Revised: May 12, 2014
Accepted: June 10, 2014
Published online: July 27, 2014
Processing time: 121 Days and 22.9 Hours
Costimulatory signals are crucial for T cell activation. Attempts to block costimulatory pathways have been effective in preventing unwanted immune reactions. In particular, blocking the CD28/cytotoxic T lymphocyte antigen (CTLA)-4/B7 interaction (using CTLA-4Ig) and the CD40/CD40L interaction (using anti-CD40L antibodies) prevents T cell mediated autoimmune diseases, transplant rejection and graft vs host disease in experimental models. Moreover, CTLA-4Ig is in clinical use to treat rheumatoid arthritis (abatacept) and to prevent rejection of renal transplants (belatacept). Under certain experimental conditions, this treatment can even result in tolerance. Surprisingly, the underlying mechanisms of immune modulation are still not completely understood. We here discuss the evidence that costimulation blockade differentially affects effector T cells (Teff) and regulatory T cells (Treg). The latter are required to control inappropriate and unwanted immune responses, and their activity often contributes to tolerance induction and maintenance. Unfortunately, our knowledge on the costimulatory requirements of Treg cells is very limited. We therefore summarize the current understanding of the costimulatory requirements of Treg cells, and elaborate on the effect of anti-CD40L antibody and CTLA-4Ig treatment on Treg cell activity. In this context, we point out that the outcome of a treatment aiming at blocking the CD28/CTLA-4/B7 costimulatory interaction can vary with dosing, timing and underlying immunopathology.
Core tip: Costimulation blockade (e.g., CD28/B7 and CD40/CD40L blockade) has been successfully used experimentally to induce tolerance to allo- or auto-antigens. Several studies suggest that effector T cells (Teff) and regulatory T cells (Treg) have different requirements regarding costimulation. While blockade of the CD40L receptor does not affect Treg cells and targets Teff cells, the effect of blocking the CD28/cytotoxic T lymphocyte antigen (CTLA)-4/B7 interaction (with CTLA-4Ig) is more difficult to predict and depends on the type, the strength and the stage of an immune process. Importantly, manipulating these costimulatory signals can therefore shift the Treg/Teff cell balance towards dominant Treg cell activity.
- Citation: Vogel IT, Gool SWV, Ceuppens JL. CD28/CTLA-4/B7 and CD40/CD40L costimulation and activation of regulatory T cells. World J Immunol 2014; 4(2): 63-77
- URL: https://www.wjgnet.com/2219-2824/full/v4/i2/63.htm
- DOI: https://dx.doi.org/10.5411/wji.v4.i2.63
Costimulatory interactions between T cells and antigen presenting cells (APCs), such as the CD28/B7 pathway and the CD40/CD40L pathway, are essential for T cell activation. As a consequence, reagents that deliberately block those costimulatory signals (e.g., the CTLA-4Ig fusion protein or antagonistic anti-CD40L antibodies) can be used to prevent unwanted or inappropriate T cell activation. Blocking costimulation, therefore, has been used to treat T cell mediated autoimmune diseases, transplant rejection or graft vs host disease (GvHD). Although anti-CD40L antibodies showed great potential in pre-clinical animal models and cytotoxic T lymphocyte antigen (CTLA)-4Ig is successfully used in clinical practice to treat rheumatoid arthritis and to prevent rejection of renal transplants, the precise mechanisms underlying their efficacy are still not fully understood. While effector T cells (Teff) clearly depend on costimulation for their activation, the costimulatory requirements of a suppressive T cell population, the regulatory T cells (Treg), are not completely clear. Several studies suggest that Treg and Teff cells have different requirements regarding costimulation. Furthermore, it has been suggested that Treg cells play an important role in the process of tolerance induction by costimulation blockade. In this review we discuss some possibilities to modulate costimulation in such a way that Teff cells are blocked but Treg cells remain active and functional. In this context, we summarize the current understanding of the costimulatory requirements of Treg cells, and elaborate on the effect of anti-CD40L antibody and CTLA-4Ig treatment on Treg cells. We point out that CTLA-4Ig has a quite complex effect on Treg cells, which should be taken into account when interfering with the CD28/CTLA-4/B7 interaction.
Immune tolerance refers to a state of specific immune non-responsiveness of the immune system to a particular antigen or a group of antigens. Tolerance to self-antigens is a hallmark of an effectively functioning immune system and disabling tolerance to self-antigens can lead to autoimmune diseases. In a similar way, an inappropriate response to a harmless environmental antigen can result in allergies. To avoid such harmful reactions, the immune system has developed several sophisticated mechanisms to induce and maintain tolerance.
During the maturation in the thymus, T cells undergo positive and negative selection. T cells which recognize a self-antigen presented by major histocompatibility complex (MHC) molecules, can be eliminated (negative selection)[1]. In this process, the signal strength with which the T cell receptor (TCR) recognizes its antigen determines the fate of the T cell. A strong signal and definite recognition of the auto-antigen leads to immediate deletion of the responding cell. A weak signal often leads to ignorance and migration to the periphery[2]. This is reasonable in order to maintain a pool of variable TCRs in the periphery. However, these cells might regain self-reactivity later on. Furthermore, some T cells escape thymic selection. Under these circumstances, peripheral tolerance induction should come into action.
Peripheral tolerance is maintained by mechanisms such as anergy (which results from a lack of sufficient activation signals)[3], deletion by apoptosis[4,5] and control by regulatory T (Treg) cells. The role of regulatory T cells, as well as the importance of costimulation for the induction and maintenance of peripheral tolerance, will be discussed in the following section.
Naïve T cells need two distinct signals in order to get fully activated[6]. The first signal is transmitted through the TCR, which recognizes an antigen presented by specialized antigen-presenting cells (APCs) on MHC molecules. This signal determines the specificity of the T cell response. The second (or accessory) signal is provided by the ligation of costimulatory receptors on the cell surface[7]. Without proper costimulation, T cells fail to become fully activated and enter a state of hypo-responsiveness (anergy)[8]. Up to now, many costimulatory signals and pathways have been identified, among which the best characterized are the CD28/CTLA-4/B7 pathway and the CD40/CD40L pathway.
The CD28/CTLA-4/B7 interaction: Mice deficient in CD28 are unable to mount an effective immune response to foreign antigens, pathogens or allografts. The CD28 receptor is a disulfide-linked homodimer, which is constitutively expressed on T cells and is engaged by both the CD80 (B7-1) and CD86 (B7-2) molecule on activated APC[9]. The monomeric CD86 ligand is constitutively expressed in low amounts on professional APC and up-regulated upon activation, while CD80 is expressed as a dimer on activated APC. The up-regulation of CD86 occurs rapidly after activation and reaches its maximum 18 to 24 h after stimulation, while the up-regulation of CD80 is delayed and reaches a maximum after 48 to 72 h[10,11]. Studies with knock-out (KO) mice have shown that CD86 is more important for initiating an immune response than CD80. Otherwise the functions of the two B7 molecules are largely overlapping[12]. Signalling via CD28 is mediated through the phosphatidylinositol 3-kinase-protein kinase B (PKB/Akt) and the growth factor-receptor-bound protein 2 (Grb2) pathways and promotes IL-2 production[13] and T cell proliferation[14] by decreasing the threshold for activation via the TCR[15]. In addition, T cell survival is strengthened by up-regulation of the anti-apoptotic factor Bcl-xL[16]. CD28 engagement also up-regulates or induces the expression of additional costimulatory receptors such as ICOS and CTLA-4[17]. While CD28/B7 signalling is crucial for the activation of naïve T cells, previously activated cells are less dependent on costimulation. After priming and differentiation are completed, the production of effector cytokines (e.g., IL-4 or IFNγ) does not require further costimulation. Only IL-2 production depends on continuous costimulatory signalling[12].
Another receptor molecule, which binds to both B7 molecules and is structurally homologous to CD28, is the “cytotoxic T lymphocyte antigen 4” (CTLA-4) or CD152. It is up-regulated on T cells upon activation with a peak at 24-48 h after initial priming[18]. However, its expression on the surface is not stable and the CTLA-4 molecule is continuously internalized in a clathrin dependent way, degraded in lysosomes and recycled to the cell surface[19]. CTLA-4 binds CD80 and CD86 with a 10-20 fold higher affinity compared to CD28[20] and consequently out-competes CD28 mediated activation[21]. Furthermore, CTLA-4 has an advantage in engaging to B7 molecules as it binds divalently, while CD28 binds monovalently[22]. In contrast to CD28 signalling, the CTLA-4 pathway has a suppressive character, and CTLA-4 deficient mice develop severe lymphoproliferative disease and die 3 to 4 wk after birth[23]. Of note, CTLA-4 KO mice deficient in B7-1 and B7-2, as well as CTLA-4 KO mice with a defective CD28 receptor are protected from this fatal disease[24,25]. This suggests that CTLA-4 selectively regulates CD28 mediated activation. Binding of CTLA-4 to its ligands recruits phosphatases (SHP-1, SHP-2 and PP2A), which inhibit TCR phosphorylation and several other pathways such as the PKB/Akt activation as well as the phosphorylation of extracellular-signal-regulated kinases (ERK) and c-Jun N-terminal kinases (JNK)[26]. This reduces the production of IL-2 and its receptor, inhibits T cell proliferation, and consequently results in termination of the immune response[18,27].
Other members of the B7 and CD28 superfamilies: Other members of the B7 superfamily, which have been studied extensively, are the inducible costimulator ligand (ICOSL, CD275, B7h or B7-PR-1), which binds to ICOS (CD278) and the programmed death ligands 1 and 2 (PD-L1 and PD-L2) which binds to programmed death 1 (PD 1). ICOS is structurally and genetically related to CD28 and up-regulated in the course of activation[28]. ICOSL is expressed on APCs and some non-hematopoietic cells (e.g., endothelial cells). Different from CD28/B7 signalling, ICOS/ICOSL interaction is not essential for T cell activation, but rather acts by fine-tuning effector T cell differentiation and cytokine production[29]. Furthermore, ICOS is crucial for germinal centre formation and class switching in B cells[30,31].
