Published online Jul 27, 2014. doi: 10.5411/wji.v4.i2.98
Revised: June 12, 2014
Accepted: June 27, 2014
Published online: July 27, 2014
Processing time: 141 Days and 17.6 Hours
Cancer vaccines to date have not broadly achieved a significant impact on the overall survival of patients. The negative effect on the immune system of the tumor itself and conventional anti-tumor treatments such as chemotherapy is, undoubtedly, a key reason for these disappointing results. Myeloid-derived suppressor cells (MDSCs) are considered a central node of the immunosuppressive network associated with tumors. These cells inhibit the effector function of natural killer and CD8+ T cells, expand regulatory T cells and can differentiate into tumor-associated macrophages within the tumor microenvironment. Thus, overcoming the suppressive effects of MDSCs is likely to be critical for cancer immunotherapy to generate effective anti-tumor immune responses. However, the capacity of cancer vaccines and particularly their adjuvants to overcome this inhibitory population has not been well characterized. Very small size proteoliposomes (VSSP) is a nanoparticulated adjuvant specifically designed to be formulated with vaccines used in the treatment of immunocompromised patients. This adjuvant contains immunostimulatory bacterial signals together with GM3 ganglioside. VSSP promotes dendritic cell maturation, antigen cross-presentation to CD8+ T cells, Th1 polarization, and enhances CD8+ T cell response in tumor-free mice. Currently, four cancer vaccines using VSSP as the adjuvant are in Phase I and II clinical trials. In this review, we summarize our work characterizing the unique ability of VSSP to stimulate antigen-specific CD8+ T cell responses in two immunocompromised scenarios; in tumor-bearing mice and during chemotherapy-induced leukopenia. Particular emphasis has been placed on the interaction of these nanoparticles with MDSCs, as well as comparison with other cancer vaccine adjuvants currently in preclinical or clinical studies.
Core tip: Very small size proteoliposomes (VSSP) is a nanoparticulated adjuvant being used in the formulation of several cancer vaccines that are currently in clinical trials. In this review we summarize the unique ability of VSSP to stimulate antigen-specific CD8+ T cell responses in tumor-bearing mice and in mice with chemotherapy-induced leukopenia, both immunosuppressive scenarios frequently found in cancer patients. As a possible mechanism of this efficacy, we have focused on the modulation of myeloid-derived suppressor cells (MDSCs) by these nanoparticles, in the context of the current knowledge about the interaction of cancer vaccine adjuvants with MDSCs.
- Citation: Fernández A, Oliver L, Alvarez R, Fernández LE, Mesa C. GM3-containing nanoparticles in immunosuppressed hosts: Effect on myeloid-derived suppressor cells. World J Immunol 2014; 4(2): 98-106
- URL: https://www.wjgnet.com/2219-2824/full/v4/i2/98.htm
- DOI: https://dx.doi.org/10.5411/wji.v4.i2.98
The central importance and complexity of the interactions between tumors and the immune system has only recently been recognized, with rapidly expanding investigations in the last decade. Tumors are not only shaped by the immune system[1,2] but actively induce impairment of antigen-presenting cells (APCs) as well as effector T lymphocytes[3,4], contributing significantly to both tumor progression and metastasis. One of the key cellular mediators of tumor-induced immunosuppression are myeloid-derived suppressor cells (MDSCs), which not only are the manifestation of the myeloid differentiation block that causes loss of mature APCs, but also actively and directly inhibit the lytic activity of both CD8+ T cells[5,6] and NK cells[7].
MDSCs are currently thought of as a heterogeneous population of immature myeloid cells with suppressive activity. In mice these cells are routinely identified by the co-expression of CD11b and Gr1 markers. More recently, two subpopulations of MDSCs have been identified with different phenotypes and mechanisms of suppression: monocytic (Mo-MDSCs) and granulocytic (G-MDSCs)[8-11]. In tumor-bearing mice, as well as in cancer patients, the G-MDSCs constitute 70%-80% of overall MDSCs, whereas Mo-MDSCs represent only 20%-30%[11-14]. Mo-MDSCs (CD11b+Ly6ChiLy6G-) are highly immunosuppressive and exert their suppression via antigen-independent mechanisms[15-18]. In comparison, G-MDSCs (CD11b+Ly6CloLy6G+) are moderately immunosuppressive, release reactive oxygen species (ROS) and require antigen-specific interaction with T cells to induce tolerance[9,11,19,20]. Several mechanisms of MDSC-mediated suppression have been described and are extensively detailed in other reviews[3,21]. Among these, the depletion of L-arginine, production of nitric oxide (NO) and generation of ROS/reactive nitrogen species have been linked to the overexpression of arginase 1 (ARG1), inducible nitric oxide synthase (NOS2) and NADPH oxidase[3,13,22]. MDSCs are also able to expand regulatory T cells (Tregs) populations[23,24] and can differentiate into tumor-associated macrophages within the tumor microenvironment[25,26]-both regulatory populations that play an important role in tumor-induced immunosuppression. Recent findings suggest that MDSCs can also facilitate tumor-progression and metastasis by increasing angiogenesis[27,28], via secretion of matrix metallopeptidases[29,30] and by aiding in the formation of the metastatic niche[27,31].
