Revised: March 12, 2012
Accepted: December 23, 2012
Published online: March 27, 2013
AIM: To study the effect of blocking the eo-2 pathway on the development and severity of experimental autoimmune encephalomyelitis (EAE).
METHODS: We produced mAb directed against eo-2, named D8. MOG35-55 induced-EAE mice were daily intravenously injected with either 25 μg or 100 μg D8, or with vehicle control alone [phosphate-buffered saline (PBS)], starting from day 0 post immunization and were monitored for EAE clinical score (n = 10 in each group). Mice were sacrificed on day 58 and their sera were assessed for the presence of anti-myelin oligodendrocyte glycoprotein (anti-MOG) antibodies autoantibodies, as well as for the profile of pro-inflammatory cytokines and chemokines. Histological analysis of brain sections was performed by hematoxylin and eosin staining.
RESULTS: Daily treatment of EAE induced mice with D8 significantly decreased the severity of EAE symptoms. Treatment with both concentrations of D8 ameliorated EAE symptoms compared to PBS treated mice, starting from day 42 post immunization (0.89 ± 0.35 in D8 25 μg and D8 100 μg treated groups vs 2.11 ± 0.38 in the PBS treated group, P = 0.03). A significant improvement in EAE clinical score compared to total IgG treated mice was observed with the higher concentration of D8 (0.81 ± 0.38 in D8 100 μg treated group vs 2.11 ± 0.31 in IgG1 treated group, on day 56 post immunization, P = 0.04). D8 treated mice with EAE did not significantly exhibit lower sera levels of anti-MOG autoantibodies compared to IgG-treated mice. However, they expressed lower sera levels of the pro-inflammatory cytokines: tumor necrosis factor (7.8 ± 0.2 pg/mL in D8 100 μg treated mice vs 19.9 ± 3.4 pg/mL in IgG treated mice, P = 0.005) and interferon-gamma (1.4 ± 0.6 pg/mL in D8 100 μg treated mice vs 3.6 ± 0.4 pg/mL in IgG treated mice, P = 0.02), as well as reduced levels of the chemokine macrophage chemoattractant protein-1 (27.2 ± 3.1 pg/mL in D8 100 μg treated mice vs 63.7 ± 12.3 pg/mL in IgG treated mice, P = 0.03). These findings indicate that blocking the eo-2 pathway in EAE may affect not only eosinophil infiltration into the central nervous system (CNS), but also have an effect on monocytes and T cells, but not humoral, mediated responses. Histological analysis of the brains of D8 treated mice with EAE support that this treatment decreases immune cells infiltrates in the CNS.
CONCLUSION: Taken together, these findings suggest a role for eo-2 in EAE pathogenesis and consequentially may support a therapeutic potential of anti-eo-2 neutralizing mAb in multiple sclerosis.
- Citation: Mausner-Fainberg K, Karni A, George J, Entin-Meer M, Afek A. Eotaxin-2 blockade ameliorates experimental autoimmune encephalomyelitis. World J Immunol 2013; 3(1): 7-14
- URL: https://www.wjgnet.com/2219-2824/full/v3/i1/7.htm
- DOI: https://dx.doi.org/10.5411/wji.v3.i1.7
Experimental autoimmune encephalomyelitis (EAE) is a T helper cell type 1 (Th1) mediated demyelinating disease of the central nervous system (CNS) that serves as an animal model for multiple sclerosis (MS)[1-3]. EAE can either be induced by active immunization with whole myelin or a variety of myelin antigens plus adjuvant, or by passive transfer of encephalitogenic T cells. During induction of EAE, T cells sensitized to myelin antigens migrate across the blood-brain barrier (BBB) into surrounding white matter[4], re-encounter antigen and become stimulated to release proinflammatory cytokines[5] and chemokines[6], for which there is compelling evidence for roles in lesion pathogenesis, including dysfunction of the BBB, demyelination, axonal injury and neurodegeneration[3].
Chemokines are chemoattractants produced under pathological conditions by tissue elements and infiltrating leukocytes[7], which were found to be involved, not only in leukocyte trafficking, but also in leukocyte maturation and renewal of circulating leukocytes[8]. During EAE, involvement and up-regulation of several CC chemokines, including macrophage inhibitory protein-1a (MIP-1a) and macrophage chemoattractant protein-1 (MCP-1), are well established[9]. In vivo neutralization studies have shown a distinct role for MIP-1a in the pathogenesis of acute EAE and for MCP-1 in relapsing EAE[10].