PD-1 is a suppressive member of the CD28 superfamily. Different from CD28 and CTLA-4, PD-1 is not expressed as a dimer and its expression is not limited to T cells. It can be found on activated T cells, but also on B cells and myeloid cells, which suggests a broader spectrum of regulation compared to CTLA-4[32]. Ligation to PD-L1 and PD-L2, which are expressed on activated APCs, inhibits cytokine production and leads to cell cycle arrest[33,34]. Furthermore, PD-1 signalling was found to be involved in CD8+ T cell differentiation and regulation[35].
The CD40/CD40L interaction: CD40 (TNFRSF5) is a type I trans-membrane protein, which clusters upon engagement to its ligand CD40L (CD154, TNFSF5, gp39, T-BAM, or TRAP)[36]. CD40 ligation further induces the recruitment of adaptor proteins (TNF-associated factors), which then in turn trigger several possible pathways including the canonical and non-canonical nuclear factor κB (NFκB) signalling pathway, the mitogen activated protein kinases (MAPK), the phosphoinositide 3-kinase (PI3K) and the phospholipase Cγ (PLCγ) pathways[37]. CD40 is constitutively expressed on APC and on many other cell types including non-hematopoietic cells (e.g., fibroblasts and epithelial cells)[38]. CD40L forms a sandwich structure composed of a β-sheet, an α-helix loop and another β-sheet and is expressed as a trimeric complex on activated T cells and platelets[39]. Under inflammatory conditions it can also be found on natural killer (NK) cells, mastocytes and eosinophils[38]. Its expression on T cells is mainly restricted to CD4+ T helper (Th) cells, but there is also a small population of CD8+ T cells and γδ T cells which can express CD40L[36]. Furthermore, it has been shown that CD40L is expressed on CD8+ T cells in the presence of IL-12 and that these cells potentially represent a CD8+ T helper cell subset[40,41].
Upon activation, CD40L is up-regulated as early as 5 to 15 min after stimulation and reaches a maximum after 6 to 8 h[36]. This fast up-regulation is made possible via preformed CD40L (pCD40L), which is stored in lysosomal compartments and can be mobilised in response to an activation signal[42].
The broad expression of CD40 suggests involvement in many different immune modulatory mechanisms. In this context, CD40L engagement to CD40 results in increased survival of APC[43], production of cytokines[44], up-regulation of B7 molecules and nitric oxide (NO) production[45] and is critical for full maturation of dendritic cells (DC)[46]. Furthermore, CD40 signalling is crucial for B cell activation and differentiation, antibody production, immunoglobulin-class switching and germinal centre formation[47,48]. CD40/CD40L KO mice do not only show hyper-IgM syndrome, but also exhibit deficiency in priming of T cells[36]. Signalling via CD40/CD40L results in enforcement of the CD28-B7 interaction and antigen presentation and is crucial for expansion and maturation of effector T (Teff) cells[38,49]. Furthermore, CD40/CD40L mediated contact between CD4+ T helper cells and professional APC (DC) is important to enable DC to subsequently prime CD8+ cytotoxic T lymphocytes (CTL)[50].
Other members of the TNF and TNFR superfamilies: Other members of the TNF/TNFR superfamily have gained importance during the last years. Among those are the interactions between the glucocorticoid-induced tumour necrosis factor related receptor (GITR) and its ligand GITR-L, between OX40 (CD134 or TNFRSF4) and OX40 ligand (OX40L, CD252 or TNFSF4), between 4-1BB (CD137 or TNFRSF9) and 4-1BB ligand (4-1BBL or TNFSF9) and CD27 (TNFRSF7) and CD70 (TNFSF7). In general, these TNF/TNFR superfamily members are up-regulated or induced upon activation on T cells and their ligands on APCs. Signalling via these pathways regulates the frequency of effector or memory cells, provides proliferation and survival signals and promotes cytokine production[51]. The expression of OX40L, 4-1BBL and CD70 on non-immune cells (e.g., endothelial cells or smooth muscle cells) further suggests a role in tissue inflammation in different disease settings[52,53]. In addition, TNF/TNFR superfamily members are expressed on natural killer (NK) and natural killer T (NKT) cells and signalling increases their effector function[51].
A subset of CD4+ T cells has regulatory capacity. In a healthy individual they constitute about 10% of circulating CD4+ T cells. Treg cells play a key role in dampening of immune responses, prevention of autoimmune and allergic diseases, as well as in tolerance after transplantation[54]. They are characterized by constitutive expression of the IL-2 receptor α-chain CD25, CTLA-4 and the forkhead transcription factor Foxp3[55,56]. The latter one is crucial for the suppressive function of Treg cells, as ectopic expression of Foxp3 can induce regulatory function in naïve T cells[57]. Loss of Foxp3 results in impairment of Treg cells and in autoimmune disorders in mice (Scurfy)[58] and humans (IPEX-syndrome)[59].
Two subgroups of Foxp3 expressing Treg cells have been identified: the so called thymus derived Treg cells (tTreg) and induced Treg cells (iTreg), which are generated in the periphery from naïve CD4+ T cells. In vitro, iTreg cells can be induced by antigenic stimulation in the presence of IL-2 and TGF-β[60,61]. Although the situation in vivo is less clear, iTreg cells are thought to be generated under non-inflammatory conditions in the presence of IL-2 and TGF-β by chronic sub-optimal antigen exposure[62-64], e.g., by recognition of an antigen on immature DC which do not provide costimulation[65]. Furthermore, a role for retinoic acid (RA), which increases TGF-β production and favors Foxp3 polarization, has been unraveled[66,67]. During an acute inflammation (e.g., in allergic or autoimmune diseases or during the course of an infection), in the presence of high amounts of inflammatory cytokines, the generation of Teff cells is favored over Treg cell induction[68].
Unfortunately it is not yet possible to distinguish tTreg and iTreg cells since both of them express CTLA-4, CD25 and Foxp3. Helios (a member of the Ikaros transcription factor family) and Neuropilin-1 (Nrp 1) have been suggested as specific markers for tTreg cells, but controversial findings regarding their expression on tTreg vs iTreg cells limit their use as reliable markers[69-72].
There are also CD4+ Treg cell subtypes induced in the periphery which do not express Foxp3. Among those are T regulatory cells 1 (Tr1), which can be induced from naïve CD4+ T cells in the presence of IL-10[73] and T helper cells type 3 (Th3), which require TGF-β[74]. Up to now, it is difficult to identify those Treg cell subsets by means of a specific surface marker. Therefore, they are predominantly defined by their cytokine profile. Tr1 cells are characterized by a high IL-10 and TGF-β production, low levels of IL-2, variable levels of IL-5 and IFN-γ and no IL-4[73]. Th3 cells produce mainly TGF-β and variable levels of IL-10 and IL-4[75].
Activation and expansion of Treg cells requires a TCR signal in vitro[76,77] and in vivo[78,79] and is consequently antigen specific. Whether or not they suppress in an antigen-specific way is still a matter of debate. A key molecule in suppression by Treg cells is CTLA-4. Mice which display a Treg-specific deficiency in CTLA-4 develop severe autoimmune diseases, and Treg cells from these mice show reduced suppressive capacity in vitro[80]. In contrast to conventional T cells, Treg cells express CTLA-4 constitutively[81] and therefore have a natural advantage over naïve T cells in terms of CD80/CD86 engagement. In addition, CTLA-4 expressed by Treg cells also has a cell-extrinsic mechanism of action. It has been demonstrated by Qureshi and coworkers that CTLA-4 engagement to the B7 molecules leads to trans-endocytosis and degradation of CD80 and CD86 on the surface of APCs[82]. This effect can only be mediated by CTLA-4 expressed on the cell surface, but not by soluble CTLA-4. As a result, the availability of B7 receptors and consequently the CD28 mediated activation of T cells are reduced. Moreover, CTLA-4/B7 interaction might lead to “reverse signalling” in APC. In the course of CTLA-4 engagement, APC start to produce indoleamine 2,3-dioxygenase (IDO), which catalyses the degradation of tryptophan and thus creates a local inhibitory environment for T cells[83]. This also induces the nuclear translocation of the transcription factor Foxo3[84], which inhibits the production of IL-6 and of tumor necrosis factor alpha (TNFα) but increases the secretion of suppressive cytokines such as IL-10[85]. Apart from mechanisms mediated by direct cell contact to APCs, Treg cells also secrete suppressive molecules such as IL-10[86], TGFβ[87] and IL-35[88] and molecules which can directly kill Teff cells, such as granzyme B and perforin[89]. Membrane-bound TGFβ[90] or production of cyclic adenosine monophosphate (cAMP), which can be transferred to Teff cells via gap junctions, can suppress Teff cells via direct cell-cell contact[91]. Other suppressive mechanisms involve CD39 and CD72 mediated degradation of adenosine monophosphate (AMP) and adenosine triphosphate (ATP) to adenosine[92] or suppression by Galectin-1[93]. Finally, Treg cells are thought to suppress Teff cells by IL-2 deprivation and subsequent apoptosis[94]. IL-2 is crucial for Treg cell generation, induction and maintenance[95], but, in contrast to Teff cells, Treg cells lack the ability to produce IL-2 and are consequently dependent on an external source[96]. Since Treg cells constitutively express the high affinity receptor for IL-2 (CD25)[55], they have an advantage over Teff cells in terms of binding IL-2. In an inflammatory setting, however, when Teff cells also up-regulate CD25, this advantage is lost. Therefore, it was suggested that suppression by IL-2 consumption is predominantly important in steady-state conditions as a feed-back mechanism to prevent Treg cell overgrowth and not in an inflammatory setting[97].
