb induced liver injury: Council for International Organizations of Medical Sciences scale for causality assessment. World J Hepatol. 2014;6:17-32. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 59. | Burns JJ, Zhao L, Taylor EW, Spelman K. The influence of traditional herbal formulas on cytokine activity. Toxicology. 2010;278:140-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 60. | Liang Q, Wu Q, Jiang J, Duan J, Wang C, Smith MD, Lu H, Wang Q, Nagarkatti P, Fan D. Characterization of sparstolonin B, a Chinese herb-derived compound, as a selective Toll-like receptor antagonist with potent anti-inflammatory properties. J Biol Chem. 2011;286:26470-26479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 61. | Reid-Adam J, Yang N, Song Y, Cravedi P, Li XM, Heeger P. Immunosuppressive effects of the traditional Chinese herb Qu Mai on human alloreactive T cells. Am J Transplant. 2013;13:1159-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 62. | Kim ME, Kim HK, Park HY, Kim DH, Chung HY, Lee JS. Baicalin from Scutellaria baicalensis impairs Th1 polarization through inhibition of dendritic cell maturation. J Pharmacol Sci. 2013;121:148-156. [PubMed] |
| 63. | Takikawa H, Takamori Y, Kumagi T, Onji M, Watanabe M, Shibuya A, Hisamochi A, Kumashiro R, Ito T, Mitsumoto Y. Assessment of 287 Japanese cases of drug induced liver injury by the diagnostic scale of the International Consensus Meeting. Hepatol Res. 2003;27:192-195. [PubMed] |
| 64. | Usui K, Oda Y, Kubota R, Negishi K, Uno K, Tsunematsu S, Kumagai N, Komiyama T. Clinical application of the leukocyte migration test and new diagnostic criteria for identifying causative agents in patients with drug-induced liver injury. Hepatogastroenterology. 2007;54:1752-1757. [PubMed] |
| 65. | Polson J, Lee WM. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41:1179-1197. [PubMed] |
| 66. | Bohan TP, Helton E, McDonald I, König S, Gazitt S, Sugimoto T, Scheffner D, Cusmano L, Li S, Koch G. Effect of L-carnitine treatment for valproate-induced hepatotoxicity. Neurology. 2001;56:1405-1409. [PubMed] |
| 67. | Giannattasio A, D’Ambrosi M, Volpicelli M, Iorio R. Steroid therapy for a case of severe drug-induced cholestasis. Ann Pharmacother. 2006;40:1196-1199. [PubMed] |
| 68. | Wree A, Dechêne A, Herzer K, Hilgard P, Syn WK, Gerken G, Canbay A. Steroid and ursodesoxycholic Acid combination therapy in severe drug-induced liver injury. Digestion. 2011;84:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
P- Reviewer: Han T, He JY, Suk KT S- Editor: Wen LL L- Editor: A E- Editor: Lu YJ