Eosinophil chemotactic protein 2 (eotaxin-2 or eo-2), also known as CC ligand 24 (CCL24) or myeloid progenitor inhibitory factor 2 (MPIF-2), is a CC chemokine which interacts with the CC chemokine receptor 3 (CCR3) to induce chemotaxis in eosinophils[11]. This chemokine was also found to be strongly chemotactic for basophils and resting T lymphocytes, and slightly chemotactic for neutrophils[12]. Eo-2 mRNA is expressed in activated T lymphocytes, GM-CSF treated macrophages[12] and dermal fibroblasts[13], indicating a possible route for cross-talk between activated T lymphocytes and macrophages with eosinophils.
The role of eo-2 in eosinophils-mediated classic disorders, such as asthma[14], chronic bronchitis[15] and allergic reactions[16], has been well established. However, it should be noted that the eo-2 receptor CCR3 expression is not restricted to eosinophils but it is also expressed on other inflammatory cells, such as monocytes[17], mast cells[18], peripheral memory T cells[19], Th2 lymphocytes[20] and immature dendritic cells[21]. This emphasizes the complexity of the eo-2/CCR3 system and raises the possibility of eo-2/CCR3 system involvement in a wide range of inflammatory and autoimmune disorders, far exceeding its role in allergy and atopy. Indeed, it has been previously shown that CCR2, CCR3 and CCR5 expression is elevated in MS CNS tissue compared to control CNS tissue, suggesting that the eo-2/CCR3 system might also be involved in MS pathogenesis[22].
We have recently demonstrated that treatment of adjuvant-induced arthritis (AIA), a commonly used animal model of rheumatoid arthritis (RA), with our developed D8 anti-eo-2 neutralizing mAb was effective in ameliorating AIA, both as a preventive treatment given before development of arthritis and as a therapeutic agent given at the time of the initial manifestation of arthritis[23].
The aims of the current study were: to evaluate the effect of blocking the eo-2 pathway on the development and severity of EAE; to study the effect of this treatment on humoral-mediated response in our EAE model, i.e., sera levels of anti-myelin oligodendrocyte glycoprotein antibody (anti-MOG) autoantibodies; and on the levels of the cytokines: interleukin-6 (IL-6), interferon-γ (IFN-γ), tumor necrosis factor (TNF), IL-12p70 and MCP-1.
We have produced several clones of monoclonal antibodies (mAbs) against eo-2, according to standard protocols. Briefly, Balb/C mice were immunized with 20 g of eo-2 (Peprotech, Rocky Hill, NJ, United States) followed by 4 additional boosts. After confirming the presence of polyclonal anti-eo-2 Abs in the sera, mice were sacrificed and their spleens were hybridized with a NS/0 myeloma line, followed by clonal screening for binding to eo-2. The hybridomas were then grown in serum-free media for 2-3 wk and media collected and loaded onto 100 kDa centricons (Biological Industries, Beit Haemek, Israel) for antibody concentration. D8 refers to the anti-eo-2 mAb clone which was selected to treat the mice with EAE. The cross-reactivity of D8 between human and murine eotaxin-2 [5 μg eotaxin-2 diluted in phosphate-buffered saline (PBS)], with Kd of 0.77 mg and 4 mg, respectively, was determined.
EAE was induced in 6-8 wk C57BL/6 female mice (Harlan Laboratories, Jerusalem, Israel) by subcutaneous immunization on days 0 and 7 at two sites with 200 μg/mouse myelin-oligodendrocyte glycoprotein peptide (MOG35-55, synthesized by Sigma-Aldrich) in 100 μL PBS. The peptide was emulsified in an equal volume of Complete Freund’s Adjuvant (CFA, from DIFCO) containing 500 μg Mycobacterium tuberculosis H37RA (MT, from DIFCO)[24]. Mice were maintained at the local animal facility and all procedures were performed under the supervision and guidelines of the Animal Welfare Committee.
EAE-induced mice were injected daily intraperitoneally with either 25 μg or 100 μg D8, or with vehicle control only (PBS), starting from the day of immunization (day 0). Animals were monitored for symptoms of EAE and scored as follows: 0, no disease; 1, tail paralysis; 2, hind limb weakness; 3, hind limb paralysis; 4, hind limb plus forelimb paralysis; and 5, moribund/death.