Since none of the above described mechanisms results in a complete absence of regulatory activity when deleted, there is most likely not one core-mechanism of suppression. In this context, Treg-specific CTLA-4 deficiency resulted in systemic autoimmune diseases[80], but transfer of CTLA-4 deficient Treg cells could prevent experimental colitis in vivo[98] and IL-10 deficient Treg cells are able to suppress auto-immunity, but cannot prevent experimental colitis[86,99]. Thus, Treg cells can compensate for defects and adapt to environmental circumstances.
Since the “second” or “costimulatory” signal is of great importance for the activation and successful differentiation of naive T cells into fully functional Teff cells[6], blocking these pathways presents a promising approach to treat T cell mediated autoimmune diseases (e.g., rheumatoid arthritis or multiple sclerosis), transplant rejections or graft vs host disease (GvHD). Compared to conventional immunosuppressive drugs, costimulation blockade provides the advantage of selective inhibition of T cell responses and has the potential of inducing long-lasting antigen-specific tolerance[100]. The most promising and best studied candidates for such manipulations are the CD28/B7 and CD40/CD40L pathways as they are both critical for T cell activation.
Up to now, the most promising candidate to achieve CD28/B7 costimulation blockade is the CTLA-4Ig fusion protein. It consists of the extracellular domain of the CTLA-4 molecule fused to the Fc-region of IgG. CTLA-4-Ig binds both B7 molecules with the same high binding affinity as CTLA-4. The effect of CTLA-4Ig has first been demonstrated in an animal model of islet transplantation, where CTLA-4Ig treatment led to long-term acceptance of xenografts[101]. Also in systems of allogeneic islet or cardiac transplantation or graft vs host disease (GvHD), CTLA-4Ig could prolong survival and reduce rejection[102-104]. Furthermore, CTLA-4Ig is a potent immunosuppressor in animal models of autoimmunity such as experimental autoimmune encephalomyelitis (EAE)[105], diabetes[106] and systemic lupus erythematodes (SLE)[107].
CTLA-4Ig has also been used effectively in clinical trials. Davies and co-worker showed that tolerizing bone marrow cells ex vivo in the presence of CTLA-4Ig prior to transplantation to a MHC-matched recipient reduces the incidence of acute and chronic GvHD[108]. Furthermore, CTLA-4Ig (abatacept) treatment in combination with cyclosporin and methotrexate prevents acute GvHD after hematopoietic cell transplantation from an unrelated donor[109]. Since 2005, CTLA-4Ig (abatacept) is approved by the FDA for the treatment of rheumatoid arthritis (RA)[110] and a second-generation molecule (belatacept) with higher binding affinity for B7-1 and B7-2 was approved in 2011 to prevent rejection after renal transplantation[111].
Antagonistic anti-CD40L monoclonal antibodies (mAb) have shown impressive effects in many animal models. Blocking CD40L prevents acute and chronic GvHD[112]. If given at the time of transplantation, anti-CD40L treatment prolongs graft survival in a model of heart, islet, liver and limb transplantation[113-116]. Targeting the CD40L receptor proved to be efficient in animal models of autoimmune diseases such as EAE, arthritis, SLE, colitis and arteriosclerosis[117]. However, clinical trials with an anti-CD40L mAb (Ruplizumab) in SLE patients have led to thromboembolic side-effects and had to be halted[118]. This effect was caused by the Fc-fragment of the antibody bound to a receptor on platelets which also express CD40L. Nonetheless, the findings in animal systems are extremely promising and, consequently, it is attempted to find alternative ways to achieve CD40L blockade. mAb with an engineered, aglycosylated or mutated Fc-part were created[119-121]. The modifications alter the antibody in a way that Fc-receptor or complement mediated platelet aggregation and subsequent thromboembolic events are prevented. Furthermore, alternative blocking reagents such as small molecules or peptides are currently explored[122,123].
The CD40/CD40L interaction can also be interrupted by targeting the CD40 receptor. A human antagonistic anti-CD40 antibody showed some effect in ex vivo studies[124,125] and proved to be safe in a Phase I clinical trial on lymphocytic leukaemia patients[126]. Another antagonistic anti-CD40 antibody, chimeric 5D12, was tested successfully in an EAE model in marmoset monkeys[127]. Furthermore, we showed that 5D12 was well tolerated in a phase I clinical trial in patients with Crohn’s disease[128]. However, CD40 is expressed on many different cell types and consequently targeting this molecule might have broad and undesired effects. Additionally, most antibodies directed against CD40 are stimulatory for APC and B cells by cross-linking the trimeric receptor.
Although CTLA-4Ig and anti-CD40L antibodies show great potential in various disease models, the combination of both is often superior. It is indeed possible that in the absence of CD40L or CD28 triggering, the T cell can still receive sufficient activation signals from other costimulatory pathways[129,130]. Especially in animal models of solid organ transplantation, combined blockade of CD28/B7 and CD40/CD40L is required for permanent tolerance induction in mice[131] and non-human primates[132]. Also, in animal models of leukaemia[133] or autoimmune diseases such as EAE[134] and SLE[135], the combination of CTLA-4Ig and MR1 (an anti-CD40L mAb) could more effectively reduce disease symptoms than both alone. We made similar observations in a fully MHC mismatch model of GvHD with allogeneic bone marrow transfer. In our study, only the combined blockade of the CD28/B7 pathway (using CTLA-4Ig) and the CD40/CD40L pathway (using MR1) prevented lethal GvHD and resulted in long-lasting tolerance and the induction of stable mixed chimerism[136].
The mechanisms of tolerance induction by costimulation blockade, in particular of the CD28/CTLA-4/B7 and the CD40/CD40L interaction, have extensively been studied in allo-responses such as GvHD or transplant rejection. In these settings, deprivation of necessary activation signals (CD28 and/or CD40 triggering) leads to T cell hypo-responsiveness[8], which is followed by peripheral clonal deletion[136,137]. Elimination of the hypo-responsive T cells is predominantly mediated by apoptosis[138-140]. In a fully miss-matched transplantation model, the tolerising effect of combined CD28/B7 (using CTLA-4Ig) and CD40/CD40L (using MR1) blockade can be reversed by the calcineurin inhibitor cyclosporine A (CsA), which prevents apoptosis[138]. In contrast, rapamycin (which favours apoptosis) acts synergistically with costimulation blockade. While activation induced cell death (AICD) seems not to be essential, passive cells death is crucial for the induction of tolerance under the cover of CTLA-4Ig and MR1. Heart allografts were rejected in Bcl-xL deficient mice despite costimulation blockade[139], but Fas-deficiency was not able to break tolerance[140]. Additionally, CTLA-4Ig has been suggested to act via reverse signalling to APCs and to induce IDO production, which contributes to creating a suppressive environment[141].
Although apoptosis of Teff cells after activation in the absence of costimulatory has been demonstrated by many research groups, complete deletion of responsive T cells takes several weeks[137] while tolerance can already be observed shortly after treatment[142]. In this context, it has been demonstrated by the group of Waldmann that CD4+ cells, which have been tolerized to allo-antigens by CD40L blockade, are not only hypo-responsive but moreover display a suppressive function[143,144]. Therefore, it has been suggested that Treg cells, at least partially, mediate tolerance until Teff cells have been eliminated. In line with this, it has been demonstrated that tolerance induction by CD40L or B7 blockade is abrogated when Treg cells are depleted. In a study performed by Taylor and co-workers, CD4+ cells were tolerized to allo-antigens ex vivo in the presence of antagonistic anti-CD40L or anti-B7 antibodies. Transfer of these cells to animals suffering from GvHD did abrogate the disease. However, if Treg cells were depleted prior to the transfer, GvHD was not suppressed[145]. Also, long-term acceptance of a skin or a heart allograft under the cover of CD40L blockade could be abrogated if recipient Treg cells were depleted[146,147]. However, Kurtz et al[148] showed that it is possible to induce mixed chimerism after allogeneic bone marrow transplantation under the cover of CD40L blockade, but they did not find evidence for an involvement of Treg in this system. In line with this, we have previously shown in a model of GvHD with allogeneic bone marrow transplantation that tolerance induction by combined CD40/CD40L and CD28/B7 blockade and the development of mixed chimerism are still possible despite the absence of donor Treg cells[136]. In both studies T cell hypo-responsiveness and deletion were the main mechanisms by which tolerance was achieved. The importance of Treg cells for tolerance induction by costimulation blockade thus might depend on the disease model. The recipient Treg cells might be important in the setting of a solid organ transplant, while in GvHD the presence of Treg cells within the donor cell transplant might not be crucial for the outcome of the disease.