Given the pro-tumor importance of MDSCs, many efforts have been undertaken to find drugs capable of reducing the number of circulating MDSCs, abrogate MDSCs suppressive function or differentiate these cells into mature APCs. For instance, it has been demonstrated that 25-hydroxy vitamin D3 and all-trans retinoic acid reduce the frequency of MDSCs by inducing their differentiation towards HLA-DR+ cells and dendritic cells (DCs), respectively, in patients with advance head and neck squamous cell carcinoma and metastatic renal cell carcinoma (RCC)[32-34]. Sunitinib, a pan-receptor tyrosine kinase inhibitor, and chemotherapeutic agents (taxanes, gemcitabine and 5-fluorouracil) also decrease circulating MDSCs in patients with RCC, melanoma, pancreatic and esophagogastric cancer[35,36]. Finally, the phosphodiesterase-5 inhibitor sildenafil diminishes the suppressive function of human MDSCs[37].
Although the pharmacological modulation of MDSCs represents a potentially important strategy for cancer treatment, none of these drugs detailed above have thus far improved the clinical outcome in cancer patients. These data suggest that inhibiting MDSCs alone (unlike the T cell checkpoint inhibitors) is not sufficient to achieve an effective anti-tumor response, and that combination with strategies to specifically activate immune responses against the cancer are needed. However, most cancer vaccines have not shown significant objective responses in clinical trials. But, the unimpressive clinical impact of active immunotherapy in cancer patients may be in turn tied to the immunosuppressive environment generated by tumors[3,4,21] as well as the aggressive chemotherapeutic treatments used in patients, which frequently induce leukopenia[38-40]. Thus, the combination of cancer vaccines with agents interfering with MDSCs number/function may be an effective approach to generate fully functional tumor-specific immune effectors. Even more desirable would be to find agents that are capable of simultaneously activating tumor-specific effector cells, inhibiting the suppressive function of MDSCs, and diminishing leukopenic period after chemotherapy. As detailed below, these are all properties of the VSSP adjuvant.
Adjuvants are critical but largely unappreciated components of vaccine formulations, necessary to potentiate the immune response specific for the nominal antigen. This is particularly important in cancer, where the vaccine antigen is often a self protein for which self-tolerance needs to be broken. In recent years the interaction of adjuvants with regulatory cells, and particularly MDSCs, have begun to be study[41-45]. This field is still in its infancy however, and there is only strong evidence for the modulation of tumor-induced MDSCs by synthetic oligodeoxynucleotides containing unmethylated CpG motifs (CpG)[44], formalin-inactivated Herpes Simplex Virus[43] and VSSP[42], while indirect evidence suggests that other adjuvants may expand MDSCs once inoculated in the hosts. Therefore, the selection of suitable adjuvants for cancer vaccines is a very complex matter, and needs to be based in the ability to overcome the immunosuppression generated by tumors and chemotherapy. In this review we summarize the immunomodulatory properties of VSSP, a novel adjuvant for cancer immunotherapy.
VSSP is a nanoparticulated adjuvant obtained through the hydrophobic incorporation of the GM3 ganglioside into outer membrane vesicles (OMPs) from Neisseria meningitidis[46]. It has been shown that VSSP contains TLR4 and TLR2 ligands, which play an important role in the immunomodulatory properties of this compound[47,48]. Immunization of mice, monkeys and humans with VSSP generated IgM and IgG antibodies specific for both GM3 and OMPs[46,49]. This adjuvant also induced DC maturation, as evidenced by the increased expression of MHCII and CD40, CD80 and CD86 costimulatory molecules (Table 1)[47]. Additionally, VSSP-treated DCs secreted inflammatory cytokines such as IL-12p40/70 and IL-6[47]. DCs from healthy donors treated in vitro with VSSP produced not only higher levels of IL-6 but also decreased amount of IL-10, in comparison to lipopolysaccharide [LPS, the prototypic TLR4 agonist (Table 1)][48]. Experiments with antigen-specific transgenic T cells demonstrated that VSSP-treated DCs induced a Th1 phenotype in stimulated naïve CD4+ T cells[47]. Furthermore, VSSP expanded CD8+ T cells specific for the co-injected antigen and promoted an effective in vivo cytotoxic T lymphocytes (CTL) response[50]. In the latter case, CD8+ T cell activation was mediated by the cross-presentation of exogenous antigens and did not require help from CD4+ T cells (Table 1)[50].
| Immune cell | Effect of VSSP | Ref. |
| DCs | Increases costimulation and MHCII expression | [47] |
| Enhances production of IL-12, IL-6, IL-18, IL-1β and reduces secretion of IL-10 | [47,48] | |
| Induces Th1-polarizing capacity | [47] | |
| Facilitates cross-presentation of protein antigens | [50] | |
| MDSCs | Expands poorly suppressive MDSCs | [42] |
| Reduces the suppressive function of tumor-induced MDSCs | [42] | |
| Impairs migration of tumor-induced MDSCs towards the tumor microenvironment | [42] | |
| Promotes differentiation of tumor-induced MDSCs into mature DCs | [42,59] | |
| Reduces the suppressive function of MDSCs generated during chemotherapy-induced leukopenia | [62] | |
| CD4+ T cells | Induces Th1 polarization | [47] |
| CTL | Potentiates CTL responses in healthy mice. Primary expansion independent of CD4+ T cell help | [50] |
| Generates similar CTL responses in tumor-free and tumor-bearing mice | [42] | |
| Increases CD8+ T cell counts, with memory phenotype, and protects CTL response in leukopenic mice | [62] |
More recently, we have found that VSSP treatment of naive mice (without a vaccine antigen) significantly increased the frequency of splenic CD11b+Gr1+ cells[42]. However, these CD11b+Gr1+ cells were poorly suppressive on both antigen-specific and allogeneic CTL assays (Table 1). The residual suppressive capacity of VSSP-derived MDSCs depended on NOS but not ARG, which was associated with a significant increase of NOS3 enzyme. Although VSSP contains TLR2 and TLR4 ligands, the interaction of these particles with the immune system appears to be more complex than can be explained by just TLR activation. For example, OMPs containing the same TLR ligands induced a significantly lower expansion of CD11b+Gr1+ cells than did VSSP, indicating that the presence of the GM3 ganglioside is also relevant for the immunomodulatory properties of this compound.