Mice were sacrificed on day 58 and their sera were assessed for the presence of anti-MOG autoantibodies. For this purpose, a flat-bottom 96-well plate (Greiner bio-one) was coated with 10 μg/mL MOG35-55 peptide (Sigma-Aldrich) in carbonate buffer (0.05 mol/L NaHCOO3, pH 9.5) overnight at 4 °C. The next day, the plate was blocked with 2% bovine serum albumin (BSA, Sigma-Aldrich) in PBS for 1 hour at room temperature. To detect serum antibodies, sera were diluted 1/25 in PBS with 0.5% BSA. The diluted sera were then added to the plates (100 μL/well in duplicates) and incubated for 2 h at room temperature. Bound antibodies were detected using 1/8000 diluted horseradish-peroxidase (HRP) conjugated goat anti-mouse IgG secondary antibody (Santa-Cruz Biotechnology, United States). 3,3’,5,5’-Tetramethylbenzidine (TMB) reagent (Chemicon-Millipore) was used as a substrate solution and the reaction was halted by the addition of 1 mol/L H2SO4. Absorbance at 450 nm was measured using a Termo Max ELISA reader (Molecular Devices microplate reader, United States).
Sera of EAE-induced mice were assessed for the presence of IL-6, IFN-γ, TNF-α, IL-12p70 and MCP-1 using the BD™ Cytometric Bead Array (CBA) Mouse Inflammation Kit, according to the manufacturer’s instructions (BD Biosciences, United States). Briefly, test samples or recombinant standards of the cytokines were incubated with beads coated with capture antibodies specific for IL-6, IFN-γ, TNF, IL-12p70 and MCP-1 proteins and PE-conjugated detection antibodies to form sandwich complexes. Samples were analyzed on a FACScan flow cytometer, using CellQuest software (Becton Dickinson).
EAE-induced mice and their healthy C57BL/6 littermates brains were removed, snap-frozen and kept at -80 °C until examination. Brains were sectioned at 8 μm and stained with hematoxylin and eosin.
Two-tailed Student’s t test was performed when 2 groups were compared. The 1-way analysis of variance (ANOVA), followed by Tukey’s test for multiple comparisons, was carried out for statistical analysis of the clinical course of EAE. P < 0.05 was considered statistically significant. Results are expressed as mean ± SEM unless otherwise specified in the text.
Monoclonal antibodies against human eo-2 were developed in our laboratory. As previously described[24], of our newly-developed monoclonal antibodies, D8 was selected for in vivo treatment since it has been demonstrated to possess neutralizing activity, i.e., to inhibit adhesion of murine and rat splenocytes as well as human peripheral blood mononuclear cells (PBMCs) to fibronectin, to inhibit their migration towards vascular endothelial growth factor (VEGF) and to reduce adhesion of HEK cells stably transfected with CCR3 to eo-2 (data not shown), indicating that D8 interferes with the CCR3/eo-2 binding interaction.
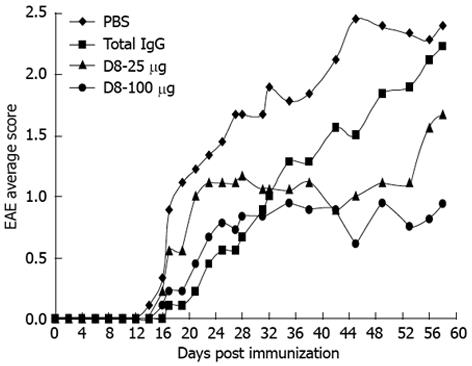
A moderate model of monophasic (progressive) EAE was achieved by immunization of C57BL/6 mice with two following subcutaneous injections of MOG35-55 peptide, emulsified in CFA, with an interval of 1 wk[25]. EAE-induced mice were injected daily intraperitoneally with 25 μg or 100 μg D8, starting from day 0 post immunization. EAE mice treated with total mouse IgG, or with vehicle control only (PBS) served as negative controls. As shown in Figure 1, all EAE-induced mice started to display clinical symptoms on days 14-17 post immunization. As expected from this monophasic model, a gradual increase in clinical score was observed in PBS-treated EAE mice until a maximal average score of 2.44 was observed on day 45, which remained constant until day 58. A similar trend, although more moderate, of a gradual increase in EAE severity, was also observed in total IgG treated mice until a maximal average score of 2.22 was observed on day 58, indicating that total IgG treatment did not significantly affect EAE severity. Interestingly, though initially both D8 doses (25 μg and 100 μg) exhibited a higher average clinical score in comparison to the total IgG treated group, this trend was inverted on day 32, from which both D8 treated groups exhibited an improved average score compared to PBS and IgG treated mice. Treatment with both concentrations of D8 led to a significant improvement in EAE clinical score compared to PBS treated mice. This significant effect was first observed on day 42, in which D8 25 μg and D8 100 μg treatment led to a decline of 57.9% in average clinical score (0.89 ± 0.35 in D8 25 μg and D8 100 μg treated groups vs 2.11 ± 0.38 in IgG treated group, n = 10 in each group, P = 0.03), and remained constant until day 58.