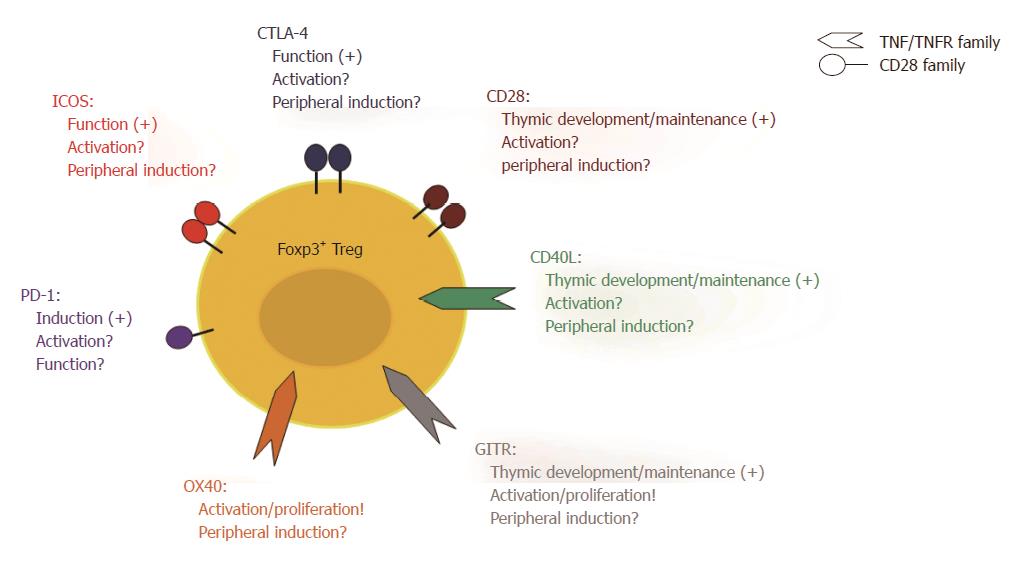
Involvement of Treg cells in tolerance induction by costimulation blockade implies that Teff cells and Treg cells have different requirements regarding costimulation. Such different requirements could result in differential modulation of Teff cells and Treg cells by costimulation blockade. Both cell types share the TCR-mediated recognition of an antigen as the first signal for activation. However, the costimulatory requirements for Treg cells are less clear than those for Teff cells (Figure 1). CD28/B7 signalling is crucial for thymic Treg cell generation and homeostasis since mice deficient in CD28 or B7 molecules have a significantly reduced number of Treg cells in the thymus as well as in the periphery[149,150]. CD40L and glucocorticoid-induced tumour necrosis factor related receptor (GITR) signalling also play an important role during thymic development of Treg cells[151-153]. Whether CD28 and/or CD40L costimulation is equally important for the activation or the induction of Treg cells in peripheral lymphoid organs as it is for Teff cells, however, is still a matter of debate. We have shown that blocking the B7 molecules using anti-B7-1 and anti-B7-2 antibodies in combination with an antagonistic anti-CD40 antibody resulted in human T cell hypo-responsiveness in vitro. This effect was associated with the induction of a T cell subset with suppressive activity, which expressed high levels of ICOS and produced IL-10[154]. Furthermore, we have shown that the beneficial effect of combined CTLA-4Ig and MR1 treatment in a mouse model of GvHD is associated with an increase in the frequency of Foxp3+ Treg cells between day 6 and 30 after T cell transfer[136]. Both findings argue for costimulation independent Treg induction and expansion. We further conducted a more detailed examination of the effect of CTLA-4Ig and MR1 on murine Treg cells in vitro. Here, we showed that Treg cells can proliferate and be activated if CTLA-4Ig and MR1 were added to the cultures at a dose where Teff cells are inhibited[155]. Also other laboratories, in which the blockade of the CD28/B7 and/or the CD40/CD40L interaction was studied, have observed an increase of functional Treg cells in vitro[145,156]. Furthermore, a selective non-cross-linking CD28 antagonist induced tolerance to renal and cardiac allografts in non-human primates and this was associated with an increased frequency of Foxp3+ Treg cells[157]. In a mouse model of heart transplantation under the cover of an anti-CD40L mAb, Treg cell were functional and crucial to prevent rejection[147]. Altogether, these findings suggests that Treg cells are less dependent on CD28/B7 and CD40/CD40L costimulation compared to Teff cells and can therefore still be activated and expand in the presence of CTLA-4Ig and MR1.
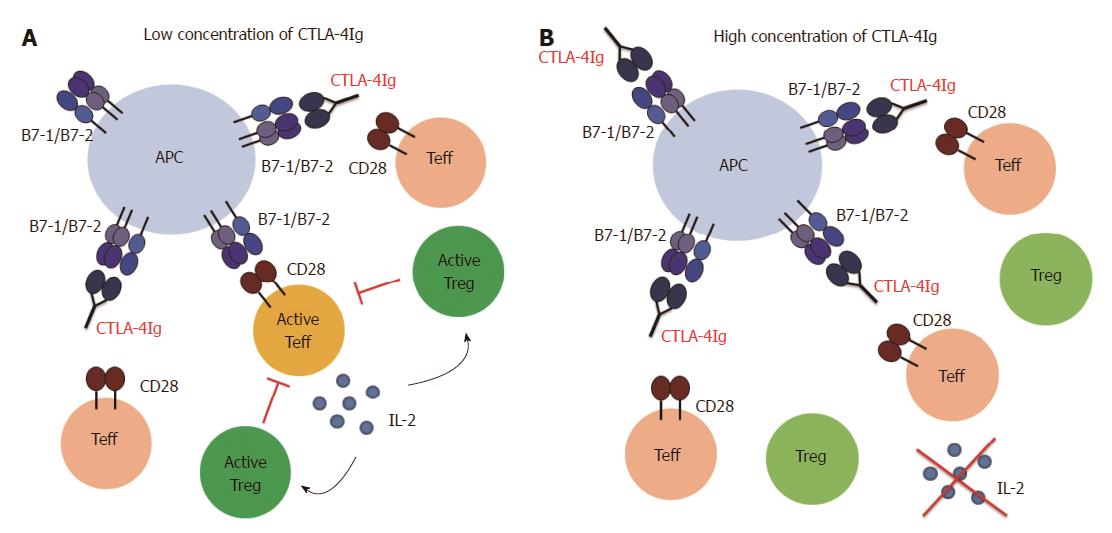
However, Treg cells are probably not completely independent of CD28/B7 and CD40/CD40L costimulation. In this context, we showed that the increase in the Treg cell frequency in vitro observed in the presence of CTLA-4Ig and MR1 is dependent on the concentration of the blocking agents. While a low dose of CTLA-4Ig and MR1, ranging between 0.125 μg/mL and 4 μg/mL, resulted in a concentration dependent increase in the frequency of Treg cells, a higher dose (between 8 μg/mL and 32 μg/mL) resulted in a concentration dependent decrease in the frequency of the Treg cells (manuscript in preparation). Thus, at a very high dose of costimulatory blocking agents, Treg cells also seem to be affected. We further explored this issue in a mouse model of GvHD. A treatment regime using 500 μg (per mouse) of CTLA-4Ig (in combination with MR1) was equally effective as a 10 times lower dose in preventing the disease. However, intermediate doses had no effect on survival. Again, the treatment with a low dose of CTLA-4Ig, but not with a high dose, was followed by an increase in Treg cell frequency (manuscript in preparation). This observation can potentially be explained assuming two separate mechanisms of action (Figure 2): treatment with a high dose blocks all the Teff cells (but also the Treg cells) and therefore prevents the disease. At a low dose, however, not all the Teff cells are blocked, but Treg cells remain activated and are able to suppress the remaining Teff cells. Intermediate doses are not effective, most likely because not all the Teff cells are blocked while at the same time Treg cells are affected and therefore not able to suppress Teff cells. It is possible that Treg cells need the same costimulatory signals as Teff cells, but have a lower threshold for activation. Another possibility is that a low dose of CTLA-4Ig and MR1 only partially blocks the Teff cells, which produce low amounts of IL-2. As Treg cells can take up IL-2 more efficiently than Teff cells due to the constitutive expression of the high affinity IL-2 receptor (CD25)[95], the low amounts of IL-2 might be sufficient to maintain Treg cells but not enough to allow for Teff cell priming and activation. This issue will have to be examined more closely in the future. If IL-2 and not costimulation is the limiting factor for Treg cell activation, expansion of Treg cells can be facilitated by adding exogenous IL-2.
Other costimulatory pathways have been suggested to be relevant for Treg cell activation and function. Triggering GITR on Treg cells increases their proliferation and enforces their suppressive activity[158]. Blocking the ICOS/ICOSL interactions in a model of ovalbumin (OVA) induced airway inflammation[159] and EAE[30] abrogated Treg activity in vitro and in vivo. An antagonistic anti-PD-1 antibody can prevent the induction of Treg cells from naive CD4+ T cell in vitro, which suggests that PD-1 signalling is important in this process[160]. Defects in or blockade of CTLA-4 leads to uncontrolled expansion of Treg cells, which suggests a cell-intrinsic effect of CTLA-4 triggering on Treg cells and an important role for CTLA-4 in regulating Treg generation in the thymus and in the periphery[161,162]. Also, CTLA-4 regulates the TCR specificity during thymic development as over-expression of CTLA-4 leads to a self-skewed TCR repertoire whereas deficiency of CTLA-4 prevents the development of a self-skewed TCR repertoire[163]. There is also evidence that CTLA-4 signalling is involved in the induction of Foxp3 in naïve T cells and promotes generation of iTreg cells in the periphery[164]. In addition, CTLA-4 is a key mediator in suppression by Treg cells as described before. Recently, the OX40/OX40L pathway has come into focus with regard to Treg cell activation and proliferation. OX40 triggering acts in concert with IL-2 and leads to extensive Treg cell expansion. In the presence of IL-2, these cells are stable and show potent suppressive activity[165].
A large body of evidence including our own studies suggests that Treg cells are not affected by CD40/CD40L blockade[120,136,143-147,155]. Although Treg cells require CD40L signalling during their development in the thymus[152,153], only about 4%-9% of Treg cells express CD40L in the periphery[166]. Up-regulation of CD40L in Treg cells upon activation is delayed compared to Teff cells, which express CD40L within the first 5 to 15 min after activation[36]. This fast up-regulation is made possible through the storage of preformed CD40L (pCD40L). Treg cells, on the other hand, are incapable of storing pCD40L and consequently have to generate it de novo[42,166]. Altogether this suggests that Treg cells are indeed not dependent on CD40L signalling concerning their activation. Therefore, CD40L blockade provides a promising target to modulate the balance between Treg cells and Teff cells in favour of Treg cell activity.