VSSP-induced expansion of MDSC numbers is not entirely unexpected, as MDSCs have also been reported to accumulate in mice treated with granulocyte and macrophage colony-stimulating factor (GM-CSF)[51,52], LPS[41], CpG[53], complete Freund’s adjuvant[45] and Bacillus Calmette-Guérin from Mycobacterium bovis[54]. Similar MDSCs expansion has been described for other conditions involving major inflammatory responses, such as superantigen vaccination[55], polymicrobial sepsis[56], after burn[57] and traumatic injuries[58]. These findings are consistent with a physiological role of MDSCs as a counter-balancing mechanism to inflammation, preventing collateral damage to the tissue caused by activated T cells once the “dangerous” antigen has been eliminated.
The effect of VSSP on the phenotype, suppressive function and differentiation status of tumor-induced MDSCs has been evaluated in mice bearing C26GM, EL4, EG.7 and MCA203 tumors (Table 1)[42]. Splenic MDSCs derived from VSSP-treated tumor-bearing mice (MDSCs-T+V) contained a higher frequency of CD11b+Gr1hi and Ly6CloLy6G+ G-MDSCs than untreated tumor-bearing counterparts (MDSCs-T). In addition, IL-4Rα is downregulated on MDSCs-T+V, and these cells showed an increase of the homing molecule CD62L. Consistent with our in vitro studies, the suppressive function of tumor-induced splenic MDSCs was significantly reduced when VSSP is given in vivo. Several different findings support this effect of VSSP. First, MDSCs-T+V were unable to suppress the hemagglutinin (HA) peptide-specific proliferation of CD8+ T cells from CL4 TCR transgenic mice, in the same experimental setting where equal number of MDSCs-T were significantly inhibitory. In vitro51Cr release CTL assays demonstrated that, as expected, MDSCs-T completely suppressed both antigen-specific and alloantigen-specific lytic activity of CD8+ T cells. In contrast, MDSCs-T+V isolated from EL4 and C26GM tumor-bearing mice only marginally affected the generation of the CTL.
The effect of VSSP on MDSCs in vivo was further examined in adoptive transfer experiments. In the first approach, MDSCs-T and MDSC-T+V were adoptively transferred into CD45.1+ B6 congenic mice, which previously received the transference of ovalbumin (OVA)-specific CD8+ T cells from OTI transgenic mice, and vaccinated with the immunodominant OVA257-264 (SIINFEKL) peptide emulsified in incomplete Freund’s adjuvant (IFA). Similar frequencies of IFN-γ+ antigen-specific CD8+ T cells were found in recipient mice transferred with MDSCs-T+V compared to control mice receiving no MDSCs, whereas transfer of MDSCs-T significantly impaired the activation of OTI lymphocytes. Additional experiments were performed to compare VSSP with other adjuvants or well-established vaccination systems. On this regard, we found that VSSP-based vaccines are more efficient than vaccination with DCs or vaccines employing the adjuvant polyinosinic:polycytidylic acid (polyI:C) in activating antigen-specific CTL responses in the presence of MDSCs-T. In fact, vaccination of BALB/c mice, which had been adoptively transferred with both congenic antigen-specific CD8+ T cells and MDSCs-T, with HA peptide in VSSP adjuvant prevented the MDSCs-T-mediated suppression of CD8+ T cell responses that was observed in mice vaccinated with HA-pulsed DCs. Also congenic OTI CD8+ T cells transferred to EG.7 tumor-bearing mice produce IFN-γ in response to VSSP admixed with SIINFEKL peptide- but not to a vaccine consisting of SIINKEKL-pulsed DCs. Importantly, the OVA-specific in vivo CTL response generated in mice with EL4 tumors by the administration of OVA/VSSP was comparable to that observed in tumor-free mice, whereas vaccination with OVA/polyI:C was unable to overcome the tumor-induced impairment of the CTL response.
In addition to TCR transgenic T cell responses to a model antigen, we have found that VSSP blunts MDSC-mediated suppression of endogenous T cell responses to native tumor antigen, by measuring the inhibition of tumor-specific CD8+ T cells by MDSCs in an ELISPOT assay. CD8+ T cells isolated from MCA203 tumor-bearing mice did not release IFN-γ when stimulated with MCA203 tumor cells, irrespective of the presence of MDSCs. In contrast, a significant frequency of CD8+ T cells derived from VSSP-treated tumor-bearing mice were activated by tumor cells and produced IFN-γ, even when MDSCs-T+V were added to the culture. Importantly, MDSCs-T maintained their ability to suppress tumor-specific CTL in this experiment.