However, a significant improvement in EAE clinical score compared to total IgG treated mice was observed only with the higher concentration of D8 on day 56, in which treatment with D8 100 μg led to a decline of 61.5% in average clinical score (0.81 ± 0.38 in D8 100 μg treated group vs 2.11 ± 0.31 in PBS treated group, n = 10 in each group, P = 0.04). Thus, it can be concluded that treatment with both concentrations of D8 ameliorated EAE severity, although it appears that treatment with the higher concentration of D8 (100 μg) is more effective.
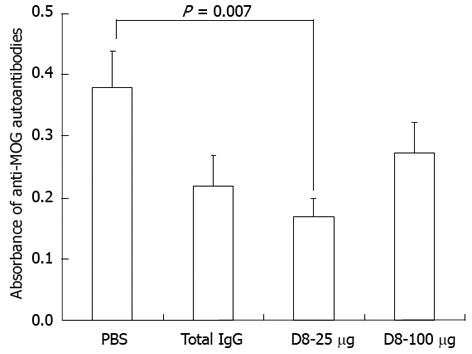
In contrast to other models, MOG35-55 protein elicited EAE is also characterized by a pathogenic antibody response. Although anti-MOG antibodies cannot induce EAE on their own, they strongly enhance T cell and macrophage-initiated demyelination and may augment disease severity[25,26]. Since it has been previously demonstrated that the severity of EAE might correlate with the presence of MOG-specific autoantibodies, our next purpose was to examine the effect of anti-eo-2 neutralizing mAb treatment on serum levels of anti-MOG autoantibodies. As demonstrated in Figure 2, although treatment with 25 μg D8 led to a significant decrease of 55.6% in the level of anti-MOG IgG antibodies compared to PBS treatment, as detected in EAE-induced mice sera on day 58 (n = 9 in each group, P = 0.007), no significant effect in the level of anti-MOG IgG antibodies was seen in both D8 treated groups compared to the IgG treated group, indicating that the clinical anti-eo-2 neutralizing mAb treatment effect in EAE is probably not mediated through the humoral anti-MOG antibodies response.
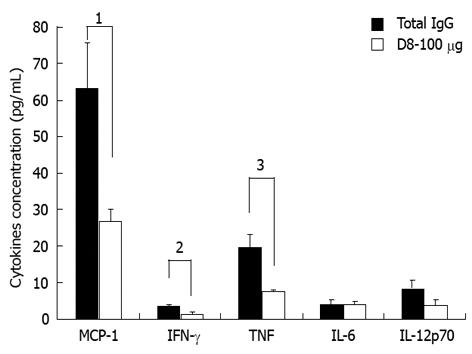
We next examined the effect of anti-eo-2 neutralizing mAb treatment on serum levels of the cytokines IL-6, IFN-γ, TNF-α, IL-12p70 and the chemokine MCP-1. As shown in Figure 3, treatment of EAE-induced mice with D8 100 μg led to a significant decrease of 57.3% in serum levels of MCP-1 compared to IgG treatment (27.2 ± 3.1 pg/mL in D8 100 μg treated mice vs 63.7 ± 12.3 pg/mL in IgG treated mice, P = 0.03), a decrease of 61.2% in serum levels of IFN-γ (1.4 ± 0.6 pg/mL in D8 100 μg treated mice vs 3.6 ± 0.4 pg/mL in IgG treated mice, P = 0.02) and a reduction of 60.8% in levels of TNF-α (7.8 ± 0.2 pg/mL in D8 100 μg treated mice vs 19.9 ± 3.4 pg/mL in IgG treated mice, P = 0.005). Although a similar trend for reduction of IL-12p70 sera levels was accepted in D8 100 μg treated mice vs total IgG treated mice, this effect was found to be non significant (3.9 ± 1.5 pg/mL in D8 100 μg treated mice vs 8.6 ± 4.2 pg/mL in IgG treated mice, P = not significant). Serum levels of IL-6 did not seem to be affected by D8- 100 μg treatment.