CTLA-4Ig has been proven to be very effective as an immunosuppressive treatment in various animal models and is successfully used in the clinic to treat rheumatoid arthritis (abatacept) and rejection after renal transplantation (belatacept)[110,111]. However, recent findings have raised concern about the use of CTLA-4Ig in systems where Treg cells are crucial for the success of the therapy. Riella and co-workers showed that CTLA-4Ig accelerates transplant rejection in a MHC class II mismatch model, in which tolerance induction and graft survival is crucially dependent on Treg cell function[167]. Furthermore, in a study in which rejection of a skin transplant could be prevented by expansion of Treg cells using IL-2/anti-IL-2 complexes, simultaneous administration of CTLA-4Ig could break tolerance induction[168]. As mentioned before, we have observed a dose dependent effect of CTLA-4Ig on Treg cells (manuscript in preparation). It is possible that the amount of CTLA-4Ig applied was indeed high enough to interfere with the Treg cells. Especially in a model where Treg cells are crucial for the outcome of the disease, a high dose might be less effective than a low dose which spares the Treg cells.
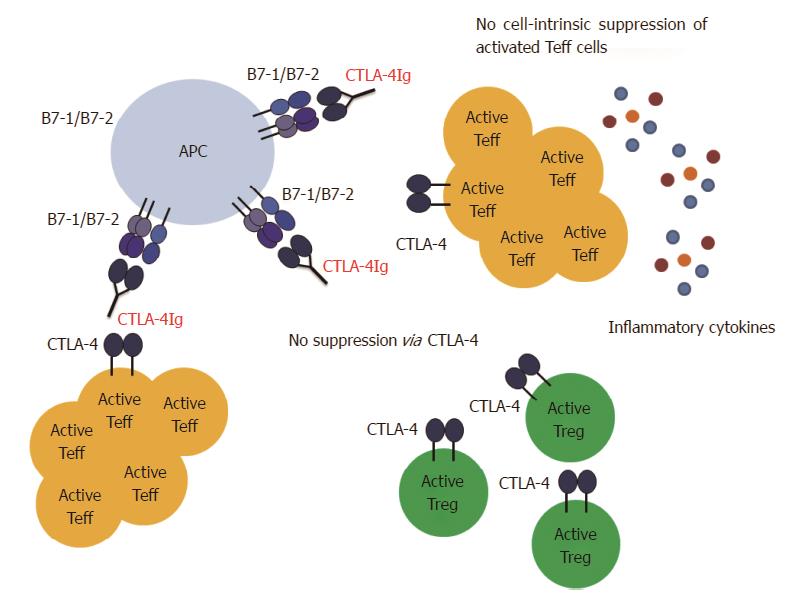
The differential sensitivity of Treg cells vs Teff cells to CD28/CTLA-4/B7 blockade is certainly not the only problem that might arise from CTLA-4Ig treatment. Another factor that has to be considered is that CTLA-4Ig does not only interfere with the CD28/B7 signaling but also with the CTLA-4/B7 signaling (Figure 3). CTLA-4 is expressed on activated Teff cells and constitutively on Treg cells, and triggering of membrane CTLA-4 leads to suppression of the corresponding T cell[18,20]. This holds true for Teff cells as well as for Treg cells[169]. Since Treg cells express CTLA-4 constitutively, CTLA-4Ig administration during priming will presumably prevent CTLA-4 mediated cell-intrinsic suppression of Treg cells and will therefore enhance their activity. In addition, CTLA-4Ig engagement to the B7 ligands leads to reverse signalling to the APCs, which results in IDO production[141]. Both mechanisms thus result in the creation of a suppressive environment. However, CTLA-4 is a also key molecule for Treg cell function[81]. Our above mentioned data argue against interference of CTLA-4Ig with Treg cell activation, but do not exclude interference with Treg function or induction. In this context, blockade of the B7 molecules with CTLA-4Ig prevents CTLA-4 mediated trans-endocytosis and degradation of the B7 molecules by Treg cells as well as “reverse signalling”via CTLA-4/B7 signalling and IDO production. Moreover, if CTLA-4Ig is given after T cell priming, Teff cells will also have up-regulated CTLA-4 and by blocking B7 molecules, the cell-intrinsic suppression of Teff cells might be blocked. This is not relevant in a setting of transplantation, when it is exactly known when T cell priming occurs. However, for patients with autoimmune diseases such as multiple sclerosis (MS), the situation is different. It is not possible to predict disease onset or a relapse episode and therefore it is not known when auto-reactive T cells are primed and activated. In such settings it might be dangerous to apply CTLA-4Ig treatment. Indeed, we have found in a model of experimental autoimmune encephalomyelitis (EAE), the mouse model for the human disease MS, that treatment with CTLA-4Ig after T cell priming leads to exacerbation of the disease. This is most likely due to interference with the CTLA-4/B7 mediated suppression (manuscript in preparation). Further studies will be required to examine if this exacerbation is a result of missing cell-intrinsic suppression of the Teff cells, interference with Treg cell function and de novo induction or both.
Based on the above discussed studies and our own results we believe that it can be possible to modulate costimulation in such a way that Teff cell activation is prevented but Treg cells can still be activated. Especially blockade of the CD40/CD40L pathway provides a promising target to manipulate the Teff/Treg cell balance in favor of Treg cell activity. However, blockade of the CD40/CD40L interaction alone is not always sufficient to guarantee full protection. Therefore, CD40/CD40L blockade must be combined with CTLA-4Ig in order to prevent CD28 mediated activation. Several factors have to be taken into account when using CTLA-4Ig as a treatment option. First, if CTLA-4Ig is given before T cell priming (e.g., in a transplant setting), the dose of the reagent is an important factor. A high dose of CTLA-4Ig can also affect the Treg cells. Careful titration is required to find the optimal dose that blocks Teff cells but spares the Treg cells (Figure 2). This might be of great importance if Treg cells are crucial for the success of the therapy. Second, it has to be considered whether CTLA-4Ig is given before or after T cell priming. CTLA-4Ig treatment after T cell priming might be dangerous as it can interfere with CTLA-4 mediated suppression (Figure 3). This can affect cell-intrinsic suppression of the Teff cells and/or affect Treg cell function and induction. Third, knowing the pathophysiology of the disease (especially concerning involvement of Treg cells) is crucial in order to find a balance between maximal suppression of Teff cells and minimal interference with Treg cells.
It will be important to more closely study the costimulatory requirements of Treg cells and the effect of blocking those signals on their activity. This will help to improve the success of a therapy involving costimulation blockade. Especially when using CTLA-4Ig, it will be necessary to know exactly which effect the treatment has in the corresponding disease setting in order to prevent undesired effects. Furthermore, the finding that Treg cells and Teff cells respond differently to costimulation blockade can potentially be exploited in a context of Treg cells based therapy. Treg cells can be expanded in vitro or perhaps even in vivo, while the outgrowth of Teff cells is prevented under the cover of costimulation blockade.
| 1. | Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1120] [Cited by in RCA: 1132] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 2. | Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5:772-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 446] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 3. | Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1052] [Cited by in RCA: 1068] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 4. | Grillot DA, Merino R, Núñez G. Bcl-XL displays restricted distribution during T cell development and inhibits multiple forms of apoptosis but not clonal deletion in transgenic mice. J Exp Med. 1995;182:1973-1983. [PubMed] |
| 5. | Lenardo MJ. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991;353:858-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 820] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 6. | Bretscher PA. A two-step, two-signal model for the primary activation of precursor helper T cells. Proc Natl Acad Sci USA. 1999;96:185-190. [PubMed] |
| 7. | Liu Y, Linsley PS. Costimulation of T-cell growth. Curr Opin Immunol. 1992;4:265-270. [PubMed] |
| 8. | Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1034] [Cited by in RCA: 1103] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 9. | Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116-126. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1234] [Cited by in RCA: 1294] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 10. | Lenschow DJ, Su GH, Zuckerman LA, Nabavi N, Jellis CL, Gray GS, Miller J, Bluestone JA. Expression and functional significance of an additional ligand for CTLA-4. Proc Natl Acad Sci USA. 1993;90:11054-11058. [PubMed] |
| 11. | Hathcock KS, Laszlo G, Pucillo C, Linsley P, Hodes RJ. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J Exp Med. 1994;180:631-640. [PubMed] |
| 12. | McAdam AJ, Schweitzer AN, Sharpe AH. The role of B7 co-stimulation in activation and differentiation of CD4+ and CD8+ T cells. Immunol Rev. 1998;165:231-247. [PubMed] |
| 13. | Jenkins MK, Taylor PS, Norton SD, Urdahl KB. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J Immunol. 1991;147:2461-2466. [PubMed] |
| 14. | Appleman LJ, Berezovskaya A, Grass I, Boussiotis VA. CD28 costimulation mediates T cell expansion via IL-2-independent and IL-2-dependent regulation of cell cycle progression. J Immunol. 2000;164:144-151. [PubMed] |
| 15. | Viola A, Lanzavecchia A. T cell activation determined by T cell receptor number and tunable thresholds. Science. 1996;273:104-106. [PubMed] |
| 16. | Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87-98. [PubMed] |
| 17. | Verbinnen B, Van Gool SW, Ceuppens JL. Blocking costimulatory pathways: prospects for inducing transplantation tolerance. Immunotherapy. 2010;2:497-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405-413. [PubMed] |
| 19. | Qureshi OS, Kaur S, Hou TZ, Jeffery LE, Poulter NS, Briggs Z, Kenefeck R, Willox AK, Royle SJ, Rappoport JZ. Constitutive clathrin-mediated endocytosis of CTLA-4 persists during T cell activation. J Biol Chem. 2012;287:9429-9440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 20. | Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7:445-450. [PubMed] |
| 21. | Carreno BM, Bennett F, Chau TA, Ling V, Luxenberg D, Jussif J, Baroja ML, Madrenas J. CTLA-4 (CD152) can inhibit T cell activation by two different mechanisms depending on its level of cell surface expression. J Immunol. 2000;165:1352-1356. [PubMed] |
| 22. | Collins AV, Brodie DW, Gilbert RJ, Iaboni A, Manso-Sancho R, Walse B, Stuart DI, van der Merwe PA, Davis SJ. The interaction properties of costimulatory molecules revisited. Immunity. 2002;17:201-210. [PubMed] |
| 23. | Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985-988. [PubMed] |
| 24. | Mandelbrot DA, McAdam AJ, Sharpe AH. B7-1 or B7-2 is required to produce the lymphoproliferative phenotype in mice lacking cytotoxic T lymphocyte-associated antigen 4 (CTLA-4). J Exp Med. 1999;189:435-440. [PubMed] |