Within the tumor microenvironment itself, VSSP treatment did not change the phenotype and functional capacity of CD11b+ sorted MDSCs. However, adoptively transferred congenic MDSCs-T had a reduced ability to infiltrate tumors in EL4 tumor-bearing mice treated with VSSP. More importantly, in these VSSP-treated mice, tumor-infiltrating transferred MDSCs-T were more differentiated into CD11c+MHCII+CD11b- phenotype characteristic of DCs, and did not differentiate towards MHCII+F4/80+ macrophages. A similar differentiation pattern was observed in vivo in the spleen and lymph nodes from VSSP-inoculated tumor-bearing mice. In a more recent work, it was demonstrated that in vitro treatment with VSSP of tumor-induced MDSCs was sufficient to differentiate this immature population towards phenotypically mature DCs and, more importantly, causes the loss of their suppressive function[59]. Since VSSP contains a TLR4 ligand, a comparison with LPS was done in the same experimental setting. Interestingly, incubation with LPS fails to differentiate tumor-induced MDSCs into DCs and, consequently, these cells retain their inhibitory activity[59]. In agreement with these results, Greifenberg et al[60] have shown that incubation of bone marrow (BM)-derived MDSCs with the combination of LPS and IFN-γ increases NO secretion, enhancing the suppressive activity of these MDSCs and impairing their maturation into DCs. These findings further suggest that VSSP’s effect on MDSCs is not a shared characteristic of all TLR4 agonists, but is a unique property of VSSP. Other authors have reported that TLR4 signaling is involved in the promotion of tumor growth associated with the recruitment of G-MDSCs, through the interaction with S100A9 protein[61]. VSSP also expands G-MDSCs subpopulation in tumor-bearing mice, however it also potentiates CTL responses and anti-tumor activity on those mice[42]. Therefore, the complexity of signals in the structure of VSSP (TLR2 agonist, GM3 ganglioside, etc.) likely makes these particles distinct from single TLR4 agonists. In fact, VSSP can induce activation of BM-derived DCs obtained from LPS hyporesponsive mice (C3H/HeJ)[47].
It has been shown in the literature that other adjuvants can also reduce the suppressive function of tumor-recruited MDSCs. For instance, intratumoral injection of CpG reduces the suppressive function of Mo-MDSCs and induces their differentiation towards macrophages with tumoricidal capability[44]. However, CpG does not modify G-MDSCs, and intratumoral injections in patients may be difficult to impossible. Formalin-inactivated Herpes Simplex Virus also decreases the suppressive function of MDSCs-T, but whether this adjuvant is able to differentiate MDSCs has not been addressed[43].
The ability of VSSP to rescue the number and functionality of relevant immune populations on mice undergoing chemotherapy-induced leukopenia has been also tested (Table 1)[62]. The widely used chemotherapy agent cyclophosphamide (CY) was used to induce the leukopenic setting for these studies. In this model, VSSP accelerated the recovery of specific leukocytes population when administered in the early stages of leukopenia. Splenic CD4+ and CD8+ T cells (with a memory CD4+CD44hi and CD8+CD44hi phenotype) and CD11c+CD11b+ DCs were some of the populations most enhanced by VSSP in leukopenic mice. Interestingly, MDSCs were also significantly expanded. However, similar to what was seen in the tumor-mediated immunosuppression setting, MDSCs from leukopenic mice treated with VSSP showed a reduced capacity to suppress T cell responses, compared to CY-induced MDSCs (Table 1). Importantly, in the same experimental setting, we found that polyI:C treatment induced none of the effects observed with VSSP inoculation.
The ability of VSSP to activate antigen-specific CD8+ T cells was also tested in leukopenic mice. In this immunocompromised scenario, vaccination with a single dose of OVA/VSSP, at the time point corresponding to the lowest CD8 counts, induced significant antigen-specific CTL responses. In comparison, vaccination with three doses of OVA/polyI:C was not capable of inducing antigen-specific effector CD8+ T cell activation. Furthermore, VSSP treatment of OVA/polyI:C vaccinated animals restored the dampened CTL responses in polyI:C-treated leukopenic mice, indicating that VSSP can function as an immunomodulator as well. This effect could be associated to the capacity of VSSP, different from polyI:C, to accelerate the recovery of effector CD8+ memory T cells and to induce the expansion of DCs and less suppressive MDSCs.
Granulocyte colony-stimulating factor (G-CSF) is the standard growth factor used in the clinic to revert chemotherapy-induced leukopenia, but also has been reported to be a tumor-derived factor that induces MDSCs generation and recruitment[63]. Therefore we assessed whether treatment with recombinant G-CSF could restore the in vivo CTL response barely induced by OVA/polyI:C vaccine in CY-treated mice[62]. Administration of G-CSF has no impact in the impaired antigen-specific CTL response, possibly due to the expansion of MDSCs but also via G-CSF-induced Th2 responses[64] and the resulting differentiation of Tregs that may impair effector T lymphocyte proliferation[65]. However, when VSSP was given with G-CSF, the ability of VSSP to restore CD8+ T cell function was not affected, which opens the possibility for their concomitant use in the clinic. Moreover, the functionality of MDSCs recruited in these experiments was additionally evaluated. As expected from previous reports, our data also demonstrated that, in leukopenic mice treated with G-CSF, the induced MDSCs were highly suppressive. Importantly, the concomitant treatment with VSSP dampened the inhibitory function of MDSCs expanded after G-CSF injection. To our knowledge, no other adjuvant has been tested in this immunosuppressive leukopenic scenario induced by chemotherapy.