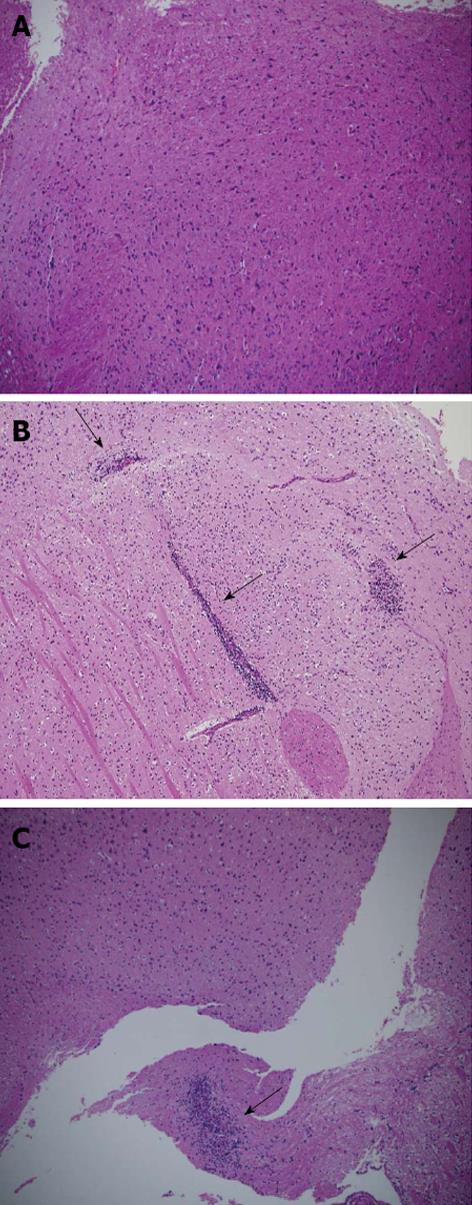
Histopathological analysis of EAE-induced mice brains, treated with either D8 100 μg or with IgG, and their healthy C57BL/6 littermates, demonstrates that the extent of cellular infiltration in the D8 100 μg treated group is very mild compared with the IgG treated group (Figure 4).
Although eosinophils have been observed in the spinal fluid of MS patients[27,28], their role in MS pathology has been poorly investigated. Gladue et al[29] reported that EAE treatment with the specific LTB4 receptor antagonist CP-105,696 selectively inhibited eosinophils recruitment into the spinal cord, without inhibition of lymphocyte infiltration into the CNS, and concomitantly prevented EAE symptoms. This finding led to the hypothesis that the role of eosinophils in EAE may have been underestimated in previous studies and that blockade of eosinophil infiltration into the CNS may represent a potential therapeutic target in MS, in addition to the well known strategy of restraining activated T cells and monocytes.
In this current study, we blocked the eo-2 pathway directly involved in eosinophil migration in EAE-induced mice, by our developed specific D8 anti-eo-2 neutralizing mAb. Treatment with D8 significantly ameliorated EAE clinical score in a trend of a dose-dependent manner. Whereas the trend of an improved clinical score in both D8 treated groups vs PBS treated group was observed during the whole experiment, ameliorated EAE symptoms in both D8 treated groups vs IgG treated group was seen only from day 32. This finding could imply that although the initial beneficial effect of D8 is probably not specific, a specific effect of blocking the eo-2 pathway, mediated by the mAb D8 occurs in later stages of the disease.
Theoretically, the clinical beneficial effect of blocking the eo-2 pathway in EAE could be explained merely by inhibiting eosinophil infiltration into the CNS[29]. We hypothesized that this therapeutic effect of D8 involves an expanded immune reaction and might also be mediated via restraining T cells and monocyte responses since MS is rarely associated with eosinophilia.
Although we found that treatment of EAE-induced mice with D8 did not significantly affect the humoral response, as examined by the level of anti-MOG IgG autoantibodies in mice sera, it had a significant impact on T cell and monocyte mediated responses, i.e., the level of the proinflammatory (Th1 type) cytokines TNF-α and IFN-γ in the sera. Histological examination of EAE-induced murine brains confirmed that D8 treatment inhibited immune cell infiltration into the CNS. Since eosinophils tend to appear in the lower area of the spinal cord in EAE near the cauda equina[29], it can be assumed that the reduced cellular infiltrates in EAE mice treated with D8 brains is a result of reduced T cells and monocyte infiltration into the CNS.