| 25. | Tai X, Van Laethem F, Sharpe AH, Singer A. Induction of autoimmune disease in CTLA-4-/- mice depends on a specific CD28 motif that is required for in vivo costimulation. Proc Natl Acad Sci USA. 2007;104:13756-13761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Bour-Jordan H, Esensten JH, Martinez-Llordella M, Penaranda C, Stumpf M, Bluestone JA. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/ B7 family. Immunol Rev. 2011;241:180-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 316] [Cited by in RCA: 315] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 27. | Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459-465. [PubMed] |
| 28. | Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1138] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 29. | Nurieva RI. Regulation of immune and autoimmune responses by ICOS-B7h interaction. Clin Immunol. 2005;115:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Dong C, Temann UA, Flavell RA. Cutting edge: critical role of inducible costimulator in germinal center reactions. J Immunol. 2001;166:3659-3662. [PubMed] |
| 31. | McAdam AJ, Greenwald RJ, Levin MA, Chernova T, Malenkovich N, Ling V, Freeman GJ, Sharpe AH. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 506] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 32. | Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765-772. [PubMed] |
| 33. | Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027-1034. [PubMed] |
| 34. | Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2021] [Cited by in RCA: 2297] [Article Influence: 91.9] [Reference Citation Analysis (0)] |
| 35. | Nishimura H, Honjo T, Minato N. Facilitation of beta selection and modification of positive selection in the thymus of PD-1-deficient mice. J Exp Med. 2000;191:891-898. [PubMed] |
| 36. | van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67:2-17. [PubMed] |
| 37. | Bishop GA, Moore CR, Xie P, Stunz LL, Kraus ZJ. TRAF proteins in CD40 signaling. Adv Exp Med Biol. 2007;597:131-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 147] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 38. | Mackey MF, Barth RJ, Noelle RJ. The role of CD40/CD154 interactions in the priming, differentiation, and effector function of helper and cytotoxic T cells. J Leukoc Biol. 1998;63:418-428. [PubMed] |
| 39. | Karpusas M, Hsu YM, Wang JH, Thompson J, Lederman S, Chess L, Thomas D. 2 A crystal structure of an extracellular fragment of human CD40 ligand. Structure. 1995;3:1031-1039. [PubMed] |
| 40. | Stark R, Hartung A, Zehn D, Frentsch M, Thiel A. IL-12-mediated STAT4 signaling and TCR signal strength cooperate in the induction of CD40L in human and mouse CD8+ T cells. Eur J Immunol. 2013;43:1511-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Frentsch M, Stark R, Matzmohr N, Meier S, Durlanik S, Schulz AR, Stervbo U, Jürchott K, Gebhardt F, Heine G. CD40L expression permits CD8+ T cells to execute immunologic helper functions. Blood. 2013;122:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 42. | Koguchi Y, Buenafe AC, Thauland TJ, Gardell JL, Bivins-Smith ER, Jacoby DB, Slifka MK, Parker DC. Preformed CD40L is stored in Th1, Th2, Th17, and T follicular helper cells as well as CD4+ 8- thymocytes and invariant NKT cells but not in Treg cells. PLoS One. 2012;7:e31296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 43. | Banchereau J, Bazan F, Blanchard D, Brière F, Galizzi JP, van Kooten C, Liu YJ, Rousset F, Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 922] [Cited by in RCA: 913] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 44. | Foy TM, Aruffo A, Bajorath J, Buhlmann JE, Noelle RJ. Immune regulation by CD40 and its ligand GP39. Annu Rev Immunol. 1996;14:591-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 491] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 45. | Van Kooten C, Banchereau J. CD40-CD40 ligand: a multifunctional receptor-ligand pair. Adv Immunol. 1996;61:1-77. [PubMed] |
| 46. | Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10867] [Cited by in RCA: 10815] [Article Influence: 386.3] [Reference Citation Analysis (0)] |
| 47. | Rousset F, Garcia E, Banchereau J. Cytokine-induced proliferation and immunoglobulin production of human B lymphocytes triggered through their CD40 antigen. J Exp Med. 1991;173:705-710. [PubMed] |
| 48. | Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T, Kikutani H. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167-178. [PubMed] |
| 49. | Grewal IS, Flavell RA. The role of CD40 ligand in costimulation and T-cell activation. Immunol Rev. 1996;153:85-106. [PubMed] |
| 50. | Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1617] [Cited by in RCA: 1649] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 51. | Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 730] [Cited by in RCA: 713] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 52. | Seko Y, Ishiyama S, Nishikawa T, Kasajima T, Hiroe M, Suzuki S, Ishiwata S, Kawai S, Tanaka Y, Azuma M. Expression of tumor necrosis factor ligand superfamily costimulatory molecules CD27L, CD30L, OX40L and 4-1BBL in the heart of patients with acute myocarditis and dilated cardiomyopathy. Cardiovasc Pathol. 2002;11:166-170. [PubMed] |
| 53. | Burgess JK, Carlin S, Pack RA, Arndt GM, Au WW, Johnson PR, Black JL, Hunt NH. Detection and characterization of OX40 ligand expression in human airway smooth muscle cells: a possible role in asthma? J Allergy Clin Immunol. 2004;113:683-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 54. | Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 827] [Cited by in RCA: 859] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 55. | Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1981] [Cited by in RCA: 2055] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 56. | Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1729] [Cited by in RCA: 1847] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 57. | Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5576] [Cited by in RCA: 5960] [Article Influence: 259.1] [Reference Citation Analysis (0)] |
| 58. | Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1827] [Cited by in RCA: 1980] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 59. | Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1371] [Cited by in RCA: 1408] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 60. | Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875-1886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3466] [Cited by in RCA: 3837] [Article Influence: 174.4] [Reference Citation Analysis (7)] |
| 61. | Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149-5153. [PubMed] |
| 62. | Knoechel B, Lohr J, Kahn E, Bluestone JA, Abbas AK. Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J Exp Med. 2005;202:1375-1386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 254] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 63. | Petrausch U, Jensen SM, Twitty C, Poehlein CH, Haley DP, Walker EB, Fox BA. Disruption of TGF-beta signaling prevents the generation of tumor-sensitized regulatory T cells and facilitates therapeutic antitumor immunity. J Immunol. 2009;183:3682-3689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 64. | Sawamukai N, Satake A, Schmidt AM, Lamborn IT, Ojha P, Tanaka Y, Kambayashi T. Cell-autonomous role of TGFβ and IL-2 receptors in CD4+ and CD8+ inducible regulatory T-cell generation during GVHD. Blood. 2012;119:5575-5583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 65. | Maldonado RA, von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol. 2010;108:111-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 407] [Cited by in RCA: 423] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 66. | Elias KM, Laurence A, Davidson TS, Stephens G, Kanno Y, Shevach EM, O’Shea JJ. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2008;111:1013-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 358] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 67. | Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, Kuchroo VK. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol. 2008;181:2277-2284. [PubMed] |
| 68. | Bilate AM, Lafaille JJ. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol. 2012;30:733-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 469] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 69. | Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433-3441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1089] [Cited by in RCA: 1110] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 70. | Verhagen J, Wraith DC. Comment on “Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells”. J Immunol. 2010;185:7129; author reply 7130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 71. | Akimova T, Beier UH, Wang L, Levine MH, Hancock WW. Helios expression is a marker of T cell activation and proliferation. PLoS One. 2011;6:e24226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 316] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 72. | Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, Xiong H, Dolpady J, Frey AB, Ruocco MG. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J Exp Med. 2012;209:1723-1742, S1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 428] [Cited by in RCA: 504] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 73. | Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2752] [Cited by in RCA: 2727] [Article Influence: 94.0] [Reference Citation Analysis (13)] |
| 74. | Miller A, Lider O, Roberts AB, Sporn MB, Weiner HL. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen-specific triggering. Proc Natl Acad Sci USA. 1992;89:421-425. [PubMed] |
| 75. | Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207-214. [PubMed] |
| 76. | Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287-296. [PubMed] |
| 77. | Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969-1980. [PubMed] |
| 78. | Yamazaki S, Iyoda T, Tarbell K, Olson K, Velinzon K, Inaba K, Steinman RM. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J Exp Med. 2003;198:235-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 675] [Cited by in RCA: 659] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 79. | Tonkin DR, He J, Barbour G, Haskins K. Regulatory T cells prevent transfer of type 1 diabetes in NOD mice only when their antigen is present in vivo. J Immunol. 2008;181:4516-4522. [PubMed] |
| 80. | Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2013] [Cited by in RCA: 2318] [Article Influence: 128.8] [Reference Citation Analysis (0)] |