Several pre-clinical studies support the anti-tumor efficacy of VSSP, whether used alone or in combination with other tumor-associated antigens different from the GM3 ganglioside. The combination of surgery and VSSP alone prevented tumor recurrence and improved survival in melanoma B16F10 tumor-bearing mice[66]. In a different tumor model, treatment of mice bearing MCA203 tumors with three doses of VSSP was sufficient to significantly delay tumor growth[42]. Of interest, GM3 ganglioside, an important component of VSSP, is highly expressed on both melanoma B16F10 and MCA203 sarcoma. Particularly in MCA203 tumor-bearing mice, treatment with VSSP alone caused a significant increase in the frequency of classical IFN-γ-producing CD8+ T cells specific for MCA203 antigens, suggesting an antigen-spreading likely induced by the initial response against the GM3 ganglioside[42]. Moreover, VSSP-adjuvanted vaccines (both peptides and whole proteins) have shown anti-tumor activity. For instance, a vaccine containing the extracellular domain of murine epidermal growth factor receptor (EGFR) and VSSP has a potent anti-metastatic effect in the Lewis lung carcinoma model[67]. In a mouse model of cervical cancer induced by Human Papilloma Virus (HPV), the immunization with an E7-derived CTL peptide from HPV 16 mixed with VSSP induced regression of established tumors[68]. Therapeutic vaccination of EG.7 tumor-bearing mice with OVA or SIINFEKL peptide adjuvated in VSSP, but not SIINFEKL emulsified in IFA, caused a significant reduction of tumor growth[42]. However, VSSP administration alone to EL4 and C26GM tumor-bearing mice, with the same schedule associated with the inhibition of MDSCs suppressive function, does not delay tumor growth. One possible explanation for the absence of an anti-tumor effect of VSSP alone in these models is the lack of a tumor-associated antigen during treatment, and consequently, the absence of antigen-specific CD8+ T cell activation. In fact, EL4 tumors express low levels of GM3 whereas an inappropriate exposure of this ganglioside on the surface of C26GM tumor cells has been observed[42]. Altogether, these data strongly suggest that the best induction of anti-tumor responses requires combining the abrogation of tumor-induced MDSCs with a specific stimulation of T lymphocytes, which can be successfully done by mixing a proper tumor-associated antigen with VSSP.
Finally, four therapeutic cancer vaccines employing VSSP as adjuvant are in clinical trials. An EGFR-based vaccine[67] is currently in Phase I clinical trials. A Phase I clinical trial in patients with advanced solid tumors using a formulation of a mutated vascular endothelial growth factor[69] and VSSP has been recently completed. In this trial, the most common adverse events were Grade 1 pain and erythema at injection site and Grade 1 fever [70]. Additionally, a gonadotropin releasing hormone-based vaccine[71] and a HPV-derived peptidic vaccine[72] are currently in Phase II trials in prostate cancer patients and women with high-grade cervical intraepithelial neoplasia, respectively. Both vaccines have previously shown to be safe and immunogenic. The most frequent adverse event in patients receiving the HPV vaccine was local pain at the vaccination site, whereas fever, tremors and cramps were seen in few cases, but none exceeded Grade 1[72]. Another Phase I trial using VSSP alone in metastatic melanoma patients demonstrated the safety of this preparation even in the presence of Montanide ISA 51, with toxicity consisting of local reaction at the site of injection and mild fever and chills[49]. In this trial both humoral and cellular responses were induced by the VSSP treatment. Additionally, an ongoing physician-lead trial is evaluating the modulation of tumor-induced MDSCs by VSSP treatment alone in RCC patients.
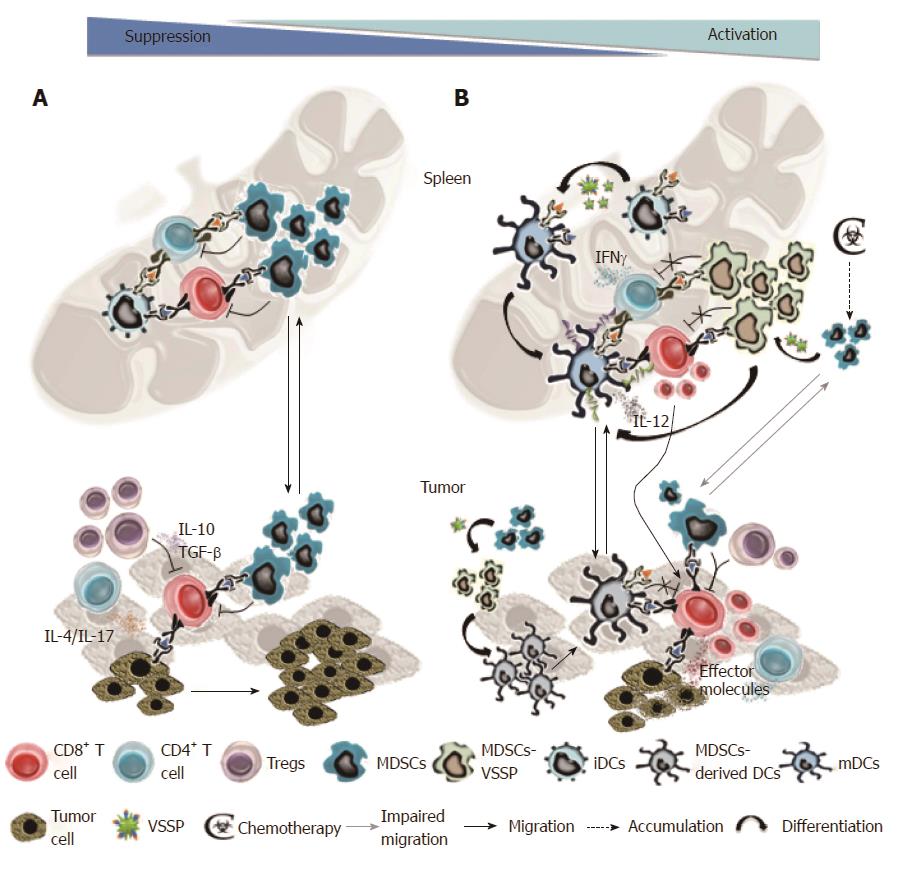
The immunomodulatory and anti-tumor properties of VSSP are summarized in Figure 1. In tumor-bearing mice, activation and effector function of tumor-specific CD8+ and CD4+ T cells are impaired, among other factors, due to ineffective antigenic presentation by immature DCs and through multiple suppressive mechanisms exerted by MDSCs. Experimental evidence suggest that VSSP-based vaccines could promote cross-presentation of the formulated antigen by DCs, drive the full maturation of the DCs and, simultaneously, inhibit tumor-induced MDSCs immunosuppression. In addition, VSSP could induce Th1 polarization on tumor-specific CD4+ T cells. All these effects may significantly enhance the proliferation and activation of tumor-specific CD8+ T cells, thus eliciting robust anti-tumor immunity. VSSP also diminishes the migration of MDSCs towards the tumor site and promotes their differentiation into DCs. Tumor-infiltrating MDSCs have been related with the recruitment and expansion of Tregs[23,24,73], in addition to an impaired migration of effector T cells[74]. Thus, within the tumor microenvironment, VSSP treatment may tip the the balance between functional T cells vs suppressive MDSCs/Tregs to favor the immune effectors that ultimately lead to an anti-tumor response. The higher frequency of DCs could additionally contribute to activate T cells specific for other tumor antigens by capturing, processing and presenting the proteins released by dying tumor cells. In chemotherapy-treated individuals, VSSP also accelerates the homeostatic recovery of CD8+ T cells and DCs, whereas the suppressive function of chemotherapy-induced MDSCs is abrogated. Altogether, these elements support the use of VSSP as a novel adjuvant or immunomodulator for active immunotherapy and, particularly, for the combination with chemotherapy in the clinical setting.