How might blocking the eo-2 pathway affect monocyte infiltration into the CNS? The answer is probably concealed in the complex cross-talk between different chemokines. Indeed, we found that by blocking the eo-2 pathway directly involved in eosinophil chemotaxis, the level of MCP-1, primarily involved in monocytes chemotaxis, significantly diminished. This finding is not surprising since it has been previously demonstrated that peripheral blood monocytes express and secrete both bioactive eo-2 and MCP-1 constitutively, and that both of these chemokines production in monocytes stimulated with LPS is regulated by IL-4[30]. Thus, a reciprocal regulation mechanism might exist in which the level of each of these CC chemokines might be influenced by the other.
The role of MCP-1 in EAE pathogenesis has been well established. It has been previously demonstrated that C57BL/6 MCP-1-null mice exhibit markedly reduced clinical and histological EAE after active immunization and do not develop clinical disease after receiving encephalitogenic T cells from wild-type animals. Moreover, disruption of the MCP-1 gene led to an attenuated Th1 pathogenic response and additionally increased the Th2 protective response[31].
The correlation between IL-6 and TH17 responses, in general as well as specifically in EAE, has been previously described[32,33]. Since we did not detect lower sera levels of IL-6 in D8 treated EAE mice, we do not believe that eo-2 blockade mode of action is mediated via restriction of TH17 pathogenic responses. Nevertheless, this aspect remains open and should be further investigated. Moreover, given the well recognized protective role of IL-10, TGF-β and IL-4 in EAE, as well as the putative role of the pro-inflammatory cytokines, IL-17 and IL-23, in EAE induction[34-38], the effect of eo-2 blockade on the levels of these cytokines in the sera should be further studied.
Our results imply that the main mode of action of eo-2 blockade is mediated via the restriction of cellular responses rather than affecting humoral responses. Therefore, we did not focus in this study on the effect of D8 treatment on the humoral responses and the effect of D8 on IgG sub-classes, such as IgG1 and IgG2a, remains unclear.
Taken together, although the exact mode of action of eo-2 blockade should be further characterized, our results indicate that eo-2 plays a critical role in EAE pathogenesis and that blocking the eo-2 pathway ameliorates EAE, either by direct inhibition of eosinophil infiltration into the CNS or by indirect impact on MCP-1 level, involved in monocyte infiltration into the CNS. Herein, these findings support a therapeutic potential of anti-eo-2 neutralizing antibody in EAE, as well as motivation for a continuing effort to study the role of the eo-2 pathway in MS.
Multiple sclerosis (MS) is a chronic, inflammatory, demyelinating disease that affects the central nervous system (CNS). Although it is still unclear how exactly MS initiates, it is well recognized that autoreactive T cells generated in the systemic compartment migrate into the CNS where they persist and induce an inflammatory cascade, which includes recruitment of macrophages and activation of local microglia. The recruitment of inflammatory cells into the CNS is mediated by chemokines. Eosinophil chemotactic protein 2 (eotaxin-2 or eo-2) is known to induce chemotaxis, primarily in eosinophils. Nonetheless, authors have previously demonstrated that our developed neutralizing mAb against eo-2, named D8, was effective in ameliorating other inflammatory diseases not classically eosinophil mediated, such as adjuvant-induced arthritis (AIA).
Experimental autoimmune encephalomyelitis (EAE) is the most commonly used animal model for testing new therapeutic agents in the field of MS. Progressive EAE, which resembles the progressive pattern of MS in humans, is induced by immunization of C57BL/6 mice with the autoantigen MOG35-55. The research hot spot was to examine the effect of inhibiting eo-2 with the neutralizing mAb, D8, on the development and severity of EAE.
Although eosinophils have only rarely been associated with MS pathogenesis, we have demonstrated that direct blockage of the eo-2 pathway may possess therapeutic properties in EAE. This effect was found to be mediated by restricting cell-mediated responses, i.e., reducing T cells and monocyte infiltration into the CNS, but not substantially affecting humoral responses. Restriction of cell-mediated responses may be derived from the observed reduced levels of pro-inflammatory cytokines, tumor necrosis factor (TNF)-α and interferon (IFN)-γ, as well as diminished levels of the chemokine macrophage chemoattractant protein (MCP)-1.
The results suggest that blockage of the eo-2 pathway by D8 may represent a new therapeutic strategy for MS. Moreover, these results raise the need for further research in order to gain a better insight of the role of eosinophils in MS pathogenesis.
A neutralizing antibody is an antibody which neutralizes or inhibits the biological activity of its antigen. Cell-mediated response is an immune response that does not involve antibodies but rather involves the activation of macrophages, antigen-specific T-lymphocytes and the release of various cytokines in response to an antigen. Humoral-mediated response is the aspect of immunity that is mediated by secreted antibodies.