| 81. | Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303-310. [PubMed] |
| 82. | Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1406] [Cited by in RCA: 1390] [Article Influence: 92.7] [Reference Citation Analysis (3)] |
| 83. | Munn DH, Sharma MD, Mellor AL. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol. 2004;172:4100-4110. [PubMed] |
| 84. | Dejean AS, Beisner DR, Ch’en IL, Kerdiles YM, Babour A, Arden KC, Castrillon DH, DePinho RA, Hedrick SM. Transcription factor Foxo3 controls the magnitude of T cell immune responses by modulating the function of dendritic cells. Nat Immunol. 2009;10:504-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 85. | Veldhoen M, Moncrieffe H, Hocking RJ, Atkins CJ, Stockinger B. Modulation of dendritic cell function by naive and regulatory CD4+ T cells. J Immunol. 2006;176:6202-6210. [PubMed] |
| 86. | Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995-1004. [PubMed] |
| 87. | Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 658] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 88. | Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1324] [Cited by in RCA: 1551] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 89. | Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, Ley TJ. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 743] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 90. | Andersson J, Tran DQ, Pesu M, Davidson TS, Ramsey H, O’Shea JJ, Shevach EM. CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-beta-dependent manner. J Exp Med. 2008;205:1975-1981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 276] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 91. | Bopp T, Becker C, Klein M, Klein-Hessling S, Palmetshofer A, Serfling E, Heib V, Becker M, Kubach J, Schmitt S. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp Med. 2007;204:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 476] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 92. | Ernst PB, Garrison JC, Thompson LF. Much ado about adenosine: adenosine synthesis and function in regulatory T cell biology. J Immunol. 2010;185:1993-1998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 155] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 93. | Pace KE, Lee C, Stewart PL, Baum LG. Restricted receptor segregation into membrane microdomains occurs on human T cells during apoptosis induced by galectin-1. J Immunol. 1999;163:3801-3811. [PubMed] |
| 94. | Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 799] [Cited by in RCA: 913] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 95. | Yu A, Zhu L, Altman NH, Malek TR. A low interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells. Immunity. 2009;30:204-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 216] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 96. | Papiernik M, de Moraes ML, Pontoux C, Vasseur F, Pénit C. Regulatory CD4 T cells: expression of IL-2R alpha chain, resistance to clonal deletion and IL-2 dependency. Int Immunol. 1998;10:371-378. [PubMed] |
| 97. | Wing JB, Sakaguchi S. Multiple treg suppressive modules and their adaptability. Front Immunol. 2012;3:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 98. | Read S, Greenwald R, Izcue A, Robinson N, Mandelbrot D, Francisco L, Sharpe AH, Powrie F. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J Immunol. 2006;177:4376-4383. [PubMed] |
| 99. | Suri-Payer E, Cantor H. Differential cytokine requirements for regulation of autoimmune gastritis and colitis by CD4(+)CD25(+) T cells. J Autoimmun. 2001;16:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 178] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 100. | Vincenti F. Costimulation blockade in autoimmunity and transplantation. J Allergy Clin Immunol. 2008;121:299-306; quiz 307-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 101. | Lenschow DJ, Zeng Y, Thistlethwaite JR, Montag A, Brady W, Gibson MG, Linsley PS, Bluestone JA. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992;257:789-792. [PubMed] |
| 102. | Lin H, Bolling SF, Linsley PS, Wei RQ, Gordon D, Thompson CB, Turka LA. Long-term acceptance of major histocompatibility complex mismatched cardiac allografts induced by CTLA4Ig plus donor-specific transfusion. J Exp Med. 1993;178:1801-1806. [PubMed] |
| 103. | Blazar BR, Taylor PA, Linsley PS, Vallera DA. In vivo blockade of CD28/CTLA4: B7/BB1 interaction with CTLA4-Ig reduces lethal murine graft-versus-host disease across the major histocompatibility complex barrier in mice. Blood. 1994;83:3815-3825. [PubMed] |
| 104. | Pearson TC, Alexander DZ, Winn KJ, Linsley PS, Lowry RP, Larsen CP. Transplantation tolerance induced by CTLA4-Ig. Transplantation. 1994;57:1701-1706. [PubMed] |
| 105. | Cross AH, Girard TJ, Giacoletto KS, Evans RJ, Keeling RM, Lin RF, Trotter JL, Karr RW. Long-term inhibition of murine experimental autoimmune encephalomyelitis using CTLA-4-Fc supports a key role for CD28 costimulation. J Clin Invest. 1995;95:2783-2789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 126] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 106. | Lenschow DJ, Ho SC, Sattar H, Rhee L, Gray G, Nabavi N, Herold KC, Bluestone JA. Differential effects of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J Exp Med. 1995;181:1145-1155. [PubMed] |
| 107. | Finck BK, Linsley PS, Wofsy D. Treatment of murine lupus with CTLA4Ig. Science. 1994;265:1225-1227. [PubMed] |
| 108. | Davies JK, Gribben JG, Brennan LL, Yuk D, Nadler LM, Guinan EC. Outcome of alloanergized haploidentical bone marrow transplantation after ex vivo costimulatory blockade: results of 2 phase 1 studies. Blood. 2008;112:2232-2241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 109. | Koura DT, Horan JT, Langston AA, Qayed M, Mehta A, Khoury HJ, Harvey RD, Suessmuth Y, Couture C, Carr J. In vivo T cell costimulation blockade with abatacept for acute graft-versus-host disease prevention: a first-in-disease trial. Biol Blood Marrow Transplant. 2013;19:1638-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 110. | Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, Birbara C, Box J, Natarajan K, Nuamah I. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353:1114-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 862] [Cited by in RCA: 896] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 111. | Charpentier B. Belatacept: a novel immunosuppressive agent for kidney transplant recipients. Expert Rev Clin Immunol. 2012;8:719-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 112. | Blazar BR, Taylor PA, Panoskaltsis-Mortari A, Buhlman J, Xu J, Flavell RA, Korngold R, Noelle R, Vallera DA. Blockade of CD40 ligand-CD40 interaction impairs CD4+ T cell-mediated alloreactivity by inhibiting mature donor T cell expansion and function after bone marrow transplantation. J Immunol. 1997;158:29-39. [PubMed] |
| 113. | Larsen CP, Alexander DZ, Hollenbaugh D, Elwood ET, Ritchie SC, Aruffo A, Hendrix R, Pearson TC. CD40-gp39 interactions play a critical role during allograft rejection. Suppression of allograft rejection by blockade of the CD40-gp39 pathway. Transplantation. 1996;61:4-9. [PubMed] |
| 114. | Bumgardner GL, Li J, Heininger M, Orosz CG. Costimulation pathways in host immune responses to allogeneic hepatocytes. Transplantation. 1998;66:1841-1845. [PubMed] |
| 115. | Molano RD, Berney T, Li H, Cattan P, Pileggi A, Vizzardelli C, Kenyon NS, Ricordi C, Burkly LC, Inverardi L. Prolonged islet graft survival in NOD mice by blockade of the CD40-CD154 pathway of T-cell costimulation. Diabetes. 2001;50:270-276. [PubMed] |
| 116. | Tung TH, Mackinnon SE, Mohanakumar T. Long-term limb allograft survival using anti-CD40L antibody in a murine model. Transplantation. 2003;75:644-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 117. | Law CL, Grewal IS. Therapeutic interventions targeting CD40L (CD154) and CD40: the opportunities and challenges. Adv Exp Med Biol. 2009;647:8-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 118. | Sidiropoulos PI, Boumpas DT. Lessons learned from anti-CD40L treatment in systemic lupus erythematosus patients. Lupus. 2004;13:391-397. [PubMed] |
| 119. | CDP7657 , a Monovalent Fab PEG Anti-CD40L Antibody, Inhibits Immune Responses in Both HuSCID Mice and Non-Human Primates. Arthritis Rheum. 2010;Suppl 10: 1245. [DOI] [Full Text] |
| 120. | Pinelli DF, Wagener ME, Liu D, Yamniuk A, Tamura J, Grant S, Larsen CP, Suri A, Nadler SG, Ford ML. An anti-CD154 domain antibody prolongs graft survival and induces Foxp3(+) iTreg in the absence and presence of CTLA-4 Ig. Am J Transplant. 2013;13:3021-3030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 121. | Daley SR, Cobbold SP, Waldmann H. Fc-disabled anti-mouse CD40L antibodies retain efficacy in promoting transplantation tolerance. Am J Transplant. 2008;8:2265-2271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 122. | Margolles-Clark E, Umland O, Kenyon NS, Ricordi C, Buchwald P. Small-molecule costimulatory blockade: organic dye inhibitors of the CD40-CD154 interaction. J Mol Med (Berl). 2009;87:1133-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 123. | Allen SD, Rawale SV, Whitacre CC, Kaumaya PT. Therapeutic peptidomimetic strategies for autoimmune diseases: costimulation blockade. J Pept Res. 2005;65:591-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 124. | Tai YT, Li X, Tong X, Santos D, Otsuki T, Catley L, Tournilhac O, Podar K, Hideshima T, Schlossman R. Human anti-CD40 antagonist antibody triggers significant antitumor activity against human multiple myeloma. Cancer Res. 2005;65:5898-5906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 125. | Luqman M, Klabunde S, Lin K, Georgakis GV, Cherukuri A, Holash J, Goldbeck C, Xu X, Kadel EE, Lee SH. The antileukemia activity of a human anti-CD40 antagonist antibody, HCD122, on human chronic lymphocytic leukemia cells. Blood. 2008;112:711-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 126. | Byrd JC, Kipps TJ, Flinn IW, Cooper M, Odenike O, Bendiske J, Rediske J, Bilic S, Dey J, Baeck J. Phase I study of the anti-CD40 humanized monoclonal antibody lucatumumab (HCD122) in relapsed chronic lymphocytic leukemia. Leuk Lymphoma. 2012;53:2136-2142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 127. | Boon L, Brok HP, Bauer J, Ortiz-Buijsse A, Schellekens MM, Ramdien-Murli S, Blezer E, van Meurs M, Ceuppens J, de Boer M. Prevention of experimental autoimmune encephalomyelitis in the common marmoset (Callithrix jacchus) using a chimeric antagonist monoclonal antibody against human CD40 is associated with altered B cell responses. J Immunol. 2001;167:2942-2949. [PubMed] |