We are grateful to Dr. Kelvin P (Roswell Park Cancer Institute, Buffalo, United States) for the language correction and critical review of the scientific content of this manuscript.
| 1. | Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4947] [Cited by in RCA: 4672] [Article Influence: 311.5] [Reference Citation Analysis (1)] |
| 3. | Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2369] [Cited by in RCA: 2948] [Article Influence: 210.6] [Reference Citation Analysis (0)] |
| 4. | Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13:739-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 981] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 5. | Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777-2790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 645] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 6. | Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828-835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 940] [Cited by in RCA: 910] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 7. | Liu C, Yu S, Kappes J, Wang J, Grizzle WE, Zinn KR, Zhang HG. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood. 2007;109:4336-4342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 266] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 8. | Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C, Geilich M, Winkels G, Traggiai E, Casati A. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol. 2010;40:22-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 449] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 9. | Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233-4244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 913] [Cited by in RCA: 996] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 10. | Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 521] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 11. | Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791-5802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1391] [Cited by in RCA: 1378] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 12. | Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182:5693-5701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 635] [Cited by in RCA: 626] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 13. | Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5480] [Cited by in RCA: 5468] [Article Influence: 321.6] [Reference Citation Analysis (1)] |
| 14. | Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61:4756-4760. [PubMed] |
| 15. | Cuervo H, Guerrero NA, Carbajosa S, Beschin A, De Baetselier P, Gironès N, Fresno M. Myeloid-derived suppressor cells infiltrate the heart in acute Trypanosoma cruzi infection. J Immunol. 2011;187:2656-2665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Lesokhin AM, Hohl TM, Kitano S, Cortez C, Hirschhorn-Cymerman D, Avogadri F, Rizzuto GA, Lazarus JJ, Pamer EG, Houghton AN. Monocytic CCR2(+) myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Res. 2012;72:876-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 315] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 17. | Nausch N, Galani IE, Schlecker E, Cerwenka A. Mononuclear myeloid-derived “suppressor” cells express RAE-1 and activate natural killer cells. Blood. 2008;112:4080-4089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Qin A, Cai W, Pan T, Wu K, Yang Q, Wang N, Liu Y, Yan D, Hu F, Guo P. Expansion of monocytic myeloid-derived suppressor cells dampens T cell function in HIV-1-seropositive individuals. J Virol. 2013;87:1477-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 141] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 19. | Choi J, Suh B, Ahn YO, Kim TM, Lee JO, Lee SH, Heo DS. CD15+/CD16low human granulocytes from terminal cancer patients: granulocytic myeloid-derived suppressor cells that have suppressive function. Tumour Biol. 2012;33:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol. 2010;185:2273-2284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 488] [Cited by in RCA: 489] [Article Influence: 30.6] [Reference Citation Analysis (7)] |
| 21. | Serafini P. Myeloid derived suppressor cells in physiological and pathological conditions: the good, the bad, and the ugly. Immunol Res. 2013;57:172-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 22. | Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1219] [Cited by in RCA: 1387] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 23. | Pan PY, Ma G, Weber KJ, Ozao-Choy J, Wang G, Yin B, Divino CM, Chen SH. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. 2010;70:99-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 369] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 24. | Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68:5439-5449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 549] [Cited by in RCA: 547] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 25. | Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439-2453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 757] [Cited by in RCA: 982] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 26. | Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, DeNardo DG, Coussens LM, Karin M, Goldrath AW, Johnson RS. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010;70:7465-7475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 542] [Cited by in RCA: 541] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 27. | Chioda M, Peranzoni E, Desantis G, Papalini F, Falisi E, Solito S, Mandruzzato S, Bronte V. Myeloid cell diversification and complexity: an old concept with new turns in oncology. Cancer Metastasis Rev. 2011;30:27-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 921] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 29. | Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegué E, Song H, Vandenberg S, Johnson RS, Werb Z. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 946] [Cited by in RCA: 905] [Article Influence: 50.3] [Reference Citation Analysis (11)] |
| 30. | Pahler JC, Tazzyman S, Erez N, Chen YY, Murdoch C, Nozawa H, Lewis CE, Hanahan D. Plasticity in tumor-promoting inflammation: impairment of macrophage recruitment evokes a compensatory neutrophil response. Neoplasia. 2008;10:329-340. [PubMed] |