The authors have previously shown that blocking the eo-2/CCR3 interaction by anti-eo-2 neutralizing mAb (D8) improves the therapeutic outcome of inflammatory diseases such as AIA. In this study, the authors took similar approaches to test this D8 mAb in another autoimmune model, EAE. They found that daily treatment of MOG35-55 induced-EAE mice with anti-eo-2 neutralizing mAb (D8) significantly decreased the severity of EAE in a dose-dependent manner. While D8 treated EAE mice did not show lower sera levels of anti-MOG autoantibody, they expressed lower levels of the pro-inflammatory cytokines, such as TNF-α, IFN-γ and the chemokine MCP-1, in the serum. They also found that blocking the eo-2 pathway by D8 affects the infiltration of eosinophils, monocytes and T cells into the CNS. These data are expected as the authors found similar results in AIA.
| 1. | Martin R, McFarland HF. Immunological aspects of experimental allergic encephalomyelitis and multiple sclerosis. Crit Rev Clin Lab Sci. 1995;32:121-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 297] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 2. | Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 750] [Cited by in RCA: 779] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 3. | Scolding NJ, Zajicek JP, Wood N, Compston DA. The pathogenesis of demyelinating disease. Prog Neurobiol. 1994;43:143-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Cross AH, Cannella B, Brosnan CF, Raine CS. Homing to central nervous system vasculature by antigen-specific lymphocytes. I. Localization of 14C-labeled cells during acute, chronic, and relapsing experimental allergic encephalomyelitis. Lab Invest. 1990;63:162-170. [PubMed] |
| 5. | Renno T, Krakowski M, Piccirillo C, Lin JY, Owens T. TNF-alpha expression by resident microglia and infiltrating leukocytes in the central nervous system of mice with experimental allergic encephalomyelitis. Regulation by Th1 cytokines. J Immunol. 1995;154:944-953. [PubMed] |
| 6. | Godiska R, Chantry D, Dietsch GN, Gray PW. Chemokine expression in murine experimental allergic encephalomyelitis. J Neuroimmunol. 1995;58:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 264] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 7. | Furie MB, Randolph GJ. Chemokines and tissue injury. Am J Pathol. 1995;146:1287-1301. [PubMed] |
| 8. | Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2088] [Cited by in RCA: 2051] [Article Influence: 73.3] [Reference Citation Analysis (4)] |
| 9. | Glabinski AR, Ransohoff RM. Chemokines and chemokine receptors in CNS pathology. J Neurovirol. 1999;5:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 136] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Karpus WJ, Kennedy KJ. MIP-1alpha and MCP-1 differentially regulate acute and relapsing autoimmune encephalomyelitis as well as Th1/Th2 lymphocyte differentiation. J Leukoc Biol. 1997;62:681-687. [PubMed] |
| 11. | White JR, Imburgia C, Dul E, Appelbaum E, O’Donnell K, O’Shannessy DJ, Brawner M, Fornwald J, Adamou J, Elshourbagy NA. Cloning and functional characterization of a novel human CC chemokine that binds to the CCR3 receptor and activates human eosinophils. J Leukoc Biol. 1997;62:667-675. [PubMed] |
| 12. | Patel VP, Kreider BL, Li Y, Li H, Leung K, Salcedo T, Nardelli B, Pippalla V, Gentz S, Thotakura R. Molecular and functional characterization of two novel human C-C chemokines as inhibitors of two distinct classes of myeloid progenitors. J Exp Med. 1997;185:1163-1172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 195] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Dulkys Y, Schramm G, Kimmig D, Knöss S, Weyergraf A, Kapp A, Elsner J. Detection of mRNA for eotaxin-2 and eotaxin-3 in human dermal fibroblasts and their distinct activation profile on human eosinophils. J Invest Dermatol. 2001;116:498-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Garcia G, Godot V, Humbert M. New chemokine targets for asthma therapy. Curr Allergy Asthma Rep. 2005;5:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Arend WP. Physiology of cytokine pathways in rheumatoid arthritis. Arthritis Rheum. 2001;45:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Kitaura M, Nakajima T, Imai T, Harada S, Combadiere C, Tiffany HL, Murphy PM, Yoshie O. Molecular cloning of human eotaxin, an eosinophil-selective CC chemokine, and identification of a specific eosinophil eotaxin receptor, CC chemokine receptor 3. J Biol Chem. 1996;271:7725-7730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 337] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 17. | Combadiere C, Ahuja SK, Murphy PM. Cloning and functional expression of a human eosinophil CC chemokine receptor. J Biol Chem. 1995;270:16491-16494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 189] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Romagnani P, De Paulis A, Beltrame C, Annunziato F, Dente V, Maggi E, Romagnani S, Marone G. Tryptase-chymase double-positive human mast cells express the eotaxin receptor CCR3 and are attracted by CCR3-binding chemokines. Am J Pathol. 1999;155:1195-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 163] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875-883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1218] [Cited by in RCA: 1223] [Article Influence: 43.7] [Reference Citation Analysis (18)] |