| 128. | Kasran A, Boon L, Wortel CH, Hogezand RA, Schreiber S, Goldin E, Boer M, Geboes K, Rutgeerts P, Ceuppens JL. Safety and tolerability of antagonist anti-human CD40 Mab ch5D12 in patients with moderate to severe Crohn’s disease. Aliment Pharmacol Ther. 2005;22:111-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 129. | Kopf M, Coyle AJ, Schmitz N, Barner M, Oxenius A, Gallimore A, Gutierrez-Ramos JC, Bachmann MF. Inducible costimulator protein (ICOS) controls T helper cell subset polarization after virus and parasite infection. J Exp Med. 2000;192:53-61. [PubMed] |
| 130. | Howland KC, Ausubel LJ, London CA, Abbas AK. The roles of CD28 and CD40 ligand in T cell activation and tolerance. J Immunol. 2000;164:4465-4470. [PubMed] |
| 131. | Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, Cho HR, Aruffo A, Hollenbaugh D, Linsley PS. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1204] [Cited by in RCA: 1158] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 132. | Kirk AD, Harlan DM, Armstrong NN, Davis TA, Dong Y, Gray GS, Hong X, Thomas D, Fechner JH, Knechtle SJ. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci USA. 1997;94:8789-8794. [PubMed] |
| 133. | Ito D, Ogasawara K, Iwabuchi K, Inuyama Y, Onoé K. Induction of CTL responses by simultaneous administration of liposomal peptide vaccine with anti-CD40 and anti-CTLA-4 mAb. J Immunol. 2000;164:1230-1235. [PubMed] |
| 134. | Schaub M, Issazadeh S, Stadlbauer TH, Peach R, Sayegh MH, Khoury SJ. Costimulatory signal blockade in murine relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol. 1999;96:158-166. [PubMed] |
| 135. | Wang X, Huang W, Mihara M, Sinha J, Davidson A. Mechanism of action of combined short-term CTLA4Ig and anti-CD40 ligand in murine systemic lupus erythematosus. J Immunol. 2002;168:2046-2053. [PubMed] |
| 136. | Verbinnen B, Billiau AD, Vermeiren J, Galicia G, Bullens DM, Boon L, Cadot P, Hens G, Dewolf-Peeters C, Van Gool SW. Contribution of regulatory T cells and effector T cell deletion in tolerance induction by costimulation blockade. J Immunol. 2008;181:1034-1042. [PubMed] |
| 137. | Wekerle T, Sachs DH, Sykes M. Mixed chimerism for the induction of tolerance: potential applicability in clinical composite tissue grafting. Transplant Proc. 1998;30:2708-2710. [PubMed] |
| 138. | Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med. 1999;5:1298-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 621] [Cited by in RCA: 601] [Article Influence: 22.3] [Reference Citation Analysis (13)] |
| 139. | Wells AD, Li XC, Li Y, Walsh MC, Zheng XX, Wu Z, Nuñez G, Tang A, Sayegh M, Hancock WW. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999;5:1303-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 463] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 140. | Li XC, Li Y, Dodge I, Wells AD, Zheng XX, Turka LA, Strom TB. Induction of allograft tolerance in the absence of Fas-mediated apoptosis. J Immunol. 1999;163:2500-2507. [PubMed] |
| 141. | Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 893] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 142. | Kurtz J, Ito H, Wekerle T, Shaffer J, Sykes M. Mechanisms involved in the establishment of tolerance through costimulatory blockade and BMT: lack of requirement for CD40L-mediated signaling for tolerance or deletion of donor-reactive CD4+ cells. Am J Transplant. 2001;1:339-349. [PubMed] |
| 143. | Honey K, Cobbold SP, Waldmann H. CD40 ligand blockade induces CD4+ T cell tolerance and linked suppression. J Immunol. 1999;163:4805-4810. [PubMed] |
| 144. | Graca L, Honey K, Adams E, Cobbold SP, Waldmann H. Cutting edge: anti-CD154 therapeutic antibodies induce infectious transplantation tolerance. J Immunol. 2000;165:4783-4786. [PubMed] |
| 145. | Taylor PA, Noelle RJ, Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J Exp Med. 2001;193:1311-1318. [PubMed] |
| 146. | Quezada SA, Bennett K, Blazar BR, Rudensky AY, Sakaguchi S, Noelle RJ. Analysis of the underlying cellular mechanisms of anti-CD154-induced graft tolerance: the interplay of clonal anergy and immune regulation. J Immunol. 2005;175:771-779. [PubMed] |
| 147. | Jiang X, Sun W, Guo D, Cui Z, Zhu L, Lin L, Tang Y, Wang X, Liang J. Cardiac allograft acceptance induced by blockade of CD40-CD40L costimulation is dependent on CD4+CD25+ regulatory T cells. Surgery. 2011;149:336-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 148. | Kurtz J, Shaffer J, Lie A, Anosova N, Benichou G, Sykes M. Mechanisms of early peripheral CD4 T-cell tolerance induction by anti-CD154 monoclonal antibody and allogeneic bone marrow transplantation: evidence for anergy and deletion but not regulatory cells. Blood. 2004;103:4336-4343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 149. | Tang Q, Henriksen KJ, Boden EK, Tooley AJ, Ye J, Subudhi SK, Zheng XX, Strom TB, Bluestone JA. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:3348-3352. [PubMed] |
| 150. | Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431-440. [PubMed] |
| 151. | Pacholczyk R, Kern J. The T-cell receptor repertoire of regulatory T cells. Immunology. 2008;125:450-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 152. | Martín-Gayo E, Sierra-Filardi E, Corbí AL, Toribio ML. Plasmacytoid dendritic cells resident in human thymus drive natural Treg cell development. Blood. 2010;115:5366-5375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 152] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 153. | McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311-323. [PubMed] |
| 154. | Vermeiren J, Ceuppens JL, Van Ghelue M, Witters P, Bullens D, Mages HW, Kroczek RA, Van Gool SW. Human T cell activation by costimulatory signal-deficient allogeneic cells induces inducible costimulator-expressing anergic T cells with regulatory cell activity. J Immunol. 2004;172:5371-5378. [PubMed] |
| 155. | Vogel I, Verbinnen B, Maes W, Boon L, Van Gool SW, Ceuppens JL. Foxp3+ regulatory T cells are activated in spite of B7-CD28 and CD40-CD40L blockade. Eur J Immunol. 2013;43:1013-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 156. | Coenen JJ, Koenen HJ, van Rijssen E, Hilbrands LB, Joosten I. Tolerizing effects of co-stimulation blockade rest on functional dominance of CD4+CD25+ regulatory T cells. Transplantation. 2005;79:147-156. [PubMed] |
| 157. | Poirier N, Azimzadeh AM, Zhang T, Dilek N, Mary C, Nguyen B, Tillou X, Wu G, Reneaudin K, Hervouet J. Inducing CTLA-4-dependent immune regulation by selective CD28 blockade promotes regulatory T cells in organ transplantation. Sci Transl Med. 2010;2:17ra10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 158. | Igarashi H, Cao Y, Iwai H, Piao J, Kamimura Y, Hashiguchi M, Amagasa T, Azuma M. GITR ligand-costimulation activates effector and regulatory functions of CD4+ T cells. Biochem Biophys Res Commun. 2008;369:1134-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 159. | Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, Berry G, DeKruyff RH, Umetsu DT. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002;8:1024-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 589] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 160. | Wang C, Li Y, Proctor TM, Vandenbark AA, Offner H. Down-modulation of programmed death 1 alters regulatory T cells and promotes experimental autoimmune encephalomyelitis. J Neurosci Res. 2010;88:7-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 161. | Verhagen J, Gabrysová L, Minaee S, Sabatos CA, Anderson G, Sharpe AH, Wraith DC. Enhanced selection of FoxP3+ T-regulatory cells protects CTLA-4-deficient mice from CNS autoimmune disease. Proc Natl Acad Sci USA. 2009;106:3306-3311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 162. | Tang AL, Teijaro JR, Njau MN, Chandran SS, Azimzadeh A, Nadler SG, Rothstein DM, Farber DL. CTLA4 expression is an indicator and regulator of steady-state CD4+ FoxP3+ T cell homeostasis. J Immunol. 2008;181:1806-1813. [PubMed] |
| 163. | Yamaguchi T, Kishi A, Osaki M, Morikawa H, Prieto-Martin P, Wing K, Saito T, Sakaguchi S. Construction of self-recognizing regulatory T cells from conventional T cells by controlling CTLA-4 and IL-2 expression. Proc Natl Acad Sci USA. 2013;110:E2116-E2125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 164. | Barnes MJ, Griseri T, Johnson AM, Young W, Powrie F, Izcue A. CTLA-4 promotes Foxp3 induction and regulatory T cell accumulation in the intestinal lamina propria. Mucosal Immunol. 2013;6:324-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 165. | Xiao X, Gong W, Demirci G, Liu W, Spoerl S, Chu X, Bishop DK, Turka LA, Li XC. New insights on OX40 in the control of T cell immunity and immune tolerance in vivo. J Immunol. 2012;188:892-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 166. | Li W, Carlson TL, Green WR. Stimulation-dependent induction of CD154 on a subset of CD4+ FoxP3+ T-regulatory cells. Int Immunopharmacol. 2011;11:1205-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 167. | Riella LV, Liu T, Yang J, Chock S, Shimizu T, Mfarrej B, Batal I, Xiao X, Sayegh MH, Chandraker A. Deleterious effect of CTLA4-Ig on a Treg-dependent transplant model. Am J Transplant. 2012;12:846-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 168. | Charbonnier LM, Vokaer B, Lemaître PH, Field KA, Leo O, Le Moine A. CTLA4-Ig restores rejection of MHC class-II mismatched allografts by disabling IL-2-expanded regulatory T cells. Am J Transplant. 2012;12:2313-2321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 169. | Tai X, Van Laethem F, Pobezinsky L, Guinter T, Sharrow SO, Adams A, Granger L, Kruhlak M, Lindsten T, Thompson CB. Basis of CTLA-4 function in regulatory and conventional CD4(+) T cells. Blood. 2012;119:5155-5163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 198] [Article Influence: 14.1] [Reference Citation Analysis (1)] |
P- Reviewer: Camara NOS, Chui YL, Haque A S- Editor: Ji FF L- Editor: A E- Editor: Wang CH