| 31. | Yan HH, Pickup M, Pang Y, Gorska AE, Li Z, Chytil A, Geng Y, Gray JW, Moses HL, Yang L. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 2010;70:6139-6149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 316] [Cited by in RCA: 321] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 32. | Kusmartsev S, Su Z, Heiser A, Dannull J, Eruslanov E, Kübler H, Yancey D, Dahm P, Vieweg J. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2008;14:8270-8278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 226] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 33. | Lathers DM, Clark JI, Achille NJ, Young MR. Phase 1B study to improve immune responses in head and neck cancer patients using escalating doses of 25-hydroxyvitamin D3. Cancer Immunol Immunother. 2004;53:422-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, Lush RM, Antonia S, Gabrilovich DI. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66:9299-9307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 446] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 35. | Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 691] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 36. | Montero AJ, Diaz-Montero CM, Kyriakopoulos CE, Bronte V, Mandruzzato S. Myeloid-derived suppressor cells in cancer patients: a clinical perspective. J Immunother. 2012;35:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 37. | Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691-2702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 533] [Cited by in RCA: 614] [Article Influence: 30.7] [Reference Citation Analysis (8)] |
| 38. | Crawford J, Dale DC, Kuderer NM, Culakova E, Poniewierski MS, Wolff D, Lyman GH. Risk and timing of neutropenic events in adult cancer patients receiving chemotherapy: the results of a prospective nationwide study of oncology practice. J Natl Compr Canc Netw. 2008;6:109-118. [PubMed] |
| 39. | Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 518] [Article Influence: 22.5] [Reference Citation Analysis (1)] |
| 40. | Todryk S. A sense of tumour for the immune system. Immunology. 2002;107:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | De Wilde V, Van Rompaey N, Hill M, Lebrun JF, Lemaître P, Lhommé F, Kubjak C, Vokaer B, Oldenhove G, Charbonnier LM. Endotoxin-induced myeloid-derived suppressor cells inhibit alloimmune responses via heme oxygenase-1. Am J Transplant. 2009;9:2034-2047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 42. | Fernández A, Mesa C, Marigo I, Dolcetti L, Clavell M, Oliver L, Fernández LE, Bronte V. Inhibition of tumor-induced myeloid-derived suppressor cell function by a nanoparticulated adjuvant. J Immunol. 2011;186:264-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Ohkusu-Tsukada K, Ohta S, Kawakami Y, Toda M. Adjuvant effects of formalin-inactivated HSV through activation of dendritic cells and inactivation of myeloid-derived suppressor cells in cancer immunotherapy. Int J Cancer. 2011;128:119-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Shirota Y, Shirota H, Klinman DM. Intratumoral injection of CpG oligonucleotides induces the differentiation and reduces the immunosuppressive activity of myeloid-derived suppressor cells. J Immunol. 2012;188:1592-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 205] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 45. | Wang Z, Jiang J, Li Z, Zhang J, Wang H, Qin Z. A myeloid cell population induced by Freund adjuvant suppresses T-cell-mediated antitumor immunity. J Immunother. 2010;33:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Estevez F, Carr A, Solorzano L, Valiente O, Mesa C, Barroso O, Sierra GV, Fernandez LE. Enhancement of the immune response to poorly immunogenic gangliosides after incorporation into very small size proteoliposomes (VSSP). Vaccine. 1999;18:190-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Mesa C, De León J, Rigley K, Fernández LE. Very small size proteoliposomes derived from Neisseria meningitidis: an effective adjuvant for Th1 induction and dendritic cell activation. Vaccine. 2004;22:3045-3052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 48. | Venier C, Guthmann MD, Fernández LE, Fainboim L. Innate-immunity cytokines induced by very small size proteoliposomes, a Neisseria-derived immunological adjuvant. Clin Exp Immunol. 2007;147:379-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 49. | Guthmann MD, Bitton RJ, Carnero AJ, Gabri MR, Cinat G, Koliren L, Lewi D, Fernandez LE, Alonso DF, Gómez DE. Active specific immunotherapy of melanoma with a GM3 ganglioside-based vaccine: a report on safety and immunogenicity. J Immunother. 2004;27:442-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 50. | Mesa C, de León J, Fernández LE. Very small size proteoliposomes derived from Neisseria meningitidis: An effective adjuvant for generation of CTL responses to peptide and protein antigens. Vaccine. 2006;24:2692-2699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 51. | Parmiani G, Castelli C, Pilla L, Santinami M, Colombo MP, Rivoltini L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol. 2007;18:226-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 233] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 52. | Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64:6337-6343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 390] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 53. | Morecki S, Gelfand Y, Yacovlev E, Eizik O, Shabat Y, Slavin S. CpG-induced myeloid CD11b+Gr-1+ cells efficiently suppress T cell-mediated immunoreactivity and graft-versus-host disease in a murine model of allogeneic cell therapy. Biol Blood Marrow Transplant. 2008;14:973-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 54. | Martino A, Badell E, Abadie V, Balloy V, Chignard M, Mistou MY, Combadière B, Combadière C, Winter N. Mycobacterium bovis bacillus Calmette-Guérin vaccination mobilizes innate myeloid-derived suppressor cells restraining in vivo T cell priming via IL-1R-dependent nitric oxide production. J Immunol. 