| 20. | Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005-2007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 808] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 21. | Beaulieu S, Robbiani DF, Du X, Rodrigues E, Ignatius R, Wei Y, Ponath P, Young JW, Pope M, Steinman RM. Expression of a functional eotaxin (CC chemokine ligand 11) receptor CCR3 by human dendritic cells. J Immunol. 2002;169:2925-2936. [PubMed] |
| 22. | Simpson J, Rezaie P, Newcombe J, Cuzner ML, Male D, Woodroofe MN. Expression of the beta-chemokine receptors CCR2, CCR3 and CCR5 in multiple sclerosis central nervous system tissue. J Neuroimmunol. 2000;108:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 154] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Ablin JN, Entin-Meer M, Aloush V, Oren S, Elkayam O, George J, Barshack I. Protective effect of eotaxin-2 inhibition in adjuvant-induced arthritis. Clin Exp Immunol. 2010;161:276-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Montero E, Nussbaum G, Kaye JF, Perez R, Lage A, Ben-Nun A, Cohen IR. Regulation of experimental autoimmune encephalomyelitis by CD4+, CD25+ and CD8+ T cells: analysis using depleting antibodies. J Autoimmun. 2004;23:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Bernard CC, Johns TG, Slavin A, Ichikawa M, Ewing C, Liu J, Bettadapura J. Myelin oligodendrocyte glycoprotein: a novel candidate autoantigen in multiple sclerosis. J Mol Med (. Berl). 1997;75:77-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 198] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Linington C, Bradl M, Lassmann H, Brunner C, Vass K. Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am J Pathol. 1988;130:443-454. [PubMed] |
| 27. | Snead OC, Kalavsky SM. Cerebrospinal fluid eosinophilia. A manifestation of a disorder resembling multiple sclerosis in childhood. J Pediatr. 1976;89:83-84. [PubMed] |
| 28. | Tanphaichitr K. Multiple sclerosis associated with eosinophilic vasculitis, pericarditis, and hypocomplementemia. Arch Neurol. 1980;37:314-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Gladue RP, Carroll LA, Milici AJ, Scampoli DN, Stukenbrok HA, Pettipher ER, Salter ED, Contillo L, Showell HJ. Inhibition of leukotriene B4-receptor interaction suppresses eosinophil infiltration and disease pathology in a murine model of experimental allergic encephalomyelitis. J Exp Med. 1996;183:1893-1898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Watanabe K, Jose PJ, Rankin SM. Eotaxin-2 generation is differentially regulated by lipopolysaccharide and IL-4 in monocytes and macrophages. J Immunol. 2002;168:1911-1918. [PubMed] |
| 31. | Huang DR, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med. 2001;193:713-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 477] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 32. | Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5130] [Cited by in RCA: 5543] [Article Influence: 277.2] [Reference Citation Analysis (0)] |
| 33. | Serada S, Fujimoto M, Mihara M, Koike N, Ohsugi Y, Nomura S, Yoshida H, Nishikawa T, Terabe F, Ohkawara T. IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2008;105:9041-9046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 283] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 34. | Bettelli E, Das MP, Howard ED, Weiner HL, Sobel RA, Kuchroo VK. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J Immunol. 1998;161:3299-3306. [PubMed] |
| 35. | Racke MK, Dhib-Jalbut S, Cannella B, Albert PS, Raine CS, McFarlin DE. Prevention and treatment of chronic relapsing experimental allergic encephalomyelitis by transforming growth factor-beta 1. J Immunol. 1991;146:3012-3017. [PubMed] |
| 36. | Ponomarev ED, Maresz K, Tan Y, Dittel BN. CNS-derived interleukin-4 is essential for the regulation of autoimmune inflammation and induces a state of alternative activation in microglial cells. J Neurosci. 2007;27:10714-10721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 333] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
P- Reviewer Azizul Haque S- Editor Cheng JX L- Editor A E- Editor Lu YJ