2010;184:2038-2047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 55. | Cauley LS, Miller EE, Yen M, Swain SL. Superantigen-induced CD4 T cell tolerance mediated by myeloid cells and IFN-gamma. J Immunol. 2000;165:6056-6066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 56. | Scumpia PO, Kelly-Scumpia KM, Delano MJ, Weinstein JS, Cuenca AG, Al-Quran S, Bovio I, Akira S, Kumagai Y, Moldawer LL. Cutting edge: bacterial infection induces hematopoietic stem and progenitor cell expansion in the absence of TLR signaling. J Immunol. 2010;184:2247-2251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 57. | Murphey ED, Lin CY, McGuire RW, Toliver-Kinsky T, Herndon DN, Sherwood ER. Diminished bacterial clearance is associated with decreased IL-12 and interferon-gamma production but a sustained proinflammatory response in a murine model of postseptic immunosuppression. Shock. 2004;21:415-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 58. | Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J Immunol. 2006;176:2085-2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 274] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 59. | Fernández A, Oliver L, Alvarez R, Hernández A, Raymond J, Fernández LE, Mesa C. Very small size proteoliposomes abrogate cross-presentation of tumor antigens by myeloid-derived suppressor cells and induce their differentiation to dendritic cells. J Immunother Cancer. 2014;2:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 60. | Greifenberg V, Ribechini E, Rössner S, Lutz MB. Myeloid-derived suppressor cell activation by combined LPS and IFN-gamma treatment impairs DC development. Eur J Immunol. 2009;39:2865-2876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 206] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 61. | Källberg E, Vogl T, Liberg D, Olsson A, Björk P, Wikström P, Bergh A, Roth J, Ivars F, Leanderson T. S100A9 interaction with TLR4 promotes tumor growth. PLoS One. 2012;7:e34207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 62. | Oliver L, Fernández A, Raymond J, López-Requena A, Fernández LE, Mesa C. Very small size proteoliposomes derived from Neisseria meningitidis: an effective adjuvant for antigen-specific cytotoxic T lymphocyte response stimulation under leukopenic conditions. Vaccine. 2012;30:2963-2972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 63. | Waight JD, Hu Q, Miller A, Liu S, Abrams SI. Tumor-derived G-CSF facilitates neoplastic growth through a granulocytic myeloid-derived suppressor cell-dependent mechanism. PLoS One. 2011;6:e27690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 206] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 64. | Pan L, Delmonte J, Jalonen CK, Ferrara JL. Pretreatment of donor mice with granulocyte colony-stimulating factor polarizes donor T lymphocytes toward type-2 cytokine production and reduces severity of experimental graft-versus-host disease. Blood. 1995;86:4422-4429. [PubMed] |
| 65. | Rutella S, Pierelli L, Bonanno G, Sica S, Ameglio F, Capoluongo E, Mariotti A, Scambia G, d’Onofrio G, Leone G. Role for granulocyte colony-stimulating factor in the generation of human T regulatory type 1 cells. Blood. 2002;100:2562-2571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 66. | Gabri MR, Mazorra Z, Ripoll GV, Mesa C, Fernandez LE, Gomez DE, Alonso DF. Complete antitumor protection by perioperative immunization with GM3/VSSP vaccine in a preclinical mouse melanoma model. Clin Cancer Res. 2006;12:7092-7098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 67. | Ramírez BS, Pestana ES, Hidalgo GG, García TH, Rodríguez RP, Ullrich A, Férnandez LE. Active antimetastatic immunotherapy in Lewis lung carcinoma with self EGFR extracellular domain protein in VSSP adjuvant. Int J Cancer. 2006;119:2190-2199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 68. | Torréns I, Mendoza O, Batte A, Reyes O, Fernández LE, Mesa C, Guillén G. Immunotherapy with CTL peptide and VSSP eradicated established human papillomavirus (HPV) type 16 E7-expressing tumors. Vaccine. 2005;23:5768-5774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 69. | Bequet-Romero M, Morera Y, Ayala-Ávila M, Ancizar J, Soria Y, Blanco A, Suárez-Alba J, Gavilondo JV. CIGB-247: a VEGF-based therapeutic vaccine that reduces experimental and spontaneous lung metastasis of C57Bl/6 and BALB/c mouse tumors. Vaccine. 2012;30:1790-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 70. | Gavilondo JV, Hernández-Bernal F, Ayala-Ávila M, de la Torre AV, de la Torre J, Morera-Díaz Y, Bequet-Romero M, Sánchez J, Valenzuela CM, Martín Y. Specific active immunotherapy with a VEGF vaccine in patients with advanced solid tumors. results of the CENTAURO antigen dose escalation phase I clinical trial. Vaccine. 2014;32:2241-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 71. | Aguilar FF, Barranco JJ, Fuentes EB, Aguilera LC, Sáez YL, Santana MD, Vázquez EP, Baker RB, Acosta OR, Pérez HG. Very small size proteoliposomes (VSSP) and Montanide combination enhance the humoral immuno response in a GnRH based vaccine directed to prostate cancer. Vaccine. 2012;30:6595-6599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 72. | Solares AM, Baladron I, Ramos T, Valenzuela C, Borbon Z, Fanjull S, Gonzalez L, Castillo D, Esmir J, Granadillo M. Safety and Immunogenicity of a Human Papillomavirus Peptide Vaccine (CIGB-228) in Women with High-Grade Cervical Intraepithelial Neoplasia: First-in-Human, Proof-of-Concept Trial. ISRN Obstet Gynecol. 2011;2011:292951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 73. | Schlecker E, Stojanovic A, Eisen C, Quack C, Falk CS, Umansky V, Cerwenka A. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J Immunol. 2012;189:5602-5611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 335] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 74. | Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, De Palma A, Mauri P, Monegal A, Rescigno M. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208:1949-1962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 443] [Cited by in RCA: 533] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
P- Reviewer: Fukuda S, Magnusson LU S- Editor: Song XX L- Editor: A E- Editor: Wang CH